Document 147944
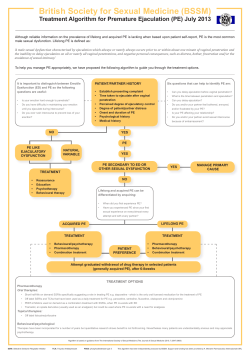
Blackwell Science, LtdOxford, UKJSMJournal of Sexual Medicine1743-6095Journal of Sexual Medicine 2005 20052S2103109Original ArticlePremature Ejaculation: The Physician’s PerspectiveSharlip 103 Diagnosis and Treatment of Premature Ejaculation: The Physician’s Perspective Ira Sharlip, MD University of California San Francisco, San Francisco, CA, USA Corresponding Author: Ira Sharlip, MD, 2100 Webster Street, Suite 222, San Francisco, CA 94115, USA. Tel: 415202-0250; E-mail: [email protected] ABSTRACT Premature ejaculation (PE) is a common condition associated with significant adverse effects on the sexual and overall quality of life of men with this condition. Behavioral therapies, such as the “squeeze” and “stop–start” techniques, and psychotherapy, have been the mainstay of PE management for many years. However, evidence of their short-term efficacy is limited while support for their long-term benefit is lacking. There are currently no medications licensed specifically for the treatment of PE. This paucity of pharmacological treatment may, in turn, contribute to the absence of systematic procedures for the identification, evaluation, and treatment of PE patients. Current “off-label” pharmacotherapeutic approaches include topical anesthetics, phosphodiesterase-5 inhibitors, and serotonin reuptake inhibitors. Of these, the serotonin reuptake inhibitors show the greatest efficacy and an increasing body of evidence is illuminating their mode of action. Nevertheless, all current “off-label” pharmacotherapeutic approaches fall short of the ideal therapy for PE. In the absence of a cure, such a treatment should be tolerable, inconspicuously used, effective from first dose, rapid in onset of action, and available as a prn-dosing regimen. It is anticipated that agents in development for the specific indication of PE will come closer to this ideal than existing pharmacotherapeutic approaches. Key Words. Premature Ejaculation; Intravaginal Ejaculatory Latency Time; Seretonin; Phosphodiesterase-5 (PDE-5) Inhibitors Introduction Evaluation of Premature Ejaculation C The lack of a universally accepted clinical definition of PE has impeded the development of systematic strategies for the identification and treatment of the condition. Indeed, there is even confusion over which healthcare practitioners are most appropriate to conduct patient evaluation and treatment. For instance, many specialties, including primary care physicians, urologists, psychiatrists, and psychologists, come into contact with potential PE patients, and all of these practitioners are therefore well placed to help manage the condition. This, in turn, raises the question of whether practitioners should proactively seek identification of the condition. For example, should primary care physicians ask urrently, strategies for the diagnosis and treatment of premature ejaculation (PE) are not widely understood or used. The American Urological Association (AUA) has recently published its guideline on the pharmacologic management of PE [1]. Most patients remain unaware that PE is a medical condition, while many physicians remain unaware of the available treatment approaches. This article provides a perspective on how the diagnosis and treatment of PE can be improved. It discusses the ways in which the condition can be identified, provide an overview of the currently available treatment approaches and discusses some of the characteristics sought in the ideal pharmacotherapy. J Sex Med 2005; Supplement 2 104 about PE, or should they wait until the patient presents with the problem? While there is no definitive answer to this question at the moment, it is very probable that, as more effective therapies become available, the number of patients seeking help will increase. There are several considerations in ensuring that an accurate evaluation and diagnosis is performed. Of primary importance is the differentiation of PE from other sexual dysfunctions. For instance, it is common for patients with ED to present with the chief complaint of PE when the actual problem is the patient’s haste to achieve orgasm before the failure of an erection. In these cases, successful treatment for ED may often resolve the secondary PE [2]. It is also important to understand that there are links between sexual function, overall health, and quality of life [3–5]. This is particularly true for PE because it can have a major impact on the sexual and overall quality of life of both the patient and his partner. Establishing an accurate sexual and psychosocial history is critical in identifying the etiology of PE and establishing an effective treatment regimen. There are several key areas that should be addressed when taking the history of a patient with possible PE. For instance, it is extremely important to establish the level of distress experienced by both the patient and his partner. Indeed, this is a central component of current diagnostic criteria [2,6]. A second important area of discussion is the patient’s perception of ejaculatory control. Specifically, it is important to determine the extent to which the patient can exert voluntary control over the ejaculation. It may also be helpful to ask patients for an estimate of their intravaginal ejaculatory latency time (IELT). While this may not be accurate, and may differ significantly from the partner’s estimate, it can provide important insights into the patient’s perception of the severity of the problem. Questions about the onset and duration of PE can help to establish whether the condition is primary or secondary. In this regard, psychosocial aspects of the patient history are very important, particularly if there is a history of childhood physical or sexual abuse. Such a history may indicate the need for psychotherapy as part of the treatment regimen. A comprehensive medical history is equally important in the evaluation of PE because of the established relationship between some medical conditions, such as diabetes and other neuropathies, with ejaculatory dysfunction [7,8]. Furthermore, PE is an acknowledged consequence of J Sex Med 2005; Supplement 2 Sharlip substance abuse, particularly during withdrawal from opiates [9,10]. Current Treatment Approaches for Premature Ejaculation Current treatment approaches for PE can be divided into two broad categories: psychotherapeutic/behavioral and pharmacotherapeutic (Table 1). Two types of behavioral therapy have been widely advocated: the “stop–start” and “squeeze” techniques. There are currently no pharmacotherapies specifically licensed for the treatment of PE, but a number of agents have been used “off label” to treat the condition. These pharmacotherapeutic approaches can be divided into three groups: topical agents; phosphodiesterase5 (PDE-5) inhibitors; and serotonin reuptake inhibitors (SRIs). Behavioral Therapy and Psychotherapy Behavioral therapy has long been a mainstay of treatment for PE and remains, in the absence of anything better, a common approach today. Part of the popularity of this approach is due to the paucity of other effective therapies. Semans was the first to advocate the squeeze technique in a report published in 1956 [11], but it was more extensively popularized in the 1970s by Masters and Johnson’s report [12]. This technique involves withdrawal of the penis during intercourse and prior to the moment of ejaculatory inevitability. The sexual partner is instructed to give a very sharp and hard squeeze to the glans penis to abort the ejaculation. However, many practitioners and patients report that this technique is unpractical. The stop–start technique has been used, with variations, for some three decades and was particularly popularized by Kaplan in 1983 [13]. This method requires the man to pause during sexual stimulation, just prior to impending ejaculation. The pause allows the patient to acclimatize to the sensation and eventually to condition himself to increased ejaculatory control. This technique may Table 1 Current treatment options for premature ejaculation Behavioral techniques Pharmacotherapies Squeeze technique [11,12] Stop–start technique [13] Topical local anesthetics Phosphodiesterase-5 (PDE-5) inhibitors Selective serotonin reuptake inhibitors (SSRIs) Psychotherapy Premature Ejaculation: The Physician’s Perspective be effective for two reasons: it heightens a man’s awareness of his sexual sensations and decreases the emphasis on coitus as the all-important activity during sexual interaction. Psychotherapy can be effective in some men, with or without the use of behavioral therapy. Some patients can learn to modulate their psychosexual responses to sexual stimulation and gain some conscious control of their ejaculatory reflexes. While behavioral and/or psychotherapeutic approaches are commonly used, they are associated with significant drawbacks. For obvious reasons, they work best in a stable relationship, as they require the committed assistance and understanding of the partner. Time must also be given to learning and using these approaches, and the cost of the psychotherapy, with the accompanying instruction, is often substantial. Furthermore, behavioral techniques are slow to be effective and, even so, there are mixed reports of efficacy: Clarke and Parry reported that 60% of men using the squeeze technique received benefit in the short term [14], while De Amicis et al. found that any success achieved by behavioral therapy was not maintained at 3-year follow-up [15]. Current Pharmacotherapeutic Options Local anesthetics have been a commonly used pharmacotherapeutic option for PE. Anesthetics such as the lidocaine–prilocaine combination and herbal preparations such as the Korean product known as SS cream are applied to the glans penis and can diminish sensitivity and delay the point of ejaculation. SS cream has been reported to improve ejaculatory control in a high percentage of patients [16]. However, there are several drawbacks that make local anesthetics a less than ideal therapeutic option. Local irritation can occur with herbal preparations [17] and significant penile hypoesthesia may occur. This latter effect can be severe enough to prevent the patient from achieving an orgasm [18]. Additionally, there are reports that the use of local anesthetics can induce mild erectile dysfunction and lowering of sexual arousal [19]. There is also a potential for transvaginal absorption of the drugs, which could induce vaginal numbness and even female anorgasmia, although these problems can be prevented with condom use [18]. Phosphodiesterase-5 inhibitors are widely used for the treatment of erectile dysfunction [20] and have also been tested in clinical studies for the treatment of PE. However, to date, there is little 105 convincing evidence to show that PDE-5 inhibition significantly increases IELT [21–23]. Furthermore, the studies of PDE-5 inhibitors in PE have used uncontrolled, open-label protocols using self-diagnosed patients and IELT estimates gathered through patient recall. A mechanism of action for a delay of ejaculation by PDE-5 inhibition has not been identified. The third class of widely used pharmacotherapeutic agents is the SRIs. The potential of antidepressants to treat PE was first suggested by Ahlenius et al. in 1979 [24]. This study showed that the tricyclic antidepressant, clomipramine (a nonselective SRI), prolonged ejaculatory latency in rats by blocking central serotonin reuptake [24] and were shortly followed by the appearance of clinical studies on the efficacy of clomipramine in PE [25,26]. However, the use of clomipramine was associated with a high incidence of adverse events, including anticholinergic effects, reduced sexual desire, and genital anesthesia [26,27]. There is now considerable neurophysiological and neuropharmacological evidence to suggest how inhibition of 5-HT uptake may delay ejaculation. The medial preoptic area (MPOA) and paraventricular nucleus (PVN) of the hypothalamus play essential roles in the integration of sexual responses in men [28,29]. Both MPOA and PVN send efferent projections to the serotonergic nucleus paragigantocellularis (nPGi) in the brainstem (Figure 1). Serotonergic projections from the nPGi exert a tonic inhibition of ejaculation via the lumbosacral motor nuclei in the spinal cord [30] making the nPGi a pivotal nucleus in the central control of ejaculation. Nevertheless, the way in which 5-HT influences the ejaculatory pathway is complex and incompletely understood. Some 14 different 5-HT receptor subtypes have been identified [31]. Of the serotonergic processes studied to date, the activity of the 5-HT1A, 5-HT2C receptors, and the serotonin transporter (5-HTT) appear to be most important for the modulation of ejaculatory function and may be appropriate pharmacotherapeutic targets for PE. The 5-HT1A receptor is principally a presynaptic (somatodendritic) autoreceptor that, when stimulated, reduces the rate of the firing of 5-HT neurones [32]. Acute administration of selective serotonin reuptake inhibitors (SSRIs) blocks the 5-HTT, leading to elevated extracellular 5-HT levels, and consequently increased activation of the 5-HT1A autoreceptor. Thus, the rate of firing of 5-HT neurones decreases, as does the release of 5-HT into the J Sex Med 2005; Supplement 2 106 Sharlip Figure 1 Central 5-HT function and premature ejaculation. Ejaculation is determined by the complex interplay of local and distal influences. Although ejaculation is a spinal sympathetic reflex generated in the lumbosacral spinal cord, it is influenced by genital sensory input and by tonic descending inhibitory serotonergic control from the nucleus paragigantocellularis (nPGi). The nPGi receives modulatory influences from the medial preoptic area and paraventricular nucleus of the hypothalamus. synaptic cleft, although this effect is counterbalanced by the ongoing blockade of 5-HTT, which allows released 5-HT to remain longer in the extracellular. The net effect is either neutral or a mild increase of 5-HT neurotransmission and a small upregulation of stimulation of postsynaptic 5-HT receptors [33]. The effects of chronic SSRI treatment are markedly different (Figure 2). The chronic blockade of 5-HTT and the resultant persistence of elevated levels of extracellular 5-HT lead to a desensitization of the 5-HT1A autoreceptor. The combined effect of this desensitization and the continued blockade of 5-HTT is to markedly Figure 2 Proposed chronic action of SSRIs on 5-HT transmission. Schematic description of the chronological sequence of neurochemical events involved in the enhancement of serotonergic neurotransmission by chronic SSRI administration. (Adapted from McMahon and Samali, 1999) [18]. J Sex Med 2005; Supplement 2 disinhibit the firing of 5-HT neurones. Thus, 5-HT neurotransmission is strongly enhanced [30,33]. The mechanism described above explains not only why chronic SSRI administration is more effective for anti-PE and antidepressant activity but also why SSRIs show limited acute activity to delay ejaculation. Interestingly, Waldinger’s group have compared the pharmacology of the SSRIs and selective 5-HT receptor agonists on ejaculatory function, concluding that the characteristics of SSRIs most closely resemble those of 5-HT2C agonists [30,33]. Evidence that this receptor subtype plays a key role in ejaculation comes from two key animal studies. Ahlenius et al. showed that nonselective 5-HT2C agonists delay ejaculation in rats, while a selective 5-HT2A agonist did not [34]. Subsequently, Foreman et al. showed that 1(2,5-dimethoxy-4 -iodophenyl)-2-aminopropane, a nonselective 5-HT2 agonist that stimulates both 5-HT2A and 5-HT2C, also delays ejaculation in rats [35]. There is evidence too of a functional interaction between 5-HT2C and 5-HT1A receptors as the behavioral response to activation of 5-HT1A agonists in rats is attenuated or completely abolished by the coactivation of 5-HT2C receptors [36]. Based on this evidence, Waldinger et al. have hypothesized that PE may be a result of a hypersensitive 5-HT1A receptor system and/or a hyposensitive 5-HT2C system and that the action of SSRIs may restore a balance between these two systems [30,33]. Following the introduction of the nonselective serotonin reuptake inhibitor clomipramine as an Premature Ejaculation: The Physician’s Perspective IELT (minutes) antidepressant, more tolerable SSRIs were developed and since the late 1980s, five SSRIs have been licensed for the treatment of depression: citalopram, paroxetine, sertraline, fluoxetine, and fluvoxamine. Waldinger et al. reported the first trial of an SSRI for PE in 1994 [37]. This randomized, double-blind, placebo-controlled study found that paroxetine significantly improved PE. Shortly thereafter, Mendels et al. described the first use of sertraline in PE in 1995 showing that sertraline increased IELT in men with PE [38]. Figure 3 shows the results of another study of sertraline in men with PE [39]. Like most of the studies of SSRIs in PE, the protocol was based on chronic, rather than prn dosing. The study enrolled 37 men with PE and randomized them in a crossover fashion to receive either placebo or sertraline (50 mg/day). At baseline the mean IELT of the men was less than half a minute. Over a period of 1–3 weeks after initiation of therapy, IELT increased to the range of 3 minutes. There was a decrease in IELT on termination of treatment and after approximately 4 weeks mean IELT was similar to pretreatment baseline. Despite a minor placebo response rate, the effect of sertraline was clearly significant (P < 0.001 at 4 weeks vs. placebo). In the crossover phase of the trial, after the 4-week washout period, the effects of sertraline mirrored the first round of therapy [39]. Evidence from a comparative randomized, double-blind, placebo-controlled trial shows that there is considerable variation in the efficacy of SSRIs in the treatment of PE [40]. The trial Time (weeks) Figure 3 Time-dependent effect of sertraline on ejaculatory latency in men with premature ejaculation. Effects of daily sertraline (50 mg/day) or placebo on intravaginal ejaculatory latency time (IELT) in minutes during 4 week dosing periods. The arrow shows the peak response, at 4 weeks, in the group given sertraline first. (Data from McMahon et al., 1998) [39]. 107 compared the efficacy of fluoxetine, fluvoxamine, paroxetine, and sertraline in 60 men with PE. At baseline, the mean IELT was approximately 20 seconds. As with the sertraline trial mentioned above, a small placebo response rate was observed. However, after 6 weeks of treatment fluoxetine, paroxetine, and sertraline all increased the mean IELT above this placebo level significantly while fluvoxamine did not. In this study, paroxetine was by far the most effective SSRI, for which the mean IELT increased from a baseline of approximately 30 seconds to over 450 seconds after 6 weeks. Apart from the variability in efficacy, there are other drawbacks with the existing SSRIs, not least that these agents are associated with adverse events such as nausea, drowsiness, cognitive impairments, and sexual side-effects including abnormal ejaculation, decreased libido, male and female sexual dysfunction, and menstrual disorders [18]. Furthermore, to work optimally, they must be administered over periods of weeks (see Figure 3). Waldinger et al. emphasized the importance of chronic administration in a comparative trial of clomipramine and paroxetine [41]. The study enrolled 30 men with PE and showed that, while on-demand treatment with clomipramine (25 mg) led to a clinically significant 4.05-fold increase [95% confidence intervals (CI): 3.26– 5.02] in IELT, a similar regimen for paroxetine (20 mg) led to a clinically meaningless 1.41-fold increase (95% CI: 1.22–1.63) in IELT. Furthermore, both therapies were associated with “mostly mild, but annoying non-sexual side-effects” [41]. A further drawback of the slow onset of action of SSRIs and relatively long half-life of these agents is the risk of accumulation and an exacerbation of SSRI-related adverse events [18]. These shortcomings emphasize the need for the development of therapies more specifically tailored for the treatment of PE. Such a therapy would be an oral formulation that is effective from the first dose. It would have a rapid onset of action and pharmacological half-life commensurate with the purpose recognized by the patient; that is the time from the beginning of sexual interest through to the conclusion of mutually satisfying sexual intercourse. Equally important, the therapy should have a low incidence of adverse events. Although a drug matching this profile is not currently available, there are candidates in the pipeline that have the potential to meet these therapeutic requirements more closely. J Sex Med 2005; Supplement 2 108 Sharlip Conclusions Behavioral techniques and psychotherapy have dominated the therapeutic approach to PE for many years, however these methods can be cumbersome and expensive, with limited long-term efficacy. The dawning of the pharmacological age of PE treatment offers hope for the many men who suffer from PE. However, maximum patient benefit will only come from improvements in the pharmacotherapy of PE if they are coupled with the development of systematic approaches to the identification and evaluation of patients with the condition. Current diagnostic criteria for PE are inconsistent. Similarly, optimum methods of evaluation and treatment have only recently been recommended [1]. Current pharmacotherapies offer some degree of efficacy for men with PE, but are far from ideal. Their clinical utility is limited by several factors, including a significant incidence of adverse events. The ideal agent for the treatment of PE would be oral, well tolerated, effective from first dose and available as a prn-dosing regimen. Agents currently in development may come closer to the ideal therapy for PE. Such tailored therapies will be a welcome addition to the armamentarium available to healthcare professionals and, most of all, to men suffering from PE and their partners. References 1 Montague DK, Jarow J, Broderick GA, Dmochowski RR, Heaton JP, Lue TF, Nehra A, Sharlip ID, AUA Erectile Dysfunction Guideline Update Panel. AUA guideline on the pharmacologic management of premature ejaculation. J Urol 2004;172:290–4. 2 American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Text revision. Washington, DC: American Psychiatric Association; 2000. 3 McCabe MP. Intimacy and quality of life among sexually dysfunctional men and women. J Sex Marital Ther 1997;23:276–90. 4 Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. JAMA 1999;281:537–44. 5 Symonds T, Roblin D, Hart K, Althof S. How does premature ejaculation impact a mans life? J Sex Marital Ther 2003;29:361–70. 6 World Health Organization. International classification of diseases and related health problems. Geneva: World Health Organization; 1994. 7 Screponi E, Carosa E, Di Stasi SM, Pepe M, Carruba G, Jannini EA. Prevalence of chronic prosJ Sex Med 2005; Supplement 2 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 tatitis in men with premature ejaculation. Urology 2001;58:198–202. El-Sakka AI. Premature ejaculation in non-insulindependent diabetic patients. Int J Androl 2003;26: 329–34. Metz ME, Pryor JL. Premature ejaculation: A psychophysiological approach for assessment and management. J Sex Marital Ther 2000;26:293–320. Clayton DO, Shen WW. Psychotropic druginduced sexual function disorders: Diagnosis, incidence and management. Drug Saf 1998;19:299–312. Semans JH. Premature ejaculation: A new approach. South Med J 1956;49:353–8. Masters W, Johnson V. Premature ejaculation. In: Masters W, Johnson V, eds. Human sexual inadequacy. Boston, MA: Little Brown & Co; 1970. Kaplan H. The evaluation of sexual disorders: Psychological and medical aspects. New York, NY: Taylor Francis; 1983. Clarke M, Parry L. Premature ejaculation treated by the dual sex team method of Masters and Johnson. Aust N Z J Psychiatry 1973;7:200–5. De Amicis LA, Goldberg DC, LoPiccolo J, Friedman J, Davies L. Clinical follow-up of couples treated for sexual dysfunction. Arch Sex Behav 1985;14:467–89. Xin Z, Choi Y, Lee S, Choi H. Efficacy of a topical agent SS-cream in the treatment of premature ejaculation: Preliminary clinical studies. Yonsei Med J 1997;38:91–5. Choi HK, Jung GW, Moon KH, Xin ZC, Choi YD, Lee WH, Rha KH, Choi YJ, Kim DK. Clinical study of SS-cream in patients with lifelong premature ejaculation. Urology 2000;55:257–61. McMahon CG, Samali R. Pharmacological treatment of premature ejaculation. Curr Opin Urol 1999;9:553–61. Slob A, van Berke A, van der Werff ten Bosch J. Premature ejaculation treated by local penile anaesthesia in an uncontrolled clinical replication study. J Sex Res 2000;37:244–7. Stief CG. Phosphodiesterase inhibitors in the treatment of erectile dysfunction. Drugs Today (Barc) 2000;36:93–9. Aversa A, Mazzilli F, Rossi T, Delfino M, Isidori AM, Fabbri A. Effects of sildenafil (Viagra) administration on seminal parameters and postejaculatory refractory time in normal males. Hum Reprod 2000;15:131–4. Mondaini N, Ponchietti R, Muir GH, Montorsi F, Di Loro F, Lombardi G, Rizzo M. Sildenafil does not improve sexual function in men without erectile dysfunction but does reduce the postorgasmic refractory time. Int J Impot Res 2003;15:225–8. Abdel-Hamid IA. Phosphodiesterase 5 inhibitors in rapid ejaculation: Potential use and possible mechanisms of action. Drugs 2004;64:13–26. Ahlenius S, Heimann M, Larsson K. Prolongation of the ejaculation latency in the male rat by thior- Premature Ejaculation: The Physician’s Perspective 25 26 27 28 29 30 31 32 33 idazine and chlorimipramine. Psychopharmacology (Berl) 1979;65:137–40. Goodman RE. An assessment of clomipramine (Anafranil) in the treatment of premature ejaculation. J Int Med Res 1980;8(3 suppl):53–9. Girgis SM, El-Haggar S, El-Hermouzy S. A double-blind trial of clomipramine in premature ejaculation. Andrologia 1982;14:364–8. Kim SC, Seo KK. Efficacy and safety of fluoxetine, sertraline and clomipramine in patients with premature ejaculation: A double-blind, placebo controlled study. J Urol 1998;159:425–7. Meisel R, Sachs B. The physiology of male sexual behavior. In: Knobil E, Neill J, Greenwald G, Markert C, Pfaff D, eds. The physiology of reproduction. Vol. 3-107. New York: Raven; 1997. Bancila M, Giuliano F, Rampin O, Mailiy P, Brisorgueil MJ, Calas A, Verge D. Evidence for a direct projection from the paraventricular nucleus of the hypothalamus to putative serotoninergic neurons of the nucleus paragigantocellularis involved in the control of erection in rats. Eur J Neurosci 2002;16:1240–8. Waldinger MD. The neurobiological approach to premature ejaculation. J Urol 2002;168:2359– 67. Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 1999;38:1083–152. Stamford JA, Davidson C, McLaughlin DP, Hopwood SE. Control of dorsal raphe 5-HT function by multiple 5-HT(1) autoreceptors: Parallel purposes or pointless plurality? Trends Neurosci 2000;23:459–65. Waldinger MD, Berendsen HH, Blok BF, Olivier B, Holstege G. Premature ejaculation and serotonergic antidepressants-induced delayed ejaculation: 34 35 36 37 38 39 40 41 109 The involvement of the serotonergic system. Behav Brain Res 1998;92:111–8. Ahlenius S, Larsson K, Svensson L, Hjorth S, Carlsson A, Lindberg P, Wilkstrom H, Sanchez D, Arvidsson LE, Hacksell U, Nilsson JL. Effects of a new type of 5-HT receptor agonist on male rat sexual behavior. Pharmacol Biochem Behav 1981;15:785–92. Foreman MM, Hall JL, Love RL. The role of the 5-HT2 receptor in the regulation of sexual performance of male rats. Life Sci 1989;45:1263–70. Berendsen HH, Broekkamp CL. Behavioural evidence for functional interactions between 5-HTreceptor subtypes in rats and mice. Br J Pharmacol 1990;101:667–73. Waldinger MD, Hengeveld MW, Zwinderman AH. Paroxetine treatment of premature ejaculation: A double-blind, randomized, placebo-controlled study. Am J Psychiatry 1994;151:1377–9. Mendels J, Camera A, Sikes C. Sertraline treatment for premature ejaculation. J Clin Psychopharmacol 1995;15:341–6. McMahon CG. Treatment of premature ejaculation with sertraline hydrochloride: A single-blind placebo controlled crossover study. J Urol 1998; 159:1935–8. Waldinger MD, Hengeveld MW, Zwinderman AH, Olivier B. Effect of SSRI antidepressants on ejaculation: A double-blind, randomized, placebocontrolled study with fluoxetine, fluvoxamine, paroxetine, and sertraline. J Clin Psychopharmacol 1998b;18:274–81. Waldinger MD, Zwinderman AH, Olivier B. On-demand treatment of premature ejaculation with clomipramine and paroxetine: A randomized, double-blind fixed-dose study with stopwatch assessment. Eur Urol 2004;46:510–15; discussion 516. J Sex Med 2005; Supplement 2
© Copyright 2026