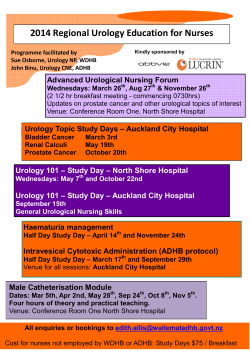
PRIMARY CARE urology for A Manual of Common Urological Conditions
urology for PRIMARY CARE A Manual of Common Urological Conditions Encountered by the Primary Care Physician Edited by Richard S. Pelman, M.D. Urology for Primary Care A Manual of Common Urological Conditions Encountered by the Primary Care Physician Edited by Richard S. Pelman, M.D. verathon.com This document, or parts hereof, may not be reproduced in any form without written permission from the author. This material was compiled by Dr. Richard Pelman and Verathon Inc. You may not resell, rent, lease, or otherwise distribute the materials in this manual, in whole or in part, in any form whether by itself (altered or unaltered) or as part of another collection. The above shall be governed by the copyright laws of the United States of America. 2007, 2010 Verathon Inc. All rights reserved. Urology for Primary Care The team at Verathon is committed to modernizing health care delivery by “Putting Patients First.” Our products support you, the health care provider, by providing the highest level of accuracy, utility, and excellence. Please contact us directly at 1.800.331.2313 (USA and Canada only) or 1.425.867.1348, if we can improve our service to you. Legal Statement Copyright© 2007, 2010 Verathon Inc. All rights reserved. This material was compiled by Dr. Richard Pelman and Verathon Inc. This manual may not be copied without the express written consent of Verathon. BladderScan, NeuralHarmonics, ScanPoint, Vmode, Verathon, and the Verathon Torch symbol are trademarks of Verathon Inc. The BladderScan technology referenced in this manual is protected by U.S. Patent Numbers 5,235,985, 6,676,605, 6,884,217 and 6,905,468. ScanPoint technology is protected by U.S. Patent Number 6,569,097. Other patents pending. Verathon Inc. 20001 North Creek Parkway Bothell, WA 98011 USA 800.331.2313 (US and Canada) 425.867.1348 Fax: 425.883.2896 www.verathon.com EU Representative Verathon Medical (Europe) B.V. Linnaeusweg 11 3401 MS IJsselstein The Netherlands +31.30.68.70.570 Fax: +31.30.68.70.512 0900-0608-10-86 Verathon Inc. 800.331.2313 www.verathon.com 2 Urology for Primary Care TABLE OF CONTENTS Foreword by Richard S. Pelman, M.D. About the Contributors Section 1 Introduction by Richard S. Pelman, M.D. Section 2 Bladder Outlet Obstruction by Richard S. Pelman, M.D. Section 3 Prostatitis by Richard S. Pelman, M.D. Section 4 Overactive Bladder by Richard S. Pelman, M.D. Section 5 The Aging Male by Mike B. Siroky, M.D. Section 6 Initial Evaluation of Hematuria for Primary Care Physicians by David S. Wang, M.D. and Richard K. Babayan, M.D. Section 7 Initial Management of Urinary Calculus Disease for Primary Care Physicians by David S. Wang, M.D. and Richard K. Babayan, M.D. Section 8 Sexual Medicine for Men: Male Erectile Dysfunction by Irwin Goldstein, M.D. Section 9 Sexual Medicine for Women: Management of Women with Desire, Arousal and Orgasm Sexual Health Concerns by Irwin Goldstein, M.D. Verathon Inc. 800.331.2313 www.verathon.com 3 Urology for Primary Care Section 10 Management of Uncomplicated Urinary Tract Infections by J. Curtis Nickel, M.D., FRCSC Section 11 Neonatal Circumcision: An American Enigma by Martin A. Koyle, M.D., FAAP, FACS Section 12 A Primer for Pediatric Urological Referrals From Primary Care Providers by Martin A. Koyle, M.D., FAAP, FACS Section 13 In Service Training: BladderScan Bladder Volume Instrument Use Appendices Appendix A: AUA Guideline Flow Diagram for Diagnosis and Treatment of BPH Appendix B: American Urological Association Symptoms Score Appendix C: Incontinence Questionnaire (UDI-6) Appendix D: Flow Rate Nomongrams Appendix E: Categories for Prostatitis Appendix F: Voiding Diary Appendix G: Frequency Volume Charts Bibliography Verathon Inc. 800.331.2313 www.verathon.com 4 Urology for Primary Care FOREWORD My purpose in writing a Urology manual for Primary Care physicians is to continue fulfilling my role as a consultant and educator. In addition to being available for referrals and providing definitive opinions about urinary disease, Urologists today have another goal: To serve as educators, both for our patients and for the wider medical community. Over the last decade, Primary Care physicians have been offered an increasing array of pharmacological options to treat various urological diseases; however, they may not have had the opportunity to receive focused training about these diseases. Consequently, through the experience of practice they have acquired the necessary knowledge to initiate diagnosis, and in some cases treatment, of these diseases. Yet Primary Care physicians may not be as familiar as Urologists with the supporting “tools of the trade,” and are thus unable to utilize as broad a database for decision-making. This manual should help define some of the Urologist’s tools for Primary Care physicians. The topics in this edition include Bladder Outlet Obstruction; Prostatitis; Overactive Bladder; The Aging Male; Hematuria; Urinary Stone Disease; Sexual Medicine for Men: Male Erectile Dysfunction; Sexual Medicine for Women: Management of Women with Desire, Arousal, and Orgasm Sexual Health Concerns; and Management of Uncomplicated Urinary Tract Infections. Future chapters will discuss subjects such as Pediatric Urology. It is my sincere hope that Primary Care physicians will find “Urology for Primary Care” to be of practical use. Richard S. Pelman, M.D. Associate of Bellevue Urology Associates Inc., Bellevue, Washington Clinical Professor of Urology, University of Washington School of Medicine Verathon Inc. 800.331.2313 www.verathon.com 5 Urology for Primary Care ABOUT THE CONTRIBUTORS Richard K. Babayan, M.D. Dr. Babayan is Professor and Chairman of the Department of Urology at Boston University School of Medicine (BUSM) and Chief of Urology at Boston Medical Center (BMC). Dr. Babayan is a graduate of Indiana University School of Medicine. He received his surgical training at Yale-New Haven Hospital, and he subsequently completed a Urology residency at Boston University Medical Center in 1980. From 1980 to 1982, he was an American Urological Association Research Scholar, performing basic science research in the field of hyperthermia at both MIT and BUSM. Dr. Babayan is a founding member of the Endourological Society and has been actively involved in minimally invasive therapies within the field of Urology. His clinical interests center around BPH, prostate cancer and urologic oncology, and endourology. He is currently one of two urologic surgeons at BMC using the daVinci Robot for robotic-assisted laparoscopic radical prostatectomy and robotic-assisted pyeloplasty. Dr. Babayan is actively involved in local and national urologic organizations. He is currently the New England Section representative to the Board of Directors of the American Urological Association. Dr. Babayan is a past president of the New England Section of the AUA and is a member of the Board of Directors of the Massachusetts Association of Practicing Urologists. Irwin Goldstein, M.D. Dr. Goldstein was on the faculty of Boston University School of Medicine (BUSM) for twentyfive years, where he was professor of Urology and Gynecology. He is founder and former director of the Institute for Sexual Medicine at BUSM. He holds a bachelor’s degree in Engineering from Brown University, with an honors thesis in Biomedical Engineering. In 1975, he graduated from McGill University Faculty of Medicine in his hometown of Montreal, Quebec, Canada. He has been involved with sexual dysfunction research since the late 1970's. Dr. Goldstein's interests include penile microvascular bypass surgery, surgery for dyspareunia, physiologic investigation of sexual function in men and women, and diagnosis and treatment of sexual dysfunction in men and women. He has authored more than three hundred publications in the field of sexual dysfunction, and his research in this area was funded by the National Institutes of Health for twenty years. Dr. Goldstein is editor-in-chief of The Journal of Sexual Medicine, the official journal of The International Society for Sexual Medicine and its regional affiliate societies. He is secretary of the International Society for the Study of Women’s Sexual Health, past president of the Sexual Medicine Society of North America, member of the executive board of the International Society for Sexual Medicine, a member of the International Academy of Sex Research, and a member of the American Association of Sex Educators, Counselors and Therapists. Verathon Inc. 800.331.2313 www.verathon.com 6 Urology for Primary Care Martin A. Koyle, M.D. Dr. Koyle is Professor of Surgery and Pediatrics and Vice-Chief, Division of Urology at the University of Colorado at Denver and Health Sciences Center, and Chairman of the Department of Pediatric Urology at the Children's Hospital in Denver, Colorado. He is native of Winnipeg, Canada and completed medical school training at the Manitoba Medical College in 1976. This was followed by general surgical training at Los Angeles County-USC Medical Center and a Urology residency at the Harvard Program in Urology in Boston. His academic career was initiated with appointment at the UCLA School of Medicine in 1984, where he was an Assistant Professor of Surgery and Director of Transplantation and Pediatric Urology at Harbor/UCLA Medical Center. In 1989, he was recruited to Denver. Dr. Koyle is a past President of the Society for Pediatric Urology and currently is President of the American Association of Pediatric Urologists. He is president-elect of the Colorado Urological Association and serves as that state's representative to the South Central section of the American Urological Association. His clinical interests are in pediatric transplantation, oncology, and complex reconstruction with emphasis in minimally invasive techniques. He has pioneered multiple techniques utilized commonly worldwide in Pediatric Urology and is utilizing the da Vinci robot in his practice. Presently he is on the editorial boards of the Journal of Urology, Journal of Pediatric Urology, Issues in Urology, and Dialogues in Pediatric Urology. J. Curtis Nickel, M.D., FRCSC Dr. Nickel is a Professor of Urology at Queen's University in Kingston, Ontario and a Staff Urologist at Kingston General and Hotel Dieu Hospitals in Kingston, Ontario. Dr. Nickel was born, educated, and completed his undergraduate, surgical, urological, and research training in Canada. He has been a member of the Department of Urology at Queen’s University since 1984 and was promoted to full Professor in 1994. Dr. Nickel’s research has been in the fields of inflammatory diseases of the urinary tract and benign diseases of the prostate gland. He has written over three hundred scientific papers, reviews, chapters and books on these subjects. He maintains a laboratory at Queen’s University, funded continuously by peer reviewed and industry grants. His Prostatitis Clinical Research Center, Interstitial Cystitis Clinical Research Center, and BPH Research Center are funded in part by five concurrent grants from the United States NIH/NIDDK. His work in prostatitis has led to new awareness of the condition, updated definitions and classsifications, validated outcome parameters, and recently, multiple clinical treatment trials in this difficult field. He has been involved in multiple clinical trials (single and multi-center) in BPH and interstitial cystitis, many times as one of the prinicipal investigators, which have helped shape the evolution of medical therapy for these diseases. Dr. Nickel participates as organizer, chairman, invited lecturer, or visiting professor, in many local, national, and international university and CME events. He has given invited lectures in over thirty-five countries. He is on the scientific or review panel of numerous granting agencies, a Verathon Inc. 800.331.2313 www.verathon.com 7 Urology for Primary Care regular reviewer for over a dozen urological and medical journals, and sits on the editorial board of eight national and international urological journals. He is a member of the Canadian Prostate Health Council, the NIH Chronic Prostatitis Collaborative Research Network, the NIH Interstitial Cystitis Collaborative Treatment Group, and the NIH Complementary and Alternative Medical Approaches to Urinary Symptoms (CAMUS) research group. He has been a member of the American Association of Genito-urinary Surgeons since 2004. He has served on the examination committee of the American Board of Urology (ABU) for four years and as an examiner for the ABU for four years. Richard S. Pelman, M.D. Dr. Pelman is an Associate Urologist at Bellevue Urology Associates in Bellevue, Washington and a Clinical Professor of Urology at the University of Washington School of Medicine. An experienced urological surgeon, Dr. Pelman has served as a member of the Active Staffs of Overlake Hospital Medical Center in Bellevue, Washington, and Evergreen General Hospital in Kirkland, Washington since 1985. Dr. Pelman graduated from the University of Washington School of Medicine in 1979. He served both as a surgical house officer from 1979 to 1982, and as a urological resident from 1982 to 1985 at Boston University Affiliated Hospitals. He holds medical licenses in the State of Washington and the Commonwealth of Massachusetts. He is a Diplomat of the National Board of Medical Examiners, and is Board Certified and Re-certified with the American Board of Urology. Dr. Pelman has served on the Executive Committee of the Washington State Urology Society since 1993, where he is the advisor regarding men’s health issues. He conceived, organized, and edited the Washington State Urology Society Guide to Men's Health, as well as authored the chapter on BPH. He belongs to the Northwest Urological Society, the American Medical Association, the American Urological Association, the American Society for Reproductive Medicine, the Seattle Surgical Society, the American Association of Clinical Urologists, and the King County Medical Society. Mike B. Siroky, M.D., FACS Dr. Siroky is currently a Professor of Urology at Boston University in Boston, MA. He also serves as Associate Chief of Urology for the Boston VA Healthcare System. Dr. Siroky earned his undergraduate degree and MD degree from Boston University in 1970. Dr. Siroky completed his post graduate training at Albert Einstein College of Medicine (Montefiore Hospital) in New York City, Harvard Medical School (Boston City Hospital), and the Boston University Training Program in Urology. Dr. Siroky is the recipient of the Grayson Carroll award from the AUA and the Zimskind award from the Urodynamics Society, and he is a three-time recipient of the Jack Lapides Prize. Dr. Siroky is a principal investigator or co-investigator in both NIH and VA funded research, as well as an invited presenter at numerous medical conferences, both nationally and internationally. Verathon Inc. 800.331.2313 www.verathon.com 8 Urology for Primary Care As author or co-author, Dr. Siroky's work has been published in many leading medical journals, including the Journal of Urology, Urology, British Journal of Urology, Neurourology, and Urodynamics. He is a Fellow of the American College of Surgeons and a member of the American Urological Association and the International Continence Society. His current research interests are in erectile dysfunction and bladder ischemia. David S. Wang, M.D. Dr. Wang is an Assistant Professor of Urology at the Boston University School of Medicine. He is an expert in the field of endoscopic and minimally invasive urologic surgery, and he has considerable experience in laparoscopic surgery, including robotic radical prostatectomy, radical nephrectomy, donor nephrectomy, partial nephrectomy, and repair of ureteropelvic junction obstruction. He has incorporated the daVinci robot into his practice to enhance his surgical repertoire. Dr. Wang is also proficient in endoscopic surgery for both benign and malignant conditions, including urinary tract stones and tumors. He has extensive experience in percutaneous kidney stone removal, ureteroscopy (endoscopy of the ureter), and shock-wave lithotripsy. Dr. Wang completed his residency training in General Surgery at the Massachusetts General Hospital/Harvard Medical School and in Urology at the Lahey Clinic Medical Center. He then completed a Fellowship in Endourology and Laparoscopic Surgery at the University of Iowa. Dr. Wang has published extensively in leading Urology journals and has presented at conferences at the national and international level. He is board-certified by the American Board of Urology and is a Fellow of the Endourological Society. He is also a member of the American Urological Association. Verathon Inc. 800.331.2313 www.verathon.com 9 Urology for Primary Care Introduction INTRODUCTION Richard S. Pelman, M.D. Associate of Bellevue Urology Associates Inc. Bellevue, Washington Clinical Professor of Urology University of Washington School of Medicine Seattle, Washington The Primary Care physician is the first person most patients approach when seeking evaluation and treatment for health concerns. This visit to the Primary Care physician may lead to further testing, referral to a specialist, or diagnosis and treatment. Today, Primary Care physicians are likely to consider initiating a treatment plan for common urological conditions that, in the past, required referral to a specialist. The availability of medications for many of these conditions has moved the Primary Care practitioner into an expanded role of care delivery. The following common urological conditions now fall within the scope of Primary Care: • Bladder outlet obstruction (BOO) • Lower urinary tract symptoms (LUTS) • Urinary tract infections (UTI) • Lower abdominal/pelvic pain • Initial diagnosis of urinary retention • Overactive bladder (OAB), including urinary urgency with or without urge incontinence, and also including urinary frequency and nocturia • Erectile dysfunction (ED) • Initial diagnosis of other urinary conditions such as hematuria, urinary calculus disease, et cetera TERMINOLOGY OF DIAGNOSTIC TOOLS A variety of diagnostic tools are available to help physicians arrive at the correct therapeutic conclusion for common urological problems. Urologists have used these tools for a long time, and Primary Care physicians are becoming increasingly aware of them. The following review covers the diagnostic measures currently used to evaluate and treat common urological problems. It is accompanied by a brief review of these conditions and will provide a solid resource for the Primary Care physician. Urologists today have adopted a more specific terminology than in the past. This has been done to prevent limiting diagnostic considerations, which was previously a problem. For example, there was a tendency to describe a patient with “obstructive symptoms” as having benign prostatic Verathon Inc. 800.331.2313 www.verathon.com 10 Urology for Primary Care Introduction hyperplasia (BPH) before confirming this diagnosis histologically. In a similar way, a patient with “irritative symptoms” was thought to have a urinary tract infection. In essence, this limited consideration of other diagnostic possibilities. The terminology of lower urinary tract symptoms (LUTS) provides a less restrictive framework and is less likely to limit consideration of other diagnostic possibilities. All urinary tract symptoms do not have a basis in obstruction or infection. More precision in terminology helps facilitate diagnosis, rather than limit thinking about urinary symptoms. First, consider the common questions in the patient history regarding the lower urinary system. In the past, many Urologists preferred to classify the symptoms referred to in these questions as “obstructive” or “irritative.” Recently, the urologic community has sought to retire these terms and replace them with the lower urinary tract symptoms of the “storage, voiding, bladder sensation, or post-micturition” type. Utilizing terms like “obstructive” and “irritative” tends to presuppose a diagnosis. Obstructive symptoms typically include: • • • • • • • Reduced force of stream Urinary hesitancy Straining to void Nocturia Urinary frequency Urinary urgency Sensation of incomplete emptying Note that included in these “obstructive symptoms” are the “irritative symptoms” of urinary urgency and frequency, which result from changes in bladder ultrastructure and the bladder outlet due to the aging of the bladder and long term obstruction. The term “irritative voiding symptoms” has traditionally been used to describe the symptoms of urinary tract infection. These symptoms include: • • • Dysuria (urinary burning) Urinary frequency Urinary urgency Urinary incontinence may or may not accompany these symptoms. While a lower urinary tract infection may cause these symptoms, their presence may be explained by other etiologies such as: • • • • • Bladder cancer Carcinoma in situ (CIS) Bladder calculus Interstitial cystitis Neuromuscular disease (Parkinson disease, diabetic neuropathy, multiple sclerosis, spinal cord disc disease and compression, cerebrovascular accident) Verathon Inc. 800.331.2313 www.verathon.com 11 Urology for Primary Care Introduction In female patients, these symptoms may arise from a loss of local estrogen, pelvic mass, retroverted (tipped) uterus, cystocele, or a urethral diverticulum. Because these “obstructive” and “irritative” symptoms overlap in both men and women in a variety of conditions, the terminology of lower urinary tract symptoms (LUTS) has been suggested as a better diagnostic framework. Utilizing LUTS to describe events associated with bladder storage, voiding, sensation, and post micturition helps to separate symptoms from initial diagnosis. The physician may be less inclined to focus on a disease entity until all components of the disorder, including symptoms, signs, examination, and lab, can unify the problems as a specific diagnosis. LUTS are defined by the International Continence Society as follows 1: Bladder Storage Symptoms: • • • • Increased daytime frequency Nocturia Urgency Urinary incontinence (stress urinary incontinence, urge urinary incontinence, mixed urinary incontinence, enuresis, continuous urinary incontinence) Voiding Symptoms: • • • • • • Slow urinary stream Splitting or spraying of the urinary stream Intermittent stream Urinary hesitancy Straining to void Terminal dribbling Bladder Sensation Symptoms: • • • • Normal Increased Reduced Absent Postmicturition Symptoms: • • 1 Feeling of incomplete emptying Postmicturition dribbling Adapted from P. Abrams et al., Neurourology and Urodynamics 2002; 21:167-178. Verathon Inc. 800.331.2313 www.verathon.com 12 Urology for Primary Care Introduction The term “overactive bladder” has been used to describe patients with urinary frequency, urgency, and nocturia, with or without urge incontinence. Overactive bladder patients may be male or female; the underlying etiology of the overactive bladder is varied in both. As described in the chapter on overactive bladder, diagnostic tools are available to help sort out this condition from other potentially responsible entities. A review of the differential diagnosis of conditions responsible for LUTS is included in Table 1. Benign prostatic enlargement (BPE) is currently utilized to describe the clinical condition previously referred to as benign prostatic hyperplasia (BPH). BPH is a histological description of the benign prostate tissue that leads to the changes associated with bladder outlet obstruction (BOO). In the clinical setting, without tissue confirmation, it is more correct to consider BPE as the working diagnostic term when referring to LUTS associated with benign prostatic obstruction leading to bladder outlet obstruction. The chapter on bladder outlet obstruction in this manual will introduce some relevant tools to help resolve LUTS secondary to BPE. The AUA Guideline Flow Diagram (see Appendix A) provides a useful review of the evaluation and treatment of this condition. This review of current terminology should help the Primary Care physician approach the patient history with a more focused view of the patient’s complaint. This will allow the physician to evaluate the patient more efficiently and develop an initial treatment plan. Verathon Inc. 800.331.2313 www.verathon.com 13 Urology for Primary Care Introduction Table 1: Differential Diagnosis of Lower Urinary Tract Symptoms 2 Prostate Benign prostatic hyperplasia Prostatitis Prostate cancer Bladder Bladder cancer Bladder stones Overactive bladder Interstitial cystits Primary bladder neck hypertrophy Radiation cystitis Urethral Urethritis - Gonococcal - Non-gonococcal Urethral stricture Neurologic and spinal cord Parkinson disease Multiple sclerosis Cerebrovascular accident Spinal cord trauma Lumbosacral disc disease Urinary tract infection Bacterial Tuberculosis Viral Fungal Metabolic Adult onset diabetes mellitus Nephrogenic diabetes insipidus Pharmacologic agents Diuretics α−Agonists Anticholinergics Pelvic Surgery 2 Adopted from H. Lepor, “Evaluating men with BPH,” Reviews in Urology 2004, Vol. 6 Suppl. 1: S10. Verathon Inc. 800.331.2313 www.verathon.com 14 Urology for Primary Care Bladder Outlet Obstruction BLADDER OUTLET OBSTRUCTION Richard S. Pelman, M.D. Associate of Bellevue Urology Associates Inc. Bellevue, Washington Clinical Professor of Urology University of Washington School of Medicine Seattle, Washington Recently, evidence-based recommendations were made concerning the diagnosis and treatment of benign prostatic hyperplasia (BPH) at the 5th International Consultation on BPH. These recommendations suggest using diagnostic tools that are evidence-based for a specific condition, in this instance a male patient with lower urinary tract symptoms (LUTS). These tools include: A clinical history incorporating the Symptom Index for BPH (see Appendix B), and a physical examination that includes a digital rectal examination (DRE) Laboratory testing, including a prostate specific antigen (PSA) and a urinalysis Additional diagnostic tools exist for evaluating and monitoring these symptoms. Some of these tools, while not incorporated into evidence-based recommendations, still provide unique information to the clinician who is developing a treatment strategy. These include: Flow rates Measurement of post-void residual bladder volume (PVR) Cystoscopy Urodynamics Although the serum creatinine (recently dropped from the evidence-based recommendations) may also be useful in numerous instances, like many other useful tools it has not been considered evidence-based because studies of adequate power have yet to be conducted. BENIGN PROSTATIC ENLARGEMENT (BPE) Benign prostatic enlargement (BPE) leading to benign prostatic obstruction (BPO) creating bladder outlet obstruction (BOO) defines the sequence of clinical events previously described using the term “benign prostatic hyperplasia” (BPH). BPH is still considered an appropriate description of the prostate when referring to the histological changes occurring in the prostate in this benign condition. BPH is a progressive, age-related condition of men, demonstrating a histological incidence of 50% at age sixty to 90% by age eighty-five. This incidence is in excess of men who are Verathon Inc. 800.331.2313 www.verathon.com 15 Urology for Primary Care Bladder Outlet Obstruction symptomatic, as by age fifty, only approximately 25% of men suffer some symptoms of the disease. The incidence of symptomatic men does increase with age. Why the disease progresses is still unclear, although there is a substantial link to testicular androgens and aging. As the prostate grows, various histologic patterns emerge. Fibro-epithelial hyperplasia is the most common pattern. The fibrous component is represented by prostatic smooth muscle that is laden with alpha-receptors. The epithelial component is less significant and responds to inhibition of testosterone metabolism by undergoing involution. Interestingly, the size of the gland correlates poorly with the degree of symptoms exhibited by the individual patient. Some patients are relatively symptom free with large glands, while others with a relatively small gland may become symptomatic. Prostate gland size does help predict the success of medical therapy, with larger glands responding more favorably to testosterone metabolism inhibition. The intrusion of the prostate into the urethra and bladder neck produces an obstructive process, which requires a higher voiding pressure to be generated by the detrusor of the bladder. This detrusor hypertrophy changes the ultrastructure of the bladder and will lead to a compromise of bladder storage function. Benign prostatic enlargement leading to benign prostatic obstruction creating bladder outlet obstruction is one scenario leading to the following troublesome lower urinary tract symptoms: Bladder Storage Symptoms: - Urinary frequency - Urinary urgency - Nocturia - Urinary urge incontinence Voiding Symptoms: - Slowing of the urinary stream - Intermittent stream - Urinary hesitancy - Straining to void - Terminal dribbling Post Micturition Symptoms: - Feeling of incomplete emptying - Post micturition dribbling Some people may have adequate urinary flow rates in the face of increased outlet resistance by developing significant detrusor hypertrophy. Alternate etiologies of increased voiding pressures may be: Bladder neck hypertrophy Bladder neck contraction (postoperative complication) Urethral stricture disease Meatal stenosis While these symptoms may be common to bladder outlet obstruction, they are not specific to this entity. Similar symptoms, including slowing of the urinary stream, hesitancy, nocturia, and frequency, may be attributed to diseases intrinsic to the bladder, neurogenic bladder, or even failure to relax when voiding (the non-relaxing voider). Verathon Inc. 800.331.2313 www.verathon.com 16 Urology for Primary Care Bladder Outlet Obstruction The American Urological Association (AUA) Symptom Score Index (see Appendix B), based on a seven-question survey, can be useful in sorting out the symptoms of male patients. The symptom score has been validated only for bladder outlet obstruction (BOO). Remember that the patient with undiagnosed diabetes can also have a very high symptom score, as can the patient with an active urinary tract infection. Score > 0 to 7 - Mild Symptoms Score > 8 to 19 - Moderate Symptoms Score > 20 to 35 - Severe Symptoms All male patients with voiding symptoms should complete a symptom score on initial assessment. Those with higher scores should then be questioned about the degree to which their symptoms interfere with quality of life, to determine a “bother” score. The bother score is the symptom score with the word “bother” added. The last question on the Symptom Index for BPH relates to bother and quality of life (see Appendix B). Scores May Not Correlate to “Bother” Factor Patients with significant symptom scores relating to bladder outlet obstruction may not be overly bothered by their symptoms. Patients with significant symptom scores and low “bother” scores might want to defer treatment, pursuing instead a policy of “watchful waiting” to see if their condition worsens. They should be monitored for progression of the disease and urinary retention. Patients who choose to proceed with treatment should have the symptom score repeated after treatment, to assess the adequacy of the treatment selected. CONDUCTING AN EXAM The physician should perform a digital examination of the prostate during the physical exam to assess the size, contour, and consistency of the prostate. Any significant asymmetry, induration, or nodularity should be referred to a Urologist for evaluation. Any rectal mass should also be noted. The rectal sphincter tone should be assessed as an indication of a possible neurological component to the patient’s condition. The prostate specific antigen (PSA) should be tested. The PSA may correlate with prostate size. If the PSA is found to be elevated or has undergone a greater than 20% increase in a year, refer the patient to a Urologist for further evaluation. Perform a urinalysis to help detect hematuria, pyuria, and bacteriuria. The occasional diabetic patient may be uncovered by significant glycosuria. The serum creatinine is useful in discovering the small percentage of patients with azotemia. A Simple Way to Determine Post-void Residual Bladder Volume (PVR) Use the BladderScan Bladder Volume Instrument to measure post-void residual bladder volume. The BladderScan bladder volume instrument provides an easy, noninvasive tool for identifying patients with significant residual volumes. In the most recent American Urological Association Verathon Inc. 800.331.2313 www.verathon.com 17 Urology for Primary Care Bladder Outlet Obstruction Guidelines on BPH, the authors note that “Large PVR volumes may indicate bladder dysfunction and predict a slightly less favorable response to treatment.” They also note that “large PVRs may herald progression of disease.” While it is noted in the guidelines that it is not feasible to establish a PVR cutpoint, they did consider the use of PVR measurements optional for men undergoing noninvasive therapy. Patients with high symptom scores and low bother scores who demonstrate high residual urine volumes and wish to be treated expectantly should be counseled regarding the possibility of a high failure rate. They should be monitored for progression of disease through symptom progression and increasing residual urinary volume. The BladderScan bladder volume instrument is also helpful in evaluating and managing patients with neurogenic bladders. The urinary flow rate, urodynamics, and cystoscopy are useful tools to help differentiate patients with lower urinary tract symptoms (LUTS) from BPE or alternative etiologies. These procedures are not generally necessary when evaluating a symptomatic patient who wants to undergo a trial of pharmacologic therapy; however, these procedures are needed for patients who fail an initial medication trial. The urinary flow rate is another noninvasive means of obtaining significant information about the degree and even the type of outlet obstruction. Flow rate nomograms (see Appendix D) have been constructed to help differentiate patients with minimal obstruction from those with more significant problems. Absolute Indications for Therapy: Acute urinary retention Chronic urinary retention with incontinence or renal insufficiency Hematuria unresponsive to medical therapy Hydronephrosis Relative Indications for Therapy: Recurrent urinary tract infections Increasing significant residual urinary volume Progressive lower urinary tract symptoms leading to reduced quality of life IF THE PATIENT PROCEEDS WITH TREATMENT... For patients electing to undergo therapy, a variety of choices are available, including medical (pharmacologic) therapy, minimally invasive therapy, and surgical therapy. Medical Therapy Medical therapy lies within the realm of the Primary Care practitioner. Three types of medical therapy are currently available: Alpha-blockers, 5-alpha reductase inhibitors, and phytotherapy (natural products). Verathon Inc. 800.331.2313 www.verathon.com 18 Urology for Primary Care Bladder Outlet Obstruction Alpha-blockers – Available alpha-blockers include terazosin (Hytrin ), doxazosin (Cardura ), tamsulosin (Flomax ), and alfuzosin (Uroxatral ). All of these medications share the potential for improving the patient’s symptom score, flow rate, and post-void residual. Some require dose titration. All share the potential side effects of orthostatic hypotension, dizziness, asthenia, headache, and nasal congestion. Retrograde ejaculation has been reported in 10% or less of men taking tamsulosin. Recent AUA guidelines have deemed these agents as appropriate treatment options for men with LUTS secondary to BPE/BPH. Most authors would suggest that if one medication (monotherapy) is to be utilized for treatment of LUTS secondary to BPE, alpha blockers are the best choice. Alpha-blockers do not change the PSA level. NOTE: In March 2000, the National Heart, Lung and Blood Institute stopped part of a large study on hypertension. The reason was that patients treated with doxazosin who also had significant underlying cardiovascular disease were 25% more likely to experience cardiovascular events. They also were twice as likely to be hospitalized for congestive heart failure if they used the alpha-blocker as compared to patients on another type of antihypertensive medication. 5-Alpha Reductase Inhibitors – Available 5-alpha reductase inhibitors include finasteride (Proscar ) and dutasteride (Avodart ). These medications lower intraprostatic DiHydro Testosterone (DHT) by inhibiting the enzyme 5-alpha reductase. This is the enzyme responsible for cleaving testosterone into the intraprostatic active congener DHT. By inhibiting the cleaving of testosterone, serum testosterone remains unchanged, while intraprostatic metabolic activity is blocked. Two isoenzymes of 5-alpha reductase exist, type 1 and type 2. Finasteride selectively inhibits type 2, dutasteride inhibits both. No long term head-to-head studies are currently available to demonstrate superior outcome in clinical efficacy and safety of either product over the other. In short term head-to-head trials (24 weeks), dutasteride demonstrated a greater reduction of serum DHT than did finasteride (94.7 to 70.8). The clinical relevance of complete isoenzyme inhibition remains to be demonstrated in long term head-to-head comparative trials. Three to six months of therapy are necessary before patients may see symptom improvement. The serum PSA must be doubled after six months of therapy to realize its value. Limited side effects are noted, with less than 10% of patients complaining of decreased libido and decreased ejaculatory volume. The 5-alpha reductase inhibitors appear to demonstrate efficacy in men with enlarged prostates. Thus, they are not considered the best initial single agent (monotherapy) for men with LUTS secondary to BPE/BPH. The AUA Guideline Committee on BPH has recommended that finasteride and dutasteride “are appropriate and effective treatments for patients with LUTS associated with demonstrable prostatic enlargement.” The four-year Proscar Long-Term Efficacy and Safety Study (PLESS) demonstrated a long term reduction in risk of BPH related surgery (55%) compared to placebo and a 57% reduction in acute urinary retention. Mean prostate volumes in this study were 55 cc +/- 25cc. This data has supported the recommendation of the AUA Guideline Committee that physicians present the option of therapy with a 5-alpha reductase inhibitor to patients with significant prostate enlargement, but without significant LUTS or bother from BPE. Verathon Inc. 800.331.2313 www.verathon.com 19 Urology for Primary Care Bladder Outlet Obstruction Included in this recommendation was the need for counseling the patient regarding side effects and the need for long term daily therapy in comparison “to a reasonable estimate of his baseline risk of progression (i.e., retention and risks associated with BPH-related surgery).” They concluded that “5-alpha reductase inhibitors are not appropriate treatments for men with LUTS who do not have evidence of prostatic enlargement.” Combination Medical Therapy – The long term Medical Treatment of Prostate Symptoms Study (MTOPS) presented a placebo-controlled prospective double blind multi-center trial over four years to evaluate the effect of medical therapy on BPE/BPH progression. Participants were randomized to placebo, doxazosin, finasteride, and a combination of finasteride and doxazosin. At the completion of the study, combination therapy demonstrated superior benefit to either single agent alone. This superiority was demonstrated not only for symptom improvement, but also for prevention of symptom progression (39% for doxazosin, 34% for finasteride, 67% for the combination of both). The combination therapy also demonstrated a reduction in risk of urinary retention (31% doxazosin, 67% finasteride, and 79% combination therapy). Therefore, the AUA Guideline Committee on BPH has recommended that, “The combination of an alpha-adrenergic receptor blocker and a 5-alpha reductase inhibitor (combination therapy) is an appropriate and effective treatment for patients with LUTS associated with demonstrable prostatic enlargement.” Appropriate consultation with the patient regarding risks and benefits was included in the recommendation. Therapy of Overactive Bladder (OAB) Symptoms Secondary to BPE – The symptoms of overactive bladder are frequently present in men with bladder outlet obstruction. These include the lower urinary tract symptoms of urinary urgency, frequency, nocturia, and urge incontinence. While the majority of patients will respond to therapy directed at the prostate, a subset of patients may receive symptom relief from therapy aimed at the bladder. This subset includes patients who have maintained good urinary flow rates in the face of obstruction with minimal residual urinary volume. As previously noted, a subset of obstructed patients may continue to demonstrate normal or even super normal flows due to significant detrusor hypertrophy. If adequate bladder emptying is demonstrated, then anticholinergic/ antimuscarinic therapy aimed at the bladder may be useful, either as primary therapy or in conjunction with therapy directed at the prostate. Patients with significantly reduced urinary flow rates or residual urinary volumes are not candidates for therapy at the bladder level. These patients may have primary bladder failure (low flow and low detrusor contracture pressure), or require relief of the obstructive process before benefiting from medications for overactive bladder. If these patients are treated at the bladder level, they may develop more severe symptoms, increase the residual urine, or possibly develop urinary retention. In the selected subset of eligible patients, urinary flow rates and residual urinary volumes must be considered when choosing therapy directed at the bladder. The residual urinary volume should be monitored after OAB therapy has been intiated. Phytotherapy – Insufficient clinical data exits to make objective recommendations concerning phytotherapy. It is important to remind patients that no data exits to suggest a prevention of Verathon Inc. 800.331.2313 www.verathon.com 20 Urology for Primary Care Bladder Outlet Obstruction progression of BPE, or prevention of prostate cancer, from the use of phytotherapies. Since phytotherapies are not FDA regulated, no recommendation can be made regarding the dosage of one product over another. Many patients do find symptom benefit from these therapies; however, patients should be encouraged to follow up with a physician for monitoring of symptoms, PSA, urinalysis, and residual urine, as appropriate. Since a prescription is not required for these remedies, a surprising number of patients are taking these therapies without supervision. Minimally Invasive Therapy Minimally invasive therapy is recommended for the symptomatic patient who: Fails to improve on medical therapy Cannot tolerate medical therapy Elects to have definitive therapy as initial treatment Thermal therapy – Thermal therapies can be accomplished in the ambulatory or outpatient setting. This kind of therapy is currently available in the form of transurethral microwave therapy (TUMT), microwave energy, interstitial laser, contact laser, and transurethral needle ablation (TUNA), which employs a high frequency radio wave. They impose fewer restrictions on activity during convalescence and can be performed under sedation with local anesthesia. The results may not last as long as those obtained with standard surgery, and patients may have significant bladder storage and voiding symptoms for a number of weeks after therapy. Approximately twenty to thirty percent may develop acute urinary retention postoperatively for a short period of time. Surgical Therapy Transurethral resection of the prostate (TURP) remains the gold standard for treatment of bladder outlet obstruction (BOO). When compared to other forms of therapy, TURP provided the most improvement in flow rates, symptom scores, and reduction of residual urine volume. In addition, TURP is still the most durable form of treatment. Transurethral incision of the prostate (TUIP), a procedure that involves making an incision along the floor of the prostate, may provide as much relief from obstructive voiding symptoms in men with smaller prostates as standard TURP. Transurethral vaporization of the prostate (TUVP) is a procedure similar to TURP; however, the prostate tissue is vaporized with electrodes rather than cut. The advantages of TUVP include less bleeding and the potential for use with patients on anti-coagulation therapy. Transurethral photoselective vaporization of the prostate (PVP) is a procedure similar to TURP; however, the prostate tissue is vaporized with a laser, rather than cut. The advantages of PVP include less bleeding and the potential for use in patients on anti-coagulation therapy. No tissue is obtained for pathological review. Verathon Inc. 800.331.2313 www.verathon.com 21 Urology for Primary Care Bladder Outlet Obstruction Monitoring Changes in Bladder Volume Primary Care practitioners should have the ability to evaluate lower urinary tract symptoms (LUTS) and to recommend and monitor therapy for many of the underlying conditions. While the Primary Care physician may not have the capability of performing studies such as cystoscopy or urodynamics, these studies are not necessary for many patients on initial presentation. The AUA Symptom Score and PSA testing are readily available to all practitioners. With regard to determining residual urine volume, the BladderScan bladder volume instrument is a noninvasive ultrasound device that offers ease of operation, portability, and reliability, making it an excellent choice for Primary Care practices. With a noninvasive means of determining residual urine volume, Primary Care practices should be able to achieve the highest quality of care in the expanded focus of urological disease. Verathon Inc. 800.331.2313 www.verathon.com 22 Urology for Primary Care Prostatitis PROSTATITIS Richard S. Pelman, M.D. Associate of Bellevue Urology Associates Inc. Bellevue, Washington Clinical Professor of Urology University of Washington School of Medicine Seattle, Washington Urologists sometimes describe prostatitis as the “low back pain” of Urology. Dealing with prostatitis often presents a challenge to both the patient and the physician. Many prostatitis patients have had long-standing symptoms and little relief. Such patients are a constant presence in the physician’s office, seeking a solution that will finally ease their symptoms. Information and education can help reassure the patient, especially in instances where the patient’s symptoms are not clearly due to prostatitis. To help focus the diagnosis, the Primary Care physician should devote attention to the four categories of prostatitis, described below. Prostatitis is described by four different presentations, or categories. Each variety has associated symptoms, diagnostic parameters, and therapeutic options. Not all prostatitis is believed to be infection-related. The National Institutes of Health (NIH) classification system includes the following categories: Acute Bacterial Prostatitis: Category 1 Patients with acute bacterial prostatitis will present with a fever and a toxic appearance. They may complain about difficulty voiding, slowing of the urinary stream, dysuria, urgency, and frequency. Upon review of the urinalysis, the urine will appear infected. A urine culture usually reveals E. coli or some other gram-negative bacteria. White blood count will be elevated. In cases of acute bacterial prostatitis, the physical exam reveals a febrile, ill patient. Although the genitalia are benign upon examination, they should be checked for acute epididymitis as the possible cause of the infection. The prostate exam will reveal a firm and indurated prostate. The physician must be gentle during the exam to avoid causing further bacteremia. The PSA should not be obtained at this time because it will be elevated and will take weeks to reach normal levels. The physician should use the BladderScan bladder volume instrument to evaluate the patient for urinary retention. NOTE: A firm and indurated prostate in the absence of a documented infection needs to be evaluated. A PSA and a Urology referral are appropriate in this instance. Therapy for Acute Prostatitis Therapy should be initiated with high dose quinolone therapy for at least a couple of weeks. Further antibiotic therapy with more quinolone or a second line antibiotic should be undertaken for a minimum of two more weeks (if quinolone is used) or longer (if a second line antibiotic such as trimethoprim-sulfamethoxazole [Bactrim] is used). A review of the prostatic secretions on Verathon Inc. 800.331.2313 www.verathon.com 23 Urology for Primary Care Prostatitis follow-up examination may help the physician determine the length of the antibiotic course required for adequate treatment. Failure to sterilize the prostate invites the risk of chronic bacterial prostatitis. To relieve that patient’s voiding difficulties, consider initiating alpha-blocker therapy. A urinary catheter should be utilized in cases of urinary retention. After treating the acute infection, the physician will need to monitor the patient for sterility of urine and prostate secretions. The physician will also need to determine if the patient is voiding without restriction or significant retention of urine. Chronic Bacterial Prostatitis: Category II Cases of acute and bacterial prostatitis account for only 5% of all patients presenting with prostatitis. Patients with chronic bacterial prostatitis are likely to mirror Category I patients, but they are usually less ill when they present at the initial onset of symptoms. These patients are likely to have suffered an episode of acute bacterial prostatitis that was incompletely treated. In cases of chronic bacterial prostatitis, the urine culture, prostatic secretions, or ejaculate should test positive for a gram-negative bacterium or other uropathic organism. The expressed prostatic secretions will yield high levels of inflammatory cells. The VB3 (Voided Bladder Urine 3 – see below for details) may yield a positive culture. Category II patients require aggressive and prolonged antibiotic therapy. Adequacy and efficiency of voiding mechanics should also be assessed. Use the BladderScan bladder volume instrument to rule out urinary retention. The status of the expressed prostatic secretions and sterility of the VB3 urine will help the physician determine whether the antibiotic course was adequate, or needs to be extended. A urine specimen obtained after prostate massage is often useful in determining whether or not the prostate is sterile. This is an initial stream of urine obtained immediately after massage. It is generally referred to as the Voided Bladder Urine 3 (VB3) to distinguish it from the Voided Bladder Urine 2 (VB2), which is a typical mid-stream urine collection to ascertain status of bladder urine. A Voided Bladder Urine 1 (VB1) is an initial stream prior to any prostate massage and is collected before the mid-stream urine. The VB1 should demonstrate the presence or absence of any urethral organisms. The expressed prostatic secretions can be collected on a slide and reviewed. To collect a sample, the prostate is massaged, a glass slide is given to the patient, and any fluid dripping from the urinary meatus is placed on the slide and reviewed. While the exact number of inflammatory cells that indicates prostatitis is debated in Urology literature, it is felt that any increase in secretions from pre-massage urine (VB2) to post-massage urine (VB3) may represent prostatitis. The presence of 10 or more white blood cells per high power field upon review of the expressed prostatic secretions may also confirm a diagnosis of prostatitis. Chronic Prostatic Pelvic Pain Syndrome of the Inflammatory Type: Category IIIA Verathon Inc. 800.331.2313 www.verathon.com 24 Urology for Primary Care Prostatitis leukocytes in the expressed prostatic secretions. Category IIIA patients differ from Category I and II patients in that the urine culture, ejaculate, and VB3 urine are sterile. The expressed prostatic secretions have a significant number of inflammatory cells. Category IIIA patients may have microorganisms not identified by current culture methods. These patients seem to benefit from long-term antibiotic treatment. The addition of antibiotics to cover chlamydia, as well as ureaplasma, has been suggested for non-responders. As with the previous category of patient, the evaluation of mechanical voiding dysfunction is warranted. Residual urine volume should be evaluated with the BladderScan bladder volume instrument. In addition to antibiotic therapy, Category IIIA patients may benefit from anti-inflammatory agents and perhaps a trial of alpha-blockers, or even phytogens such as Saw Palmetto. Chronic Prostatic Pelvic Pain Syndrome of the Non-Inflammatory Type: Category IIIB Patients in this category are to be distinguished from the prior category of patient (Category IIIA) in that, although they may present with similar symptoms, the expressed prostatic secretions are normal. Category IIIB patients lack a pathological increase in inflammatory cells. The urine culture and the culture of the secretions and ejaculate, as well as the VB3 urine, are lacking any identifiable pathogen. These patients should be evaluated for any mechanical problem associated with the emptying of the lower urinary tract. Use the BladderScan bladder volume instrument to check for residual urine. Urodynamic evaluation should be contemplated. A PSA should be obtained and, if abnormal, the patient should be referred to a Urologist for evaluation. Remember that the classic boggy prostate has very little to do with any particular pathology. The presence of an indurated prostate or nodule is a key area of concern and requires referral. Pelvic Floor Syndromes Some Category IIIB patients may suffer from pelvic floor syndromes. These are problems related to the supporting musculature of the urogenital diaphragm, or levator muscles. This condition is analogous to back problems involving muscle spasms, where relaxation of the muscles will result in relief of symptoms. Thus, patients with pelvic floor syndromes may benefit from antiinflammatory agents or muscle relaxers. Others may benefit from biofeedback training in relaxation of the pelvic floor. If there is consideration of bladder outlet obstruction (BOO), as in a mechanical voiding problem, a trial of alpha-blocker therapy is warranted. Long-term antibiotic therapy does not seem to have a place in the treatment of these patients. Consideration of referred pain might be given to the refractory patient. After completing your evaluation, it may be helpful to reassure and educate your patient about these syndromes. This will help to relieve the patient’s fear of more serious underlying problems, such as prostate cancer. Category IV: Subclinical Prostatitis Category IV patients are asymptomatic. In such cases, prostate biopsy, a surgical specimen (TURP chips), or investigation of the expressed prostatic secretions can reveal an inflammation of the prostate; however, this particular category of patient does not generally require therapy or evaluation. Verathon Inc. 800.331.2313 www.verathon.com 25 Urology for Primary Care Prostatitis SUMMARY OF PROSTATITIS Any febrile urinary tract infection (UTI) should be considered complicated. The source of most febrile infections usually lies in the kidney, prostate, testis, or epididymis in the male patient, and in the kidney in female patients. In the male patient, confusion regarding the etiology of the infection (between kidney and prostate) may lead to partial treatment. Acute pyelonephritis may be incorrectly diagnosed, instead of acute prostatitis. Acute pyelonephritis usually responds to two weeks of quinolone therapy, while acute prostatitis requires a longer course of antibiotic treatment to sterilize the prostate gland, as previously discussed. If you diagnose the problem correctly when the patient presents, you will be able to prevent recurrent infections due to partial or incomplete treatment. You will also avoid the prolonged use of antibiotics for patients lacking an infectious etiology for their symptoms. See Appendix E for more information about the categories of prostatitis. Verathon Inc. 800.331.2313 www.verathon.com 26 Urology for Primary Care The Overactive Bladder THE OVERACTIVE BLADDER Richard S. Pelman, M.D. Associate of Bellevue Urology Associates Inc. Bellevue, Washington Clinical Professor of Urology University of Washington School of Medicine Seattle, Washington The International Continence Society (ICS) previously described the overactive bladder as a disorder of bladder filling and storage, in which involuntary bladder contractions are demonstrated while the patient is attempting to inhibit such contractions. If the patient is successful in inhibiting the contractions, the patient will experience urinary urgency without incontinence. If the patient cannot inhibit the contractions, then urinary urgency and incontinence will result (urge incontinence). Urinary frequency may also be associated with this condition. Currently, the International Continence Society 2002 edition of the Standardization of Terminology of the Lower Urinary Tract Function describes overactive bladder syndrome as urinary urgency with or without urge incontinence, usually with frequency and nocturia, in the absence of pathologic or metabolic conditions that might explain these symptoms. Thus, a patient with diabetes may have symptoms of overactive bladder, but does not have the syndrome of overactive bladder. The syndrome of overactive bladder is exclusive of any underlying metabolic or pathologic conditions. This current definition of overactive bladder attempts to define the condition by patient symptoms, rather than urodynamic findings. Stress incontinence occurs through a different mechanism and is not considered to be a component of the overactive bladder, though some patients experience urinary incontinence from both mechanisms. Patients who experience both stress and urge incontinence are described as having mixed urinary incontinence. If the patient’s overactive bladder symptoms are the result of a neurological condition, then the uninhibited bladder contractions may be termed neurogenic detrusor overactivity (previously termed detrusor hyperreflexia), implying a neurogenic bladder. In many instances, the symptoms of overactive bladder occur without any underlying neurological disorder. The involuntary detrusor activity is then classified as idiopathic detrusor overactivity (previously termed detrusor instability). The most common symptoms of the overactive bladder are: Urinary urgency Frequency Nocturia Urge incontinence These symptoms, while relating to the overactive bladder, may have origins in many different types of conditions. Verathon Inc. 800.331.2313 www.verathon.com 27 Urology for Primary Care The Overactive Bladder DIFFERENTIAL DIAGNOSIS OF OVERACTIVE BLADDER As noted in the introduction, diseases intrinsic to the bladder may cause the symptoms of overactive bladder. These diseases include: Urinary tract infection (UTI) Bladder cancer Carcinoma in situ (CIS) of the bladder Bladder calculus Interstitial cystitis Diseases extrinsic to the bladder may also cause the symptoms of overactive bladder. In the male patient, the extrinsic disorder most often responsible for detrusor instability is bladder outlet obstruction (BOO). Disorders extrinsic to the bladder in the female patient include urethral diverticulum, retroverted uterus, pelvic prolapse (including cystocele), gravid uterus, and loss or reduction of estrogen. Disorders extrinsic to the bladder common to both men and woman include pelvic mass, physiologic nocturnal diuresis, and polyuria caused by factors such as excessive fluid intake, diuretic use, or diabetes. Neuromuscular disorders may also account for the overactive bladder. Such neurological disorders include Parkinson disease, multiple sclerosis, cervical stenosis, spinal cord injury, diabetic neuropathy, and hydrocephalus. Bladder aging may also account for these symptoms. A patient history of pelvic trauma, pelvic radiation, or bladder, prostate, or urethral surgery should also be considered when seeking to determine the etiology of the overactive bladder. Transient Causes of Overactive Bladder Transient conditions that may cause symptoms of the overactive bladder can be remembered using the DIAPERS acronym (as developed by N. M. Resnick): Delirium Infection Atrophic urethritis or vaginitis Pharmaceuticals and psychological problems Excessive urine output Restricted mobility Stool impaction Verathon Inc. 800.331.2313 www.verathon.com 28 Urology for Primary Care The Overactive Bladder OTHER TYPES OF INCONTINENCE Urinary incontinence may or may not have its origins in the overactive bladder. Overflow incontinence is associated with overdistension of the bladder resulting in the involuntary loss of urine. Patients may complain of urinary urgency, frequency, nocturia, incontinence, or urinary dribbling. In males, bladder outlet obstruction is the most common cause of overflow incontinence. Diseases causing the detrusor to become hypotonic, such as multiple sclerosis, diabetes, lower spinal cord injury, and medication effect, can also be considered as possible etiologies for this condition. Stress incontinence is defined as urine loss accompanied by an increase in intra-abdominal pressure in the absence of a detrusor contraction. In females, the most common cause of stress incontinence is hypermobility of the urethra with associated compromise of the urinary sphincter. This refers to movement of the urethra during periods of increasing abdominal pressure. By displacing the urethra below the point where it can receive concomitant increases in pressure, such hypermobility results in a net loss of urine. MECHANISMS OF URINARY CONTROL The mechanisms of urinary continence differ in the male and female. They both involve the integration of multiple mechanisms, and they continue to evolve with respect to anatomical and neurophysiologic relationships. The female continence mechanism is complex in that it requires the following features: Anatomic integrity of the bladder and urethra with respect to the levator muscle group, endopelvic fascia, and pelvic ligaments. The male patient maintains continence through both the proximal urethral and distal urethral mechanisms. Either one of these mechanisms is sufficient to keep the male patient continent. The proximal mechanism incorporates the bladder neck and preprostatic sphincter. The distal urethral mechanism incorporates periurethral smooth muscle in the membranous urethra. Both male and female continence mechanisms require urethral coaptability. This is the folding and compression of the mucosa and submucosa. Any injury to the urethra, such as prior surgery, radiation, trauma, or atrophy, may compromise this mechanism. Bladder compliance is also integral to normal bladder storage. The normal vesico-elastic property of the bladder allows for urine storage at low intravesicle pressures. Neurophysiologic integrity of the brain, spinal cord, bladder, and urethra complete the requirements for normal bladder storage and emptying. Bladder storage requires a functional cerebrocortical inhibitory center in the cerebrum and midbrain, coordinated with intact spinal cord pathways involving hypogastric sympathetic outflow from the thoracolumbar region. Numerous local feedback loops are further integrated into the storage mechanism, allowing detrusor stability, sphincter integrity, and normal bladder storage in the face of bladder filling and rising intravesicle pressures. Verathon Inc. 800.331.2313 www.verathon.com 29 Urology for Primary Care The Overactive Bladder EVALUATING THE PATIENT WITH OVERACTIVE BLADDER The evaluation of the patient with overactive bladder should integrate a patient history, physical exam, laboratory data, and the use of tools such as voiding diaries, pad counts, and ultrasound bladder volume measurement (BladderScan bladder volume instrument) to help sort out the underlying cause of the patient’s symptoms. Patient History The patient history should focus on important risk factors, including: History of pelvic surgery or pelvic radiation Trauma Medical conditions Neurological problems There should be an update of the medical history and a medication review. genitourinary history should take into account: A focused Frequency of urination Urgency Amount of urine produced in response to the urge to void (sensation which is disproportionate to the amount of urine produced) Sensations of incomplete emptying Duration of urge events and time of day they occur (i.e., does the patient complain of daytime frequency but sleep all night?) A review of any incontinent episodes, including information about the amount of urine lost, the association of urinary urgency, and any activities or events surrounding the incident Stress incontinence is typically associated with some type of activity, and the amount of urine loss can be quantified (e.g., a drop resulting in a quarter-size spot, fifty-cent size spot, or soaking the undergarment or pad). The number of pads used each day is a helpful measure in determining the severity of the problem. You can also compare pad counts before and after treatment to assess the effectiveness of the therapy. Use of Voiding Diary or Frequency Volume Charts The voiding diary (see Appendix F) and frequency volume chart (see Appendix G) are very useful tools when taking a patient genitourinary history. In addition to recording the number of voiding episodes, the patient tracks associated sensations, volume of voided urine, amounts of associated leakage, and the activities or events surrounding the episode, over a three to four day period. The frequency and volume of the patient’s fluid intake are also documented. Verathon Inc. 800.331.2313 www.verathon.com 30 Urology for Primary Care The Overactive Bladder The classic frequency volume chart, as developed by Klevmark and modified by Abrams, contains five categories: Type 1 Type 2 Type 3A Type 3B Type 4 Type 5 Normal volume and normal frequency, as seen over a normal 24-hour period in a normal patient Normal volume and increased frequency. This may indicate an increased 24-hour urine production secondary to increased oral intake. Also consider the possibility of diabetes insipidus or uncontrolled diabetes mellitus. Reduced, fixed volumes day and night. Consider intrinsic bladder pathology. Reduced, varying volumes day and night. Consider detrusor instability. Normal morning voiding, reduced and varying daytime volume, no nocturia. Consider psychosomatic issues or stress incontinence. Nocturnal polyuria, defined as passing more than 30% of the total 24-hour urine volume during eight hours of rest, with normal daytime volume. Consider congestive heart failure. This condition may also be idiopathic. Physical Exam The physical exam is directed toward a general assessment of the patient’s overall health. The abdominal, genitourinary, and neurological exam should search for any evidence of significant pathology. The female genital exam should assess the local hormonal environment, the position of the pelvic organs, and the urethra. The patient may be asked to cough and strain. The movement and support of the bladder and urethra can then be assessed. Observe and document urinary leakage. This can be done while the patient lies supine and then repeated while she is in a standing position. For male patients, the physical exam should evaluate prostate size, symmetry, and consistency. For both male and female patients with significant incontinence, the status of the surrounding tissue should be assessed for evidence of skin breakdown and ulceration. The rectal exam will reveal the quality of sphincter tone. Note and evaluate the presence of any rectal mass. Laboratory Investigation Lab work should include a urinalysis to check for signs of infection. The presence of white blood cells may or may not indicate infection. Sterile pyuria should be evaluated through upper and lower tract investigation. In the absence of infection, the presence of red blood cells should also lead to a similar evaluation. The presence of glucosuria will perhaps explain urinary frequency as a symptom of diabetes. Proteinuria also requires further evaluation to exclude renal parenchyma disease. Residual Urine Assessment The patient should be evaluated for residual urine noninvasively, through use of the BladderScan bladder volume instrument. The ability to determine which patients are suffering from overflow incontinence and which patients are suffering from hypotonic detrusor function, detrusor Verathon Inc. 800.331.2313 www.verathon.com 31 Urology for Primary Care The Overactive Bladder areflexia, or sensory urgency (the feeling of pressure and desire to void in the face of an empty bladder) will help the physician to correctly stratify patients into appropriate categories of bladder function. THERAPY FOR THE OVERACTIVE BLADDER Treatment for the overactive bladder currently falls into several categories, including pharmacologic therapy, behavioral therapy, electrical stimulation, catheterization, and bladder augmentation. Pharmacologic Therapy Other than use of absorbent pads, pharmacologic treatment is by far the most common form of therapy for the overactive bladder. Pharmacologic treatment is achieved by peripheral targeting of the bladder, urethra, and prostate. The introduction of current concepts regarding cholinergic receptors has led to the development of antimuscarinic agents, which inhibit the transmission of acetylcholine in smooth muscle, the peripheral nervous system, and the central nervous system. Currently available agents demonstrate selectivity and nonselectivity for the bladder receptors. Some, while nonselective for bladder receptors, are still uroselective due to the molecular design of the agent. These may share less side effects than other, nonselective antimuscarinic agents. Examples of the multiple, currently available overactive bladder medications include: Oxybutynin (Ditropan , Ditropan XL, Oxytrol ) Ditropan XL – This agent first appeared as a 5 mg tablet for immediate release, administered every six to eight hours. More recently, it has been made available as an extended release formulation in 5 mg and 10 mg tablets. The initial starting dosage in adults is 5 mg or 10 mg once daily, with incremental dose titration of 5 mg, adjusted to achieve a balance of efficacy and tolerability to up to 30 mg per day. Pediatric patients six years of age and older may start at 5 mg once daily and titrate upward in a 5 mg dose adjustment (based on a balance of tolerability and efficacy) to a total of 20 mg. Due to the patented oral osmotic (OROS) delivery system, the tablet should be taken whole without chewing, crushing, or dividing it. Ditropan is metabolized by the cytochrome P 450 enzyme system in the liver and small intestinal wall. This is described as first pass metabolism and results in the active metabolite Ndesethyloxbutynin. This metabolite is largely responsible for the side effect of dry mouth. Since Ditiropan XL is released in a steady state over twenty-four hours, releasing a greater quantity in the colon than the immediate release variety, a decrease in the effects of first pass metabolism is realized. Co-administration of other potent cytochrome P450 3A4 enzyme inhibitors (e.g., itraconazole, miconazole, erythromycin, clarithromycin, protease inhibitors and others) should be used with caution. An increase in side effects is realized with concomitant administration of other anti-cholinergic agents. Verathon Inc. 800.331.2313 www.verathon.com 32 Urology for Primary Care The Overactive Bladder Caution and appropriate dose reduction should be exercised in accordance with the patient’s general health status and with the use of other medications, such as tricyclic antidepressants. The use of this medication is contraindicated in patients with urinary retention, gastric retention and other gastrointestinal conditions with severely decreased motility, uncontrolled narrow-angle glaucoma, and in patients who are at risk for these conditions. Please review the package insert for more complete contraindications, cautions and a listing of adverse events. The most common side effects are dry mouth, constipation, headache, asthenia, and somnolence. Behavioral cognitive changes should be monitored, particularly in the elderly. Caution is recommended when considering use in patients with hepatic and renal impairment. Oxytrol (Oxybutynin Transdermal System) – In this delivery system, a transdermal patch is utilized to administer oxybutynin in a continuous and consistent manner over a three to four day interval. Because little of the cytochrome P 450 CYP3A4 enzyme pathway is found in skin, transdermal administration thus significantly reduces first pass metabolism of oxybutynin and limits formation of metabolite N-desethyloxybutynin and its troubling side effect of dry mouth. Pediatric safety and efficacy have not been established. The major side effect is application site reactions, such as erythema, rash, and pruritus. Dry mouth and constipation were also noted. Pharmacokinetic studies were not performed with patients receiving concomitant cytochrome P450 enzyme inhibitors. Similar contraindications are suggested as with other anticholinergic medications. Tolterodine (Detrol , Detrol LA): Initial launch of the immediate release formulation (2 mg po bid) in 1998 was heralded as a anticholinergic/ antimuscarinic with less affinity for the salivary glands. Thus, patients experienced less dry mouth than those receiving immediate release oxybutynin. The more recently released (2001) extended release formulation, Detrol LA, continues to demonstrate more uroselective behavior than other agents, contributing to it’s improved tolerability. Uroselective behavior suggests high affinity for the bladder muscarinic receptors and less for those in the salivary glands. The ability of the extended release formulation (Detrol LA 4 mg po q day) to achieve steady peak plasma concentrations over a twenty-four hour period allows for excellent and consistent plasma levels, while reducing serum level peak and valley variability. This results in consistent efficacy and tolerability. The CNS penetration of this agent may be limited, secondary to the bulky nature of the molecule and its decrease in lipophilicity and charge as compared to oxybutynin. All tertiary amines can cross the blood brain barrier. The safety and efficacy in pediatric patients has not been established. Similar precautions regarding cytochrome P450 CYP3A4 pathway are suggested, as with other agents. Solifenacin (VESIcare ): This agent was launched in the United States after FDA approval in January 2005. It is rapidly gaining acceptance in the overactive bladder marketplace due to its remarkable dry rate (51% control of urinary urge incontinence in clinical studies presented to the FDA). This medication is available as Vesicare 5 mg q day recommended starting dose. It can be titrated to 10 mg (available as a 10 mg capsule) as required, regarding a balance of clinical efficacy and tolerability. Like tolterodine, it seems to demonstrate uroselective behavior, making it very tolerable while Verathon Inc. 800.331.2313 www.verathon.com 33 Urology for Primary Care The Overactive Bladder being efficacious. The significant improvement in the overactive bladder wet population makes for high patient satisfaction. The medication should be taken whole and should not be split, crushed or chewed. Safety and efficacy in the pediatric population have yet to be determined. Similar precautions regarding cytochrome P450 CYP3A4 pathway are recommended, as with other agents. Darifenacin (Enablex ): This agent received FDA approval in 2004 and was launched in the first quarter of 2005. It is a competitive antimuscarinic receptor antagonist, as are other agents in this category. It differs from other agents in demonstrating greater affinity for the M3 receptor. The M3 receptor has been demonstrated to be active in bladder contractility. This is supplied as a 7.5 mg po extended release tablet. Safety and efficacy in the pediatric population have not been established. No dose adjustment is recommended for patients with renal impairment. Dose adjustment is recommended for patients with hepatic impairment. Adverse effects were noted to be dry mouth, constipation, headache, and dyspepsia. Additional adverse events, reactions, and contraindications can be found in the package insert. Similar precautions regarding the cytochrome P450 CYP3A4 pathway are recommended, as with other agents. Tropsium (Sanctura ): This agent has been available in Europe for some time. Recently launched in the United States in 2004, tropsium is the only currently available antimuscarinic/ anticholinergic agent that is a quaternary amine. Quaternary amines do not cross the blood brain barrier with any significant levels. This medication is supplied as a 20 mg tablet and the suggested dosage is 20 mg po bid. For geriatric patients over seventy-five years of age, it is recommended that the dose be decreased to 20 mg po q day. Similar dose reduction is suggested for patients with renal impairment. The cytochrome P450 does not appear to contribute significantly to the elimination of tropsium. Leading adverse reactions include dry mouth, constipation, headache, and dyspepsia. Other adverse events and contraindications should be reviewed in the package insert. All medications in this category are contraindicated in patients with urinary retention, gastric retention and impaired bowel motility, uncontrolled narrow-angle glaucoma, and in patients who are at risk for these conditions. Other precautions regarding dosage adjustment, drug-drug interactions, adverse events, and contraindications should be thoroughly reviewed for each product prior to prescribing. Behavioral Therapy Behavioral therapy has been demonstrated to alter a patient’s voiding habits due to overactive bladder. While behavioral therapy and pharmacological therapy each demonstrate statistically significant improvement in the symptoms of overactive bladder, the combination of the two is superior to any one therapy. Behavior modification may include education of the patient regarding modification of fluid intake and lower urinary tract function. The introduction of a voiding diary or frequency volume chart may facilitate this aspect of behavioral modification. Some patients may benefit from a voiding schedule, rather than being allowed to void by demand. Verathon Inc. 800.331.2313 www.verathon.com 34 Urology for Primary Care The Overactive Bladder Timed voiding and voiding deferment have been demonstrated to be excellent strategies in improving bladder storage, particularly when coupled with pharmacologic therapy. Kegel exercises will benefit patients with urinary urgency and urinary urge incontinence, as well as those with stress urinary incontinence. Some patients will also find biofeedback to be helpful. Electrical Stimulation Therapy Currently, electrical stimulation comes in many forms. Some patients will find surface stimulation from lower extremity pathways helpful. More severe cases require sacral electrode implantation. This has been shown to be effective for patients refractory to pharmacologic therapy for the overactive bladder. Botox Intravesical injection of Botox is currently being studied as a possible treatment for overactive bladder patients refractory to oral medication and behavioral modification. Initial study data demonstrates significant improvement in bladder storage with reduction in urinary urgency and frequency when Botox is administered in this manner. The Botox injections require repeated application over time. Catheterization Patients may be treated temporarily or on a permanent basis with either a urethral catheter or a suprapubic tube. This form of treatment is less favored, due to the associated risks of chronic infection, calculus formation, and, if used for long-term treatment, bladder malignancy. Bladder Augmentation Patients whose bladders have decreased in capacity secondary to injury, or have significant loss of compliance, may benefit from augmentation cystoplasty. In this surgical procedure, either small bowel or colon tissue is added to the bladder to increase its capacity and compliance. CONCLUSION After consideration of a patient’s symptoms, verification of a benign urinalysis, and determination of minimal residual urine, a trial of pharmacologic therapy is appropriate. Patients with pure stress incontinence or patients who have more complex presentations may always be considered for referral to the appropriate specialist for further evaluation. Verathon Inc. 800.331.2313 www.verathon.com 35 Urology for Primary Care The Aging Male THE AGING MALE Mike B. Siroky, M.D. Professor of Urology, Boston University School of Medicine Director of Neuro-Urology, Boston University Medical Center Chief of Urology, VA BHS Staff Urologist, VA BHS Boston, MA It is commonly accepted that the proportion of elderly individuals in our society is growing. In many Western countries, it is projected that one-third of the population will be aged at least sixty by the year 2025. Prolonged life span will likely produce increased consultations by men concerned about age-dependent complaints. In contrast to the female menopause, the process of aging in the male genital system is slow and highly variable between individuals. Impairment of spermatogenesis is a continuous, decadeslong process that may never reach the point of causing infertility. Men do not experience a sudden fall in Leydig cell or seminiferous tubular function as they age; rather, there is a slow and persistent decrease in sperm production with aging. Only one-third of men over age sixty and 50% of men over age eighty are completely infertile. Men have fathered children even when over ninety years of age. Similarly, testosterone production falls slowly rather than precipitously. Clinical signs of male aging include decreased muscle mass and strength, reduced sexual hair, and diminished libido and sexual performance. THE ROLE OF TESTOSTERONE Although it has androgenic properties, testosterone (T) is best regarded as a testicular prohormone that is converted into two major products – dihydrotestosterone (DHT) and 17ß estradiol (E). For the conversion of T to DHT, the required enzyme is 5α reductase while for conversion to E, it is the enzyme aromatase found in fat, muscle, liver, and kidney. DHT binds to the same receptor as T, but at much higher affinities. Thus, lack of 5α reductase reduces DHT levels and produces a relative androgen deficiency. The 5α reductase type 2 inhibitor finasteride produces reduction in prostate size and occasional complaints of reduced libido and ejaculatory volume, but no change in muscle mass or bone density. The role of E in the male is uncertain, but it seems to be involved in closure of bone epiphyses and, along with androgens, in maintaining bone mass. TESTICULAR MORPHOLOGY IN AGING MEN With advancing age, the seminiferous tubules gradually become atrophic and increasing disturbances of spermatogenesis can be observed by electron microscopy. Sertoli cells are also affected, as they become increasingly vacuolated due to their role in phagocytizing degenerated germ cells. In addition, the number of Leydig cells declines with advancing age. This correlates with the well-known decrease in free testosterone and total testosterone as males age. Verathon Inc. 800.331.2313 www.verathon.com 36 Urology for Primary Care The Aging Male ENDOCRINE CHANGES Advancing age is generally accompanied by a progressive decline in the synthesis of testosterone by Leydig cells, as well as a decline in the number of functioning Leydig cells. This induces an increase in leutinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Although lower sex hormone concentrations may be evident as early as the fifth to sixth decade of life, there is great variability, and testosterone levels in older men may well be within the normal range for younger men. It is only after the seventh decade of life that an age-associated decrease of testosterone becomes statistically significant. In addition, the level of free testosterone falls with aging. Normally, about 98% of testosoterone is bound by sex hormone binding globulin (SHBG), and only 2% of testosterone is free and thus biologically active. After the age of seventy, serum SHBG levels begin to rise, resulting in lower concentrations of free testosterone. Elevated SHBG levels in elderly men are caused by an increase of free estradiol due to aromatization of androgens, especially in the peripheral fatty tissue. Therefore, apart from decreased synthesis of testosterone, higher levels of SHBG reduce the availability of biologically active free testosterone. At the hypothalamo-pituitary level, elevated LH secretion partly compensates for reduced testosterone synthesis by the Leydig cells; however, LH levels are not high enough to normalize free testosterone levels. Furthermore, high amplitude LH pulses from the pituitary gland are significantly less frequent. Finally, the normal circadian rhythm of high serum testosterone levels in the early morning disappears in the aging male. Male aging is also accompanied by a dramatic decline in adrenal androgens, e.g. dehydroepiandrostertone (DHEA). Peak DHEA secretion occurs at about thirty years of age and declines rapidly thereafter. Males of seventy years or more have DHEA levels that are 10-15% of their peak values. No specific receptor for DHEA has been identified, leading to the idea that it serves as a precursor for other more specific hormones, such as estrone. Whether the decline in DHEA levels represents a disease, or is merely an aging-associated phenomenon, is still unclear. DHEA supplementation is widely available in the United States without prescription and has been touted as an anti-aging agent. No long-term studies are available to support this claim, but one-year studies using 50 mg DHEA/day seem to indicate no harmful effects. THE “MALE CLIMACTERIC” In 1939, Wermer described men aged fifty years or older who complained of impaired memory, lack of concentration, tiredness, nervousness, and lowered resistance to stress. A sudden reduction in plasma testosterone levels was postulated and the syndrome was termed the “climacterium virile.” The existence of a true male climacteric has been debated over the years. None of the symptoms attributed to the so-called male climacteric have been demonstrated to be of statistically significant prevalence in men at the fifth or sixth decade of life, as compared to younger men. Studies have shown a poor correlation between such symptoms and lowered plasma testosoterone levels in the aging male. The fact that administration of androgenic compounds may ameliorate some of these symptoms does not necessarily support the hypothesis, as androgens have well-known psychotropic effects. In recent years, terms such as partial androgen deficiency Verathon Inc. 800.331.2313 www.verathon.com 37 Urology for Primary Care The Aging Male of aging men (PADAM) and andropause have been suggested to characterize the psychosexual problems of aging men. SEXUALITY AND LIBIDO IN AGING MEN A decline in frequency of intercourse is observed after the age of forty and becomes more pronounced between the fifties and seventies. However, it must be remembered that many factors influence the frequency of intercourse, including sexual desire, opportunity, general health, mobility, and medications, among others. While only 15% of men over sixty years of age claim to have no interest in sexual activity, the corresponding rate in women is several times higher. Thus, lack of interest on the part of the spouse may induce the male to give up on regular sexual activity. According to the Massachusetts Male Aging Study, complete loss of erectile function is reported by 5% of men at age forty, and by 15% of men aged seventy years, while moderate erectile dysfunction occurs in 17% and 34%, respectively. Endocrine insufficiency, including hyperprolactinemia, is causative in less than 5% of men with erectile dysfunction (ED). Erectile dysfunction in older men is, in fact, multifactorial in origin. The most important cause is peripheral vascular disease, but diabetes mellitus, peripheral neuropathy, pelvic surgery, impaired fat metabolism, and renal insufficiency may also contribute. Cigarette smoking is an independent risk factor, as well as an indirect cause of ED, due to its deleterious effect on cardiovascular disease. Drugs may also impair sexual function, including antihypertensive agents (beta blocking agents and angiotensin-converting enzyme inhibitors), psychopharmacological agents, antiandrogens, antihistamines, diuretics, and chemotherapeutic agents. In many cases, erectile dysfunction is the first indicator of significant cardiovascular disease. The patient who presents with new onset ED to his Primary Care physician should be carefully investigated for symptoms and signs of cardiovascular disease. In many cases, erectile dysfunction is the first clinical sign of a significant cardiovasculopathy, especially coronary artery disease. TREATMENT OF ED A discussion of the many options available today for ED therapy is beyond the scope of this chpater; however, a few comments on the use of sildenafil in patients with cardiovascular disease are in order. Sildenafil citrate is the initial treatment of choice in most cases of organic erectile dysfunction. Overall, it is an extremely safe and well tolerated drug. The most common side effects are headache, flushing (due to vasodilation), and dyspepsia (due to gastroesophageal reflux). Nevertheless, there are two major areas of concern in using sildenafil citrate. The first concern is the risk to the cardiovascular patient of myocardial ischemia as a consequence of increased sexual activity. Prior to considering sildenafil citrate or any other treatment, the Primary Care physician should assess the risk of increased exertion posed by sexual intercourse. The risk of coronary complications during sexual activity is low. Of more than 1700 men with myocardial infarction, only 3% had had intercourse within two hours before the heart attack. Nevertheless, prescribing sildenafil is inadvisable to patients with unstable angina pectoris, Verathon Inc. 800.331.2313 www.verathon.com 38 Urology for Primary Care The Aging Male patients with significant cardiac failure, and patients within six months of acute myocardial infarction. The second concern is the increased risk of hypotension when sildenafil citrate is prescribed along with certain nitrovasodilating agents. There are reports of serious hypotension or circulatory shock when sildenafil citrate is given to patients taking nitrovasodilating drugs. It is well known, even by patients, that sildenafil is contraindicated in patients receiving any kind of nitrate medication. This is especially true for nitric oxide donors such as glyceryl trinitrate and isosorbide dintrate. Vasodilators such as diltiazem may be taken with sildenafil safely. CHANGES IN MUSCLES AND BONES With advancing age, the male skeleton begins to lose mass. Starting approximately at age thirty, there is a continuous reduction in the amount of bone, and by age seventy, approximately 30% of the original skeletal mass is lost. Compared with osteoporosis in women, loss of bone in men is less dramatic. Due to the greater initial bone mass in men, the risk of osteoporotic fracture is significantly lower in men than in women. Factors that may contribute to osteoporosis in men are genetic disposition, endocrine changes, immobilization, and nutrition. In adults, bone density at any given age is determined by the peak bone mass achieved at sexual maturity and the rate of bone loss thereafter. At puberty, there is a marked increase in testosterone levels that is followed closely by an increase in alkaline phosphatase activity and subsequently of cortical bone density. Peak bone mass in men is reached during the mid-twenties. Thereafter, bone density declines linearly with aging. It is clear that hypogandism plays a major role in osteoporosis, but the exact mechanism is unclear. In castrated young men, progressive bone loss has been observed with increasing years after orchidectomy. Both androgen and estrogen receptors have been demonstrated in osteoblasts. Androgens may also interact with calcium regulatory hormones such as calcitonin. In addition, human bone cultures have been shown to convert testosterone to dihydrotestosterone (DHT), indicating that DHT may be the active androgen in bone. Finally, locally active cytokines may also play an important role in bone metabolism, including prostaglandins, TGF-β and insulin-like growth factor 1, which stimulates collagen production by osteoblasts. THE USE OF ANDROGEN SUPPLEMENTATION IN THE AGING MALE Androgen supplementation in the aging male remains controversial and a careful risk-benefit assessment should be made in each case. The major benefits claimed for androgen supplementation are improved libido, increased energy, and improved bone density and/or muscle mass. Several possible drawbacks to androgen supplementation have been proposed, but the major one is the possibility of accelerated growth of undetected prostatic cancer. Verathon Inc. 800.331.2313 www.verathon.com 39 Urology for Primary Care The Aging Male Other possible drawbacks include: 1. 2. 3. 4. Changes in LDL/HDL ratio to increase cardiovascular risk Stimulating effect on erythropoiesis with a risk of polycythemia More rapid development of benign prostatic hyperplasia Development of sleep apnea Hajjar et al (1997) compared long-term testosterone replacement in 45 older hypogonadal men who received 200 mg testosterone enanthate or cypionate IM every two weeks for at least two years, with a control group consisting of 27 hypogonadal men taking no testosterone. After two years, there was improved libido and an elevation in hematocrit in the testosterone-treated group. In contrast to prior studies, no significant increase in prostate-specific antigen level was noted. Twenty-four percent of the testosterone-treated subjects developed polycythemia sufficient to require intervention. In a meta-analysis of androgen supplementation by Tenover in 1997, it was concluded that improvement in bone and muscle mass are reliably seen. Improved sexual function is also seen in most cases. Evaluation The patient complaining of symptoms suggestive of andropause should undergo: Complete history, including social history, marital history, prescription and recreational drug use, prior urologic surgery, and characterization of current complaints. In patients complaining of ED, the physician should assess the libido, the frequency and quality of erection, and ejaculatory ability and volume. Physical examination, including assessment of prostate size and consistency by digital rectal examination as well as assessment of testicular size and consistency. Blood work, including prostate specific antigen (PSA) level, complete blood count, blood sugar, BUN, serum creatinine, lipid profile, and liver function tests. Total testosterone and serum LH should be obtained. In patients with low normal or below normal T levels, free testosterone and serum prolactin should be measured. Bone density measurement may be considered as a baseline value. Treatment As discussed above, androgens are involved in a wide variety of different biological processes and the levels required to maintain them are currently unknown. In other words, is it necessary to achieve “normal” levels of serum testosterone to support bone mass, muscle mass, libido, erectile function, and ejaculation? There is evidence that about 50% of normal serum levels is sufficient to maintain libido and erectile function. The levels required to sustain other androgenic effects is unknown and may be quite different. In this situation, it is advisable to carefully monitor serum T and LH levels during therapy, especially just prior to the next scheduled administration of androgen. The LH level is probably the best indicator available to assess the adequacy of androgen replacement. Methods of testosterone supplementation include intramuscular injection of testosterone esters as the enanthate or cypionate, testosterone gel or transdermal systems, and oral administration of Verathon Inc. 800.331.2313 www.verathon.com 40 Urology for Primary Care The Aging Male testosterone undecanoate or methyltestosterone. Transdermal delivery has the advantage of maintaining physiologic levels of testosterone over the treatment period. Table: Some commonly used testosterone preparations Agent Dose Range How Supplied Possible Adverse Effects testosterone enanthate 50-400 mg IM q syringe containing cholestatic jaundice, liver 2-4 weeks 1 ml or 200 mg dysfunction hepatocellular cancer, serum cholesterol, polycythemia testosterone gel 5-10 G daily to non-scrotal skin 1% dermal gel same as above plus local skin irritation testosterone transdermal 2.5 - 5 mg daily to non- scrotal skin 2.5 mg, 5 mg skin patch same as above plus pruritis, skin irritation methyltestosterone 10 - 50 mg PO daily 10 mg capsules same as above The risks of long-term testosterone replacement therapy, especially in the area of cardiovascular disease and prostate cancer, are unknown. At this point, the use of androgens should probably be limited to men with total and free testostorone levels below the normal range. Patients with biopsy proven prostate cancer, elevated PSA, or severe voiding symptoms should not be considered for testosterone replacement therapy. CONCLUSION Current knowledge regarding the aging male remains inadequate. Androgen deficiency in the aging male will likely increase as the population continues to age and the average life span increases. The Primary Care physician will continue to be in the forefront of dealing with these problems and must apply available knowledge to help his or her patients reach reasonable decisions regarding treatment. Verathon Inc. 800.331.2313 www.verathon.com 41 Urology for Primary Care Hematuria INITIAL EVALUATION OF HEMATURIA FOR PRIMARY CARE PHYSICIANS David S. Wang, M.D. Assistant Professor of Urology Department of Urology Boston University School of Medicine Boston, Massachusetts Richard K. Babayan, M.D. Professor and Chief of Urology Department of Urology Boston University School of Medicine Boston, Massachusetts INTRODUCTION Hematuria (blood in the urine) may originate from any part of the urinary tract and may signify serious underlying disease. Whether microscopic or gross, hematuria may arise from a variety of potentially life-threatening etiologies, such as malignancy, and should never be ignored. Therefore, it is important to recognize which patients with hematuria require further investigation and urologic evaluation. MICROSCOPIC HEMATURIA Microscopic hematuria is usually detected initially on a urine dipstick test, in which oxidation caused by hemoglobin results in a color change. However, false-positive dipstick readings are common, and a positive dipstick reading must be confirmed by microscopic urinalysis of the centrifuged urine sediment before proceeding to additional testing. Microscopic hematuria is defined as the presence of >3 erythrocytes per high power field (HPF) of centrifuged sediment examined microscopically. Full urologic evaluation is indicated when asymptomatic microscopic hematuria is confirmed in 2 out of 3 properly collected urine specimens. A freshly voided, cleancatch midstream urine sample is a properly collected specimen. In patients with risk factors for urologic disease (see below), full urologic evaluation is warranted with one urinalysis containing >3 red blood cells (RBC)/HPF. Evaluation should be considered in those at high risk for urothelial carcinoma with the presence of 1 to 2 RBC/HPF. Verathon Inc. 800.331.2313 www.verathon.com 42 Urology for Primary Care Hematuria Risk factors for the presence of significant urologic disease include the following: Age >40 years Smoking history Occupational exposure to chemicals or dyes Prior history of urologic disease History of recurrent urinary tract infections Gross hematuria Irritative voiding symptoms in the absence of infection History of analgesic abuse History of pelvic irradiation The presence of proteinuria, RBC casts, renal insufficiency, or dysmorphic RBCs in the urine should alert the physician to the possibility of renal parenchymal disease and the need for appropriate evaluation and/or referral to a Nephrologist. GROSS HEMATURIA The presence of blood in the urine visible to the naked eye is defined as gross hematuria. The probability of identifying significant urologic pathology increases with the degree of hematuria. Gross hematuria nearly always warrants a full urologic investigation with upper tract imaging and cystoscopy to exclude the possibility of urologic malignancy or renal calculus disease. Gross hematuria often occurs in the presence of a urinary tract infection (UTI). Because tumors of the bladder and urothelial carcinoma in situ may present as a UTI, it is important that patients with UTI who develop gross hematuria undergo urologic evaluation following resolution of the UTI. Gross hematuria should never be dismissed as merely the result of a UTI; instead, proper urologic evaluation must be performed to exclude the presence of malignancy or urolithiasis. Often, patients who are on anticoagulants (warfarin [Coumadin ], heparin, or clopidogrel) develop gross hematuria. Still, full urologic evaluation is indicated in these patients, as a clinically significant urologic condition may be uncovered by the anticoagulation. UROLOGIC EVALUATION Urologic evaluation is required in all adults with gross hematuria, and in adults with asymptomatic microscopic hematuria without characteristics suggesting renal parenchymal disease. Evaluation consists of a careful history and physical examination, appropriate laboratory analysis, urine cytology, radiographic upper tract imaging, and cystoscopy. History and Physical Examination In evaluating hematuria, several key elements should be ascertained. The degree of hematuria (microscopic or gross) should be determined. The timing of the hematuria (beginning, end, or Verathon Inc. 800.331.2313 www.verathon.com 43 Urology for Primary Care Hematuria throughout the entire stream) may suggest the location of the source of bleeding. The presence of pain in association with hematuria may indicate stone disease or obstruction. In addition, the presence and shape of clots may suggest the location of the source of bleeding and the degree of bleeding. Associated symptoms, such as flank pain, lower urinary tract symptoms, obstructive voiding symptoms, and irritative voiding symptoms may help elucidate the etiology of the hematuria. Risk factors for significant urologic disease are obtained from the history. Smoking is a known risk factor for the development of urothelial carcinomas. In addition, prior exposure to chemicals and dyes is associated with urothelial carcinomas. Those patients with previous urologic disease are also at risk for urologic disease in association with hematuria. Urine Cytology Urine cytology is useful for detecting aggressive cancers of the bladder. It is highly specific; therefore, a positive cytology nearly always indicates the presence of a urologic malignancy. Although the sensitivity of urine cytology for low-grade lesions is moderate, urine cytology detects most high-grade transitional cell carcinomas of the bladder and also carcinoma in situ of the bladder, both aggressive life-threatening forms of bladder cancer. Urine cytology is also commonly utilized to follow patients with a history of bladder cancer. Upper Tract Imaging Radiologic imaging of the kidneys and ureters is a standard component of the urologic evaluation for hematuria. There are several options for upper urinary tract imaging: 1. Computed Tomography (CT) scan – Abdominal/pelvic CT scan (with and without contrast) has become the imaging modality of choice for detecting small lesions of the kidney and renal pelvis. CT has the highest sensitivity for detecting stones in the urinary tract and also has the highest sensitivity for detecting small renal masses. Improvements in CT scanning technology, including CT urography and angiography, have allowed for better imaging of the ureters and renal vasculature, respectively. CT scanning is more cost effective and widely available than MRI. Thus, the CT scan is considered by most to be the best initial diagnostic study of the upper tract for patients with hematuria. 2. Intravenous pyelography (IVP) – IVP has long been considered to be the best imaging study for initial evaluation of the upper urinary tract for hematuria. Although it is widely available, limitations of IVP for evaluation of hematuria include the limited ability to detect small renal lesions and the difficulty of distinguishing solid from cystic renal masses. However, IVP is superior to ultrasonography in detecting urothelial tumors of the kidney or ureter. Improvements in CT scanning technology have resulted in the CT scan supplanting the IVP in most centers. IVP can be performed in conjunction with renal ultrasound to improve the sensitivity for detection of renal lesions. Verathon Inc. 800.331.2313 www.verathon.com 44 Urology for Primary Care Hematuria 3. Abdominal Ultrasound – Ultrasonography is excellent for distinguishing renal cysts from solid renal lesions, but has limited utility in the evaluation of the ureters and renal pelvis. Although easily able to detect lesions >2.5 cm, ultrasound will often miss lesions of smaller size. In patients at risk for significant urologic disease or malignancy, ultrasound alone is not adequate for evaluation. 4. Retrograde pyelography (RPG) – RPG provides excellent visualization of the ureters and renal pelvis, but requires cystoscopic evaluation and is often not well tolerated by patients. RPG also has limited utility in detecting small renal lesions. Therefore, RPG is not considered an initial upper tract imaging study. Cystoscopy Cystoscopy (endoscopic visualization of the bladder) is required to complete the urologic evaluation of hematuria. This can be performed in the office setting with only topical lidocaine gel. Improvements in endoscopic instrumentation, particularly with the flexible cystoscope, have allowed this procedure to be easily performed with minimal discomfort to the patient. Cystoscopy is required to exclude the possibility of significant bladder or urethral pathology, such as bladder tumors, bladder stones, benign prostatic hypertrophy, or urethral stricture. FOLLOW-UP A negative initial evaluation for microscopic or gross hematuria is often sufficient and follow-up is not necessary. However, some patients with a negative initial evaluation for hematuria eventually develop significant urologic disease. Thus, follow-up is generally recommended, particularly in those at high risk for the development of bladder cancer, such as smokers and those with occupational exposures. Follow-up should consist of repeat urinalysis and urine cytology at 6, 12, 24, and 36 months. Urine cytology has a relatively high sensitivity for detecting high-grade lesions and carcinoma in situ of the urinary tract, which are considered the most life-threatening malignancies of the urothelial tract. The presence of a suspicious or positive urine cytology requires immediate urologic evaluation. Repeat urologic evaluation should be performed if a patient develops an additional episode of gross hematuria, especially if the second episode occurs over one year following the first. Patients who develop irritative voiding symptoms in the absence of infection should undergo repeat evaluation. Occasionally, repeat urologic evaluation should be considered in patients with persistent microscopic hematuria and at high risk for developing urothelial carcinoma who have had an initial negative urologic survey. If after three years the patient does not have or has not developed any of the above conditions, then no further urologic monitoring is required. Evaluation for renal parenchymal disease should be considered if the hematuria is associated with hypertension, proteinuria, RBC casts, or dysmorphic RBCs. Verathon Inc. 800.331.2313 www.verathon.com 45 Urology for Primary Care Hematuria SUMMARY Hematuria of any degree, particularly in the adult, can be the initial sign of serious underlying urologic disease, including malignancy. Gross hematuria always warrants full urologic evaluation, even if it occurs in the presence of UTI. Microscopic hematuria, defined as >3 RBC/HPF, requires full urologic evaluation if present in 2 of 3 specimens. In those at risk for significant urologic disease (including adults >40 years), strong consideration should be given for full urologic evaluation after one properly performed urinalysis with >3 RBC/HPF. Verathon Inc. 800.331.2313 www.verathon.com 46 Urology for Primary Care Initial Management of Urinary Calculus Disease INITIAL MANAGEMENT OF URINARY CALCULUS DISEASE FOR PRIMARY CARE PHYSICIANS David S. Wang, M.D. Assistant Professor of Urology Department of Urology Boston University School of Medicine Boston, Massachusetts Richard K. Babayan, M.D. Professor and Chief of Urology Department of Urology Boston University School of Medicine Boston, Massachusetts INTRODUCTION Urinary stones have plagued humans since the beginning of recorded history. Archaeologists have uncovered urinary stones in the mummified remains of Egyptians estimated to be more than 7,000 years old. The treatment of stones of the urinary tract has changed dramatically over the past twenty years. Historically, the majority of renal and ureteral stones required open surgical intervention. Today, the vast majority of stones can be effectively treated with minimally invasive surgical alternatives, such as endourologic techniques and extracorporeal shock wave lithotripsy (ESWL). This has eliminated the need for open surgery in the vast majority patients; hence, open surgery for urolithiasis is exceedingly rare. EPIDEMIOLOGY OF STONES In industrialized nations, stones affect one to five percent of the adult population. In the United States, stone disease accounts for more than 400,000 hospital visits annually. The peak incidence occurs in the third to fifth decades. Men are affected three times more commonly than women, and whites are affected five times more commonly than blacks. In a patient who has passed one stone, the likelihood of passing another is 15% by 3 years and 30% by 5 years. CLINICAL PRESENTATION A renal calculus is usually asymptomatic until the stone moves and produces either hematuria or urinary obstruction. Renal colic occurs in the presence of obstructing urinary tract calculi and is characterized by the sudden onset of severe flank pain. The pain typically begins in the flank, courses laterally around the abdomen, and may radiate to the groin area. The pain may be intermittent and associated with nausea, vomiting, and urinary symptoms. Chronic obstruction may be asymptomatic. Hematuria, gross or microscopic, almost always accompanies an acute episode of stone colic; however, microscopic hematuria may be absent in up to 15% of cases, and the absence of hematuria does not exclude urinary calculi. Microscopic inspection of the Verathon Inc. 800.331.2313 www.verathon.com 47 Urology for Primary Care Initial Management of Urinary Calculus Disease urinary sediment may reveal crystals that may suggest the type of stone present. In 25% of cases, patients give a family history of stone disease. DIAGNOSIS The initial evaluation of renal colic (often in the emergency department) should include a serum white blood count, creatinine, urinalysis and urine culture (if urinalysis is positive), and a plain film of the abdomen. Approximately 80% of urinary calculi are radiopaque and can be seen on abdominal films. If available, renal ultrasonography (US) can be obtained to exclude hydronephrosis. Historically, intravenous pyelography (IVP) was the study of choice for evaluating patients with suspected renal colic. However, the preferred initial study of choice in nearly all centers is the noncontrast helical CT scan of the abdomen/pelvis, which has a sensitivity of over 95%. The spiral CT scan is rapid and does not require oral contrast, bowel preparation, or intravenous contrast. The study confirms the presence of calculus disease in the urinary tract, demonstrates the degree of obstruction, and can identify other intra-abdominal pathology. ACUTE TREATMENT Acute treatment of the acute episode depends on the size and location of the stone, degree of obstruction, and the patient’s clinical status. 1. Ureteral colic – The most important goal in the management of ureteral colic is obtaining adequate pain relief. This can be accomplished with parenteral narcotics or nonsteroidal antiinflammatory drugs (NSAIDs). Morphine sulfate and meperidine are commonly used parenteral narcotic analgesic agents. Ketorolac tromethamine (Toradol ) is effective as well for renal/ureteral colic. Antiemetic agents, such as metoclopramide HCl and prochlorperazine, should be used as needed. Patients tolerating oral medication may be treated with 1-2 oral narcotic/acetaminophen tablets every four hours as needed for pain, 600-800 mg of ibuprofen every eight hours, and 30 mg of extended release nifedipine once daily to induce ureteral smooth muscle relaxation. 2. Indications for immediate urologic intervention – Most patients who present with ureteral colic can be managed with adequate analgesics and undergo definitive stone management at a later date. However, there are indications for immediate urologic consultation and urgent intervention, usually in the form of a ureteral stent or a percutaneous nephrostomy tube: Suspected urinary tract infection – may lead to life-threatening sepsis if urinary tract obstruction is not relieved High-grade obstruction Solitary functioning kidney Significant acute renal insufficiency as determined by serum creatinine Pre-existing renal insufficiency Persistent pain not relieved by oral analgesics Verathon Inc. 800.331.2313 www.verathon.com 48 Urology for Primary Care Initial Management of Urinary Calculus Disease DEFINITIVE MANAGEMENT OF RENAL OR URETERAL CALCULI For most patients, immediate intervention is unnecessary and definitive management of renal or ureteral calculi may be accomplished on an elective basis. Several management options exist: 1. Expectant management – Patients with small stones (<5 mm on CT) and minimal hydronephrois may be treated conservatively as outpatients with oral hydration and analgesics. Nearly 90% of ureteral stones <4 mm in size will pass spontaneously, whereas only 20% of stones >6 mm in size will pass without intervention. In the absence of highgrade obstruction or infection, conservative nonsurgical management is indicated when the stone is <4 mm in diameter. The patient is instructed to drink large quantities of fluid, strain the urine, and save any stone fragments. Four to six weeks should be allowed for stone passage. Follow-up imaging is required with at minimum an abdominal film and renal ultrasound to confirm stone passage and exclude silent hydronephrosis. Alternatively, an IVP or CT scan can be obtained. It is critical to obtain follow-up imaging studies even when the patient has passed the stone. Occasionally, a small fragment of the original stone will pass but a significant fragment will remain and ultimately require urologic intervention. Nonoperative management is often an option for patients with urinary tract stones composed of uric acid. Such stones can be seen on CT scan but are not visible on abdominal plain film. When no indications exist for immediate intervention, patients with uric acid stones may be treated with urinary alkalinization. The patient is given potassium citrate or sodium bicarbonate to maintain the urine pH >6.5. Often, urinary alkalinization alone causes uric acid stones to completely dissolve, even large stones. Follow-up imaging with a CT scan is necessary to monitor treatment. 2. Extracorporeal shock wave lithotripsy (ESWL) – ESWL was developed in Germany in the early 1980s and has had an enormous impact on the treatment of stones. The treatment is based on the propagation of focused shock waves through the body which fragment the stones. The patient is positioned in a lithotripsy machine and the stone fragmented with focused acoustic shock waves delivered from outside of the body. ESWL is almost universally performed on an outpatient basis with intravenous sedation alone. ESWL is indicated for the treatment of most renal and upper ureteral calculi up to 1.5 to 2 cm in size. Success rates for upper tract and renal stones are 80% to 90% percent, and for ureteral stones 60% to 70%. Contraindications to ESWL include pregnancy, urinary tract infection, anticoagulation, and stones >2.5 cm in size. 3. Ureteroscopic management – Advances in instrumentation have allowed for the majority of ureteral stones to be treated successfully by ureteroscopy, or direct endoscopic visualization of the ureter. Currently, rigid and flexible ureteroscopes are available which are <2.5 mm in diameter, allowing for direct access to the ureter. Ureteroscopy is indicated for most midureteral and distal ureteral calculi. Flexible ureteroscopy allows for direct fragmentation of stones in the ureter and even the kidney. Ureteroscopic lithotripsy can be accomplished using a variety of energy sources; the most commonly used energy source today is the holmium laser. Often, an internal stent is placed at the conclusion of Verathon Inc. 800.331.2313 www.verathon.com 49 Urology for Primary Care Initial Management of Urinary Calculus Disease ureteroscopy and is removed in the office after one week. Currently, nearly all ureteroscopic procedures are performed on an outpatient basis with >95% success. 4. Percutaneous nephrolithotomy (PCNL) – PCNL involves gaining direct access to the kidney through a small puncture site through the flank. PCNL is indicated for large stones >2 cm in size or for staghorn calculi occupying the entire renal pelvis. Using rigid and flexible nephroscopes and a variety of fragmenting tools (ultrasonic lithotripsy is the most common), approximately 85% of patients can be rendered stone-free at three months. Long-term results are comparable with those of open surgery, although many large stones will require staged procedures. Even full staghorn calculi can be successfully removed with PCNL. 5. Open stone surgery – Once the most common method of treating urinary stone disease, open surgery is virtually never required today, given the advances in minimally invasive surgery and ESWL. In the past, ureterolithotomy and open nephrolithotomy (anatrophic nephrolithotomy) were common urologic procedures, but today are rarely performed. 6. Laparoscopic surgery has been performed in select patients with large (>2 cm) stones in the proximal ureter, although experience is limited. BLADDER STONES In the Western world, bladder stones are most often found in male patients and are caused by bladder outlet obstruction or foreign bodies (portions of catheters, sutures, or objects inserted through the urethra). Ureteral stones that reach the bladder can also act as a nidus for bladder stone formation. Bladder stones are composed of variable proportions of calcium oxalate, uric acid, and ammonium urate. If uric acid is a major component, bladder stones may be radiolucent. Patients with longstanding bladder stones are at risk for squamous metaplasia or carcinoma. Clinical presentation may include suprapubic or genital pain, intermittent stream, dysuria, and hematuria. Patients may also present with recurrent UTIs. Diagnosis is confirmed by abdominal film, ultrasound, and cystoscopy. The majority of bladder stones are successfully treated by endoscopic techniques, though occasionally open bladder stone removal (cystolithotomy) is required. METABOLIC EVALUATION A metabolic or environmental etiology of nephrolithiasis may be found in up to 90% of patients evaluated for stone disease; however, most patients do not require a full metabolic evaluation after a single stone episode. A limited evaluation includes a stone analysis and serum chemistries. Patients with abnormalities discovered after limited metabolic evaluation and repeat stone formers require a full metabolic evaluation, with urologic or nephrologic consultation. In addition, patients with risk factors for urinary stone disease warrant a full metabolic evaluation. Verathon Inc. 800.331.2313 www.verathon.com 50 Urology for Primary Care Initial Management of Urinary Calculus Disease Risk factors for urinary stone disease: Recurrent stone disease (two or more episodes) Staghorn (complete) renal calculi Family history of stones Bone or gastrointestinal disease Gout Chronic UTI Nephrocalcinosis GENERAL MEASURES OF STONE PREVENTION Although prevention of stone formation is largely dependent on the composition of the stone as well as any underlying metabolic abnormalities in the patient, several general preventative measures are suggested. 1. Hydration – Because urinary stone formation results from the supersaturation of urinary substrates, hydration is effective in preventing stone formation. Patients should measure their 24-hour urinary output once a week and adjust their fluid intake to maintain urine output of at least 3 liters per day. 2. Diet – Historically, patients with recurrent calcium stones were instructed to severely reduce dietary calcium ingestion. While excessive dietary calcium intake may be associated with increased risk for stone formation, it is now clear that marked dietary calcium restriction is actually associated with an increased risk of urinary stone formation because of gastrointestinal calcium loss and increased bone resorption. Thus, the current recommendation regarding dietary calcium intake is to maintain a moderate calcium intake. In patients at risk for osteoporosis, dietary calcium restriction is especially inappropriate. 3. Sodium – Increased dietary sodium intake results in excess natriuresis, which causes hypercalciuria. Thus, avoiding excessive sodium is a reasonable strategy for preventing recurrent calcium oxalate stone formation. 4. Other dietary modifications which have been shown to decrease stone formation include limiting daily meat intake, substituting whole wheat bread for white bread, and eating natural fiber cereals. All dietary changes should be performed in moderation. Dietary measures for preventing stone formation may be beneficial to general health and may reduce the risk of hypertension, cardiovascular disease, and colon disease. 5. Urinary alkalinization – In patients who form recurrent uric acid stones, alkalinizing the urine to maintain a urine pH >6.5 is highly effective in preventing recurrent uric acid nephrolithiasis. Potassium citrate and sodium bicarbonate are the most frequently used oral agents for alkalinizing the urine. Verathon Inc. 800.331.2313 www.verathon.com 51 Urology for Primary Care Initial Management of Urinary Calculus Disease 6. Medications – A variety of medications are used to reduce the incidence of stone formation, but a full metabolic evaluation is always required prior to starting therapy. Specific medications include thiazide diuretics, orthophosphates, sodium cellulose phosphate, allopurinol, citrates, and oral alkalizination agents. In general, medications are prescribed following urologic or nephrologic consultation. SUMMARY Once a significant source of morbidity, urinary stone disease today has become a very manageable urologic problem. Nearly all urinary tract stones can be treated using minimally invasive approaches with excellent results; open stone surgery is virtually never required. Acute treatment for renal colic includes adequate hydration, analgesic, and determining when immediate intervention is required. The majority of stones can be definitively managed on an elective basis. A limited metabolic evaluation and preventative measures regarding diet and fluid intake are sufficient in the majority of first time stone formers. A full evaluation is reserved for recurrent stone formers and those at high risk for stone disease. Verathon Inc. 800.331.2313 www.verathon.com 52 Urology for Primary Care Male Erectile Dysfunction SEXUAL MEDICINE FOR MEN: MALE ERECTILE DYSFUNCTION Irwin Goldstein, M.D. Editor-in-Chief, The Journal of Sexual Medicine www.irwingoldsteinmd.com INTRODUCTION The field of sexual medicine is rapidly evolving and will one day take its place as a parallel and independent part of the medical school. Sexual medicine may be considered as the medical discipline that embraces the study, diagnosis, and treatment of the sexual health concerns of men and women.1 It is estimated from multiple, population-based epidemiologic studies that approximately half of adult men and women will experience sexual health problems.2, 3 Based on these prevalence data and the well-recognized psychologic and biologic burdens associated with sexual health issues, it is inevitable that sexual medicine will experience an expanding role in medical health care management, research, and education.2, 3 Since health is a fundamental human right, the sexual health rights of all persons must be respected, protected, and fulfilled. Sexual health involves a state of physical, emotional, mental, and social well-being. Sexual health also involves the right to sexual pleasure, an important source of physical, psychological, intellectual, and spiritual well-being.4 The contemporary use of safe and effective, FDA-approved oral selective phosphodiesterase-5 (PDE-5) inhibitors for erectile dysfunction (ED) has transformed the management of this highly prevalent male sexual health problem.5, 6 Due to the efficacy, safety, and ease of administration of these oral erectogenic medications, the number of men with ED seeking treatment has increased dramatically. A decade ago, sex therapy and urologic specialists exclusively managed ED patients. Today, ED is commonly diagnosed and treated by many general practitioners, family physicians, and internal medicine physicians.7 The rapid developments in the knowledge of sexual health problems have emphasized the importance of continuing medical education in sexual medicine.7 For health care practitioners who wish to learn more about the field of sexual medicine, there are several multi-disciplinary international societies dedicated to the field of sexual medicine. In particular, there is the International Society for Sexual Medicine (ISSM). The regional U.S. affiliate is the Sexual Medicine Society of North America (SMSNA). It should be the goal of all health care practitioners, and in particular those not specialists in sexual medicine, to provide each of their patients (and their respective partners) with maximal sexual health medical care through evidence-based, safe, and effective management strategies.8 To that end, the aim of this chapter is to provide evidence-based medical education in the rapidly evolving field of sexual medicine. Information is provided on such areas in male erectile dysfunction as definitions, epidemiology, anatomy, physiology, pathophysiology, diagnosis, and Verathon Inc. 800.331.2313 www.verathon.com 53 Urology for Primary Care Male Erectile Dysfunction treatment. It is beyond the scope of this chapter to discuss other male sexual health problems fully, including ejaculation/orgasmic disorders, Peyronie’s disease, and priapism. In all patients (and partners) with sexual health concerns, the holistic approach needs to be followed, utilizing a multidimensional, multidisciplinary management plan for mind, body, and relationship concerns.8 The success experienced with the availability of safe and effective PDE-5 inhibitors does not exclude the need for management of any associated psychosocial and interpersonal relationship issues that may be concurrently present. The health care provider should always be familiar with the true objective of the management of sexual health concerns in men, women, and couples; that is, to maximally restore a satisfactory sex life and the opportunity for sexual pleasure. Definition Erectile dysfunction (ED) is considered as the consistent or recurrent inability of a man to attain and/or maintain a penile erection sufficient for sexual activity.3, 9 Some of the relevant aspects of this definition are as follows. The words “consistent and recurrent” denote an important aspect of the definition. Men with ED should have a persistent problem in order to qualify for the diagnosis. If the erectile problem occurs over a short period of time, for example related to a brief loss of erectile rigidity during a weekend experience, this is not considered sufficient to qualify as ED. A minimum duration of three months with erectile problems is generally accepted for establishment of the diagnosis of ED. The words “attain and/or maintain” denote another significant aspect of the definition. Some men can easily obtain the erection and their problem is in sustaining the rigidity. Finally, the words “sufficient for sexual activity” in the definition are also noteworthy. The erection needs to be functional, but the quality of the erection needed for successful sexual activity is best determined by the patient and his partner. Considering the above, ED is primarily based on patient self-report. Although the diagnosis may be supported by objective testing (or partner’s reports), these measures cannot substitute for the patient’s own thoughts and opinions. The necessary reliance on the individual implies that cultural factors and patient-physician communication factors are important. In the end, if the man believes that he has a consistent (> 3 months) problem with his penile erection such that function is not sufficient for sexual activity, then the man has ED.9 Epidemiology Multiple epidemiological questionnaire and survey studies in both community and clinic-based samples indicate that ED is widespread, involves approximately 20-30% of adult men and the prevalence increases with increasing age, especially after forty years.3 The probability of complete ED increases from about 5% at the age of forty to 15% at seventy years.10 In a U.S. study, the incidence of ED increased with increasing age such that the incidence was 12.4 per 1000 men between ages forty and forty-nine years, 29.8 per 1000 men between ages fifty and fifty-nine years, and 46.4 per 1000 men between ages sixty and sixty-nine years.11 Multiple cardiovascular risk factors are associated with ED. These include hypertension, diabetes mellitus, cerebrovascular accidents, myocardial infarction, heart disease, hyperlipidemia, low serum levels of high density lipoproteins (HDL), arteriosclerosis, and peripheral vascular disease. Other Verathon Inc. 800.331.2313 www.verathon.com 54 Urology for Primary Care Male Erectile Dysfunction recognized risk factors for ED include depression and medication use for depression, medication use for diabetes, hypertension and cardiovascular diseases, and other factors such as surgery and trauma.3, 10 – 13 Anatomy The penis is composed of three cylindrical bodies of erectile tissue including the paired corpora cavernosa and the single corpus spongiosum. The corpora cavernosa are the chambers where axial penile rigidity is developed during erection, while the corpus spongiosum is the chamber that regulates the engorgement of the glans penis during erection. The proximal portions of the paired corpora cavernosa, the crura, are firmly anchored to the ischio-pubic ramus. This internal anatomic appreciation should provide concern for bicycle riders who may excessively compress their perineum during riding.14 The tunica albuginea is the fibroelastic covering of the three corpora. The tunica albuginea surrounding the corpora cavernosa consist of thick outer longitudinal and inner transverse layers with many sublayers. There is one thin layer of tunica surrounding the corpus spongiosum. The anatomical difference provides the structural basis for the high pressure in the corporal cavernosa, approaching mean arterial pressure during erection, and the low pressure in the corpus spongiosum, approaching 20 mmHg during erection. Buck’s fascia is a thick, elastic layer surrounding the corpus spongiosum and the two corpora cavernosa. The remaining superficial layers of the penis consist of subcutaneous cellular tissue, the superficial penile fascia (Dartos fascia), and the skin.14 The arterial blood supply to the penis is primarily provided by the pudendal artery, a branch of the internal iliac artery. It divides into the bulbourethral, dorsal, and cavernosal arteries. Accessory pudendal arteries from the external iliac or obturator arteries also contribute to the arterial blood supply of the penis. The bulbourethral artery supplies the urethra and corpus spongiosum. The paired dorsal penile arteries proceed distally on the tunica albuginea along with the dorsal nerves and supply superficial structures and the glans penis, as well as the corpora cavernosa via circumflex arteries. The cavernosal arteries enter the corpora cavernosa at the hilum in the proximal portion of the crura and continue distally alongside the septum between the two corporal bodies.14 The erectile tissues within the corpora cavernosa contain numerous helicine arterioles, branches of the cavernosal artery, that regulate the amount of arterial blood inflow. The corpora cavernosa also contain numerous interconnecting vascular spaces, sinusoids, or lacunae within a fibromuscular framework. Helicine arterial inflow is directed into these endothelium-lined vascular spaces. There are no veins per se in the corpora cavernosa. On the periphery of the corporal erectile tissue are draining venules. These venules exist in the subtunical space between the tunica albuginea and the periphery of the erectile tissue. The subtunical and emissary veins are compressed during erection, resulting in the process of corporal veno-occlusion that enables a sustained and rigid penile erection. Emissary veins traverse the tunica and empty into the circumflex veins and, ultimately, into the deep dorsal vein beneath Buck’s fascia. Venous drainage then proceeds into the periprostatic venous plexus in the pelvis. The superficial venous Verathon Inc. 800.331.2313 www.verathon.com 55 Urology for Primary Care Male Erectile Dysfunction system consists of superficial dorsal veins, which drain the skin and subcutaneous tissue above the Buck’s fascia.14 The ischiocavernosus and bulbocavernosus are striated muscles partially wrapped around the crura of the corpora cavernosa and bulb of the spongiosum, respectively. The ischiocavernosus provides the extra rigidity to the penis during the rigid erection phase of erection, and the bulbocavernosus assists in the expulsion of semen during ejaculation.14 Parasympathetic fibers arrive from the sacral erection center, S 2, 3 and 4. Sympathetic fibers arise in the thoracolumbar area (T10 – L2) and travel in the preaortic plexus to the hypogastric plexus. Preganglionic parasympathetic fibers from the sacral erectile center integrate with sympathetic fibers from the hypogastric plexus in the pelvic plexus. Postsynaptic fibers travel in the cavernosal nerves to the penis. The cavernosal nerve travels close to the prostate and can be damaged during radical prostatectomy surgery for prostate cancer. In the corpora, the nerve fibers travel in the trabeculae to directly innervate the corporal smooth muscle and helicine arterioles.14 Somatosensory innervation is derived from the dorsal nerve of the penis, a terminal branch of the pudendal nerve. The sensory afferent information reaches the dorsal roots of the S2, 3 and 4 sacral segments, from where it is transferred via anterolateral spinothalamic pathways to the integrative medial preoptic area (MPOA) and paraventricular nucleus (PVN) in the hypothalamus. The pudendal nerve also has efferent motor fibers that travel to the musculature of the pelvic floor, innervating the bulbocavernosus and ischiocavernosus muscles.14 Vascular Physiology In the flaccid state, sympathetic tone to the helicine arterioles and to the corporal erectile tissue predominates. Arterial blood inflow is low and the trabecular smooth muscle is contracted. The sinusoids contain only a small amount of blood.14, 15 Erection is coordinated in the central nervous system, thus making the brain the most important sex organ. Sexual stimulation is induced by erotic stimuli originating in a variety of different centers in the brain, such as visual, auditory, olfactory, tactile, or imaginative stimuli. These excitatory stimuli trigger a release in the brain of neurotransmitters such as dopamine and oxytocin. Inhibitory messages travel to the penis via the sympathetic system and are based on release of neurotransmitters such as noradrenaline and serotonin. There is a constantly changing balance between the excitatory and inhibitory erectile factors. Excitatory and inhibitory sexual stimuli are integrated in the hypothalamus and the resultant message is transmitted to the penis via the spinal cord and sacral erection center. The state of penile flaccidity or penile erection depends on the equilibrium between excitatory and inhibitory messages.14, 15 Physiologically, erections are continuously inhibited by basal sympathetic nervous system tone, allowing the penis to remain flaccid at rest. A tonic sympathetic neural input is the main mechanism leading to smooth muscle contraction in the penis. The neurotransmitter of this mechanism is noradrenaline released from sympathetic nerve endings, which binds to alpha-1 receptors on vascular and corporal smooth muscle cells to induce contraction. Noradrenaline acts by binding to alpha-1 receptors on the surface of smooth muscle cells, increasing the activity of Verathon Inc. 800.331.2313 www.verathon.com 56 Urology for Primary Care Male Erectile Dysfunction the membrane-bound enzyme phospholipase C (PLC). Ultimately, the enzyme protein kinase C (PKC) opens the calcium channel, leading to calcium influx into the cell. This results in a rise in the cytoplasmic Ca++ concentration, inducing smooth muscle contraction. Other local factors released from vascular endothelium and local nerve endings inducing vascular smooth muscle tone include neuropeptide Y, endothelin, and prostanoids such as PGF2 alpha.14, 15 Sexual arousal results from both increased parasympathetic activity and decreased sympathetic activity. Activation of the parasympathetic nervous system via the sacral erectile center and the pelvic plexus results in an increase in arterial inflow in the cavernosal arteries and helicine arterioles and relaxation of the corporal smooth muscle fibers surrounding the lacunar spaces. The most important parasympathetic neurotransmitter acting on vascular and trabecular smooth muscle of the penis is nitric oxide (NO). The two major sources of NO in the penis are from parasympathetic nerve endings and endothelium of the corporeal blood vessels and sinuses stimulated by acetylcholine. Clinical evidence supporting the role of NO in the induction of erection includes the finding that intracavernosal injection of nitric oxide donors, such as nitroprusside and linsidomine, can produce erection.14, 15 Nerve endings do not innervate every smooth muscle fiber in the corpora cavernosa. Penile smooth muscle cells communicate between each other by intercellular channels called “gap junctions” that allow the transfer of chemicals among cytoplasm of individual cells such as cGMP, K+ or Ca++. In this fashion, penile smooth muscle cells contract and relax efficiently at the same time, allowing a coordinated corpus cavernosum response to individual stimuli.14, 15 Neurotransmitters, either from neuronal or endothelial origin, act on corporal and vascular smooth muscle by changing the intracellular calcium (Ca++) concentration. Smooth muscle cells contract when the intracellular Ca++ concentration rises, and relax when intracellular Ca++ concentration falls. Nitric oxide crosses the cell membrane of the smooth muscle cell and stimulates guanylate cyclase, converting guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP), the active second messenger that lowers intracellular calcium resulting in smooth muscle relaxation.14, 15 cGMP is inactivated by phosphodiesterase, an enzyme that converts cGMP into the inactive guanosine monophosphate (GMP) and has no ability to change intracellular calcium (Ca++) concentration. There are eleven different types of PDE in humans. The classification of PDEs is based on their structure and regulatory properties. Several types of PDE also appear to be present in penile smooth muscle. Current evidence suggests that PDE 5 is the most important isoenzyme in the physiological control of normal penile erectile activity. PDE 5 is also found in tissues other than the penis, such as vascular smooth muscle and platelets.14, 15 The expanding erectile tissue within the tunica compartment compresses the subtunical venous plexus retaining the blood inside the corpora cavernosa, allowing the penis to expand to full erection. During erection, the intracavernosal pressure reaches the mean arterial blood pressure and the penis is fully expanded. The blood flow into and out of the corpora cavernosa is minimal, approximately 3-5 ml/min. During sexual activity, the intracavernosal pressure can reach several times the systolic pressure due to the compression of the ischiocavernosus muscle on the base of the penis.14, 15 Verathon Inc. 800.331.2313 www.verathon.com 57 Urology for Primary Care Male Erectile Dysfunction Peripheral control of penile erection is dependent upon both neuronal and local factors influencing the tone of smooth muscle fibers in cavernosal arteries and erectile tissue. When these fibers are contracted, the penis is flaccid. Smooth muscle tone is determined by the balance between contractile and relaxing factors that are both neuronal and local, originating within the vascular endothelium. Of note, intracavernosal administration of alpha-adrenergic blockers facilitates erection, while intracavernosal administration of alpha-adrenergic agonists causes detumescence. These pharmacologic actions further confirm the role of alpha-adrenergic agonists and antagonists in the regulation of penile smooth muscle tone and therefore erection.14, 15 Return of helicine and corporal smooth muscle tone to baseline contractility occurs after orgasm or after cessation of sexual stimulation. The resultant increase in arterial constriction reduces inflow, while the resultant increase in corporal smooth muscle contractility reduces subtunical venule compression and reopens venous drainage from the corpora, leading to detumescence.14, 15 Endocrine Physiology Testosterone is the most important plasma androgen. Circulating testosterone acts centrally and is important for normal sexual desire and nocturnal penile erections. Testosterone also acts locally and plays an important role in maintaining the structure and function of the erectile tissues, including enzymes such as endothelial and neural nitric oxide synthase and phosphodiesterase type 5. Testosterone is secreted primarily by the testes, with a small contribution from the adrenals. Testosterone has multiple anabolic actions that are important for non-genital tissue functions, including bone growth, hair growth, and mental health.16 Prolactin is another hormone that is important for sexual health and may influence testosterone and androgen synthesis. Prolactin acts in part by impairing the production of Gonadotropin Releasing Hormone (GnRH) in the hypothalamus, leading to suppression of LH and FSH, and thereby resulting in low testosterone.16 Vasculogenic Pathophysiology Erectile dysfunction may be due to a number of different pathophysiologic processes. In the past, it was thought that most men had a psychogenic cause for their ED. An improved understanding of the physiologic mechanisms leading to penile erection has emerged and it is now appreciated that many cases of ED have an organic cause, most commonly of vasculogenic pathophysiology. Maximizing sexual health care leads to the appreciation that there is frequent coexistence of organic and psychogenic factors and that both need to be managed in individual patients.17 Two types of vasculogenic ED, arteriogenic with reduced arterial inflow and venous leak erectile dysfunction with impairment of the veno-occlusive mechanism, can be shown to exist. Arteriosclerosis is the most common cause of vasculogenic erectile dysfunction; however, traumatic injury of the common penile arteries secondary to pelvic fracture or blunt perineal trauma, such as from bicycle riding, may also occur. Reduced arterial inflow leads to ED, in part because of the direct negative hemodynamic effect of reduced blood flow. In addition, the relative ischemia of the cavernosal tissue induced by reduced blood flow leads to impaired erectile tissue oxygenation, subsequent smooth muscle dysfunction and diminished expansion of cavernosal Verathon Inc. 800.331.2313 www.verathon.com 58 Urology for Primary Care Male Erectile Dysfunction sinusoids. This, in turn, prevents adequate compression of the subtunical venules inside the tunica albuginea, with failure of the veno-occlusive mechanism. Venous leakage develops, resulting in excessive drainage of blood from the corpora cavernosa, decrease in erectile rigidity, and early loss of erection.17 Erectile dysfunction occurs in one-half of all diabetic men. Erectile dysfunction usually develops during the course of the disease but can also be a presenting complaint. The likelihood of ED is related to the degree of blood glucose control. The pathophysiology is multifactorial, including diabetic arterial disease, diabetic neuropathy, and the direct action on smooth muscle cells of hyperglycemia that both increases the contractile response to noradrenaline, and reduces the relaxation response to nitric oxide.17 Aging is the most important risk factor for ED. The age related changes develop at varying rates in individual men. Aging is associated with atherosclerosis and vascular changes; a relative loss of nerves within the corpora cavernosa, including decreased levels of neurotransmitters such as nitric oxide; loss of cavernosal compliance due to decreased elasticity and smooth muscle fibers of the erectile tissue with increased collagen connective tissue; imbalance of inhibitory and excitatory erectile neural tone with an increase in sympathetic relative to parasympathetic tone; and a gradual decline in bioavailable testosterone with a gradual increase in sex hormone binding globulin resulting in a decrease in total testosterone.17 Endocrine Pathophysiology Adult-onset hypogonadism is a clinical and biochemical syndrome frequently associated with advancing age and characterized by a deficiency in serum androgen levels, with or without changes in receptor sensitivity to androgens. Androgen insufficiency may affect the function of multiple organ systems and result in impairment of quality of life, including major alternations of sexual function. Hypogonadism is associated with low energy level, sexual dysfunction, low frequency of sexual events, low libido (desire, motivation), increased visceral fat, decrease in muscle mass, and mild depression.16 Primary hypergonadotropic hypogonadism is associated with the endocrine pathology occuring at the level of the testis. Examples of testicular failure to produce testosterone are as follows: Klinefelter’s syndrome, cryptorchidism, vanishing testes syndrome, bilateral torsion, orchitis, orchiectomy, chemotherapy or toxic damage from radiation, alcohol, or heavy metals.16 Secondary hypogonadotropic hypogonadism is associated with the endocrine pathology occuring at the level of the hypothalamus or pituitary. Examples of hypothalamic-pituitary failure to stimulate testicular testosterone production are as follows: Gonadotropin or GnRH deficiency consistent with idiopathic or Kallmann’s Syndrome, or pituitary-hypothalamic injury from tumors, trauma, or radiation.16 A third classification is the result of a combination of endocrine pathology at the level of the hypothalamus or pituitary and testis and is associated with aging, sickle cell disease, alcoholism, hemochromatosis, chronic illnesses (HIV), and medications.16 Verathon Inc. 800.331.2313 www.verathon.com 59 Urology for Primary Care Male Erectile Dysfunction Aging-related androgen insufficiency is not only associated with lower synthesis of testosterone, but also diminished bioavailable testosterone. Increasing age is associated with increasing serum levels of sex hormone binding globulin (SHBG). SHBG tightly binds testosterone and thus decreases bioavailable or free unbound testosterone. It is the free form of testosterone that enters the genital smooth muscle cells and directs genomic therapy that is critical in preserving and maintaining genital structure and function. Data are developing that testosterone synthetic functions are essential in the mechanism of corporal veno-occlusive function.16 Hyperprolactinemia is a rare cause of ED, usually accompanied by decreased sexual interest, generally secondary to a pituitary adenoma. Gynecomastia is often present.16 Psychogenic Pathophysiology Emotional problems, anxiety, depression, and stress are significantly associated with ED. Psychological erectile dysfunction may be secondary to a past event such as sexual abuse, or due to specific situations such as performance anxiety and/or partner relationship problems. Psychogenic signals may inhibit activation of NO-mediated parasympathetic nerves while excessive sympathetic outflow may increase the constriction effect on penile smooth muscle tone and maintain penile flaccidity.18 Neurogenic Pathophysiology Disruption of central neural networks or the peripheral nerves involved in sexual functions can cause neurogenic ED. It is thought that only 10%-20% of cases of ED are due to a neurogenic cause. Neurogenic ED can be classified as central or peripheral.17, 19 Central causes of neurogenic ED include spinal cord injury, multiple sclerosis, and stroke. Supraspinal central neurogenic ED may be considered an imbalance between inhibitory and excitatory factors where the inhibitory influences predominate. Suprasacral spinal cord central neurogenic ED is associated with reflex erections where minimal tactile stimulation can trigger erection, typically of short duration, whereas sacral spinal cord central neurogenic ED is typically associated with absent erections.17, 19 Peripheral causes of neurogenic ED include pelvic injury, surgery, and diabetic neuropathy. Peripheral lesions can be secondary to disruption of the sensory afferent nerves carrying information from the penis to the central nervous system or disruption of efferent nerves that mediate arterial and corporal smooth muscle relaxation.17, 19 Iatrogenic Pathophysiology One form of iatrogenic pathophysiology is erectile dysfunction that follows radical pelvic surgery. Radical pelvic surgery, especially for treatment of cancer of the bladder, prostate, or rectum, as well as following such procedures as aorto-iliac surgery, surgery for priapism, Peyronie’s disease surgery, or urethroplasty repair, can cause ED. Spinal surgery for lumbosacral back pain can also potentially damage the nerves and/or arteries that are essential for erection. In particular, the Verathon Inc. 800.331.2313 www.verathon.com 60 Urology for Primary Care Male Erectile Dysfunction prevalence of erectile dysfunction after radical prostate cancer surgery was virtually 100% in the past, but has been improved slightly with the introduction of nerve-sparing procedures. Maintenance of erectile capacity (typically still only 20%) varies depending on the surgical technique (no electrocautery), the clinical and pathological staging of the tumor, and the patient’s age. Erectile function can slowly recover during the period of up to one year after radical prostate cancer surgery. Early treatment with PDE 5 inhibitors may improve the probability of recovering erectile function, but surgical technique is a more relevant determinant.17, 19 Another form of iatrogenic pathophysiology is radiation to the pelvis for cancer. The pathogenesis is not clear, but it is believed that radiotherapy may cause vasculitis leading to scar damage of small cavernosal vessels and nerves, and loss of vascular smooth muscle. The prevalence of ED following radiotherapy for prostate cancer appears to increase over time.17, 19 An additional form of iatrogenic pathophysiology is ED secondary to medication use. Various kinds of drugs induce ED. The mechanism is not always clear and includes antihypertensive drugs that may decrease arterial blood flow to the penis, and psychotropic drugs that increase inhibitory neurotransmitters (serotonin) in the central nervous system.17, 19 DIAGNOSIS Medical, Sexual, and Psychosocial History The first step in the management of the patient with ED is to facilitate the patient’s and partner’s understanding of ED, explain the diagnostic assessment plan and identify the patient’s and partner’s needs, expectations, priorities, and preferences from the variety of available safe and effective treatment options. All men with ED should be evaluated with sensitivity toward cultural, ethnic, and religious factors. It is important to assess the degree to which patients and partners are bothered or distressed by the condition, as this influences ED management plans.20, 21 The basic evaluation or assessment in all ED patients should consist of a sexual, medical, and psychosocial history, physical examination, and focused laboratory testing. Optional tests are left to the clinical judgment of the treating physician. The rationale for testing and potential impact of a positive test should be explained to the patient.20, 21 Arguably, history-taking, including medical, sexual, and psychosocial aspects, is the most important and basic element in the evaluation of men with ED. During history-taking in the individual ED patient, the identification and recognition of medical and psychological factors associated with the sexual problems should be emphasized. The medical history should establish the presence of risk factors that are associated with ED. These include the patient’s age, history of cardiovascular disease including hypertension, diabetes, cigarette smoking, hyperlipidemia, heart disease, claudication, family history of cardiovascular disease, sedentary lifestyle, history of myocardial infarction or cerebrovascular disease, etc. Medications used by the patient are very important, specifically antihypertensive agents, antidepressants, and medications for diabetes.20, 21 Verathon Inc. 800.331.2313 www.verathon.com 61 Urology for Primary Care Male Erectile Dysfunction A sexual history is critical. The duration and nature of the ED problem, as well as the functional significance of the ED in terms of satisfaction with sexual activity, should be assessed. Is the erection rigid? Is it easy to obtain? How easy is it to sustain the erection? Is the patient’s interest in sex satisfactory? Are his orgasms satisfactory? Is he able to control the ejaculation? Individuals frequently present with several types of sexual dysfunction (e.g. lack of erection, early ejaculation, and Peyronie’s disease). The presence or absence of nocturnal erectile activity is useful information. If the erection curves, how significant is the curvature? Is the erection painful? A validated sexual function questionnaire, such as the Sexual Health Inventory for Men (SHIM) or International Index of Erectile Function (IIEF), should be provided, as this establishes baseline function allowing subsequent treatment outcome to be followed objectively.20, 21 The psychosocial evaluation is performed to assess whether individuals may have concomitant psychological or interpersonal problems. Questions include the following: Are you stressed at home or at work? What is your social situation? Do you have a partner? Have you ever experienced sexual abuse? What is the sex life of your partner? Are there sexual problems with the partner? Are there problems at work or at home and do you think these relate to your sexual problem? Do you have any health care professional working with you or any family member for any mental health concerns? When psychosocial assessment reveals the presence of significant psychological distress or partner conflict, further evaluation and management may be necessary either prior to, or in conjunction with treatment of ED. Referral to an appropriate mental health professional may be indicated.20, 21 Physical Examination A focused genital physical examination should be carried out in each patient presenting with erectile dysfunction. The following examinations should be considered: General appearance and secondary sexual characteristics of an individual with hypogonadism; a cardiovascular examination, including blood pressure and peripheral pulses; a genital examination, including inspection of the prepuce and glans, size and consistency of the testicles, size and shape of the penis and fibrotic plaques in the stretched penis consistent with Peyronie’s Disease; a digital rectal examination in patients over fifty years to evaluate the prostate size, shape, consistency and presence of masses; neurological exam for perineal and penile sensitivity; and assessment of anal sphincter tone and bulbocavernosus reflex where compression of the glans triggers anal contraction, as palpated during rectal examination.20, 21 LABORATORY TESTING Blood tests The physician must tailor the laboratory work-up based on the patient’s complaints and risk factor exposure, such as diabetes (obtain blood glucose and hemoglobin A1C), hyperlipidemia (obtain lipid profile and endothelial function blood tests), hypertension (obtain blood pressure and pulse, serum creatinine and BUN) or coronary artery disease (obtain ECG).20, 21 Verathon Inc. 800.331.2313 www.verathon.com 62 Urology for Primary Care Male Erectile Dysfunction Total testosterone and free testosterone should also be measured in the morning in men under age forty-five to fifty years, especially with erectile dysfunction and low libido. Diurinal variation of testosterone is less pronounced in men over age sixty, so their testosterone blood tests can be performed even in the afternoon. Since sex hormone binding globulin (SHBG) increases with increasing age, and since SHBG tightly binds to testosterone and thus decreases bioavailable or free unbound testosterone, the more reliable measure of the presence of hypogonadism is the measurement of free androgen index defined by the ratio of total testosterone to SHBG. Assays for total testosterone, particularly in the middle-aged man, may not reflect the man’s true androgenic status.16 Other endocrine blood tests that may be useful to diagnose the endocrine milieu include luteininzing hormone (LH), follicle stimulating hormone (FSH), and prolactin. In addition, thyroid stimulating hormone (TSH) may identify an unsuspected hypothyroid state. Estradiol determination may be relevant in men with elevated body mass indexes, as there is high aromatase activity in men with excess adipose tissue. Dehydroepiandrosterone sulphate and androstenedione may help assess the adrenal androgen status. Dihydrotestosterone may be useful to diagnose the integrity of the 5-alpha reductase enzyme. Prostate specific antigen (PSA) blood tests and a digital rectal examination are useful in men over age fifty years to diagnose prostate cancer.16 Office Intracavernosal Injection Test A variety of special investigations are available to provide more detailed understanding of the erectile process. These tests are of limited value in managing the majority of patients and should be reserved for selected patients, such as young men with ED secondary to blunt penile or perineal trauma.20 - 22 Intracavernosal injection of a vasodilator (generally prostaglandin E1 or a combination of prostaglandin E1, papaverine, and/or phentolamine) is used as a test to differentiate arterial inflow vasculogenic from venous leak vasculogenic ED. If a sustained erection is achieved after injection of the intracavernosal drug, then venous insufficiency is unlikely. If a full erection is not seen, or if the erection lasts only a short time, the patient is allowed to stimulate himself in an attempt to improve the response. Patients with severe anxiety or needle phobia may not respond to this test. The integrity of the arterial perfusion pressure is not able to be assessed with the office intracavernosal injection test. A man with arterial insufficiency may still be able to achieve a functionally rigid erection with this test if the veno-occlusive mechanism is functioning satisfactorily. Arterial integrity is best assessed using a duplex Doppler examination, or by directly recording cavernosal arterial perfusion pressure during dynamic 20 - 22 pharmacocavernosometry. Vascular Testing Color duplex Doppler ultrasound testing in conjunction with intracavernosal vasoactive agent injection may provide further information over injection alone in identifying vascular ED. This may be useful to a man with ED who is not yet aware that he may have an ongoing atherosclerotic process. If this test is positive, cardiovascular consultation may be indicated. After Verathon Inc. 800.331.2313 www.verathon.com 63 Urology for Primary Care Male Erectile Dysfunction intracavernosal injection of a vasoactive agent, the penis can be scanned by a dorsal or ventral approach at the base, with the probe held transversely or in an oblique longitudinal position. The velocity of blood in the cavernosal artery during systolic and diastolic phases is recorded within five minutes of injection and repeated frequently. In general, peak systolic velocity values less than 30 cm/sec are consistent with arterial pathology. A high resistance vascular bed, such as the engorged sinusoids of the erect penis, would only allow inflow during the high pressure systolic portion of the cardiac cycle. During diastole, the pressure would be insufficient to overcome the peripheral vascular resistance and the diastolic flow would be low.20 - 22 Determination of the cavernosal artery systolic perfusion pressure and comparison of this value to the brachial artery systolic perfusion pressure is another method of determining arterial vascular integrity in selected patients. This procedure is best performed during dynamic pharmacocavernosometry. If the ED patient is young and has a history of blunt perineal/penile trauma with an intact veno-occlusive mechanism, reduced peak systolic velocity values (for his age) and reduced cavernosal artery systolic occlusion pressures, he becomes a candidate for selective internal pudendal arteriography, especially if he is considering undergoing the treatment option of microvascular arterial bypass surgery.20 – 23 TREATMENT Step-care Principles The most rational model of treatment of men with ED is a step-care model.7 – 9 In this model of care, a step-wise process is utilized with regard to the degree of invasiveness of treatment procedures, and in the degree of involvement of Primary Care and non-Primary Care specialists. Treatment levels are graded from first, second, and third line interventions. First line therapies also include modifications of reversible causes. In this step-care model, there are clear guidelines for follow-up and referral to sexual medicine specialists when treatment strategies exceed the knowledge and skills of the health care provider.7 – 9 The three steps of treatment options are chosen based on matching the patient’s needs and preferences with the available treatment options. The choice of therapy is strongly influenced by personal, cultural, ethnic, religious and economic (affordability) factors. Treatment options should be carefully reviewed with the patient and the patient’s partner, if available. The goal of therapy should be viewed as restoration of a satisfactory sexual life.7 – 9 In addition to offering first-line therapy for ED, the physician providing good medical practice recognizes the value of modifying existing risk factors. Although usually insufficient to reverse ED completely, this step may be of great health value in selected patients. Since ED may be a marker of underlying cardiovascular, metabolic, or psychologic illness, these comorbidities should be addressed whenever possible. Efforts should be directed to modify lifestyle factors such as obesity, cigarette smoking, alcoholism, or substance abuse, as well as psychosocial factors such as relationship issues secondary to partner conflict, mood problems, and depression. Furthermore, efforts should be directed to modify drug dosages or classes concerning prescription and nonprescription drug use, such as antihypertensive agents, including diuretics or beta-blockers, and Verathon Inc. 800.331.2313 www.verathon.com 64 Urology for Primary Care Male Erectile Dysfunction psychotropic drugs, including antidepressants or neuroleptics, well-recognized to be associated with ED. Alterations in drug administration should be coordinated with the primary physician wherever possible.2, 3, 9, 10 In parallel to offering first-line therapy for ED, good medical practice recognizes the noninvasive nature and broadly applicable value of providing counseling and patient (and partner, if available) education opportunities concerning sexual function, anatomy, physiology, and pathophysiology. Providing information concerning sexual functioning or typical and expected changes in sexual functioning with aging (for men and women) seems to enhance treatment outcome. In addition, expanding patients’ knowledge concerning variations in sexual technique may salvage otherwise unsuccessful biologic-focused ED patient care.18 Sexual counseling and education for individuals or couples is aimed at improving overall patient sexual satisfaction. Addressing relevant psychological and interpersonal relationship concerns are essential to achieving sexual satisfaction goals.18 These may include addressing anxiety over erectile performance problems, or abnormal relationship interaction patterns, or any co-existing sexual conditions, such as early ejaculation or sexual problems in the partner, such as low desire or dyspareunia.24, 25 Psychosexual counseling should be considered prior to or in conjunction with medical therapy, whenever indicated. Sex therapy may improve the results of direct medical therapies for ED and maximize the patient’s sexual health. Sex therapy can help address psychological reactions to biologic-based therapies that may be initially uncomfortable for the patient and/or partner. Disadvantages of sex therapy include unpredictable efficacy in the treatment of ED, limited acceptability by the patient, limited availability of qualified providers, and cost.18 First-Line – Oral Pharmacologic Therapy Despite efforts at modifying lifestyle, increasing patient knowledge and education, and offering sex therapy counseling, the reality is that the vast majority of ED patients will need to consider direct medical treatment with regular patient follow-up. The most common medical treatment is oral pharmacologic therapy with PDE 5 inhibitors.5 – 7 It is important that the decision to initiate treatment is a shared one, as much as possible. The benefits, risks, and costs of the medical treatment strategies should be discussed with the patient and partner both actively participating in the decision. This is especially important if the partner has sexual concerns independent of the patient’s ED.24, 25 One of the most important issues to resolve prior to the institution of any medical therapy is cardiovascular safety. Will the subsequent resumption of sexual activity, especially the exercise of sexual activity, adversely affect the patient’s overall cardiovascular condition? If there are any questions, cardiovascular evaluation and intervention may be needed prior to resuming sexual activity.9 Finally, to maximize sexual health care, it is critical that reassessment and follow-up be conducted at regular intervals for patients receiving ED treatment. Equal opportunity to try all the three PDE 5 inhibitors is one aspect of patient follow-up. In this fashion, health care providers can maximize Verathon Inc. 800.331.2313 www.verathon.com 65 Urology for Primary Care Male Erectile Dysfunction sexual health care delivery and patient communication. Patients may have concerns regarding the oral medications, treatment of other sexual dysfunctions, partner issues, lifestyle factors, or concomitant medical conditions. In addition, general medical and psychosocial reassessment should occur at regular intervals, depending upon the patients’ physical and psychosocial health. Follow-up also provides an additional opportunity for necessary patient education.7 – 9 First-Line – PDE 5 Inhibitors During sexual stimulation, the smooth muscles of the genital tissues change tone from the contracted state at baseline flaccidity to the relaxed state during penile erection. Penile smooth muscle cell cytoplasm undergoes a significant drop in intracellular calcium concentration directed in large part by increased cGMP. The more sustained the increased levels of intracellular cGMP during sexual stimulation, the more sustained the low levels of intracellular calcium, the more sustained the penile smooth muscle relaxation, and assuming other biologic variables are intact, the more rigid the penile erection. Physiologic detumescence is initiated by lowering the level of intracellular cGMP by both diminished synthesis of cGMP (stopping sexual stimulation) and by on-going hydrolysis of cGMP in the catalytic site of the enzyme PDE 5 into the inactive form 5’ GMP. When intracellular calcium values increase, then penile smooth muscle regains baseline contractile tone.14, 15 PDE 5 inhibitors are widely used, safe, and effective therapies for ED. More than 30 million men utilize PDE 5 inhibitor therapy around the world26 to pharmacologically maximize intracellular levels of cGMP and to promote maximal lowering of intracellular calcium and maximal genital tissue smooth muscle relaxation and engorgement. Although there are few head-to-head trials, evidence-based data would suggest that at the maximum doses, the three oral PDE 5 inhibitors have relatively similar overall cardiovascular safety and clinical efficacy in men with ED.6 PDE 5 Inhibitors – Concomitant Use with Alpha-blockers There is a level playing field among the three PDE 5 inhibitors as it concerns concomitant use of PDE 5 inhibitors with alpha-blocker agents. A more global FDA message is now advised. Caution should be advised when any of the three PDE 5 inhibitors (sildenafil, tadalafil, and vardenafil) are co-administered with ANY alpha-blocker (alfuzosin, doxazosin, tamsulosin, terazosin).27 – 29 In some patients, the use of two vasodilator drug classes together may result in a symptomatic lowering of blood pressure. The key for the physician initiating PDE 5 therapy for ED in the patient already on an alpha-blocker is to assess hemodynamic stability. If hemodynamic stability exists (e.g. an asymptomatic patient on 0.4 mg tamsulosin for 3 weeks), the physician may consider any of the three PDE 5 inhibitors at the lowest recommended dose. If there is hemodynamic instability (e.g. a postural hypotensive patient on 1 mg terazosin for 3 days) there is an increased risk of symptomatic hypotension with concomitant use of any of the three PDE 5 inhibitors. Under such conditions, no use of concomitant PDE 5 therapy, even at the lowest recommended dose, should be initiated.27 – 29 Verathon Inc. 800.331.2313 www.verathon.com 66 Urology for Primary Care Male Erectile Dysfunction PDE 5 Inhibitors – Equal Opportunity to Try All Three Inhibitors How should health care practitioners select PDE 5 inhibitors for treatment of men with ED? Since sexual health is a fundamental human right, it should be the goal of health care practitioners to provide each of their patients with maximal sexual health medical care through evidence-based, safe, and effective managment strategies. While there are many similarities among the three PDE 5 inhibitors as a vasodilator class, there are multiple evidence-based differences in biochemical potency,30 time of onset, food effect, duration of action, selectivity to other PDEs, cumulative probability of first effect,31 – 33 side effect profile, and data concerning partner benefit. These differences translate into subtle preference choices of one PDE 5 inhibitor over another that are not predictable by the heath care provider. To maximize sexual health outcome, health care practitioners should provide equal opportunity to all three agents for a minimum two month clinical preference trial. It is good medical practice to empower the patient (and his partner) to identify their own preference(s). Evidence-based differences among the three PDE 5 inhibitors have recently emerged. To better appreciate equal opportunity for each patient to all three PDE 5 inhibitors, it is necessary to observe the results of a prospective, independent, two-site crossover design head-to-head preference study performed over a one-year period with the data presented at a recent international meeting.34 The three PDE 5 inhibitors were administered in different sequences to over 500 men with ED, all of whom were previously naïve to PDE 5 inhibitors. At the end of the trial, all PDE 5 inhibitors, sildenafil, tadalafil, and vardenafil, were preferred equally and one-third of the time.34 Of interest, criteria such as age, etiology of the ED and severity of the ED could predict which PDE 5 inhibitor would be preferred by ED patients.34 While such criteria predicted a group of men with ED, the correct drug for each individual patient was not able to be identified by any strategy other than their trying all three over a reasonable minimal clinical time trial (>2 months) and selecting their preference. This important observation re-emphasizes the fact that good medical practice in this field empowers the patient (and his partner) to self-identify preference(s) by trying all three PDE 5 inhibitors in two month trials. PDE 5 Inhibitors – Biochemical Potency There are evidence-based differences among the three PDE 5 inhibitors in biochemical potency or the combination of the onset time (affinity of the inhibitor to attach to the catalytic site) and offset time (dissociation of the inhibitor from the catalytic site). The higher the biochemical potency, the less amount of drug is required to inhibit the enzyme. The concentration of a PDE 5 inhibitor that inhibits PDE 5 enzyme activity by 50% is the IC50. In one NIH-funded laboratory investigation, all three PDE 5 inhibitors were studied under similar conditions. The biochemical potency of vardenafil was the highest, as expressed by the lowest IC50 at 0.09 nM. The IC50 for tadalafil required twice the vardenafil concentration (1.8 nM). The IC50 for sildenafil required more than four times the concentration (3.7 nM).30 For sildenafil to achieve relatively similar clinical efficacy to vardenafil, in part due to its lower biochemical potency, sildenafil requires five times the clinical dose (100 mg maximal dose sildenafil to vardenafil 20 mg maximal dose). For tadalafil to achieve relatively similar clinical Verathon Inc. 800.331.2313 www.verathon.com 67 Urology for Primary Care Male Erectile Dysfunction efficacy to vardenafil, in part due to its lower biochemical potency, tadalafil requires a half-life four times the half-life of vardenafil and sildenafil (17 hours versus 4 hours). In a separate study, cGMP accumulation in human erectile tissue was investigated. The baseline cGMP (1 picomol cGMP/mg) was significantly increased (approximately 2 picomol cGMP/mg) when vardenafil 3 nM was added with the NO-donor sodium nitroprusside to the tissue. Ten times the amount of sildenafil (30 nM) was required to signficantly increase cGMP to approximately 2 picomol cGMP/mg. This ten-fold difference further establishes the evidencebased differences in biochemical potency among the PDE 5 inhibitors.30 PDE 5 Inhibitors – Time of Onset There are evidence-based differences among the three PDE 5 inhibitors in time of onset, in part based on the biochemical potency differences. Since the IC50 is lowest for vardenafil, it would make sense that vardenafil is a fast acting PDE 5 inhibitor. In a prospective, double-blind placebo controlled stopwatch study in men with ED and their partners, using successful intercourse as the outcome, vardenafil 10 mg showed significant differences from the placebo at 10 minutes. At 25 minutes following pill ingestion, approximately 50% of couples were having successful intercourse. For sildenafil to have similar time of onset and efficacy, the dose of 100 mg is required. Tadalafil is slower in onset with approximately 25% of couples at 25 minutes having successful intercourse at the maximal dose of 20 mg. At 10 mg, tadalafil had no significant differences from placebo at 30 minutes.35 – 37 Thus, an individual patient and his partner may prefer to have spontaneous sexual activity without any significant advance notice. In this setting the ideal drug may be the fastest onset drug. In such clinical setting circumstances, there are evidence-based differences among the three drugs. PDE 5 Inhibitors – Food Interactions There are evidence-based differences among the three PDE 5 inhibitors in food effect. Food interactions with drugs are important to patients. Mean plasma vardenafil and tadalafil concentration-time profiles are unaffected by fatty meals, whereas absorption of sildenafil is slowed by food. Food is important to many couples for whom a romantic dinner may precede sexual activity. Thus, an individual patient and his partner may prefer to have spontaneous sexual activity without any interference from food interactions. In such clinical setting circumstances, there are evidence-based differences among the three drugs.27 – 29 PDE 5 Inhibitors – Duration of Action There are evidence-based differences among the three PDE 5 inhibitors in duration of action. Duration of action of a drug is based in part on the drug’s pharmacokinetic profile, especially the drug’s half-life. There are evidence-based differences among the three PDE 5 inhibitors in halflife. The half-life of tadalafil is approximately 17 hours, and the half-lives of sildenafil and vardenafil are approximately 4 hours.27 – 29 Verathon Inc. 800.331.2313 www.verathon.com 68 Urology for Primary Care Male Erectile Dysfunction In several prospective double-blind, placebo-controlled studies using successful intercourse as the outcome, the duration of action of tadalafil is 36 hours in some patients.38 In a separate prospective double-blind, placebo-controlled, stopwatch study, using successful intercourse as an outcome, men with ED who were randomly assigned to vardenafil had an approximately 70% positive intercourse rate at 8 to 10 hours after taking vardenafil, compared to approximately 34% of those assigned a placebo.39 Why does the duration of action of vardenafil last at least 8 to 10 hours, when the half-life is 4 hours? The duration of action of a drug is based in part on biochemical potency. To better understand this, a separate study was performed examining the rate of dissociation of each inhibitor from the PDE 5 enzyme. In one NIH-funded laboratory investigation, all three PDE 5 inhibitors were radio-labeled with tritium, and their dissociation from the PDE 5 enzyme was studied under similar conditions as loss of radioactivity over time. Over 60% of vardenafil remained bound to the enzyme after 30 minutes, compared with approximately 25% for tadalafil, and approximately 12% for sildenafil. Thus, vardenafil has the slowest dissociation from the PDE 5 enzyme of the three PDE 5 inhibitors, followed by tadalafil, then sildenafil.30 Based on the slow dissociation, a separate research study was performed in an NIH-funded laboratory based on a personal communication from Dr. Jackie Corbin. Preliminary results suggest vardenafil, in part due to the low dissociation rate, is actually concentrating inside the cell. The drug level in one experiment was 100 nM intracellularly and 10 nM extracellularly. The high intracellular concentration is an expression of the slow dissociation from the PDE 5 enzyme and thus explains vardenafil’s 8 to 10 hour duration of action. PDE 5 Inhibitors – Selectivity to Other PDEs There are evidence-based differences among the three PDE 5 inhibitors in selectivity to the other PDE enzymes. There is a family of eleven PDE enzymes; drugs designed to inhibit PDE 5 may also inhibit other PDE enzymes. Sildenafil has a relative higher selectivity to PDE 6 compared to the other PDE 5 inhibitors. This is clinically manifest when sildenafil induces blue color vision from inhibition of PDE 6 in the rods and cones of the retina. Tadalafil, on the other hand, has a relatively higher selectivity to PDE 11 compared to the other PDE 5 inhibitors. The clinical manifestation of tadalafil inducing a clinical symptom from inhibition of PDE 11 is not yet appreciated. The PDE 5 inhibitor that shows the least selectivity for the other PDE enzymes is vardenafil.27 – 29 PDE 5 Inhibitors – Cumulative Probability of First Effect There are evidence-based differences among the three PDE 5 inhibitors in cumulative probability of first effect, an important concern among PDE 5 naïve patients, since repeated failures breed frustration and encourage withdrawal from PDE 5 inhibitor therapy for the safe and effective treatment of ED. In a post-hoc analysis of data from multiple double-blind placebo-controlled trials, including those used for pivotal FDA trials, the cumulative probability of achieving first success of sexual intercourse with vardenafil is approximately three administrations. Similar data for sildenafil are approximately six administrations.27 – 29 Verathon Inc. 800.331.2313 www.verathon.com 69 Urology for Primary Care Male Erectile Dysfunction PDE 5 Inhibitors – Side Effect Profile There are evidence-based differences among the three PDE 5 inhibitors in terms of side effects. The side effects of sildenafil in order of presentation include headache, flushing, dyspepsia, and rhinitis. The side effects of tadalafil in order of presentation include headache, back pain/muscle pain/limb pain, dyspepsia, rhinitis, and flushing. The side effects of vardenafil in order of presentation include headache, flushing, rhinitis, and dyspepsia. Thus, the patient who experiences flushing with vardenafil should consider tadalafil, the patient who experiences dyspepsia with tadalafil should consider vardenafil, while the patient who experiences back pain with tadalafil should consider either sildenafil or vardenafil, not usually associated with such side effects.27 – 29 PDE 5 Inhibitors – Data Concerning Partner Benefit There are evidence-based differences among the three PDE 5 inhibitors in data concerning partner benefit. In a randomized, double-blind, placebo-controlled, multi-institutional comparison of vardenafil versus placebo performed in 229 couples (treated man with ED > 6 months and untreated woman partner), vardenafil significantly increased least square mean scores in intercourse satisfaction, total female sexual function and sexual desire, subjective arousal, lubrication, orgasm, and satisfaction domains scores in untreated women partners.40, 41 In a separate study, Heiman et al.42 reported on changes in sexual function and satisfaction of women whose male partners with ED were treated with sildenafil. Women partners of men with ED treated with sildenafil reported improvements in sexual enjoyment and satisfaction; however, no changes in the levels of sexual desire, arousal, or orgasm were observed among women partners of men with ED treated with sildenafil. PDE 5 Inhibitors – Drug Interactions All three PDE-5 inhibitors are strictly contraindicated in ED patients receiving organic nitrates and nitric oxide donors. PDE 5-inhibitors undergo hepatic metabolism via ccytochroe P450CYP3A4. CYP3A4 inhibitors such as erythromycin, ketoconazole, and protease inhibitors can increase the levels of PDE 5-inhibitor. In patients taking these drugs, consider administering PDE 5-inhibitors at the lowest available dosage.27 – 29 Conclusions – PDE 5 Inhibitors The advantages of oral drug therapies include broad patient acceptance, ease of administration and relative efficacy. The disadvantages include specific contraindications with the concomitant use of nitrates and the relative cost. In clinical practice, discontinuation rates while on PDE 5 inhibitors may be higher than clinical trials for a number of reasons, including inadequate patient education and follow-up, cost, and possible psychological factors. Good patient information about the optimal ways of administering the drug treatment, such as dosage and need for sexual stimulation, is important for the success of treatment and patient satisfaction. Equal opportunity to try all three PDE 5 inhibitors for a minimum two month clinical trial is the most rational strategy to achieve the goal of maximizing sexual health care to ED patients. The introduction of Verathon Inc. 800.331.2313 www.verathon.com 70 Urology for Primary Care Male Erectile Dysfunction oral therapy for ED has been a revolution in clinical management; however, ED remains a multifaceted process and introduction of a rapid and easy pharmacological solution does not eliminate the need for management of any associated psychological or other sexual health issues. It should be remembered that the objective of sexual health treatment is to restore a satisfactory sex life.7 – 9 First-Line – Androgen Therapy In men with ED and/or diminished sexual interest, a clinical picture of hypogonadism together with biochemical evidence of hypogonadism should exist prior to initiation of androgen therapy. Contraindications such as prostatic cancer or severe lower urinary tract symptoms, such as bladder neck obstruction, should be ruled out. Testosterone can be given transdermally with gel or patch or intramuscularly by injection. Since androgen therapy may be considered as chronic or lifelong, it is important that all patients receiving androgen therapy be followed on a regular basis. The treating physician must be familiar with the diagnostic, therapeutic, and monitoring aspects of androgen therapy. The ED patient with hypogonadism should be monitored closely for possible side effects or contraindications, such as abnormal liver function, hyperlipidmia, polycythemia, prostate abnormalities (prostate cancer or sever bladder outlet obstruction), hyperactivity or aggressive behavior, and sleep apnea. Inadequate therapeutic response or the appearance of significant adverse effects call for reassessment of treatment indications.16 First-Line – Vacuum Constriction Therapy Vacuum constriction devices apply a negative pressure to the corpora cavernosa that increases blood flow and produces an erection despite absence of physiologic corporal smooth muscle relaxation. A vacuum constriction device consists of three components, including a plastic cylinder, a vacuum pump to produce the negative pressure, and a constriction ring to maintain the erection. The cylinder is placed over the penis and held firmly against the pubis to obtain an airtight seal. Suction is then applied to the vacuum pump to produce a negative pressure. After the corporal cavernosa are engorged, a constriction ring is slipped from the cylinder onto the base of the penis to decrease venous drainage and maintain erection. The vacuum is then released via a valve and the cylinder removed. The usual time to achieve a vacuum erection is generally about 2 to 3 minutes.43 The constriction ring is a critical component in this process. Constriction rings may have various shapes, including those with a notch that fits over the urethra to facilitate ejaculation. The ring should not be left on for more than 30 minutes to avoid the penis appearing blue and cold and becoming painful with time. Contraindications to the use of vacuum constriction devices include bleeding conditions and anticoagulant treatment. Vacuum constriction devices are effective for satisfactory sexual intercourse in 70% to 80% of users. There is a high drop-out rate of approximately 60% after one year. Despite being cumbersome, requiring good manual dexterity, interfering with ejaculation, and being associated with a cold-feeling penis, the therapy is a first line therapy that is relatively noninvasive compared to second-line (injections) and third-line (surgery) treatment options. Furthermore, the vacuum constriction device is effective for virtually all ED pathophysiologies.43 Verathon Inc. 800.331.2313 www.verathon.com 71 Urology for Primary Care Male Erectile Dysfunction Second-Line – Intracavernosal Pharmacological Therapy Intracavernosal injection therapy was introduced in 1983, some fifteen years prior to oral PDE 5 inhibitor use. Intracavernosal injection therapy was a historic milestone in the treatment of ED, since it was the first time that a safe and highly effective pharmacologic treatment was available for men with ED. Prior to 1983, the most safe and effective treatment for men with an organic form of ED was surgery. Three drugs for intracavernosal injection therapy have been used most frequently. The most widely used is prostaglandin E1 (PGE1), but the others include papaverine hydrochloride and phentolamine mesylate.44, 45 PGE1 acts primarily via specific receptors on the surface of the penile smooth muscle cell to stimulate the enzyme adenylate cyclase. This enzyme converts adenosine triphospate (ATP) into cyclic adenosine monophosphate (cAMP) into 5’ adenosine monophosphate (5’ cAMP). Injection of PGE1 therefore causes a rise in intracellular cAMP, causing a fall in intracellular calcium, thereby inducing smooth muscle relaxation. Intracavernosal PGE1 therapy is associated with good efficacy and tolerability in most forms of ED. Adverse events associated with prostaglandin injection therapy are primarily local effects and include penile pain (15%) and priapism (1%) and, in the longer term, penile scarring (1.7%).44, 45 Papaverine hydrochloride, the first drug to be widely used clinically for intracavernosal injection therapy, is now rarely administered alone, but is mainly utilized in combination with phentolamine (bimix) and/or PGE1 (trimix) in ED patients refractory to oral therapy. Papaverine is a non-selective inhibitor of the enzyme phosphodiesterase (PDE) in the penis, inhibiting the breakdown of both cyclic GMP and cyclic AMP, resulting in a fall in the smooth muscle cell cytoplasm calcium concentration and leading to smooth muscle relaxation.44 Phentolamine mesylate has a synergistic action when combined with drugs such as papaverine and PGE1. Phentolamine acts as an inhibitor of both alpha-1 and alpha-2 adrenoceptors antagonizing the action of noradrenaline on the cell.44 Intracavernosal Injection Therapy Technique Patients must be taught to inject themselves safely and effectively, first in the clinic and later at home. The technique is easy to master and relatively painless. The solution of vasoactive drugs (determined by the physician) is injected slowly with a fine needle (30 gauge, 5/16 inch) into the side of the midshaft or base of the shaft of the penis with an insulin syringe (1 ml or 0.5 ml) held perpendicular to the skin. When the injection has been administered, the needle is removed and pressure applied to the injection site for three minutes, typically by squeezing the penis in the man’s fist. Erection normally occurs within 10 minutes. Some patients may have difficulty performing self-injections because of poor manual dexterity, poor visual acuity, or secondary to obesity. Injection by the partner or use of an auto-injector may be helpful.44, 45 The dose of the initial intracavernosal injection can be estimated. Men with neurogenic or psychogenic ED can respond to small doses (2.5 – 5 micrograms) of PGE1. Patients with severe vasculogenic ED may respond with a trimix combination of papaverine and/or phentolamine or both. The duration of erection response following intracavernosal injection may be between fifty Verathon Inc. 800.331.2313 www.verathon.com 72 Urology for Primary Care Male Erectile Dysfunction minutes and two hours. The patient must be advised that if the penile erection lasts for more than about four hours, he should promptly seek medical attention. Intracavernosal administration of phenylephrine (300 g repeated as often as needed, every 5 – 10 minutes) usually resolves the prolonged erection.44, 45 Side effects from intracavernosal injection therapy include priapism, Peyronie’s disease, penile scarring, and bleeding. The use of anticoagulants is not an absolute contraindication, but extra care must be taken to avoid excessive bruising. The success rate is about 70% - 80%. In time, a large proportion of patients drop out and stop treatment. There are a number of reasons for this high drop-out rate, including ineffective therapy, patient dissatisfaction, and partner dissatisfaction.44, 45 Second-Line – Intraurethral Pharmacological Therapy Alprostadil in the form of a small pellet can be administered into the urethra that is permeable to multiple drugs. The administration system is called MUSE (medicated urethral system for erection). The transfer of active substances (PGE1) to the corpora cavernosa occurs primarily via venous channels that communicate between the corpus spongiosum and the corpora cavernosa. MUSE is available in a range of doses of PGE1 from 125 to 1000 g. Erection starts within 15 to 30 minutes and lasts 30 to 60 minutes. The strength of erection can be improved by application of a constriction ring in order to reduce proximal absorption. Although 80% of the drug is absorbed from the urethra within 10 minutes of application, the level of PGE1 in the ejaculate could be dangerous to pregnant women, in which case a condom should be used. The MUSE applicator is inserted into the urethral meatus up to the collar. The injector button is pushed down to release the pellet containing PGE1 into the urethra. The patient then massages the penis to help the distribution of the medication. The method is safe and efficacious in approximately half of cases. The obvious advantage is that a penile injection may be avoided, but the disadvantages include penile pain, rare dizziness, and variable efficacy.44 Third-Line – Penile Prostheses Penile prostheses are the last resort therapy when all other means of ED treatment have failed or are contraindicated. There are two types of prostheses, malleable and inflatable devices. Malleable devices consist of a pair of flexible silicone rods that are surgically implanted inside the corpora cavernosa. They provide adequate axial penile rigidity for vaginal penetration and, even though they maintain their rigidity, they can be bent downward for cosmetic appearance when not in use. Compared to malleable devices, inflatable prosthesis are more “natural,” being the most rigid and wide in girth in the erect state and the most soft and small in the flaccid state. Inflatable penile prostheses are composed of a pair of penile cylinders, a pump, and a reservoir. The twopiece inflatable device combines the reservoir holding approximately 10 ml of saline and the pump. The three-piece inflatable device offers more rigidity because the pump component can transfer approximately 100 ml saline from the reservoir into the cylinders. Malleable devices are less widely used than inflatables, less expensive, and technically easier to insert into the penis. In the inflatable device, the pump component is surgically placed in the scrotum and the reservoir component is surgically placed in the retroperitoneal space lateral to the prostate, beneath the rectus abdominus muscles.46 Verathon Inc. 800.331.2313 www.verathon.com 73 Urology for Primary Care Male Erectile Dysfunction The most feared complication of both malleable and inflatable prostheses is post-operative infection that occurs in 1-5% of cases. Higher infection rates are observed in patients with diabetes, autoimmune diseases, and prosthesis revision. Infection requires prosthesis removal. Newer, antibiotic-coated penile prosthetic devices should reduce post-operative infection rates. Mechanical failures are less than 5% in the first year, about 20% at five years, and 50% at ten years. Post-operative pain is usually resolved after several weeks, and satisfaction rates from penile prosthesis surgery range from 70% to 90% for patients.46 Third-Line – Vascular Surgery The objective of microvascular arterial bypass surgery is to increase the cavernosal arterial perfusion pressure, and thus to increase the spontaneity of initiating the erection and increase the rigidity of the erection. Candidates for this surgery must have reduced cavernosal arterial perfusion pressure secondary to a focal occlusion in the distal internal pudendal artery, common penile artery or proximal cavernosal artery. The occlusive arterial pathology is presumed secondary to an episode of blunt perineal trauma such as bicycle riding. Candidates for surgery need to have a normal corporal veno-occlusive mechanism, as well as normal psychologic, neurologic, and endocrinologic integrity. Microvascular arterial bypass surgery is only indicated in highly selected patients and should be performed by experienced specialists. The surgical technique involves anastomosing the inferior epigastric artery to the dorsal penile artery. Candidates for microvascular arterial bypass surgery include young men <50 years of age with a history of trauma (perineal or penile), documented arterial insufficiency, and normal corporal veno-occlusive function as demonstrated by Doppler, dynamic pharmacocavernosometry, and arteriography, and no other risk factors for ED. It is estimated that 60% to 70% of these highly selected patients have restored erectile function.43, 47, 48 CONCLUSIONS ED is a highly prevalent medical disorder with multiple risk factors and multiple safe and effective therapies that achieve the goal of sexual satisfaction. To better understand the field, the essentials of definitions, anatomy, epidemiology, physiology, pathophysiology, diagnosis, and treatment of erectile dysfunction have been reviewed. Good clinical practice requires the use of clear and specific guidelines for diagnostic assessment and testing, and a rational, evidence-based step-care model for treatment selection that is both patient (and partner) and goal-directed, and offers men with ED the maximum opportunity for sexual health care. What does the future hold in the field of sexual dysfunction? Some possible directions include new pharmacologic agents for ED and other sexual dysfunctions, including women’s sexual health medications that are in various stages of development. By the year 2010, it is anticipated that several additional agents will be approved for safe and effective treatment of men’s and women’s sexual health problems. Verathon Inc. 800.331.2313 www.verathon.com 74 Urology for Primary Care Male Erectile Dysfunction It is also predicted that there will be Departments of Sexual Medicine in medical schools, which will engage in multidisciplinary research and patient care in the field, educating a new class of sexual medicine trained physicians. There will be new research on the molecular biological basis of sexual function that may lead, in the future, to broader therapies for prevention or treatment of men’s and women’s sexual health problems. Finally, it is anticipated that greater awareness of the importance of sexual medicine in the overall health and well-being of all patients will be recognized. Sexual health is an important element in the physical health and psychological well-being of most patients, and should be routinely addressed in all medical care settings. References 1. Goldstein I. The inaugural issue of The Journal of Sexual Medicine (JSM). J Sex Med. 2004;1(1):1-2. 2. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. JAMA. 1999;281(6):537-544. 3. Lewis RW, Fugl-Meyer KS, Bosch R, et al. Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1(1):35-39. 4. http://www.who.int/reproductive-health/gender/sexual_health.html 5. Goldstein, I., Lue, T., Padma-Nathan, H., Rosen, R., Steers, W. and Wicker, P.: Oral sildenafil in the treatment of erectile dysfunction. New Engl. J. Med. 338: 1397 - 1404, 1998. 6. Padma-Nathan H, Christ C, Adaikan G, et al. Pharmacotherapy for erectile dysfunction. J Sex Med. 2004;1(2):128-140. 7. Hatzichristou D, Rosen RC, Broderick G, Clayton, A, Cuzin B, Derogatis L, Litwin M, Meuleman E, O’Leary M, Quirk F, Sadofsky R and Seftel A. Clinical Evaluation and Management Strategy for Sexual Dysfunction in Men and Women. J Sex Med 1:1, 49 – 57, 2004. 8. Montague, D.K., Padma-Nathan, H., Rosen, R., Sadovsky, R., Segraves, R.T. and Shabisgh, R.: The process of care model for evaluation and treatment of erectile dysfunction. Int. J Impot. Res. 11: 59 - 74, 1999. 9. Lue TF, Giuliano F, Montorsi F, Rosen RC, Andersson KE, Althof S, Christ G, Hatzichristou D, Hirsch M, Kimoto Y, Lewis R, McKenna K, MacMahon C, Morales A, Mulcahy J, Padma-Nathan H, Pryor J, Saenz de Tejada I, Shabsigh R and Wagner G. Summary of the Recommendations on Sexual Dysfunctions in Men. J Sex Med 1:1, 6 – 23, 2004. 10. Feldman, H.A., Goldstein, I. Hatzichristou D.G., Krane R.J. and McKinlay J.B.: Impotence and it's medical and psychosocial correlates: Results of the Massachusetts Male Aging Study. J. Urol. 151: 54 - 61, 1994. 11. Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts Male Aging Study. J Urol 163: 460-463, 2000. 12. Araujo, A.B., Durante, R., Feldman, H.A., Goldstein, I. and McKinlay, J.B.: The relationship between depressive symptoms and male erectile dysfunction: Cross-sectional results from the Massachusetts Male Aging Study. Psychosom. Med., 60: 458 - 465, 1998. 13. Marceau L, Kleinman K, Goldstein I, McKinlay J. Does bicycling contribute to the risk of erectile dysfunction? Results from the Massachusetts Male Aging Study (MMAS). Int J Impot Res. 2001 Oct;13(5):298-302. 14. Sáenz de Tejada I, Angulo J, Cellek S, González-Cadavid N, Heaton J, Robert Pickard R, and Simonsen U Physiology of Erectile dysfunction and Pathophysiology of Erectile Dysfunction. In: Sexual Medicine Sexual Dysfunctions in Men and Women, Editors TF Lue, R Basson, R Rosen, F Giuliano, S Khoury, F Verathon Inc. 800.331.2313 www.verathon.com 75 Urology for Primary Care Male Erectile Dysfunction Montorsi 2nd International Consultation on Sexual Dysfunction. Health Publications Paris, France Edition 2004, 287 - 344. 15. Saenz de Tejada I, Angulo J, Cellek S, et al. Physiology of erectile function. J Sex Med. 2004;1(3):254-265. 16. Morales A, Buvat J, Gooren LJ, Guay AT, Kaufman JM, Tan HM and Torres LO. Endocrine Aspects of Sexual Dysfunction in Men. J Sex Med 1:1, 69 – 81, 2004 17. Sáenz de Tejada I, Angulo J, Cellek S, González-Cadavid N, Heaton J, Robert Pickard R, and Simonsen U. Pathophysiology of Erectile Dysfunction. J Sex Med 2:1 26 - 39, 2005 18. Althof, SE, Leiblum SR, Chevret-Measson M, Hartman U, Levine SB, McCabe M, Plaut M, Rodrigues O, and Wylie K. Psychological and Interpersonal Dimensions of Sexual Function and Dysfunction J Sex Med 2005 in press 19 McKenna K, Adam MA, Bivalacqua T, Coolen L, Gonzalez-Cadavid N, Hedlund P, Park K, Pescatori E, Rajfer J, Sato Y. Experimental Models for the Study of Male Sexual function In: Sexual Medicine - Sexual Dysfunctions in Men and Women, Editors TF Lue, R Basson, R Rosen, F Giuliano, S Khoury, F Montorsi 2nd International Consultation on Sexual Dysfunction. Health Publications Paris, France Edition 2004, 241 286. 20 Lue TF, Giuliano F, Montorsi F, Rosen RC, Andersson KE, Althof S, Christ G, Hatzichristou D, Hirsch M, Kimoto Y, Lewis R, McKenna K, MacMahon C, Morales A, Mulcahy J, Padma-Nathan H, Pryor J, Saenz de Tejada I, Shabsigh R and Wagner G. Summary of the Recommendations on Sexual Dysfunctions in Men. In: Sexual Medicine - Sexual Dysfunctions in Men and Women, Editors TF Lue, R Basson, R Rosen, F Giuliano, S Khoury, F Montorsi 2nd International Consultation on Sexual Dysfunction. Health Publications Paris, France Edition 2004, 605 - 681. 21 Rosen R, Hatzichristou D, Broderick G, Clayton, A, Cuzin B, Derogatis L, Litwin M, Meuleman E, O’Leary M, Quirk F, Sadofsky R and Seftel A. Clinical Evaluation and Symptom Scales: Sexual dysfunction Assessment in Men. In: Sexual Medicine - Sexual Dysfunctions in Men and Women, Editors TF Lue, R Basson, R Rosen, F Giuliano, S Khoury, F Montorsi 2 nd International Consultation on Sexual Dysfunction. Health Publications Paris, France Edition 2004, 173 - 220. 22. Pescatori, E.S., Hatzichristou, D.G. Namburi, S. and Goldstein, I.: A positive intracavernosal injection test implies normal veno-occlusive but not necessarily normal arterial function: A hemodynamic study. J. Urol. 151: 1209 - 1216, 1994. 23. Hatzichristou, D.G., Saenz de Tejada, I., Kupferman, S., Namburi, S., Pescatori, E.S., Udelson, D., and Goldstein I.: In vivo assessment of trabecular smooth muscle tone, It's application in pharmacocavernosometry and analysis of intracavernous pressure determinants. J. Urol. 153: 1126 - 1135, 1995 24. Basson R, Brotto LA, Laan E, Redmond G, and Utian, WH. Assessment and Management of Women's Sexual Dysfunctions: Problematic Desire and Arousal. J Sex Med 2 (3): 291 - 300, 2005. 25. Weijmar Schultz W, Basson R, Binik Y, Eschenbach D, Wesselmann U, and Van Lankveld J. Women's Sexual Pain and its Management. J Sex Med 2 (3): 301 - 316, 2005. 26. Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84 (1): 50-56. 27. Viagra® sildenafil citrate[package insert]. Viagra®, New York, NY: Pfizer, Inc.; 2005. 28. Levitra® vardenafil HCl [package insert]. Levitra®, Research Triangle Park, NC and Kenilworth, NJ: GlaxoSmithKline and Schering-Plough Corporation; 2005. 29. Cialis® tadalafil [package insert]. Cialis®, Indianapolis, IN: Eli Lilly and Company; 2005. 30. Blount MA, Beasley A, Zoraghi R, et al. Binding of tritiated sildenafil, tadalafil, or vardenafil to the phosphodiesterase-5 catalytic site displays potency, specificity, heterogeneity, and cGMP stimulation. Mol Pharmacol. 2004;66(1):144-152. 31. McCullough AR, Barada JH, Fawzy A, Guay AT, Hatzichristou D. Achieving treatment optimization with sildenafil citrate (Viagra) in patients with erectile dysfunction. Urol 2002;60(2 Suppl 2):28-38. Verathon Inc. 800.331.2313 www.verathon.com 76 Urology for Primary Care Male Erectile Dysfunction 32. Schulman CC, Shen W, Stothard DR, Schmitt H. Integrated analysis examining first-dose success, success by dose, and maintenance of success among men taking tadalafil for erectile dysfunction. Urol 2004;64(4):7838. 33. Montorsi F, Hellstrom WJ, Valiquette L, et al. Vardenafil provides reliable efficacy over time in men with erectile dysfunction. Urol 2004;64(6):1187-1195. 34. Claes HIM, Van Poppel H. An independent, prospective, 2-site, randomized, open-label crossover study consisting of six treatment arms to evaluate preference among sildenafil, tadalafil and vardenafil use in men with erectile dysfunction naive to PDE 5 inhibitor therapy. Submitted to JSM 2005. 35. Eardley I, Ellis P, Boolell M, Wulff M. Onset and duration of action of sildenafil for the treatment of erectile dysfunction. Br J Clin Pharmacol. 2002;53 Suppl 1:61S-65S. 36. Montorsi F, Padma-Nathan H, Buvat J, et al. Earliest time to onset of action leading to successful intercourse with vardenafil deteremined in an at-home setting: a randomized, double-blind, placebo-controlled trial. J Sex Med. 2004;1:168-178. 37. Rosen RC, Padma-Nathan H, Shabsigh R, Saikali K, Watkins V, Pullman W. Determining the earliest time within 30 minutes to erectogenic effect after tadalafil 10 and 20 mg: a multicenter, randomized, double-blind, placebo-controlled, at-home study. J Sex Med. 2004; 1 (2) :193-200. 38. Porst H, Padma-Nathan H, Giuliano F, Anglin G, Varanese L, Rosen R. Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: a randomized controlled trial. Urol 2003;62(1):121-125; 39. Valiquette L MF, Auerbach S. Vardenafil is effective for 8 hours administered in a flexible-dose regimen in patients with erectile dysfunction: EXTEND. Presented at the Sexual Medicine Society of North America (SMSNA) fall meeting November 2005. New York, NY; 2005. 40. Fischer WA, Rosen RC, Eardley I, Sand M, Goldstein I. Sexual experience of female partners of men with erectile dysfunction: The Female Experience of Men's Attitudes to Life Events and Sexuality (FEMALES) Study. J Sex Med. 2 (5): 675 - 684, 2005 41. Goldstein I, Fisher, WA, Sand M, Rosen RC, Mollen M, Brock G, Karlin G, Pommerville P, Bangerter K, Bandel TJ, Derogatis LR. Women's Sexual Function Improves When Partners Are Administered Vardenafil For Erectile Dysfunction: A Prospective, Randomized, Double-Blind Placebo-Controlled Trial. J Sex Med 2 (6) in print, 2005 42. Heiman JR, Talley DR, Bailen JL, Shumel B, Pace CR, Creanga DL, Bavendam T. Female partners of men with erectile dysfunction treated with sildenafil citrate report greater satisfaction and enjoyment from sexual intercourse: A multicentre, randomized, double-blind, placebo-controlled study. J Urol 2005;173(4):S5. 43 Mulcahy JJ, Austoni E, Barada JH, Choi HK, Hellstrom WJG, Krisnamurti S, Moncada I, Shultheiss D, Sohn M and Wessells H. Implants, Mechanical Devices and Vascular Surgery for Erectile Dysfunction In: Sexual Medicine - Sexual Dysfunctions in Men and Women, Editors TF Lue, R Basson, R Rosen, F Giuliano, S Khoury, F Montorsi 2nd International Consultation on Sexual Dysfunction. Health Publications Paris, France Edition 2004, 469 - 502. 44 Padma-Nathan H, Christ C, Adaikan G, et al Pharmacotherapy for Erectile Dysfunction In: Sexual Medicine - Sexual Dysfunctions in Men and Women, Editors TF Lue, R Basson, R Rosen, F Giuliano, S Khoury, F Montorsi 2nd International Consultation on Sexual Dysfunction. Health Publications Paris, France Edition 2004, 503 - 566. 45 Mulhall, J.P., Jahoda, A.E., Cairney, M., Goldstein, B., Leitzes, R., Woods, J., Payton, T., Krane R.J. and Goldstein, I.: The causes of patient dropout from penile self-injection therapy for impotence. J. Urol. 162: 1291 - 1294, 1999. 46. Mulcahy JJ, Austoni E, Barada JH, Choi HK, Hellstrom WJG, Krisnamurti S, Moncada I, Shultheiss D, Sohn M and Wessells H. The Penile Implant for Erectile Dysfunction. J Sex Med 1:1, 98 – 109, 2004 47. Munarriz RM, LaSalle MD, Goldstein I. Penile revascularization for treatment of erectile dysfunction secondary to blunt perineal trauma. Urol. 2003 Jan;61(1):222-3. Verathon Inc. 800.331.2313 www.verathon.com 77 Urology for Primary Care Male Erectile Dysfunction 48. Hakim, L.S., Nehra, A., Kulaksizoglu, H. and Goldstein, I: Penile microvascular arterial bypass surgery. Microsurgery 16: 296 - 308, 1995. Verathon Inc. 800.331.2313 www.verathon.com 78 Urology for Primary Care Sexual Medicine for Women SEXUAL MEDICINE FOR WOMEN: MANAGEMENT OF WOMEN WITH DESIRE, AROUSAL AND ORGASM SEXUAL HEATH CONCERNS Irwin Goldstein, M.D. Editor-in-Chief, The Journal of Sexual Medicine www.irwingoldsteinmd.com INTRODUCTION At the present time, there are limited numbers of health care professionals offering comprehensive psychologic and biologic sexual health care for women. This is likely only a temporary situation. Over the next few years, health care professionals will be increasingly engaged in the sexual heath care of women (and their partners) for several reasons. First, multiple population-based studies reveal a high prevalence of sexual health concerns in women of all ages.1 – 11 A woman’s sexual problems can cause her significant personal distress, including a diminution of self-worth and self-esteem, a reduction in life satisfaction, and a decline in the quality of her relationship with her partner. Women with sexual health concerns are becoming more empowered to seek medical consultations for assistance.2 – 5 Second, sexual health, like general health, is a fundamental human right. Sexual health, referring to a state of physical, emotional, mental, and social well-being related to sexuality, must also be a basic human right. All women have the right to safe, pleasurable sexual experiences that are a source of physical, psychological, intellectual, and spiritual satisfaction and that are free from coercion, discrimination, and violence. Promotion of women’s sexual health requires a positive, respectful approach to sexuality and sexual relationships. For sexual health to be attained and maintained, the sexual rights of all persons need be respected, protected, and fulfilled.12 Many investigations have shown that a satisfying sex life is important to most women throughout most of their lives. While the frequency of sexual intercourse declines with age, a study of 1300 older adults reported that 66% of women aged sixty years or older who had sex at least once a month regarded an active sex life to be important to their relationships.13 Third, as life expectancy increases, there are increasing numbers of aging women. Sexual health concerns are more common among aging women than in younger women. In the year 2000, the number of U.S. women older than age fifty years was almost 42 million.13 Since there are approximately 150 million women in the U.S., it can be assumed that approximately 1 out of every 3 women in the U.S. are over fifty years of age. Every year, another 2 million women in the United States reach the age of fifty years. The number of postmenopausal women worldwide in the year 2000 was 569 million; that number is projected to grow to 967 million by 2020 and to 1.2 billion by 2030.14 With the current controversy concerning hormone therapy that emerged following the release of the Women’s Health Initiative report in the summer of 2002, millions of menopausal women altered and or discontinued hormone therapy practices.15 Since estradiol has a critical role in genital physiology16, many women experienced subsequent vaginal dryness, dyspareunia, and lowered sexual interest. As the decline in estrogen production begins in Verathon Inc. 800.331.2313 www.verathon.com 79 Urology for Primary Care Sexual Medicine for Women transition and menopause, sexual dysfunction is common in aging women and often interferes with women’s interest in, and satisfaction with, sexual activity. Fourth, there have been only limited clinical and basic science research investigations in the area of women’s sexual health concerns.16 In the past, the sexual health problems of women have been associated with multiple theories and anecdotes, with little documentation in evidence-based medicine. Randomized, multi-institutional, multicultural, controlled treatment outcome studies in the management of women with sexual dysfunctions are, unfortunately, limited. This field of medicine is “wide-open” for evidence-based investigation. The existence of the International Society for the Study of Women's Sexual Health (ISSWSH), an international, multidisciplinary, academic, clinical, and scientific organization, should be noted. The purposes of ISSWSH are to provide opportunities for communication among scholars, researchers, and practitioners about women's sexual health; to support the highest standards of ethics and professionalism in research, education, and clinical practice relative to women's sexual health; and to provide the public with accurate information about women's sexual health. Interested healthcare professionals should visit the organization’s Web site, http://www.isswsh.org, for more information. The aim of this chapter is to provide the interested health care professional with relevant, evidence-based clinical information to help identify and treat specific biologic-based pathophysiologies. It is not within the realm of this chapter to thoroughly discuss psychologic management of women’s sexual health issues or management of women with sexual pain disorders. Even though health care professionals need to be holistic in managing women (and men) with sexual dysfunction, the goal is educate the biologic-focused health care professional on how to identify the biologic pathology or pathologies associated with the sexual health concern, and to provide evidence-based, safe, and effective biologic management strategies. Discussed in this chapter are first, the population-based epidemiologic studies in women with sexual health; second, the concerns for the critical physiologic role of sex steroid hormones such as estradiol and testosterone on genital structure and function; and finally, the biologically focused management strategies for women with desire, arousal, and orgasm sexual health concerns. The resultant clinical, biologic-based paradigm is founded on results derived from emerging basic science and evidence-based data derived from clinical research. EPIDEMIOLOGY There are limited population-based epidemiologic studies in women with sexual health concerns.1 – 11 Measurements of women’s sexual problems have varied, including how sexual problems are defined, how they are classified, and which instruments are utilized. It should be noted that the appropriateness of current women’s sexual health definitions developed at recent international consensus meetings are still being discussed by experts.17 – 19 The type of definitions selected in any study will obviously affect the epidemiologic prevalence estimates of women’s sexual problems. Based on current published information, the following is the most contemporary, international consensus on the classification of women’s sexual problems. Verathon Inc. 800.331.2313 www.verathon.com 80 Urology for Primary Care Sexual Medicine for Women Women’s Sexual Interest/Desire Disorder: “Absent or diminished feelings of sexual interest or desire, absent sexual thoughts or fantasies, and a lack of responsive desire. Motivations (here defined as reasons/incentives), for attempting to become sexually aroused are scarce or absent. The lack of interest is considered to be beyond a normative lessening with life cycle and relationship duration.” Subjective Sexual Arousal Disorder: “Absence of, or markedly diminished feelings of, sexual arousal (sexual excitement and sexual pleasure) from any type of sexual stimulation. Vaginal lubrication or other signs of physical response still occur.” Genital Sexual Arousal Disorder: “Complaints of absent or impaired genital sexual arousal. Self-reports may include minimal vulval swelling or vaginal lubrication from any type of sexual stimulation and reduced sexual sensations from caressing genitalia. Subjective sexual excitement still occurs from non-genital sexual stimuli.” Combined Genital and Subjective Arousal Disorder: “Absence of, or markedly diminished feelings of, sexual arousal (sexual excitement and sexual pleasure) from any type of sexual stimulation, as well as complaints of absent or impaired genital sexual arousal (vulval swelling, lubrication).” Persistent Sexual Arousal Disorder: “Spontaneous, intrusive and unwanted genital arousal (e.g. tingling, throbbing, pulsating) in the absence of sexual interest and desire. Any awareness of subjective arousal is typically but not invariably unpleasant. The arousal is unrelieved by one or more orgasms and the feelings of arousal persist for hours or days.” Women’s Orgasmic Disorder: “Despite the self-report of high sexual arousal/excitement, there is either lack of orgasm, markedly diminished intensity of orgasmic sensations, or marked delay of orgasm from any kind of stimulation.” Dyspareunia: “Persistent or recurrent pain with attempted or complete vaginal entry and/or penile vaginal intercourse.” Vaginismus: “Persistent difficulties to allow vaginal entry of a penis, a finger, and/or any object, despite the woman’s expressed wish to do so. There is variable involuntary pelvic muscle contraction, (phobic) avoidance, and anticipation/fear/experience of pain. Structural or other physical abnormalities must be ruled out/addressed.” Sexual Aversion Disorder: “Extreme anxiety and/or disgust at the anticipation of, or attempt to have, any sexual activity.” Laumann et al., in a U.S. population-based study of more than 1700 women, observed that in excess of 40% reported sexual health concern.1, 2 In that investigation, the most common sexual health concern was lack of sexual desire, followed by inability to achieve orgasm, lack of pleasure from sex, and pain during sex. Poor health, emotional problems or stress, decline in social status, and having ever been forced sexually were among the factors associated with low desire, arousal disorder, and sexual pain. A worldwide survey in more than 27,000 men and women aged forty to Verathon Inc. 800.331.2313 www.verathon.com 81 Urology for Primary Care Sexual Medicine for Women eighty years assessed the relevance of sex and the prevalence of sexual dysfunction.7 In that survey, women’s sexual health concerns were classified into five subcategories: Lack of interest in sex, inability to achieve orgasm, lubrication difficulties (dryness), sex not pleasurable, and pain during sexual intercourse (dyspareunia). The percentage of women who reported having at least one sexual health concern on a frequent or persistent basis was 39%. The percentage of women who reported having only one of the five sexual health concerns was ranged from 10% for “dyspareunia” to 21% for “lack of interest in sex.”20 Another study among women in their late reproductive years showed that significant predictors of decreased libido were: 1) children under the age of 18 living at home; and 2) depression.21 Contemporary international prevalence data concerning women’s sexual health problems are represented: For problems with sexual desire or sexual interest, the reported estimates range from 7% current to 31% lifetime prevalence. For problems with excitement or pleasure, estimates for a one-year prevalence are 23% and for lifetime prevalence are 20%. For lubrication difficulties or vaginal dryness, prevalence estimates are 20% for a oneyear prevalence and range from 19 – 23% for lifetime prevalence. For infrequent orgasm or difficulties in reaching orgasm, lifetime prevalence estimates range from 4% to 41%. For an inability to experience orgasm for several months, one population-based study estimated the prevalence to be 16% of women, while another reported that 18% had problems with orgasm for two months or more and 25% had trouble reaching orgasm more than 50% of the time. For pain during or after sex, prevalence estimates range from 3% to 48% and lifetime estimates from population-based studies range from 17% and 19%, while clinic-based studies report prevalence estimates of 10-20%. For women experiencing a problem with desire, arousal, orgasm, or pain, two population-based surveys, using similar instruments, estimate a prevalence of 33% and 35%. For women experiencing a current sexual problem, one national study estimated a onemonth prevalence of 45%. For any sexual problems among adult women, the prevalence is estimated at 43%. PHYSIOLOGY AND PATHOPHYSIOLOGY Changes in the Female Genitalia With Aging The structure and function of a woman's genitalia are highly dependent on the sex steroid hormonal milieu.6, 16, 22 – 26 As a woman ages or a young woman is exposed to medicines that Verathon Inc. 800.331.2313 www.verathon.com 82 Urology for Primary Care Sexual Medicine for Women interfere with the hormonal milieu (e.g. oral contraceptives, tamoxifene), her supply of sex steroid hormones (estradiol, testosterone, and progesterone) diminishes significantly.6 It is important to note that not all women have absent estradiol synthesis in menopause. During menopause, ovarian estradiol production ceases in all women. However, estrogen continues to be synthesized in the periphery (e.g. skin, adipose tissue, bone, muscle) in postmenopausal women through conversion of androstenedione to estrone and testosterone to estradiol. The amount of estradiol synthesized depends, in part, on the enzymatic activity of aromatase. Estrogens and androgens are required for genital tissue structure and function.16 These hormones act on estrogen and androgen receptors, respectively, which exist in high numbers in genital tissues, including the epithelial/endothelial cells and smooth muscle cells of the vagina, vulva, vestibule, labia, and urethra. Diminished estrogen production for natural or iatrogenic reasons renders women’s genital tissues highly susceptible to atrophy. Physical examination27, 28 of the postmenopausal woman’s genitalia shows clitoral atrophy, phimosis, and nearly absent labia minora. The appearance of a woman's labia minora mirrors her level of estradiol, because these labia are exquisitely sensitive to estradiol. The urogenital area termed the vestibule is very important in female sexual function because it contains organs that are sensitive to both estrogen and androgen. For example, the clitoral tissues and prepuce are androgen sensitive. The minor vestibular glands, which are located in the labial-hymenal junction, are embryologically derived from the glands of Littre, which are also androgen dependent. The glands of Littre are located on the anterior surface of the urethra. A host of structural changes and cellular dysfunctions occur in women’s genital tissues as a result of estrogen deficiency.27 – 32 For example, estrogen deficiency specific to the vagina leads to vaginal atrophy. One consequence is an alteration in the normally acidic vaginal pH that discourages the growth of pathogenic bacteria. The change to an alkaline pH value in the atrophic vagina leads to a shift in the vaginal flora, increasing the likelihood of discharge and odor. In an estrogen-rich environment, glycogen from sloughed epithelial cells is hydrolyzed into glucose and then metabolized to lactic acid by normal vaginal flora. In postmenopausal women, however, epithelial thinning reduces the available glycogen. In addition to vaginal atrophy and a reduction in organ size, other signs of a decline in sex hormones in women include vaginal dryness; no secretions or lubrication; pale or inflamed tissue; petechiae; epithelial/mucosal thinning; organ prolapse; changes in external genitalia; decreased tissue elasticity; and loss of smooth muscle. Symptoms of women’s sexual health concerns that the clinician may elicit when taking a history in a menopausal woman are dyspareunia; vaginismus; coital anorgasmia; vaginal and/or urinary tract infections (pH imbalance); and overactive bladder/incontinence.27 – 32 Effects of Sex Steroid Hormones on the Vagina The human vagina consists of three layers of tissue: The epithelium (composed of squamous cells), the lamina propria, and the muscularis (inner circular and outer longitudinal smooth muscle). The epithelium undergoes mild changes during the menstrual cycle. The lamina propria is replete with tiny blood vessels that become engorged with blood during sexual arousal, leading Verathon Inc. 800.331.2313 www.verathon.com 83 Urology for Primary Care Sexual Medicine for Women to lubrication. The smooth muscle of the muscularis enables the vagina to dilate and lengthen during penile penetration. Relaxation of that muscle leads to arousal. These three layers of tissue may function in an interrelated way. It is hypothesized that the blood vessels in the lamina propria that allow for lubrication are dependent on growth factors, and that the growth factors are derived from the muscularis. Postmenopausal atrophy of vaginal tissues may be due to decreased synthesis of these growth factors, resulting in a diminished number of critical blood vessels in the lamina propria. Controlled studies employing a rat model of vaginal atrophy demonstrated the effects of different dosages of estrogen, progestin, testosterone, and various combinations of these hormones on various physiologic and anatomic outcome parameters.33 These measures included organ wet weight, vaginal blood flow, epithelial height, muscularis thickness, and vaginal innervation. In each study, the effects on the vagina of different dosages of the hormone being tested were compared between rats that had undergone sham ovariectomies and rats that had actually had both ovaries removed. The hormones (or saline) were delivered through a pump inserted into the back of the neck of each animal. A Doppler probe inserted into the vagina was used to record blood flow after electrical stimulation of a nerve next to the vagina. All animals were then euthanized and vaginal tissue was removed for biochemical or histologic studies. In those animal studies, removal of the ovaries reduced the wet weight of the uterus; the weight of the uterus rose with the administration of estrogen because the uterus is a very estrogen-sensitive organ, much more so than the vagina. Increased vaginal blood flow is an indicator of sexual arousal. Genital swelling and lubrication are responses to increased clitoral and vaginal perfusion; increased length and diameter of the vaginal canal and clitoral corpora cavernosa; engorgement of the vagina wall, clitoris, and labia major and minora; and transudation of lubricating fluid from the vaginal epithelium. In the animal studies just described, blood flow to the vagina was greatly reduced in the oophorectomized rats compared with the intact rats. Contrary to what one might expect, subphysiologic doses of estradiol increased vaginal blood flow in oophorectomized rats more than either physiologic or supraphysiologic doses.33 Ovariectomy deprived the rats of estradiol, causing the vaginal epithelium to thin down to a single layer. Subphysiologic doses of estradiol increased the thickness of the vaginal epithelium the most because the oophorectomized rats had more estrogen-alpha receptors in the epithelium than the intact animals. A small amount of estradiol delivered to tissue with many estrogen-alpha receptors produced a huge response. Thus, estradiol regulates estrogen receptors through a negative feedback system. The more estradiol that is available, the fewer estrogen receptors there are. The muscularis, the muscle that enables the vagina to lengthen and widen during sexual arousal, also atrophies without estrogen. In postmenopausal women who do not take hormone therapy, the vaginal epithelium, lamina propria blood vessels, and muscularis all decrease. Like the epithelium, the muscularis responds to estradiol by increasing in thickness.33 Verathon Inc. 800.331.2313 www.verathon.com 84 Urology for Primary Care Sexual Medicine for Women DIAGNOSIS History There is limited consensus on management paradigms for the diagnosis of women with sexual health complaints. The cornerstone of the physical-based diagnosis of women with sexual dysfunction is a history and physical examination performed by the biologic-focused health care professional. Specifically, obtaining a careful history is crucial, since this aspect of the diagnosis establishes impressions and forms the basis of the emphasis during the physical exam.27, 28 The history of a woman with sexual health concerns includes sexual, medical, and psychosocial aspects, so that the many physical and psychological factors that often contribute to the sexual health difficulty can be characterized. It is advised that women with sexual health concerns undergo a separate and concomitant psychologic interview by a psychologic-focused health care professional to broaden the psychosocial information derived during the interview process.27, 28 The sexual history should begin with the patient describing the sexual problem. The following questions may be utilized to help obtain maximal descriptive information: What is the sexual problem? When did the sexual problem manifest? How long have you had the sexual problem? Does the sexual problem happen all the time? Does the sexual problem occur during partner-related sexual activity? Does the sexual problem occur during self-stimulation? In which situations is the sexual problem minimized? In which situations is the sexual problem maximized? Did you ever experience full capabilities for sexual interest, sexual arousal, and sexual orgasm? How many years were you at peak sexual function? What is your current sexual functioning in terms of interest, arousal, and orgasm compared to when you were at peak sexual function? Is the sexual problem associated with any degree of discomfort, tenderness, soreness, or pain? If so, can you localize the site of the pain on a schematic diagram of a woman’s genitalia? What tests have you already had in the evaluation of your sexual health concern? What treatments have you already received and what are the outcomes of the various treatments? How does the sexual problem affect you? How is your partner affected by the sexual problem? Does the sexual problem cause you to withdraw from partner-related sexual activity, from self-stimulated sexual activity, or from the relationship? How would you feel if the sexual problem were cured? Verathon Inc. 800.331.2313 www.verathon.com 85 Urology for Primary Care Sexual Medicine for Women It is relevant to inquire after the sexual health of the partner. For women with men, there may exist male sexual dysfunctions such as erectile dysfunction, early ejaculation, or an anatomical concern, such as Peyronie’s disease. Data on the importance of the male partner to the sexual function of the woman are expanding exponentially. Current knowledge indicates that in a committed heterosexual relationship, the male partner’s sexual performance is linked to his female partner’s sexual function and sexual satisfaction. Therefore, if the male partner has some form of sexual dysfunction, such as erectile dysfunction, the woman's desire, arousal, ease of achieving orgasm, and satisfaction are reduced. This information is critical to obtain during history-taking. The use of validated, reliable, standardized self-rated questionnaires34 – 37 is a very useful clinical starting point to assist in identification of: 1) the presence or absence of a sexual problem; and 2) which of the sub-types of women’s sexual dysfunction (disorders of desire, arousal, orgasm and/or sexual pain) are involved. Such self-report measures are valuable screening tools that are easy to administer and score and have normative values for populations of women with and without sexual dysfunction. Common self-rated questionnaires include the Female Sexual Function and the Sexual Function Questionnaire. As in all areas of clinical medicine, the use of screening tools for clinical diagnosis has recognized limitations. The determination of particular psychological contributors or confounds, contextual conditions, and other features and characteristics that cause individual women their unique sexual concerns requires more traditional assessment through structured history and physical examination. The medical history should include focused questions on any accompanying medical/surgical illnesses and/or the use of medications. Topics of importance in the medical history may include: i) ii) iii) iv) v) vi) vii) viii) ix) x) Chronic/medical illnesses such as diabetes, anemia, or renal failure; Neurological illnesses such as spinal cord injury, multiple sclerosis, or lumbosacral disc disease; Endocrinologic illnesses such as hypogonadism, hyperprolactinemia, or thyroid disorders; Atherosclerotic vascular risk factor exposure such as hypercholesterolemia, hypertension, diabetes, smoking, or family history; Non-hormonal medication/recreational drug use such as antihypertensives, SSRI antidepressants, over the counter drugs, street drugs, alcohol, or cocaine; Hormonal medication use, such as combined oral contraceptives, infertility drugs, or leuprolide acetate; Pelvic/perineal/genital trauma, such as pelvic fracture or bicycling injury; Gynecologic history, such as childbirth, abortions, episiotomy, abnormal PAP smears, sexually transmitted diseases, pelvic inflammatory disorder, endometriosis, fibroids, hysterectomy with or without oophorectomy, menopause; Urologic history, such as incontinence, frequent urinary tract infections, interstitial cystitis, pelvic surgeries; Surgical history, such as laminectomy, colon/anal surgery, vascular bypass surgery; and Verathon Inc. 800.331.2313 www.verathon.com 86 Urology for Primary Care Sexual Medicine for Women xi) Psychiatric history, such as depression, panic, or anxiety. Since sex steroid hormones are critical for genital structure and function, the medical history should routinely probe and evaluate for symptoms of estrogen deficiency, such as vaginal dryness, vaginal bleeding with minimal sexual contact, pain and soreness after sexual activity, hot flashes, and night sweats. Symptoms of androgen deficiency include fatigue, lack of energy, diminished skeletal muscle strength, depressed mood, falling asleep after meals, decreased athletic performance, or lack of interest in sexual activity. The psychosocial history should assess such issues as social factors, past sexual beliefs, past sexual abuse and trauma, emotional concerns, and interpersonal relationship matters. Any past history of mood or psychiatric disorders should be identified. There are multiple caveats to history-taking in women with sexual health concerns. First, the health care professional should consider history-taking as having more significance than the first diagnostic step towards resolution of the sexual problem. Indeed, history-taking may actually be viewed as the beginning of treatment for the women with sexual health concerns. Women are often empowered following the detailed discussion of their sexual health, since they have now taken the first step in overcoming their past failures to take action in this area. It is not uncommon for the discussion with the health care provider to become a model of what is possible about sexual health conversation. Many patients will thereafter initiate a sexual health conversation with their partner, a close friend, or a family member. Second, the health care professional should be cognizant that, while the women may have a specific complaint (i.e., lack of interest), there may be additional and more complex mind, body, and relationship issues in the overall pathophysiology. For example, a woman may experience sexual pain during intercourse. She may be so psychologically distracted by the discomfort, throbbing, stinging, aching, soreness, burning, and/or tenderness, that physiologic desire, arousal, and orgasm responses during sexual stimulation are not able to manifest. It is possible that this woman may present with a primary sexual complaint such as lack of interest or orgasm, whereas more detailed history and physical examination may yield the concomitant longstanding genital sexual pain problem. Physical Examination The physical examination for a woman with sexual health concerns should be tailored to the sexual medicine complaint obtained on history-taking. For example, if during history-taking genital itching is identified as a major sexual health problem, a careful assessment would follow for the presence of a genital dermatitis condition. If a woman with sexual health problems is under the age of fifty and has sexual pain, a careful physical examination should evaluate for the presence or absence of vulvar vestibulitis syndrome/vestibular adenitis. Similar complaints of sexual pain in a woman over fifty years of age should assess for the presence of vaginal atrophy with dryness, loss of rugae, mucosal thinning, pale hue, and lack of shiny vaginal secretions. The physical examination should be performed ideally without menses and without intercourse or douching for twenty-four hours before the exam. If dysfunction occurs at a specific time, such as mid-cycle dyspareunia, the physical examination should be scheduled at the time of the sexual Verathon Inc. 800.331.2313 www.verathon.com 87 Urology for Primary Care Sexual Medicine for Women problem. Such scheduling may require two visits: One for history-taking and one for the physical examination.27, 28 The genital-focused examination should be considered routine in the diagnosis of women’s sexual health problems, but its personal character demands that a rational explanation exist for its inclusion in the diagnostic process. A focused peripheral genital examination is recommended in women with sexual dysfunction for complaints of dyspareunia, vaginismus, genital arousal disorder and combined arousal disorder, orgasmic disorder, pelvic trauma history, and any disease affecting genital health such as herpes or lichen sclerosis. For women with suspected neurological disorders, the examiner may also assess for anal and vaginal tone, voluntary tightening of anus, and bulbocavernosal reflexes. It is particularly important that the patient with sexual dysfunction has full communication with the health care provider and final authority during the physical examination to terminate at any time, to ask questions, to have control over who is in attendance, and to understand the extent of the assessment. It is vital that the patient is aware of the purpose. Inclusion of the sexual partner, with permission of the patient, is advantageous and provides needed patient support. Allowing the patient (and the partner) to observe any pathology via mirrors or digital photography is often therapeutic, allowing, for the first time in many cases, an illustration and connection of a detected physical abnormality with the sexual health problem. If there exists a genital sexual pain history, the patient should point with her finger to the locations of the discomfort during the physical examination. Independent of the gender of the examining health care provider, it is strongly recommended that a female chaperone health care provider be present in the examination room. The following equipment should be available: Examination table Hospital gown Bed sheet Disposable absorbing chucks Patient covering sheet Surgical loupes or magnifying glass Examination light source Examination gloves Gauze Lubricant Q-tips cotton swabs Speculum pH paper Glass slide Saline Microscope Verathon Inc. 800.331.2313 www.verathon.com 88 Urology for Primary Care Sexual Medicine for Women The patient should wear a hospital gown and a sheet should cover her lower torso. The patient should be placed in the lithotomy position and the examining health care provider, using magnified vision and a carefully focused light source, should be sitting comfortably. The first part of the examination involves inspection. If appropriate, lubricant should be placed on the vulva. Gauze can be grasped between thumb and index finger and used to retract the labia majora for a full inspection of the vestibular contents. Two gloved fingers are placed on either side of the clitoral shaft and using an upward force in the cephalic direction, the prepuce is retracted to gain full exposure of the glans clitoris, corona, and right and left frenulum emanating at 5:00 and 7:00 from the posterior portion of the glans clitoris. Using gauze to retract the labia minora, the labial-hymenal junction is identified. A Q-tips cotton swab test is performed, gently applying pressure on the minor vestibular glands, documenting the quality of the discomfort or pain. The Q-tips cotton swab may also be placed at multiple locations in the vulva and vestibule. Palpation is next performed using a single digit examination. This procedure occurs before speculum insertion or bimanual searches for vaginismus. Single digit palpation is achieved by gently placing a finger into the vaginal opening and depressing the bulbocavernosus muscle. The test is positive if there is hypertonicity and pain. The goals of the physical examination for a woman with sexual health concerns are to confirm normal architecture, detect any existing pathology or abnormalities, educate the woman about normal anatomy and physiology and, if pain is a feature, to reproduce and localize the pain to potential tender areas of the vulva, vagina, or pelvis, or hypertonicity of the pelvic floor. A bimanual examination and evaluation of the pelvic floor may be subsequently performed if indicated. Two fingers are placed against the lateral walls and the levators and underlying obturator are assessed for tenderness or taut bands. In addition, a bimanual examination can evaluate the integrity of the fornices, bladder, and urethral bases and pelvic organs. A rectovaginal examination and speculum examination can be performed if indicated. For the speculum examination, a warm, lubricated speculum is used. The vaginal wall is examined for estrogen milieu integrity, inflammation of the walls, and any vaginal lesions. The physician should also perform a complete physical, such as examining for a thyroid goiter, to rule out other co-morbid conditions that might be causing sexual dysfunction. A general physical exam is highly recommended in women with chronic illnesses and as part of good medical care, including evaluation of blood pressure, heart rate, and a thorough breast exam.27, 28 Laboratory Testing There is currently no consensus regarding recommended routine laboratory tests for the evaluation of women with desire, arousal, and orgasm sexual health concerns. Blood testing should be dictated by clinical suspicion, especially by the results of the history and physical examination. If appropriate, the health care clinician may assess multiple androgen and estrogen values such as dehydroepiandrosterone sulphate, androstenedione, total testosterone, free testosterone, sex hormone binding globulin, dihydrotestosterone, estradiol and estrone. Pituitary function may be Verathon Inc. 800.331.2313 www.verathon.com 89 Urology for Primary Care Sexual Medicine for Women measured by obtaining luteinizing hormone, follicle stimulating hormone and prolactin. Thyroid stimulating hormone should be measured to exclude subclinical thyroid disease.38 – 41 There are multiple problems with the determination of serum hormone levels. The normal ranges of testosterone concentration values for women of different age groups without sexual dysfunction are also not well defined. Testosterone levels reach a nadir during the early follicular phase, with small but less significant variation across the rest of the cycle. Testosterone assays are not uniformly sensitive or reliable enough to accurately measure testosterone at the low serum concentrations typically found in women. Free testosterone is clinically more important than total testosterone in sexual function because the vast majority of testosterone is bound to sex hormone binding globulin (SHBG), and only a small amount of total testosterone is biologically available. The measurement of sex hormone binding globulin is not controversial and is relatively simple to perform with good reproducibility. Equilibrium dialysis is a highly sensitive assay for free testosterone, however, this method is not feasible for clinical practice. Measurement of free testosterone by analog assays is unreliable. Free androgen may also be calculated using the free androgen index, defined as total testosterone (nmol/L) divided by SHBG (nmol/L). Calculated free testosterone may be determined and takes into account total testosterone, SHBG, and albumin. A calculator for this free testosterone is available online at http://www.issam.ch/freetesto.htm.38 – 41 Dehydroepiandrosterone sulphate is commonly measured because the half-life is much longer than dehydroepiandrosterone, resulting in more stable levels. The immunoassay for dehydroepiandrosterone sulphate is relatively robust and simple to perform. Dehydroepiandrosterone sulphate does not vary in concentration within the various phases of the menstrual cycle. Multiple investigators have shown consistent decline curves for dehydroepiandrosterone sulphate with aging. Although there is a lack of clinical consensus as to the value, specificity, and sensitivity of individual hormone blood tests, there are evidence-based, placebo-controlled, double-blind data supporting the efficacy of exogenous sex steroid hormone treatment in women with sexual health concerns. As such, the prudent physician may wish to discuss with the patient the strategy of serial blood test surveillance testing to address safety concerns during such treatment. A most common and controversial question concerns the “normal range” for sex steroid blood test values in women with sexual health problems. Guay et al. examined androgen values in women “without sexual dysfunction” as determined by a validated Sexual Function Questionnaire. Androgen concentrations were highest in women aged twenty to twenty-nine years, decreased at approximately age thirty, and remained relatively constant thereafter. The free androgen index in women “without sexual dysfunction” was approximately 3.7 for women ages twenty to twentynine years and 2.0 for women between the ages of thirty and thirty-nine.38 – 41 Serum levels of sex steroids can only measure deficiency or excess. Sex steroid hormone actions are quite complex and involve critical enzymes and critical hormone receptors that also determine tissue exposure, tissue sensitivity, and tissue responsiveness. For example, in individuals there are variations in the amount and activity of critical enzymes such as 5 alpha-reductase and aromatase. In addition, individuals have variations in individual sex steroid hormone receptor sequencing. Thus, independent of the values of sex steroid hormones, the unique individual Verathon Inc. 800.331.2313 www.verathon.com 90 Urology for Primary Care Sexual Medicine for Women variations in critical enzymes and sex steroid hormone receptors result in individual differences in tissue exposure, tissue sensitivity, and tissue responsiveness. More research is needed in the blood testing of sex steroid hormones in women with sexual health concerns. TREATMENT The goal of this section is to discuss the biologic-focused management of women who have sexual health issues, especially relating to desire, arousal, and orgasm. The ideal management of all women with sexual health concerns is holistic, engaging psychologic and biologic strategies. The inclusion of health care professionals with expertise in physical therapy is especially relevant for sexual pain issues but may also be helpful with orgasmic issues. It is not within the scope of this chapter to fully discuss management of sexual pain disorders. Hormonal Sex steroid hormones are critical for sexual structure and function.16, 25, 38 – 41 A number of studies have demonstrated that hormone therapy using systemic or local preparations improves sexual desire, arousal, orgasm, and frequency of sexual activity. There is no one hormonal intervention that will be effective in all women with desire, arousal, and orgasm sexual health concerns secondary, in part, to sex steroid hormone deficiency states. Studies have consistently reported that androgen sex steroid hormone values decline gradually and estradiol values decline abruptly in menopausal women. Hormone insufficiency states may also be caused by a number of clinical conditions and medications, including the use of oral contraceptive therapy. The use of systemic testosterone and systemic and/or local estrogen with or without systemic progesterone must be individualized to each patient’s desires, wishes, requirements, and expectations.28 Systemic hormone therapy can successfully improve hot flashes, night sweats, and sleep disturbances that can otherwise markedly diminish afflicted women’s body image and mood. Local hormone therapy can successfully improve vaginal lubrication dryness and dyspareunia. Alleviation of hormone deficiency-induced symptoms by systemic and/or localized sex steroid hormones can increase quality of life, desire, arousal, and orgasmic function. Although not yet specifically approved for clinical use in women with sexual health concerns, sex steroid hormones may eventually provide a safe and effective treatment option. However, more research is indicated. The following represents a clinical paradigm currently used in our outpatient sexual health clinic for the evidence-based biologic-focused treatment of women with desire, arousal, and orgasm sexual health concerns. As discussed above, treatment is holistic and is based on the history, physical examination, and laboratory tests. Verathon Inc. 800.331.2313 www.verathon.com 91 Urology for Primary Care Sexual Medicine for Women Phase 1 It is important to emphasize that no single type of hormonal intervention or regimen will be effective in all women with desire, arousal, and orgasm sexual health concerns. Women who seek treatment may have symptoms of sexual health problems due, in part, to the onset of natural menopause or menopause induced by chemotherapy or surgery. Or, they may be younger women whose labia minora have begun to atrophy because of the adverse effects of oral contraceptives on the sex steroid hormonal milieu. Infertility or endometriosis treatments may also disturb the sex steroid hormone milieu. The hormonal abnormalities that are identified will determine which of the following four biologic treatment options women are offered in Phase 1. Based on blood test results, systemic androgen treatment may be achieved with DHEA alone, or systemic testosterone alone, or a combination of both. Based on the history and physical examination, local estrogen treatment may be achieved with vestibular estradiol alone, or intravaginal estradiol alone, or a combination of both. Systemic androgens (DHEA and/or testosterone) have been shown to improve mood, energy, stimulation, sensation, arousal, and orgasm.42 - 44 Limited clinical trials have examined the effects of DHEA therapy on sexual function in women. Baulieu and colleagues administered DHEA (50 mg) or placebo to 140 women between the ages of sixty and seventy-nine years for twelve months. DHEA treatment produced approximately a doubling of serum total testosterone concentration and also significantly increased skin hydration and bone density. Libidinal interest was increased after six months of treatment, and sexual activity and sexual satisfaction were increased after twelve months.42 - 44 Testosterone has been used to treat women with sexual dysfunction for more than fifty years.45 Transdermal patches or gels, which are more consistently absorbed and avoid first pass through the liver, are currently being studied for their safety and efficacy in reducing sexual symptoms associated with testosterone insufficiency.46, 47 Recently, transdermal testosterone patches were compared with placebo in estrogenized women who had undergone oophorectomy and hysterectomy.46 The study results showed that the 300-microgram testosterone patch was significantly more effective than the 150-microgram patch or placebo in improving frequency of sexual activity, pleasure, and fantasy during a 12-week period. Typically, testosterone is used in gel form (FDA-approved delivery system for men) and is applied to the back of the calf. One tenth of the dose used in men is appropriate to start therapy for women. One benefit of administering DHEA and testosterone is that a certain percentage of these hormones will aromatize to an estrogen, thus relieving hot flashes and night sweats without the administration of systemic estrogen. Local estrogen, whether it is prescribed in the form of vaginal estradiol or a vestibular cream, improves perfusion, lubrication, tissue tone, and elasticity, and restores the normal pH and vaginal health.29, 48, 49 Vaginal estradiol also relieves dyspareunia, atrophic vaginitis, and vaginismus. Some systemic absorption occurs with all local estrogens, but less than with oral therapy. Daily application of a film of vestibular estrogen is recommended as well, because it promotes the Verathon Inc. 800.331.2313 www.verathon.com 92 Urology for Primary Care Sexual Medicine for Women health of the frenulum (the most sensitive part of the external genitalia), labia minora, urethral meatus, hymenal tissue, and vestibular glands. Women with sexual dysfunction who are placed on Phase 1 treatment need to undergo surveillance blood tests after three months of therapy to monitor their levels of estradiol, progesterone, DHEA, testosterone, androstenedione, dihydrotestosterone, follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, and thyroid-stimulating hormone (TSH) as indicated. Women with desire, arousal, and orgasm sexual health concerns whose symptoms of distress are not resolved satisfactorily with Phase 1 treatments may consider progressing to Phase 2 treatments. Phase 2 In Phase 2, women receive systemic estrogen and/or systemic testosterone. Several clinical trials have shown that the distressing symptoms of vaginal atrophy associated with low estrogen states are ameliorated after estrogen therapy. Low doses of systemic bioidentical non-synthetic 17-beta estradiol reduced vaginal atrophy compared with placebo in healthy menopausal women.50 Systemic estrogen therapy can also successfully improve hot flashes, night sweats, and sleep disturbances that negatively affect body image, mood, and sexual desire. All efforts are made to keep serum estradiol levels between 30 – 50 ng/dL. Risks of systemic estrogen use include breast cancer, heart attack, and stroke. The concept of maintaining estradiol values at low levels is to reduce side effect risk while achieving a minimum efficacious dose. In women with an intact uterus, systemic estrogen should always be opposed by a progestogen. All efforts are made to use a bioidentical non-synthetic progesterone and keep values in the range of 1 ng/dL. Phase 3 In Phase 3, attention is given to the possible role of dopamine agonists in facilitating desire and orgasm sexual responses. Sexual motivation is encouraged, sustained, and ended by a number of central nervous system neurotransmitter and receptor changes induced, in part, by the action of sex steroids, androgens, estrogens, progestins, and the central neurotransmitter dopamine.51 – 53 The activation of dopamine receptors may be a key intermediary in the stimulation of incentive sexual motivation and sexual reward. These neurotransmitter and receptor changes, in turn, activate central sexual arousal and desire. Contemporary animal research reveals that dopamine neurotransmitter systems may play a critical intermediary role in the central regulation of sexual arousal and excitation, mood, and incentive-related sexual behavior, in particular, in the motivational responses to conditioned external stimuli. In summary, the complex central neurochemical actions of steroid hormones stimulate sensory awareness, central sexual arousal, mood, and reward, and relate them to relevant individual sexual experiences involving a partner, a place, and an action. Verathon Inc. 800.331.2313 www.verathon.com 93 Urology for Primary Care Sexual Medicine for Women Bupropion may have a beneficial effect on women with hypoactive sexual desire disorder. In a placebo-controlled trial, bupropion produced an increase in desire and frequency of sexual activity compared with placebo.52, 53 However, frequency was correlated to total testosterone level at baseline and during treatment. A traditional starting dose is 100 mg bupropion per day, generally taken in the morning. Vasodilators Basic science studies investigating the physiology of sexual function utilizing female animal models support the role of nitric oxide-cyclic guanosine monophosphate – phosphodiesterase type 5 pathways in the peripheral arousal physiology of the clitoral corpus cavernosum,54, 55 corpus spongiosum, vaginal epithelium, and vaginal lamina propria. There have been several clinical studies on selective phosphodiesterase type 5 inhibitors over the last few years, conducted with either premenopausal or postmenopausal women with arousal sexual health concerns, as well as healthy women without sexual dysfunction. Many studies did not take into account the hormonal milieu of the subjects in the inclusion and exclusion criteria. An important point in treating women with arousal sexual health concerns is that an adequate sex steroid (androgen and estrogen) hormonal milieu is critical for benefits from selective phosphodiesterase type 5 inhibitor treatment.16 The following studies did assess safety and efficacy of selective phosphodiesterase type 5 inhibitors in subjects with a normal hormonal milieu: - A double-blind, crossover, placebo-controlled safety and efficacy study with a selective phosphodiesterase type 5 inhibitor was performed in premenopausal women with normal ovulatory cycles and normal levels of steroid hormones who were affected by female sexual arousal disorder without hypoactive sexual desire disorder. Subjects were observed to benefit from treatment with the active selective phosphodiesterase type 5 inhibitor, showing improvement in sexual arousal, orgasm, frequency, and enjoyment of sexual intercourse versus placebo.56 - A double-blind, placebo-controlled safety and efficacy study with a selective phosphodiesterase type 5 inhibitor was performed in postmenopausal women with female sexual arousal disorder who had adequate serum estradiol and free testosterone values. Women with female sexual arousal disorder without hypoactive sexual desire disorder who were assigned the active drug had a significantly greater improvement in sexual arousal, orgasm, intercourse, and overall satisfaction with sexual life compared with placebo. No efficacy was shown for women with concomitant hypoactive sexual desire disorder.57 - A randomized double-blind crossover, placebo-controlled safety and efficacy study with a selective phosphodiesterase type 5 inhibitor was performed in premenopausal women asymptomatic for sexual disorders, with normal ovulatory cycles, and with normal levels of steroid hormones. The selective phosphodiesterase type 5 inhibitor improved general sexual behavior, including sexual arousal, orgasm, and enjoyment versus placebo. Adverse events associated with the use of selective phosphodiesterase type 5 inhibitors were related to vasodilatation, such as headache, or to gastrointestinal events with nausea, or to visual effects. Verathon Inc. 800.331.2313 www.verathon.com 94 Urology for Primary Care Sexual Medicine for Women The study suggested that selective phosphodiesterase type 5 inhibitors act on different sexual pathways in healthy women, improving their sexual experience, including multiple orgasms.57 - Selective phosphodiesterase type 5 inhibitor use has been studied as an antidote to psychotropic-induced sexual dysfunction in women. In one study, women reported significant improvements in all domains of sexual function, with improvement in overall sexual satisfaction, after selective phosphodiesterase type 5 inhibitor treatment. Significant improvements were reported regardless of psychotropic medication type. Patients taking selective serotonin re-uptake inhibitors reported less improvement in arousal, libido, and overall sexual satisfaction than did other patients, whereas patients taking benzodiazepines reported significantly more improvement in libido and overall sexual satisfaction.59 - Another study was performed utilizing selective phosphodiesterase type 5 inhibitors in women with psychotropic-induced sexual dysfunction. Women who had normal premorbid sexual function and who had developed sexual dysfunction, particularly anorgasmia with or without other sexual disturbances, i.e., loss of libido, lubrication difficulties, uncomfortable or painful intercourse, were treated with a selective phosphodiesterase type 5 inhibitor. The subjects showed improvement of the presenting condition, usually depression, anxiety, or both, and experienced sexual side effects continuously for more than four weeks. Patients took selective phosphodiesterase type 5 inhibitors and reported a complete or very significant reversal of their sexual dysfunction. This included return of effective duration and intensity of adequate arousal, lubrication, and orgasmic function.60 In conclusion, selective phosphodiesterase type 5 inhibitors seem to be effective in highly selected populations of premenopausal and menopausal women with sexual arousal disorder. Selection criteria best associated with success included having normal sex steroid hormonal milieu and no hypoactive sexual desire disorder. Selective phosphodiesterase type 5 inhibitors may have a role as an antidote to psychotropic-induced sexual dysfunction. CONCLUSIONS Physicians face a challenge in finding the time and opportunity to discuss sexual health concerns with their female patients. There are increasing and growing demands for other preventive care issues for our aging population. There are especially significant financial pressures of providing “managed care.” Of importance, many physicians have limited training in the diagnosis and treatment of women with sexual health concerns. Many medical schools devote only limited time in the curriculum to sexual medicine issues. Currently, 68% of American medical schools devote less than 10 hours to sexual medicine education.61 Physicians may be hesitant to introduce patient issues that are not within with their comfort level or knowledge background. In addition, patients are often unwilling or too uncomfortable to initiate a discussion about their own sexual problems with a physician, even if these problems cause distress. In a U.S. telephone survey of five hundred adults, 71% of respondents said they would be very or somewhat concerned that their physician would dismiss any sexual problem they might mention. A total of 68% said they would be concerned that their physician would be uncomfortable talking about sexual problems, and 76% said they would be concerned that no medical treatment exists for their problem.62 There are other issues. Sexual medicine is complex. It is often difficult to separate psychologic issues from Verathon Inc. 800.331.2313 www.verathon.com 95 Urology for Primary Care Sexual Medicine for Women inter-personal relationship concerns from biologic disorders. There is only limited evidencebased data available. The basic premise of biologically focused management of women’s desire, arousal, and orgasm sexual health concerns is that physiologic processes can be altered by pathology. How specific medical conditions modulate woman’s sexual health requires much investment in basic science investigation. From the perspective of biology-focused clinicians, the essential principle guiding their medical decision-making is identification of the underlying pathophysiology of the sexual dysfunction. If the biologic basis of the desire, arousal, and orgasm dysfunction can be diagnosed, management outcome may be successfully directed to the source pathophysiology. Two of the many challenges facing healthcare professionals today are improving their ability to accurately diagnose women with desire, arousal, and orgasm sexual health concerns and ensuring that they offer women the best evidence-based treatment options available. To achieve these goals, biologically-focused clinicians need to be familiar with evidence-based, state-of-the-art, data and current, biologically-focused management strategies. Verathon Inc. 800.331.2313 www.verathon.com 96 Urology for Primary Care Sexual Medicine for Women References 1. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999; 281: 537-544. 2. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. In Laumann EO, Michael RT, eds. Sex, Love, and Health in America: Private Choices and Public Policies. Chicago: University of Chicago Press, 2001. 3. Öberg, K. On Swedish Women’s Distressing Sexual Dysfunctions, Some Concomitant Conditions and Life Satisfaction. J Sex Med 2:2, 2005 4. Basson, R. Assessment and Management of Women's Sexual Dysfunctions: Problematic Desire and Arousal. J Sex Med 2:3, 2005 5. Weijmar Schultz, W. Women's Sexual Pain and its Management. J Sex Med 2:3, 2005 6. Nappi, R, Salonia, A, Traish, AM, van Lunsen, R. H.W., Vardi, Yoram, Kodiglu, A and Goldstein, I. Clinical Biologic Pathophysiologies of Women's Sexual Dysfunction J Sex Med. 2: 4 - 25, 2005 7. Lewis RW, Fugl-Meyer KS, Bosch R, Fugl-Meyer AR, Laumann EO, Lizza E, Martin-Morales A. Epidemiology/Risk Factors of Sexual Dysfunction. J Sex Med 2004;1:35-39. 8. Hayes, R. The Impact of Aging on Sexual Function and Sexual Dysfunction in Women: A Review of Population Based Studies. J Sex Med 2:3, 2005. 9. Rosen RC, Taylor JF, Leiblum SR, Bachmann GA. Prevalence of sexual dysfunction in women: results of a survey study of 329 women in an outpatient gynecological clinic. J Sex Marital Ther 1993; 19: 171-188. 10. Kadri N, Mchichi Alami KH, Mchakra T. Sexual dysfunction in women: Population based epidemiological study. Arch Womens Ment Health 2002;5:59-63 11. Salonia A, Zanni G, Nappi R, et al. Sexual dysfunction is common in women with lower urinary incontinence: Results of a cross-sectional study. Europ Urol 2004;45:642-648. 12. http://www.who.int/reproductive-health/gender/sexual_health.html 13. National Council on the Aging. Press Room: News Archive. Half of Older Americans Report They Are Sexually Active; 4 in 10 Want More Sex, Says New Survey (September 28, 1998). Available at: http://www.ncoa.org/content.cfm?sectionID=105&detail=128. 14. Menopause: One Woman’s Story, Every Woman’s Story: A Resource for Making Healthy Choices. Gaithersburg, MD: National Institute on Aging, US Dept of Health and Human Services; 2001. NIH Publication No. 01-3886. 15. Bossouw JE, Anderson GL, Prentice RL; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333. 16. Giraldi A., Marson L., Nappi R., Pfaus J., Traish A.M., Vardi Y. and Goldstein I.: Physiology of Female Sexual Function: Animal models. J Sex Med 1: 237 – 253, 2004. 17. Basson, R, Leiblum, S, Brotto, L, Derogatis, L, Fourcroy, J. Fugl-Meyer, K, Graziottin, A, Heiman, JR, Laan, E, Meston, C, Schover, L, van Lankveld, J. Schultz WW. Revised Definitions of Women’s Sexual Dysfunction. J Sex Med (2004) 1:40-48. Verathon Inc. 800.331.2313 www.verathon.com 97 Urology for Primary Care Sexual Medicine for Women 18. Basson R, Althof S, Davis S, Fugl-Meyer K, Goldstein I, Leiblum S, Meston C, Rosen RC and Wagner G. Summary of the Recommendations on Sexual Dysfunctions in Women. J Sex Med 1:1, 24 – 34, 2004. 19. Heiman JR, Guess MK, Connell K, Melman A, Hyde JS, Segraves RT and Wyllie MG. Standards for Clinical Trials in Sexual Dysfunctions of Women: Research Designs and Outcomes Assessment. J Sex Med 1:1, 92 – 97, 2004. 20. Nicolosi A, et al. Sexual behavior and sexual problems after age 40: the Global Study of Sexual Attitudes and Behaviors. Urology 2004; 64: 991-997. 21. Gracia CR, Sammel MD, Freeman EW, et al. Predictors of decreased libido in women during the late reproductive years. Menopause. 2004;11:144-150. 22. Bixo M, Backstrom T, Winblad B, Andersson A. Estradiol and testosterone in specific regions of the human female brain in different endocrine states. J Steroid Biochem Mol Biol 1995; 55:297-303. 23. Roselli CE, Resko JA. Aromatase activity in the rat brain: hormone regulation and sex differences. J Steroid Biochem Mol Biol 1993; 44:499-508. 24. McEwen BS. Estrogen action throughout the brain. Recent Prog Horm Res 2002; 57:357-384. 25. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77:660–665. 26. Traish AM, Huang YH, Min K, Kim NN, Munarriz R, Goldstein I. Binding characteristics of [3H]delta(5)androstene-3beta,17beta-diol to a nuclear protein in the rabbit vagina. Steroids. 2004 Jan;69(1):71-8. 27. Hatzichristou D, Rosen RC, Broderick G, Clayton, A, Cuzin B, Derogatis L, Litwin M, Meuleman E, O’Leary M, Quirk F, Sadofsky R and Seftel A. Clinical Evaluation and Management Strategy for Sexual Dysfunction in Men and Women. J Sex Med 1:1, 49 – 57, 2004. 28. Basson R, Althof S, Davis S, Fugl-Meyer K, Goldstein I, Leiblum S, Meston C, Rosen RC and Wagner G. Summary of the Recommendations on Sexual Dysfunctions in Women. J Sex Med 1:1, 24 – 34, 2004. 29. Bachmann, GA, and Leiblum,SR. The impact of hormones on menopausal sexuality: a literature review. Menopause. 2004; 11(1):120-130. 30. Reynolds RF and Obermeyer CM. Age at natural menopause in Spain and the United States: Results from the DAMES project. Am. J. Hum. Biol. 17:331-340, 2005. 31. Kovalevsky, G. Hormones and Female Sexual Dysfunction in Postmenopasual Women. Menopausal Medicine. 2004; 12(1):1-5. 32. Freedman, MA. Quality of Life and menopause: The Role of Estrogen. J Women’s Health. 2002; 11(8):703718. 33. Pessina MA, Hoyt RF, Goldstein I, Traish AM. Differential Effects of Estradiol, Progesterone, and Testosterone on Vaginal Structural Integrity submitted Endocrinology 2005 34. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191-208 35. Meston CM. Validation of the Female Sexual Function Index (FSFI) in women with female orgasmic disorder and in women with hypoactive sexual desire disorder. J Sex Marital Ther 2003;29:39-46 Verathon Inc. 800.331.2313 www.verathon.com 98 Urology for Primary Care Sexual Medicine for Women 36. Wiegel M, Meston C, Rosen RC. The Female Sexual Function Index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther 2005;31:1-20 37. Quirk FH, Heiman JR, Rosen RC, et al. Development of a sexual function questionnaire for clinical trials of female sexual dysfunction. J Womens Health Gend Based Med 2002;11:277-89. 38. Davis SR, Guay AT, Shifren JL, and Mazer NA. Endocrine Aspects of Female Sexual Dysfunction. J Sex Med 1:1, 82 – 86, 2004 39. Guay A, Munarriz R, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: part A. Serum androgen levels in women aged 20-49 years with no complaints of sexual dysfunction. Int J Impot Res 2004;16:112–120. 40. Guay A, Jacobson J, Munarriz R, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: part B: Reduced serum androgen levels in healthy premenopausal women with complaints of sexual dysfunction. Int J Impot Res 2004;16:121–129. 41. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction. World J Urol 2002;20:106–110. 42. Baulieu E-E. Dehydroepiandrosterone (DHEA): a fountain of youth? J Clin Endocrinol Metab 1996;81:3147– 3151. 43. Baulieu E-E, Thomas G, Legrain S, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEA Study to a sociobiomedical issue. Proc Natl Acad Sci U S A 2000;97:4279–4284. 44. Arlt W, Callies F, Van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Eng J Med 1999; 341(14):1013-1020. 45. Loeser A. Subcutaneous implantation of female and male hormone in tablet from in women. The British Medical Journal 1: 479-482, 1940. 46. Shifren JL, Braustein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function and oophorectomy. N Engl J Med 2000;343:682–688. 47. Lobo RA, Rosen RC, Yang HM, Block B, Van Der Hoop RG. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003 Jun;79(6):1341-52. 48. Buckler, H, Al-Azzawi, F, The UK VR Multicentre Trial Group. The effect of a novel vaginal ring delivering oestradiol acetate on climacteric symptoms in postmenopausal women. BJOG. 2003;110:753-759. 49. Barentsen, R, van de Weijer, PHM, Schram, JHN. Continuous low dose estradiol released from a vaginal ring versus estriol vaginal cream for urogenital atrophy. Eur J Obstet Gynecol Reprod Biol. 1997;71:73-80. 50. Rioux JE, Devlin C, Gelfand MM, Steinberg WM, Hepburn DS. 17beta-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause 2000;7:156-61. 51. Giuliano F, Allard J. Dopamine and male sexual function. Eur Urol 2001;40:601-8 52. Clayton AH, Warnock JK, Kornstein SG, Pinkerton R, Sheldon-Keller A, McGarvey EL. A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry. 2004;65:62-7. 53. Segraves RT, Clayton A, Croft H, Wolf A, Warnock J. Bupropion sustained release for the treatment of hypoactive sexual desire disorder in premenopausal women. J Clin Psychopharmacol 2004;24:339-42. Verathon Inc. 800.331.2313 www.verathon.com 99 Urology for Primary Care Sexual Medicine for Women 54. Park K, Moreland RB, Goldstein I, Atala A, Traish A. Sildenafil inhibits phosphodiesterase type 5 in human clitoral corpus cavernousum smooth muscle. Biochem Biophys Res Commun 1998; 249:612-7 55. Burnett AL, Calvin DC, Silver RI, Peppas DS, Docimo SG. Immunohistochemical description of nitric oxide syntheses isoforms in human clitoris. J Urol 1997; 158:75-8. 56. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, crossover, placebo-controlled study. Br J Obstet Gynaecol 2001; 108:623-8 57. Berman JR, Berman LA, Toler SM, Gill J, Haughie S. Safety and efficacy of sildenafil citrate for the treatment of female sexual arousal disorder: a double-blind, placebo controlled study. J Urol 2003; 170:2333-8 58. Caruso S, Intelisano G, Farina M, Di Mari L, Agnello C. The function of sildenafil on female sexual pathways: a double-blind, cross-over, placebo-controlled study. Eur J Obstet Gynecol 2003; 110:201-6 59. Salerian AJ, Deibler WE, Vittone BJ, et al. Sildenafil for psychotropic-induced sexual dysfunction in 31 women and 61 men. J Sex Marital Ther 2000;26:133-40. 60. Nurnberg HG, Lauriello J, Hensley PL, et al. Sildenafil for sexual dysfunction in women taking antidepressants. Am J Psychiatry 1999; 156:1664 61. Solursh DS, Ernst JL, Lewis RW, et al.The human sexuality education of physicians in North American medical schools. Int J Impot Res. 2003;15 Suppl 5:S41-S45. 62. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999; 281:2173-2174. Verathon Inc. 800.331.2313 www.verathon.com 100 Urology for Primary Care Management of Uncomplicated UTIs MANAGEMENT OF UNCOMPLICATED URINARY TRACT INFECTIONS J. Curtis Nickel, M.D., FRCSC Department of Urology, Queen’s University Kingston, Ontario, Canada UNCOMPLICATED URINARY TRACT INFECTIONS: A CLINICAL PROBLEM Urinary tract infections (UTIs) in premenopausal, sexually active women with anatomically normal urinary tracts are considered uncomplicated UTIs (uUTIs). Complicated UTIs are urinary tract infections associated with anatomical or functional abnormalities of the urinary tract. uUTIs are not perceived as a major clinical problem by the medical community, but this perception is a mistake. UTIs account for more than seven million visits to physicians’ offices per year (1.2% of all office visits by women). uUTI results in considerable short term morbidity and curtailment of personal and employment related activities. The financial impact of UTIs, including recurrent and uncomplicated UTIs, is greater than one billion dollars per year in the United States alone. For premenopausal, healthy, and active females, uncomplicated UTIs are a major health care concern. TREATMENT BEFORE ANTIBIOTICS Physicians have become complacent in their attitude regarding uUTIs since the introduction of effective antimicrobials. It is always interesting to see how our predecessors managed cystitis before antibiotics were introduced. Historical management of bladder inflammation in the 1800’s included initial conservative treatments (bedrest, warm herbal compresses, baths, opiate-based enemas), while more aggressive therapy was reserved for patients who did not improve or deteriorated during initial therapy. Aggressive treatment with mustard or ammonia based plasters, oral alkali, amylnitrite, quinine, lead, opium, and if these failed, then bleeding (cupping, leeches or direct bleeding), was employed. If the woman did not develop high fever, kidney infection, or abscess (progression to a complicated UTI) and did not succumb to the physicians ministrations, recovery was to be expected within several weeks. Beginning at the turn of the century, trials with chemotherapeutic agents such as hexamine, pyridium, hexylresorcinol, and mercurochrome were undertaken, initially with some success and then later with some pessimism. Sulfanilamide, introduced in 1937, ushered in the era of modern antimicrobial therapy for UTIs. EVALUATION OF WOMEN WITH UNCOMPLICATED UTIS Diagnosis and treatment of uUTI is usually straightforward. Classic symptoms include burning with urination (dysuria) and increased frequency and urgency. Symptoms are characteristic enough that women are highly reliable in self-diagnosis (perhaps even more accurate and reliable than a physician based clinical diagnosis). In addition, the microbiology of infection is consistent, with Escherichia coli isolated from 85% to 90% of episodes. Verathon Inc. 800.331.2313 www.verathon.com 101 Urology for Primary Care Management of Uncomplicated UTIs Urinalysis Microscopy is a valuable adjunctive diagnostic tool for patients with urinary symptoms and, if available, should be considered for patients presenting with cystitis. Limitations exist in detecting microscopic bacteriuria and pyuria because of lack of standardization in terms of the microscope itself (including magnification) and the volume of urine that can be observed. There is also no standardization on whether the urine should be spun (usual) or unspun, stained (usual) or unstained. However, if microscopy is performed, the absence of pyuria should cause the physician to reconsider the diagnosis of UTI. An indirect dipstick test for bacteriuria (nitrite) or pyuria (leukocyte esterase) can be helpful. While these tests are less sensitive than microscopic examination of the urine, they provide additional confirmation of a UTI when contemplating empiric therapy or while waiting for culture results. Urine Culture Urine culture is a criterion standard for the diagnosis of UTI, however problems exist for this “gold standard.” Urine must be collected properly (midstream or catheterized specimen), cultured quickly, or refrigerated. Traditionally, significant bacteriuria is noted when the bacterial colony forming unit counts reach 105/ ml. Problems with this somewhat artificial cut-off is that many women with symptomatic uUTIs present with bacterial counts much lower than this (such as 102/ ml), and patients with asymptomatic bacteriuria can have counts much higher than this. Testing of cure cultures (cultures after empiric therapy instituted) is only indicated in patients who do not respond to empiric therapy and in those in whom the initial culture grew a resistant organism. Organisms isolated from community-acquired uncomplicated urinary tract infections (uUTIs) Bacterial species Percentage of isolates Escherichia coli >90 Klebsiella spp Enterobacter spp Proteus mirabilis Citrobacter spp Pseudomonas aeuruginosa Other 8-10% Should Cultures Be Done At All in Women with Uncomplicated UTIs? The characteristic symptoms and consistent microbiology support a management approach of empiric antimicrobial therapy initiated promptly with symptom onset and targeted to E. coli, without obtaining pretherapy urine culture. It can be argued that it would be cost effective in patients with recurrent uUTIs to first establish the presence of significant bacteriuria associated with an episode of symptomatic cystitis, and then document that both symptoms and bacteriuria resolve with antimicrobial therapy. Once this has been confirmed in a specific patient, it is probably not necessary to repeat a urine culture at each episode. Urine culture, however, would be mandatory if the patient does not respond to empiric therapy. Verathon Inc. 800.331.2313 www.verathon.com 102 Urology for Primary Care Management of Uncomplicated UTIs Other Investigations Many patients with uUTIs undergo both radiologic investigations and cystoscopy. Radiological studies are unnecessary for the evaluation of the typical healthy premenopausal, sexually active woman. Similarly, cystoscopy will rarely pick up significant pathology that would change the outcome of therapy. However, further investigations are important if a patient is believed to have a complicated UTI suggested by a history of calculi, obstruction (upper or lower urinary tract), neuropathic bladder, recent genito-urinary surgery or catheterization, unusual organisms (such as tuberculosis, fungus or urea splitting organisms), immunocompromised patients, diabetic patients, and/or patients with renal failure. Similarly, investigations are required in patients who do not respond to appropriate antimicrobial therapy after five to six days of treatment. These patients should undergo some form of imaging study and be considered for urological referral. The excretory urogram has been the traditional routine examination to evaluate patients with complicated UTI and still remains useful, but is not the ideal screening test in the contemporary period. Renal ultrasonography is probably the most important urinary tract imaging technique because it is noninvasive, easy to perform, and is relatively inexpensive. CT and MRI offer the best anatomical detail (because of cost they may not always be the most appropriate screening procedures). Since residual urine is a risk factor for chronic, recurrent, and complicated UTI, ultrasound bladder volume measurement can determine if the patient is emptying her bladder. Evaluation of a women with a suspected diagnosis of uUTI: - History (voiding symptoms, sexual history, genitourinary history – specifically history of previous UTI) - Physical examination (including pelvic examination) - Urinalysis (dipstick for leukocyte esterase and nitrites, microscopy for white blood cells) - Culture (not mandatory in women with previous culture documented uUTI) - Optional (in patients with a suspected diagnosis of complicated UTI or who fail appropriate antimicrobial therapy) o Bladder volume measurement (BladderScan bladder volume instrument) o Ultrasound o Intravenous urogram o CAT scan Verathon Inc. 800.331.2313 www.verathon.com 103 Urology for Primary Care Management of Uncomplicated UTIs TREATMENT OF uUTI Antimicrobial therapy is the mainstay of treatment for uUTI. Variables considered in antimicrobial selection include efficacy, adverse effects, cost, and potential for future resistance. Trimethoprim-sulfamethoxazole (TMP/SMX), or trimethoprim (TMP) has been first line therapy for uUTI for several decades. For women with infection with susceptible E. coli, cure rates of 90-95% are achieved with three days therapy. For most other susceptible organisms, these agents are also effective as three days therapy. Adverse reactions, particularly sulfa allergy, may occur and are occasionally serious. Nitrofurantoin has been used in treatment of uUTIs for over fifty years with continued safety and efficacy. Nitrofurantoin is a narrow spectrum antimicrobial with no systemic activity (almost all excreted in the urine), and its only indication is for treatment of uUTI caused by E. coli and Staphylococcus saprophyticus. It should be noted, however, that these two pathogens are isolated from 95% of all uUTIs. Early formulations were associated with substantial gastrointestinal adverse effects, but the current macrocrystalline formulation is better tolerated. For uUTI, nitrofurantoin achieves 85-90% cure with a seven-day course, but only 70-80% cure when given as a three-day course. Flouroquinolones, including norfloxacin, ciprofloxacin, ofloxacin, levofloxacin, and gatifloxacin are effective as three days therapy. For susceptible organisms, outcomes with three days flouroquinolone therapy are similar to TMP/SMX, with 90-95% cure. Single dose therapy is not recommended. This class of antimicrobials is well tolerated. Fosfomycin, given as a single dose, is also marketed for uUTI in North America, but it is somewhat less effective than other first line agents, with cure rates of about 70%. While cephalosporins have a role in treating urinary infection, particularly in pregnant women, they are not recommended for empiric therapy because of relatively high rates of resistance and lower efficacy. FIRST LINE TREATMENT OF uUTI Antimicrobial TMP/SMX 95% Efficacy Nitrofurantoin 85-90% Fluoroquinolones 95% Indications in uUTI Effective against most uropathogens Narrow spectrum: efficacious against E. coli and Staphylococcus saprophyticus Broad spectrum, effective against most uropathogens Effect on flora Eradicate uropathogens in fecal and vaginal flora No effect on fecal or vaginal flora Eradicates uropathogens in fecal and vaginal flora Side effects Significant side effects Few side effects if duration short: potential serious complications with long-term use Excellent side-effect profile Resistance Increasing resistance Little resistance over 50 years, not increasing. Limited, but increasing resistance Verathon Inc. 800.331.2313 www.verathon.com 104 Urology for Primary Care Management of Uncomplicated UTIs Dose TMP 160mg/ SMX 800mg BID 100 mg QID Macrocrystalline formulation 100mg BID Noroxin 400mg BID Ciprofloxacin 250mg BID (or modified release 500mg OD) Ofloxacin 300mg BID Levofloxacin 250mg OD Gatifloxacin 400mg OD 3 days 7 days 3 days Duration of therapy TMP/SMX = trimethoprim/sulfamethoxazole; OD = once daily; BID = twice daily; QID = four times daily RECURRENT uUTIs Over 80% of women who have had previous uUTIs have recurrent infections following an episode. Of these recurrent infections, three quarters are caused by reinfection with different organisms, rather than the same organism. Women with frequent reinfections have a rate of 0.13 0.22 UTIs per month (1.6 to 2.6 infections per year). Traditionally, it was taught that significant bacteriuria had to be confirmed on culture before antibiotics were prescribed. Employing this traditional approach (still taught based on misguided evidence based approach, FDA guidelines, and interpretation of cost-benefit ratios), each UTI episode in young women was associated on average with 6.1 days of symptoms, 2.4 days of restricted activity, 1.2 days unable to attend class/work and 0.4 days in bed. Contemporary strategies appear to be more cost effective for both the physician and patient. Prevention of Recurrent uUTIs Conservative Approach to Prevention: A number of risk factors, including sexual activity, have been identified in patients with recurrent UTIs. Contraceptive strategies employing diaphram and/or spermicides (including spermacide-covered condoms), as well as tampon use, have been associated with increased risk of UTIs. Contraception should be changed, spermicidal agents should be discontinued, and patients should consider using pads instead of tampons. The ingestion of cranberry juice or cranberry extract appears to be a safe and possibly effective method to reduce the frequency of recurrent UTI’s in some women. Attempting to change the vaginal flora by douching with lactobacilli has been suggested but not proven. It is very probable that in the future, some form of immunization program will be the key to prevention of recurrent UTIs. It is probably appropriate to suggest that the patients stay hydrated, void regularly, avoid some feminine hygiene products (such as vaginal douches and scented bubble baths), and practice proper toileting habits (including early post-coital voiding), but none of these maneuvers has ever proven to be truly effective in reducing the incidence of recurrent UTIs. Conservative Measures that may help prevent recurrent UTIs: - Discontinue spermicides Use pads instead of tampons Cranberry juice or extract Stay hydrated Verathon Inc. 800.331.2313 www.verathon.com 105 Urology for Primary Care Management of Uncomplicated UTIs - Void regularly Avoid feminine hygiene products Proper toileting habits Antimicrobial Prophylaxis: The two contemporary strategies employing a prophylactic antibiotic regime to prevent recurrent UTIs include long-term low dose prophylactic antimicrobial treatment or post-coital antibiotic treatment. Unfortunately, these antibiotic regimes do not appear to alter the patient’s basic susceptibility to infections. Antimicrobial agents used for long-term low dose prophylaxis include TMP-SMX (or TMP alone), nitrofurantoin, cephalexin, and the fluoroquinolones. The dose is usually about ¼ the usual daily dose. Nitrofurantoin (because of rapid absorption in the upper intestinal tract) produces minimal fecal resistance and less vaginitis; however, it is associated with more adverse reactions with long-term use (acute pulmonary reactions, allergic reactions, liver problems). Most of these long-term adverse reactions occur in older patients who were taking full dose nitrofurantoin. TMP-SMX is a powerful prophylactic agent in preventing reinfections in the female by clearing Enterobacteriaceae from the rectal and vaginal flora. However, this comes with the problem of poor tolerance, potentially life threatening side effects, and the development of TMP-SMX resistant strains within the gut flora. Fluoroquinolones are probably the most effective agent for UTI prophylaxis, but should probably be restricted to women with acute symptomatic cystitis in areas where there is significant antimicrobial resistance, or patient intolerance to TMP-SMX, TMP, or nitrofurantoin. Prophylactic therapy in women with recurrent UTIs decreases recurrence by 95%. Acute cystitis is more common in sexually active women, and a number of studies have shown that post-intercourse (post-coital) therapy with antimicrobials, such as nitrofurantoin, cephalexin, and TMP-SMX taken as a single dose, effectively reduces the incidence of reinfection. An antibiotic taken immediately after intercourse presumably kills or arrests the growth of sensitive bacteria that have entered the bladder during coitus, before they reach that critical concentration required to establish an infection in a susceptible individual. This approach appears to be not only clinically effective, but also cost effective and well tolerated by the patients. The infection rate seen with post-coital TMP-SMX drops by 90%. Depending on the frequency of intercourse, postcoital prophylaxis usually results in less antibiotic use than does continuous low dose antimicrobial prophylaxis Patient Directed Self-treatment of Recurrent UTIs: It has been convincingly shown that women can self diagnose their own uUTIs, and if they immediately initiate self-treatment with three days of fluoroquinolone (or other) antibiotics, they can reduce their reinfection rate by almost 90%. After determining that a patient has suffered a culture-documented uUTI and has responded accordingly to a first-line antimicrobial, it seems very reasonable to prescribe antimicrobials that the patient can take for three days when she is sure she is developing another UTI. Studies have noted that women can accurately diagnose a bacterial cystitis in 85-95% of occurrences, and the success rate (cure of uUTI) is achieved in over 90% employing this strategy. Verathon Inc. 800.331.2313 www.verathon.com 106 Urology for Primary Care Management of Uncomplicated UTIs Antimicrobial strategies for the prevention of recurrent uUTI: - Physician-directed episodic antimicrobial therapy - Low dose prophylactic antimicrobial therapy - Post-coital prophylactic antimicrobial therapy - Patient-initiated antimicrobial therapy THE EMERGING PROBLEM OF ANTIMICROBIAL RESISTANCE Resistance in community uropathogenic bacteria has evolved with sequential introduction and widespread use of different antimicrobials over the last five decades of antimicrobial therapy. Resistance of E. coli to ampicillin/amoxicillin, once standard therapy for uUTI, now approaches 50% in most regions of North America. TMP/SMX subsequently became the first-line empiric treatment for more than thirty years; however, the resistance prevalence of E. coli to TMP/SMX has increased during the past decade, with prevalence now exceeding 20% in some regions of North America. Resistance to nitrofurantoin in E. coli isolates from uUTI remains low, despite over fifty years of widespread use. The low resistance rate is likely due to restricted indications (uUTI only), limited systemic absorption, and the need for multiple genetic mutations for the bacteria to develop resistance. Resistance to flouroquinolones, such as ciprofloxacin, remains relatively low in North America; however, there has been a recent disturbing increase in the prevalence of E. coli resistance to ciprofloxacin. Some areas of Europe, such as Spain and Portugal, have a resistance prevalence in E. coli strains from uUTI approaching 20%. There is a disturbing trend for increasing resistance of uropathogenic bacteria to the firstline antimicrobials used in the management of uUTI. PRACTICAL MANAGEMENT OF UUTI For premenopausal women, particularly sexually active women, it may be appropriate to clinically investigate the patient with history, physical examination, urinalysis, and culture (at least for the first episode), followed by empiric therapy with a first-line antimicrobial (TMP/SMX, TMP, nitrofurantoin, fluoroquinolone) for three (TMP/SMX and fluoroquinolones) to seven (nitrofurantoin) days. Test of cure cultures are not required unless the patients does not respond to first-line antimicrobials. If that is the case, further investigation and treatment is indicated. The traditional dogmatic approach of physician directed investigation, culture, and antibiotic prescription is not required for sexually active young women with no risk factors who develop recurrent uUTI (particularly if they have had a positive culture in the past with a similar episode). Antimicrobial prophylaxis is effective for women who develop a frustrating constellation of very Verathon Inc. 800.331.2313 www.verathon.com 107 Urology for Primary Care Management of Uncomplicated UTIs frequent UTIs. For sexually active women with frequent UTIs (>4 UTIs/year), post-coital antimicrobial therapy appears to successfully reduce the frequency of symptomatic recurrent episodes of cystitis. For motivated and active women who suffer two to four UTIs per year, a patient-initiated treatment strategy for the recurrent UTIs should be considered. Verathon Inc. 800.331.2313 www.verathon.com 108 Urology for Primary Care Management of Uncomplicated UTIs A proposed management algorithm for premenopausal women presenting with uncomplicated UTI (reprinted with permission*) First UTI Episode Physician Diagnosis Culture Empiric Antimicrobial Therapy Recurrence of UTI Life Style Changes1. Cranberry juice/extract Recurrent UTI >4 UTI/year Constellations of UTIs Low dose Antimicrobial Prophylaxis2. <4 UTI/year Episodic UTIs Post-coital Antimicrobial Prophylaxis3. Patient initiated Antimicrobial Therapy4. 1. Lifestyle changes - discontinue spermacides, feminine hygiene products, toileting practices Culture for breakthrough UTI; change antibiotic 3. Patient initiated therapy for breakthrough UTI 4. Culture if no response by 48 hours; change antibiotic 2. * Reprinted with permission of MedReviews, LLC, from: Nickel, J.C. “Practical Management of Recurrent Urinary Tract Infections in Premenopausal Women.” Reviews in Urology. 2005; 7 (1): 11-17. Reviews in Urology is a copyrighted publication of MedReviews, LLC. All rights reserved. Verathon Inc. 800.331.2313 www.verathon.com 109 Urology for Primary Care Neonatal Circumcision: An American Enigma NEONATAL CIRCUMCISION: AN AMERICAN ENIGMA Martin A. Koyle, M.D., FAAP, FACS Professor of Surgery and Pediatrics Vice-Chief, Division of Urology The University of Colorado at Denver and Health Sciences Center Chairman, Department of Pediatric Urology The Children’s Hospital Denver Colorado INTRODUCTION Without a doubt, there is no other “medical” subject that stimulates greater controversy than that regarding the merits (or lack thereof) of newborn circumcision. The most recent statements from the American Academy of Pediatrics (AAP) suggest that there is insufficient data in the medical literature to support that this procedure be recommended as a routine for all newborn males. Still, both passionate support and antagonism for neonatal circumcision are evident in our respected medical literature. HISTORY The origins of circumcision are thought to date back over 6000 years to Egyptian times. Pictorially, this procedure has been depicted at Saqqara at the tomb of Ankh-Mahor. Cultural, rather than medical circumcision, has been practiced for generations by varied groups from Australian Aborigines to Black Africans. It is a rite of passage for Muslims. Likewise, the “Brit Milah,” or Jewish ritual circumcision, originated from the passage in Genesis (17:10) where it is written that “every young man among you shall be circumcised.” How then did circumcision become accepted for medical reasons? This concept originated relatively recently, at the turn of the 19th century. It was claimed by Remondino that circumcision essentially could cure all that ails from hernias to masturbation, enuresis to alcoholism, and virtually any health problem in between. This unproved practice was adopted and propagated by American health practitioners and was promoted, with little opposition, in the English speaking world. Over the last 50 years or so, the concept finally was challenged. It is interesting that when Great Britain first adopted its National Health Service, medical leaders could find little support to continue the practice of routine circumcision. By 1975, a similar philosophy was ultimately adopted in other Western countries such as Australia and Canada. Even in the USA, the AAP was stimulated to review this subject. Their Task Force on Circumcision in 1971 and 1975 found no valid reason for neonatal circumcision. However, this policy was softened by continued controversy and new data implicating the role of the retained foreskin in male infant urinary tract infection. The more centered AAP statement on this subject, published in 1989, suggests that there are both risks and benefits to this procedure and the most recent recommendations of this committee from 1999 supports the concept of disseminating proper education to the parents and obtaining informed consent. Verathon Inc. 800.331.2313 www.verathon.com 110 Urology for Primary Care Neonatal Circumcision: An American Enigma THE PREPUCE (FORESKIN) It is important to understand that the anatomy of the prepuce is variable, yet virtually all neonatal foreskins are “physiologically” rather than “anatomically” phimotic (cannot be retracted). The oft accepted tenet of trying to break down the prepucial-glanular adhesions manually and retract this normal prepucial physiologic transition should be avoided, and trauma to the foreskin as a result will be negated. By letting “nature” do this retraction instead, by virtue of the child’s intermittent erections, accumulation of smegma, and penile growth, 90% or more of foreskins will be retractable by the time the child reaches his fourth birthday. The application of 0.05% Betamethasone cream in cases of persistent phimosis has been associated with resolution of the problem in two-thirds to 95% of cases. In the United States, circumcision is still performed in approximately two-thirds of all male infants. The tendency is for this procedure to be more commonly performed in Caucasian males compared to Blacks and Hispanics. It is of interest that circumcision is performed more commonly in the offspring of better educated mothers, although other socio-economic factors, such as the availability and constraints of health insurance, must be taken into account. Regardless, neonatal circumcision for non-ritual reasons continues to be performed much more commonly in the United States than in other Western countries, including its neighbor Canada, where this procedure is performed less than half as often as in the U.S. ADVANCES IN NEONATAL CIRCUMCISION Perhaps our greatest advance in performing neonatal circumcision has been our understanding that this is not a painless procedure. The evidence clearly suggests that the infant does experience pain if coincidental analgesia is not utilized. Physiologically, data has accrued to show that there is a clinical response to this pain as evidenced by significant changes in blood pressure, pulse, and respiratory status. Furthermore, stress hormone levels such as serum cortisol are found to be elevated in response to this procedure. Interestingly, Mohels (Jewish Ritual Circumcisers) have used sweet wine to “medicate” these children. In support of this practice are observations that suggest that sucrose on a pacifier provides more efficacy than a pacifier alone. It has become accepted to use topical or injectable analgesia as a routine when performing neonatal circumcision. Topical EMLA has been shown to be effective if it is applied 60 minutes before the procedure. It is customary to apply 1-2 g of this lidocaine/prilocaine mixture to the distal penis and then wrap it with Tegaderm. It can be somewhat messy and the major negative is the time required for effect. Still, it is safe but is contraindicated in children who are taking other drugs that might potentiate methemoblobinemia (a metabolite of the prilocaine component has the potential to oxidize hemoglobin to methemoglobin). Injection techniques clearly offer immediate action and thus more flexibility when compared to topical creams. Using a 27 or 30 gauge needle, 1% lidocaine without epinephrine can be injected either as a subcutaneous circumferential ring block or alternatively injected as a penile dorsal nerve block. Usually 0.8-1.0 cc is all that is necessary. Even with a small needing, some degree of bruising and edema can occur. Verathon Inc. 800.331.2313 www.verathon.com 111 Urology for Primary Care Neonatal Circumcision: An American Enigma CIRCUMCISION TECHNIQUES It is imperative to obtain informed consent before proceeding with circumcision. In most cases the child must be placed on a restraining board, which can be unsettling for those families that prefer to be involved whilst the procedure is performed. A minor skin preparation is performed prior to injecting the local anesthetic or, if EMLA is used, after the Tegaderm is removed and the extra cream is wiped off. The old carpenter’s adage of “measure twice, cut once” cannot be over emphasized. Although the perfect result is desirable, it is better to leave extra skin than to remove too much. I thus recommend using a pen to ensure that one does not cut below the line. I also make sure that there is adequate ventral skin in particular, in order to avoid “scrotalizing” the ventral penis and leaving a hidden penis. It is essential to free the internal adhesions between the glans and inner prepuce completely and to assure that the glans is normal and that the urethra is midline and orthotopic. Approximately 4% of all hypospadias cases will be the megameatus intact prepuce variety, in which they have a complete foreskin, rather than a dorsal hood. The latter is also a contraindication to standard neonatal techniques unless a urethral anomaly has been excluded. The practitioner must remember that the technical goal of circumcision is to assure that phimosis is treated but that paraphimosis is prevented. The 3 most common instruments used to perform circumcision are: 1) the Plastibell, 2) Gomco clamp, and 3) Mogen clamp (used my Mohels as well for non-religious circumcisions). The latter 2 must be left in place long enough to tamponade the tissue in order to prevent hemorrhage. None of the 3 are devoid of associated complications. Thus, each practitioner must become comfortable and familiar with the technique that he/she prefers most. POTENTIAL COMPLICATIONS OF CIRCUMCISION Complications can be separated into those identified early and those evaluated later in the child’s life. Many reports describing complications only deal with those that are evident in the newborn nursery. This grossly underestimates the true complication rate that might be referred later to the Pediatric Urologist. Early Complications: When performing circumcision, the practitioner must be prepared for the unexpected. Bleeding is the most common complication reported and it usually is minor (<1%). Severe bleeding may suggest a previously undocumented bleeding or clotting disorder. In most cases, pressure alone controls bleeding. Surgicel or topical thrombin, topical or injectable epinephrine can be utilized as needed. Rarely, electrocautery or a suture is required to control the worst situations where simpler methods cannot control the hemorrhage. The usual culprit that requires control is the frenular artery, just below the glans ventrally. Care must be taken when addressing bleeding at this location, as overaggressive treatment at this area can lead to acquired hypospadias and/or urethrocutaneous fistulae. These complications are not further addressed until the child is older and elective general anesthesia becomes less of a concern. Verathon Inc. 800.331.2313 www.verathon.com 112 Urology for Primary Care Neonatal Circumcision: An American Enigma Infection is rare, but is the second most reported complication of circumcision. Local wound care, with or without antibiotics, is more than adequate in most cases. Regardless, close evaluation is mandatory once infection has been identified, as severe infections including fatal sepsis and necrotizing infections have been reported. The most evident early complication is trauma to the glans and/or penis. Electrocautery, especially when a Gomco device is used, can transmit an electrical current through the penis and “ablate” it, a true disaster. A more common situation is total, or more often, partial glans amputation, particularly when a Mogen clamp has been utilized. Immediate replantation has been associated with excellent outcomes. Migration of the Plastibell due to inappropriate sizing or application is seen frequently. Occasionally, ischemia can occur and lead to an urethrocutaneous fistula. More commonly, we are asked to remove a retained Plastibell, often with some excess skin, 2 weeks or so after its placement. Late Complications: In this area, the spectrum again ranges from the simple to the severe. Problems that often lead to evaluation after the acute period are recurrent phimosis and excess skin. The former often can be associated with the hidden or concealed penis. In its most severe form, where the glans cannot be released, urine can be trapped and infection can ensue. We have had several such instances where urination was not possible and urgent drainage was necessary. In the other variant of excess skin, it has been estimated that almost 10% of children who have had a neonatal circumcision might be evaluated for re-circumcision and removal of excess skin. This is primarily a cosmetic issue where the initial expectations of the family have not been met. Skin bands and bridges between the glans and mucosal collar sometimes occur due to fusion between the raw, denuded glans surface and the healing shaft area after the circumcision. Tethering of the penis with erections can ensue in the worst circumstances. Also during healing, the skin edges may roll in or smegma may become trapped during healing. This can lead to inclusion cysts. Meatal problems are common problems associated with infants in diapers who have had the protective foreskin removed. Some reviewers feel that meatitis, or inflammation and irritation of the meatus, which can lead to meatal stenosis, can occur as often as one in three infant boys who are circumcised. Meatal stenosis is usually not recognized until potty training when the urinary stream is deflected dorsally from the horizontal. Cysts and bridges, as well as meatal problems, can often be treated as an office procedure using local anesthetic, although sometimes a visit to the operating room is advised. MIGHT THERE BE BENEFITS TO CIRCUMCISION?: THE CONTROVERSY There are four issues that proponents of circumcision argue as benefits: Reducing the need for circumcision in the future, reducing the risk of penile cancer, reducing the risk of sexually transmitted disease, and the recent data demonstrating that circumcision in the neonatal period reduces male infant urinary tract infections. Verathon Inc. 800.331.2313 www.verathon.com 113 Urology for Primary Care Neonatal Circumcision: An American Enigma Balanitis, phimosis and paraphimosis, as well as a cosmetic preference, might be indications for later life circumcision. Other than the cosmetic desire for a circumcised appearance, it is thought that <5% of all men will eventually require a circumcision for medical necessity. Oftentimes this procedure requires general anesthetic, and hence the cost is high and the procedure is associated with small but finite risks. Attention to penile hygiene, avoiding forceful retraction of the prepuce, and judicial use of Betamethasone can minimize the need for surgical involvement. In the past, the risk of Squamous cell carcinoma of the penis has been thought to be up to 40 times more common in men who are uncircumcised. Indeed, in countries such as Israel, there is virtually no penile cancer. On the other hand, Danish data clearly have shown that by practicing good body (penile) hygiene, the incidence of penile cancer can be reduced to levels reported in the circumcised populations. This suggests that hygiene is paramount in reducing the incidence of this tumor. The foreskin provides a warm, moist environment that may be more conducive to the growth of certain infectious agents. In studies from Africa, there have been reports suggesting an 8-fold increased risk in men who are uncircumcised, compared to those circumcised, of contracting HIV. Other sexually transmitted diseases such as syphyllis, condyloma, chancroid, and herpes have purportedly occurred at higher rates in the uncircumcised population. Poorly controlled studies have also shown that cervical cancer is lower in cohorts of Moslem and Jewish men, that is, circumcised men. This has been translated into those men carrying and transmitting less human papilloma virus, the vector deemed responsible for the development of cancer of the cervix. One of the problems in most of these studies related to sexually transmitted diseases is that educated, safe sexual practices are not taken into account, and this behavior may be in itself the biggest risk factor. The clearest benefit of neonatal circumcision is probably in the area of preventing urinary tract infections in males less than 1 year of age. The question, however, is how many circumcisions, with their own attendant complication rates, are required in order to prevent one urinary tract infection? It has been estimated that between 80-100 circumcisions are required to prevent 1 urinary tract infection (UTI). Wiswell has demonstrated in his observed population of more than 200,000 infant males that the incidence of infection was 0.11% in the circumcised group versus 1.12% (a ten-fold difference) in those with an intact foreskin. Schoen has reported similar data with the bulk of infections occurring in the first 3 months of life. In the International Reflux Study, when comparing American children (38% uncircumcised) and European children (95% uncircumcised), investigators showed that the risk of infection was substantially increased in the European children with vesicoureteral reflux compared to American counterparts (24% versus 10%). SUMMARY Without a doubt, we have not heard the last about circumcision. Long term data are lacking as is quality control. With the arrival of the technical era, strong discussions of the pros and cons regarding circumcision by various advocacy groups can be found on the Internet daily. Culture, heritage, and personal preference often challenge medical reason, especially when it comes to issues like circumcision. As practitioners, our challenge is to advise parents and families about what we know regarding circumcision, despite knowing that they already have preconceived Verathon Inc. 800.331.2313 www.verathon.com 114 Urology for Primary Care Neonatal Circumcision: An American Enigma notions about what they wish done in any event. At the same time, it is imperative that we understand how important education is in the area of caring for the penis where the prepuce is left intact. Lastly, if performing circumcision, the practitioner must not take on this task casually, as complications occur far more frequently than published, and the vast majority of such complications are preventable by adhering to meticulous detail in performing this age-old procedure. Verathon Inc. 800.331.2313 www.verathon.com 115 Urology for Primary Care A Primer for Pediatric Urological referrals from Primary Care Providers A PRIMER FOR PEDIATRIC UROLOGICAL REFERRALS FROM PRIMARY CARE PROVIDERS Martin A. Koyle, M.D., FAAP, FACS Professor of Surgery and Pediatrics Vice Chief, Division of Urology The University of Colorado at Denver and Health Sciences Center Chairman, Department of Pediatric Urology The Children’s Hospital Denver, Colorado URINARY TRACT INFECTIONS Approximately 40% of all children experience UTIs. Vesicoureteral Reflux is the most common associated clinical finding. Ultrasound alone is a poor screening exam for the diagnosis of reflux (due to its high false negative rate), as it is noninvasive with virtually little chance of false positives. There is controversy as to what should be the first screening test, a kidney scan (DMSA) or a voiding cystourethrogram (VCUG). Some investigators advocate performing a VCUG only if the DMSA scan demonstrates renal involvement, whereas the standard paradigm is to perform a VCUG routinely, especially in pre-potty trained children. Regardless, no child should have any study performed unnecessarily, that is without documentation of a culture-proven UTI. In our own practice, as many as 50 % of children referred to us with UTI have never had a documented UTI. VESICOURETERAL REFLUX This common finding is actually a spectrum, not a single entity. As such, the therapy must be individualized. The natural history is for VUR to resolve spontaneously with time. Since in the past VUR was demonstrated in the evaluation of childhood UTIs, the mainstay of therapy was low dose prophylactic antibiotics while awaiting spontaneous resolution. “Pandora’s Box” was opened, in a manner of speaking, when children without UTI were found to have VUR when VCUGs were obtained in asymptomatic children with hydronephrosis, or in the screening of the sibling of a patient known to have VUR or offspring of a parent with VUR. This resulted in the continuing dilemma of whether or not patients without UTI should be treated the same way as patients with UTI. Moreover, it has now been found that dysfunctional elimination (DES) plays a pivotal role in many children with recurrent UTI and in those with primary reflux. The advent of injectable materials to correct reflux has added another option, but also further controversy into the armamentarium of interventional therapies to cure persistent severe VUR. Some view it as an alternative to antibiotic prophylaxis, and others feel that it can minimize the need for open or laparoscopic repair of VUR. Verathon Inc. 800.331.2313 www.verathon.com 116 Urology for Primary Care A Primer for Pediatric Urological referrals from Primary Care Providers HYDRONEPHROSIS Hydronephrosis is not a disease process, but rather an X-ray finding that has become more prevalent with the adoption of routine maternal ultrasonography. As many as 1:100 fetuses imaged in the end of the second trimester will have dilation of the collecting systems of the fetal kidney. Although this may be transient and inconsequential medically when the baby reaches term in the majority of patients, in a sparse number, it may represent a fatal anomaly. Ureteropelvic junction obstruction, or blockage where the kidney drainage system joins the ureter, represents 1/3- ½ of all significant findings of hydronephrosis that ultimately come to surgery. Other forms of obstruction can occur at the ureterovesicle junction (where the ureter enters the bladder), or be due to blockage of the ureter by anomalies such as a ureterocele or ectopic ureter, or in males who have blockage in the urethra from posterior urethral valves. VUR and Prune Belly Syndrome are other entities that may be found in the investigation of hydronephrosis. In severe cases of obstruction at any level, the kidneys may be damaged at various stages of development with a spectrum of severity. In the most extreme scenario, the kidneys never mature and a multicystic, dysplastic kidney is the result. DYSFUNCTIONAL ELIMINATION AND URINARY INCONTINENCE Dysfunctional elimination and urinary incontinence also form a spectrum that ranges from simple nocturnal enuresis to severe dysfunctional elimination. It is of primary importance to exclude organic diseases and structural anomalies in these children, such as neurogenic bladder, ectopic ureter, urinary obstruction with overflow, and female epispadias. When these are excluded, functional issues are usually the finding and often respond to behavioral approaches with the rare need for medications or surgery. Thus, a careful history, physical, and urinalysis with culture as necessary are mandatory first steps before unwarranted X rays are ordered and improper therapy rendered. HYPOSPADIAS AND OTHER PENILE ANOMALIES Hypospadias, like many other anomalies, represents a diverse spectrum. Common findings include a dorsal hood or incomplete foreskin, abnormality of the shaft skin, and abnormal bent erections (chordee). Be warned that what truly lies beneath can be much worse that what one anticipates. Most repairs are now done safely in 1 stage, as an outpatient at 6 months of age. In the more severe cases, more than 1 surgery might be required. Other common issues that may be addressed are angulation anomalies of the penis, the hidden penis, penoscrotal webbing, and the megaprepuce syndrome. In any of these anomalies, it is important that neonatal circumcision is avoided until evaluation has been completed by a Pediatric Urologist. THE SCROTUM AND INGUINAL CANAL Hernia, hydrocele, and the undescended testis represent the most common anomalies of this region. The former two entities are different pathologically from the entities of the same name seen in adults. In children, they represent various degrees of persistence of the processus vaginalis. The hydrocele allows peritoneal fluid to seesaw between the abdominal cavity and the groin or as far as the scrotum, whereas a hernia is a wider, more open communication where abdominal structures may potentially fit into the opening, become trapped (incarcerated), and Verathon Inc. 800.331.2313 www.verathon.com 117 Urology for Primary Care A Primer for Pediatric Urological referrals from Primary Care Providers perhaps even suffer vascular insufficiency (strangulation). Incarcerated and strangulated hernias are true emergencies, requiring immediate evaluation and likely treatment. Most hydroceles will resolve within the first year or two of life. The undescended testis is now routinely addressed within the first 6-12 months of life, knowing that descent beyond 2-3 months of life is unlikely and that after the first birthday damage to the testis is definitely occurring. The undescended testis is also found to occur at a second peak at around 3-5 years of age and may be called a secondary ascended or gliding variant. This must be differentiated from the common retractile testis, a nonpathological entity where the testis intermittently ascends from the scrotum due to the cremasteric reflex. An empty scrotum should raise suspicion of an intersex condition as should a single undescended testis with any degree of hypospadias. Thus, a careful newborn exam and appropriate genetic and laboratory testing can avoid disaster. The finding of acute swelling and pain in the scrotum, especially if associated with nausea, vomiting, and an absent cremasteric reflex, is torsion of the testis until proved otherwise. If this diagnosis is suspected, immediate exploration is recommended rather than imaging, as any delay in treatment may lead to a lost testicle. Other causes of the acute scrotum such as torsion of a testicular or epididymal appendage, or epididymitis, are treated supportively without surgery. Varicose veins to the left testicle, varicocele, is now found in 15% of adolescents. Its significance is uncertain but because of the association of this disorder with infertility in older men, evaluation may be important. At this time, the most common indication for varicocele correction in the young boy is testicular hypotrophy (atrophy), or significant difference in testicular size of the testis ipsilateral to the varicocele. SUMMARY The urinary tract and genital systems of children can be affected by a myriad of congenital and acquired disorders. As a result, the Primary Care provider is in a unique position to affect appropriate evaluation and, as necessary, referral of these interesting patients. Verathon Inc. 800.331.2313 www.verathon.com 118 BladderScan In-Service Training BladderScan Bladder Volume Instruments BladderScan bladder volume instruments from Verathon are portable, easy to use ultrasound devices that measure bladder volume noninvasively. BladderScan bladder volume instruments provide accurate results in a manner that is safe and comfortable for the patient. In this In-service Training section, you’ll find brief guidelines for using the BladderScan bladder volume instruments, along with a competency assessment to help you verify what you’ve learned. Reasons for Using the BladderScan bladder volume instruments: • Help prevent unnecessary urethral catheterization • Help reduce rates of catheter-associated urinary tract infections • Help reduce the risk of hydronephrosis (upper urinary tract damage) • Eliminate unnecessary patient trauma and discomfort, preserve patient dignity • Save staff time Clinical Applications: • Measurement of post-void residual bladder volume • Evaluation and diagnosis of urinary retention • Determination of need to catheterize following the discontinuation of a Foley catheter or during an intermittent catheterization schedule • Verification that a Foley catheter is draining properly • Verification of an empty bladder • Checking for bladder overdistention • Monitoring patients’ hydration status Note: Your facility will provide you with the policies and procedures for using BladderScan instruments with your patient population. Verathon Inc. 800.331.2313 www.verathon.com 119 BladderScan In-Service Training In-Service Training: BladderScan Use Follow the instructions below for the BladderScan bladder volume instrument (BVI) model used at your facility (BVI 3000, BVI 6100, BVI 6400 or BVI 9400). BACKGROUND: The BladderScan bladder volume instrument determines bladder volume noninvasively. It uses ultrasonic reflections within the patient’s body to differentiate the urinary bladder from surrounding tissue and create a three dimensional ultrasound image of the bladder. Based on this image, the BladderScan bladder volume instrument automatically calculates and displays bladder volume on an easy-to-read LCD screen. EQUIPMENT: • • • • BladderScan BVI 9400 or BVI 3000 with Probe and onboard printer, BladderScan BVI 6100, or Mobile BladderScan BVI 6400 Sontac ultrasound transmission gel Tissue Alcohol preps PROCEDURE FOR USING THE BLADDERSCAN BVI 9400: A. Prepare for the Exam 1. Watch the BladderScan BVI 9400 video on the instrument and read this manual before attempting to scan a patient. Before using the BladderScan BVI 9400, personalize the instrument with the name of your facility and the correct date and time. This information will be shown on all printouts. 2. Turn on the BVI 9400 by pressing the On/Off button. Make sure the Probe is plugged in and the battery is adequately charged. Check to make sure there is enough paper in the printer. 3. Explain to the patient the BladderScan procedure and the reason for measuring bladder volume. BladderScan BVI 9400 4. If the patient is being scanned for post void residual (PVR) determination, have the patient void 10 to 15 minutes before the test is performed. Verathon Inc. 800.331.2313 www.verathon.com 120 BladderScan In-Service Training 5. Have the patient lie flat with head elevated on a pillow. During the procedure, ask the patient not to flex her/his abdomen or make any movement that could affect the results. 6. Position BladderScan instrument so that the screen is easily viewed. If educating the patient about bladder volume, position machine so that the patient can also view the screen. 7. Expose the lower abdomen. The bladder lies in the pelvis directly behind the symphysis pubis (pelvic bone). 8. Before using the Probe, clean the rounded end by wiping it with an isopropyl alcohol pad. B. Scan the Patient 1. Select gender. If the female patient has not had a hysterectomy, press the FEMALE button. For all other patients (male or female), press the FEMALE button again to clear the gender icon from the LCD screen. 2. Palpate the patient's symphysis pubis (pubic bone). Place an ample quantity of gel (with as few air bubbles as possible) midline on the patient's abdomen, approximately one inch (3 cm) above the symphysis pubis. 3. Standing at the patient’s right side, place the Probe on the gel and aim toward the expected location of the bladder. For most patients, this means tilting the Probe slightly toward the coccyx (tailbone) so the scan clears the pubic bone. 4. Press the SCAN button, located on the underside of the Probe. As the scan progresses, sections of the bladder will appear on the console screen. When you hear the end-scan tone, the scan is complete. C. Verify Aim and Print Exam Results 1. If the scan is “on target” all 8 arrows will flash on the Probe screen, and the bladder will be shown in the center of the crosshairs on the Console screen. Since no re-aiming is needed, no arrows will appear on the Console screen. 2. If the scan is “off target” the Probe will show an arrow indicating the direction to move the Probe to be “on target.” If the arrow is solid, it means you are slightly “off target.” If the arrow is flashing, it means you are significantly “off target” and must re-aim and rescan. On the Console, the bladder will not be on the crosshairs, and there will be an arrow pointing in the direction for re-aiming. 3. To re-aim, note that the small dot at “6 o’clock” on the Console target represents the feet of the patient. The “12 o’clock” position represents the head of the patient and the upper left quadrant (9-12 o’clock) represents the right shoulder of the patient. This orientation should help you in re-aiming the Probe to capture the complete bladder in the ultrasound “cone.” Verathon Inc. 800.331.2313 www.verathon.com 121 BladderScan In-Service Training 4. You may also see a screen that indicates the pubic bone is “inside” the ultrasound cone. If this occurs, you may want to re-aim and re-scan. Although the bladder may be shown as centered in the ultrasound cone, and your measurement could be complete, there is a possibility that the pubic bone is obscuring some of the bladder. By re-aiming, you can ensure you have captured the bladder fully inside the ultrasound cone. 5. To save the exam, you must annotate it. To annotate, press and release the RECORD button on the Console. When you see the RECORD button icon turn to a STOP button icon, record your patient information by speaking into the Probe microphone. Press the STOP button on the Console. When the hourglass icon disappears, press the LISTEN button to replay the annotation. To review the images of your scan, press the REVIEW button (you must first save the exam before you can review it). 6. To print exam results via on-board printer, press the PRINT button. To perform another exam, press the HOME button. Finish exam. 7. Once you have completed the scan, wipe the ultrasound gel off the patient and the Probe. For ScanPoint subscribers, log on to ScanPoint to transfer and save your annotated exams. TROUBLESHOOTING Instrument Does Not Turn On: If the instrument does not turn on, this is usually due to a dead or discharged battery and can be remedied by replacing the dead battery with a charged battery. Check the battery icon in the upper right corner of the LCD display. If the battery icon does not display any power segments, replace the battery. When the battery charge is too low to allow normal operation (but not too low to permit operation of the internal circuitry) the device displays the following message: BATTERY CHARGE LEVEL IS TOO LOW FOR INSTRUMENT OPERATION. RECHARGE BEFORE NEXT USE. In this case, the battery must be recharged or replaced with a charged one. Battery Power Level: The battery is fully charged. Partially discharged. Between 25% and 50% charged. Almost depleted. Can power a few more scans. Replace immediately. Verathon Inc. 800.331.2313 www.verathon.com 122 BladderScan In-Service Training No Paper: The BladderScan BVI 9400 senses the presence of paper and automatically displays an “out of paper” screen when the printer is out of paper. For instructions on loading paper, please refer to the User’s Manual. Too Hot: The BVI 9400 displays the message TOO HOT if the print head overheats. In this case, turn off the BVI 9400 immediately. This condition may be the result of a paper jam. To clear the paper jam, see the following paragraph. Clearing A Paper Jam: If the paper will not advance through the printer, open the printer door to clear the paper jam. PROPER CARE AND MAINTENANCE To Clean the BladderScan BVI 9400: Use a soft cloth dampened with isopropyl alcohol (or an appropriate hospital cleaning agent) to wipe the Probe until it is thoroughly cleaned. If you use a detergent solution to clean the instrument, remove all residual detergent. Dry the instrument with a clean, soft cloth. Alternatively, dampen a soft cloth in any glutaraldehydebased hospital disinfectant solution such as Cidex or Cidex 7 from Advanced Sterilization Products, or Sporocidin from Sporocidin International. Wipe the instrument with the dampened cloth. To remove all traces of disinfectant solution and wipe the instrument with a clean soft cloth dampened in sterile water or potable tap water. Verathon recommends wiping the device three separate times to remove all residual disinfectant. Thoroughly dry the instrument with a clean, soft cloth before using. ♦ Do not immerse the instrument in disinfectant solution. ♦ Do not use CidexPlus to disinfect the instrument. CidexPlus will damage the plastic enclosure. ♦ Do not subject any part of the instrument to steam sterilization or ethylene oxide sterilization. Verathon Inc. 800.331.2313 www.verathon.com 123 BladderScan In-Service Training Regular Inspections and Maintenance: Verathon Medical recommends that the BVI 9400 be certified by an authorized BladderScan Service Center once a year. Certification service includes comprehensive inspection and testing of the instrument to ensure accurate performance in clinical use. For more information, please contact your authorized BladderScan Service Center, your local BladderScan distributor, or Verathon Medical Customer Care Department at 1.800.331.2313 (North America only. International customers, please refer to the contact information in the User’s Manual). Verathon Inc. 800.331.2313 www.verathon.com 124 BladderScan In-Service Training PROCEDURE FOR USING THE BLADDERSCAN BVI 3000: A. Prepare for the Exam 1. Watch the BladderScan BVI 3000 training video before attempting to scan a patient. Before using the BladderScan BVI 3000, personalize the instrument with the name of your facility and the correct date and time. This information will be shown on all printouts. 2. Turn on the BVI 3000 by pressing the On/Off button. Make sure the Probe is plugged in and the battery is adequately charged. Check to make sure there is enough paper in the printer. BladderScan BVI 3000 3. Explain to the patient the BladderScan procedure and the reason for measuring bladder volume. 4. If the patient is being scanned for post-void residual (PVR) determination, have the patient void 10 to 15 minutes before the test is performed. 5. Have the patient lie flat with head elevated on a pillow. During the procedure, ask the patient not to flex her/his abdomen or make any movement that could affect the results. 6. Position the BladderScan BVI 3000 so that the screen is easily viewed. If educating the patient about bladder volume, position machine so that the patient can also view the screen. 7. Expose the lower abdomen. The bladder lies in the pelvis directly behind the symphysis pubis (pelvic bone). 8. Before using the Probe, clean the rounded end by wiping it with an isopropyl alcohol pad. B. Scan the Patient 1. From the BVI 3000 main menu, press the Scan button. 2. Press the gender button to select the MALE or FEMALE setting. The FEMALE option allows the BladderScan BVI 3000 to exclude the uterus from the measurement. Use the FEMALE option only for female patients who have not had the uterus removed (hysterectomy). For female patients who have had the uterus removed, use the MALE setting when scanning. Verathon Inc. 800.331.2313 www.verathon.com 125 BladderScan In-Service Training 3. Palpate the patient’s symphysis pubis (pubic bone) and apply a generous amount of ultrasound gel (2 tablespoons) to the patient’s abdomen (suprapubic area) over the area you are going to scan. 4. The use of ultrasound gel is very important. Smooth the gel to remove any air bubbles, which may block ultrasound transmission. When using conventional ultrasound transmission gel for very thin or obese patients, more gel is required. If a patient has a large amount of hair on the area being scanned, then more gel is also needed. If you scan with a dry Probe, you will get inaccurate readings. 5. Position the Probe about 1.5 inches (or 4 cm) above the symphysis pubis/pelvic bone (bladder or suprapubic area), applying light pressure on the Probe to ensure good contact with the patient’s skin. 6. The Probe must be aligned properly in order for the aiming screen to work. Locate the “stick figure” icon on the side of the Probe and line it up with the patient’s body, so the head of the stick figure points in the same direction as the head of the patient, and the feet of the stick figure point toward the feet of the patient. Note: If a scar is present on the area being scanned, scan to the side, above, or below scar tissue. The same applies for bandages. 7. Press and release the SCAN button on the Probe. (You do not need to hold the scan button in during the scan.) 8. Hold the Probe steady during the scan. Moving or rolling the Probe while the scan is taking place will cause an inaccurate reading. It is recommended that you look at the Probe during the scan. An image cannot be seen until the scan is complete. You will hear a beep when the scan is finished. C. Verify Aim and Print Exam Results 1. The Aiming screen displays a cross-section of the bladder, as viewed when looking down into the patient’s abdomen: 2. If the light-colored bladder image is not centered on the target-shaped aiming icon and the LCD screen shows a “greater than” (>) symbol next to the bladder volume, then the bladder was not within full view of the Probe and the volume is higher than what is displayed. Recheck the Probe for correct position and scan again. 3. Use the image on the aiming icon to guide you when you re-aim: For example, if the bladder image is located toward the left side of the aiming icon, then re-aim the Probe so it projects ultrasound waves further to the left. Note: If the volume shown is greater than 999, then you have the bladder in full range and can print out an accurate reading. 4. When you are satisfied that the measurement is correct, press the DONE button. The scan results will be displayed. Verathon Inc. 800.331.2313 www.verathon.com 126 BladderScan In-Service Training 5. When you are ready to print out an accurate scan, press the print button twice to start the printing process. 6. Once you have completed the scan, wipe the gel off the Probe and the patient. TROUBLESHOOTING “NO PROBE” is displayed: No Probe or an incorrect Probe is installed. “Battery charge level is too low for instrument operation. Recharge before next use.”: Battery power is too low, recharge unit. Paper jam: Lower the print head, release lever located adjacent to the paper advance thumb wheel. Gently pull the paper either forward or backward to clear the paper jam. Battery appears dead: Check the battery icon in the upper-right corner of the console’s LCD screen. If the battery icon is not darkened, replace the battery with a freshly charged one to see if that solves the problem. Scanner reading “0”: Apply more ultrasound gel to Probe and rescan. PROPER CARE AND MAINTENANCE The machine and Probe may be cleaned with a soft cloth dampened in isopropyl alcohol or any standard hospital cleaning solution that does not contain aromatic hydrocarbons. Always inspect the Probe for cracks. Keep the instrument and Probe on the rolling cart to prevent damage to the instrument. Verathon Inc. 800.331.2313 www.verathon.com 127 BladderScan In-Service Training PROCEDURE FOR USING THE BLADDERSCAN BVI 6100 AND BVI 6400 A. Prepare for the Exam 1. Watch the BladderScan BVI 6100 training video before attempting to scan a patient. Note: The BVI 6400 (“Mobile BladderScan”) can store 10 or more voiceannotated exams. For instructions on saving multiple exams, consult the documentation that came with your BVI 6400 scanner. The procedure for performing a single scan with the BVI 6400 is the same as the procedure for scanning with the BVI 6100. 2. If the scanning instrument is in sleep mode, turn it on by pressing any button. Check the battery icon to make sure the scanning instrument is sufficiently charged for patient exams. BladderScan BVI 6100 3. Explain to the patient the procedure and the reason for measuring bladder volume. 4. If the patient is being scanned for post-void residual (PVR) determination, have the patient void 10 to 15 minutes before the test is performed. 5. Have the patient lie flat with head elevated on a pillow. Ask the patient not to flex her/his abdomen during the procedure or make any other movement that could affect the results. 6. Expose the lower abdomen. The bladder lies in the pelvis directly behind the symphysis pubis (pelvic bone or suprapubic area). Mobile BladderScan BVI 6400 7. Before using the Probe unit, clean the rounded end by wiping it with an isopropyl alcohol pad. Verathon Inc. 800.331.2313 www.verathon.com 128 BladderScan In-Service Training B. Scan the Patient 1. Palpate the patient’s symphysis pubis (pubic bone). 2. Apply ultrasound gel to the abdomen over the area you are going to scan (approximately 1.5 inches/ 4 cm above the symphysis pubis). Smooth the gel to remove any air bubbles trapped beneath, which may interfere with ultrasound transmission. Note: If you scan with a dry Probe, you will get inaccurate readings. 3. Use the Gender (Top) button to select the female option for female patients who have not had the uterus removed (hysterectomy). When the female option is selected, a female icon appears on the LCD screen. The female option excludes the uterus from the measurement. For all other patients, use the Gender (Top) button to toggle between options until no icon is displayed. 4. Place the Probe on top of the gel, applying light pressure to insure contact with the patient. Note: You may need to scan around scar tissue on some patients. Scan to the side, above, or below scar tissue on these patients. The same applies for bandages. 5. Press and release the Scan button, located on the underside of the Probe’s handgrip. Hold the Probe steady during the scan. Moving or rolling the Probe while the scan is taking place will cause an inaccurate reading. When the scan is complete, the instrument beeps and displays a measurement. C. Verify Aim 1. If flashing aiming arrows appear below the bladder volume measurement, then the Probe was not aimed properly and part of the bladder was outside of its field of vision. Re-aim the Probe in the direction indicated by the flashing arrow and scan again. Repeat until a solid arrow or no arrow appears, indicating that you have achieved an accurate measurement. Note: A solid aiming arrow indicates an aiming suggestion. Solid arrows appear when the instrument senses that the bladder was contained but not completely centered in its field of vision. You may re-aim if you wish, but this is not required. 2. Chart the measurement or use ScanPoint to save and print the exam result from your personal computer. 3. When you have completed the scan, wipe the ultrasound gel off the Probe and the patient. Place the instrument in its cradle when not in use. Verathon Inc. 800.331.2313 www.verathon.com 129 BladderScan In-Service Training TROUBLESHOOTING Scanning instrument does not turn on: Usually due to a dead or discharged battery. Place the scanning instrument in its cradle and charge for a minimum of fifteen minutes. Then use the activation stylus to press the reset button, located above the scan button. After fifteen minutes, sufficient power will be restored to perform a patient exam; however, fully charging the battery may take up to six hours. Scanning instrument is charged but will not scan: Either (a) the battery has some power remaining, but not enough to perform patient exams, or (b) you need to calibrate the scanning instrument. If (a), place the instrument in its cradle to recharge the battery. If (b), use ScanPoint to calibrate your instrument. To display the number of days remaining until your instrument requires calibration, press and hold down the Gender button for five seconds. A flashing arrow appears below the bladder volume measurement: The scanning instrument was not properly aimed and part of the bladder is outside its field of vision. Reaim the scanning instrument so it projects ultrasound waves in the direction indicated by the arrow, and scan again. A solid arrow appears below the bladder volume measurement: The solid arrow indicates an aiming suggestion. Solid arrows appear when the scanning instrument senses that the bladder was not completely centered in its field of vision. In this case, you may reaim if desired, but this is not necessary. For patients with oblong or extremely large bladders, a solid arrow often accompanies a good measurement. A greater than (>) symbol appears beside the bladder volume measurement: The greater than symbol indicates that the bladder is too large to fit in the scanning instrument’s field of vision and the true bladder volume is larger than displayed. In such cases, re-aiming the scanning instrument won’t help; however, this situation occurs almost exclusively in patients with extremely high bladder volumes, and such measurements are clinically useful even though they underestimate the actual volume. PROPER CARE AND MAINTENANCE Clean and disinfect the BladderScan BVI 6100 and BVI 6400 with a soft cloth dampened in isopropyl alcohol or an appropriate hospital cleaning agent (do not use Cidex Plus). Once a week, you should inspect the Probe for physical faults or cracks. You must calibrate the scanning instrument every 6 to 12 months using ScanPoint. For details, consult the User’s Guide that came with your instrument. Store the scanning instrument in its cradle when not in use. This ensures that the battery is always sufficiently charged for patient exams. Verathon Inc. 800.331.2313 www.verathon.com 130 BladderScan In-Service Training Important Points to Remember: • The BladderScan bladder volume instrument is dependent on the user to achieve the highest level of accuracy. You may see a slight deviation in your readings, but this is normal and your results will be useful for clinical applications. • If a female patient has had a hysterectomy, do not select the female setting, which causes the instrument to differentiate for uterine tissue. • The application of ultrasound gel is very important. If you scan with a dry Probe, you will obtain inaccurate readings. • After you measure bladder volume, an aiming icon (either an aiming target or aiming arrows, depending on your BladderScan model) will appear on the LCD screen. If the aiming icon indicates that the BladderScan instrument was not properly aimed (i.e., the bladder was not centered within the range of the Probe), re-aim and scan again as necessary. • If the LCD screen shows a “greater than” symbol next to the bladder volume measurement, then you do not have the bladder within full range of the Probe and the patient’s true bladder volume is greater than the volume displayed on the screen. An exception occurs when the volume shown is greater than 999 cc; in this case, the bladder is within full range of the instrument and the reading displayed is accurate. • The BladderScan instrument automatically saves the highest volume measured, because in most scenarios the highest measurement is the most accurate. Exceptions occur when the operator moves the Probe during the scan or fails to select the female gender when applicable. In such situations, clear the scan results screen, make sure the gender setting is correct, and then re-scan the patient. • Urinary catheters, scar tissue, sutures, incisions, surgical staples and fluid-filled cysts may all interfere with the accuracy of the BladderScan bladder volume instrument and should be assessed on an individual basis. You may need to scan beside, above or below scar tissue on some patients if scanning the tissue directly is painful for the patient or prevents you from obtaining an accurate measurement. The same applies for bandages. • Remember that ultrasound cannot be transmitted through bone. Make sure you are not resting the Probe on the pubic bone of your patient. If the aiming indicator shows that the Probe needs to be aligned lower on your patient’s abdomen and you are already near the bone, simply aim the Probe so it points into the pelvic cavity. Verathon Inc. 800.331.2313 www.verathon.com 131 BladderScan In-Service Training Competency Assessment for the BladderScan Bladder Volume Instrument Name___________________________________________ Unit____________________________________________ Date____________________________________________ OBJECTIVE: THE USER WILL DEMONSTRATE THE ABILITY TO ACCURATELY ASSESS A PATIENT’S BLADDER VOLUME BY OBTAINING AN ULTRASOUND MEASUREMENT AND PRINTING A COPY TO ATTACH TO THIS FORM. COMPETENCY WILL BE DEMONSTRATED BY VIEWING THE BLADDERSCAN BLADDER VOLUME INSTRUMENT TRAINING VIDEO, OBTAINING AN ACCURATE BLADDER VOLUME READING, AND ACHIEVING A SCORE OF 80% OR HIGHER ON THE POST TEST. POST TEST: MARK THE FOLLOWING STATEMENTS TRUE OR FALSE 1._____ To ensure the highest degree of accuracy, the BladderScan BVI 3000 instrument automatically saves the average bladder volume measurement from any series of scans. 2._____ Moving the Probe while scanning will result in artificially high bladder volume measurements. In this case, the operator should clear the scan results and re-scan the patient. 3._____ The female gender should be selected for all female patients, because women’s bladders are shaped differently than men’s. 4._____ A greater than symbol always indicates that the bladder is too large to scan accurately. In this situation, repositioning the Probe and re-scanning the patient won’t help. 5._____ An ample quantity of ultrasound gel is necessary to obtain the highest degree of accuracy. 6._____ The bladder does not need to be centered in the scan plane (range of the Probe) for best results. 7._____ The aiming icon on the BladderScan LCD screen helps guide the operator to optimal Probe placement. If indicated by the aiming icon on the scan results screen, the operator should readjust the aim of the Probe and re-scan the patient to ensure maximum accuracy. 8._____ Urinary catheters, scar tissue, incisions, sutures, surgical staples, and fluid-filled cysts may all interfere with the accuracy of the BladderScan instrument. The BladderScan instrument should not be used on these patients. 9._____ If the Probe needs to be aimed further downward, but is too close to the symphysis pubis, the operator should tilt the Probe so it points down into the pelvic cavity. This is because ultrasound cannot be transmitted through bone. 10.____ You must hold down the scan button until the scan is complete. Verathon Inc. 800.331.2313 www.verathon.com 132 BladderScan In-Service Training Competency Assessment for the BladderScan Bladder Volume Instrument (Answer Key) POST TEST: MARK THE FOLLOWING STATEMENTS TRUE OR FALSE 1. __F__ To ensure the highest degree of accuracy, the BladderScan BVI 3000 instrument automatically saves the average bladder volume measurement from any series of scans. The BladderScan BVI 3000 automatically saves the largest bladder volume measurement from any series of scans, because in most cases, this is also the most accurate. 2. __T__ Moving the Probe while scanning may result in artificially high bladder volume measurements. In this case, the operator should clear the scan results and re-scan the patient. 3. __F__ The female gender should be selected for all female patients, because women’s bladders are shaped differently than men’s. The female gender should be selected ONLY for female patients who have not undergone a hysterectomy. The female option allows the BladderScan bladder volume instrument to differentiate for the presence of the uterus, which may resemble the bladder ultrasonically. 4. __F__ A greater than symbol always indicates that the bladder is too large to scan accurately. In this situation, repositioning the Probe and re-scanning the patient won’t help. In most cases, the greater than symbol indicates that the Probe was not properly aimed and the bladder was not fully within range of the BladderScan instrument; thus, the bladder volume measured is lower than the true bladder volume, and the Probe must be re-aimed and the scan repeated. If the bladder volume is greater than 999 cc, then the bladder is too large to be fully contained within the range of the Probe, and the measurement displayed is accurate and clinically useful, even though it is less than the true bladder volume. 5. __T__ An ample quantity of ultrasound gel is necessary to obtain the highest degree of accuracy. 6. __F__ The bladder does not need to be centered in the scan plane (range of the Probe) for best results. For best results, the Probe must be properly aimed so that the bladder is centered within the scan plane. 7. __T__ The aiming icon on the BladderScan LCD screen helps guide the operator to optimal Probe placement. If indicated by the aiming icon on the scan results screen, the operator should readjust the aim of the Probe and re-scan the patient to ensure maximum accuracy. 8. __F__ Urinary catheters, scar tissue, incisions, sutures, surgical staples, and fluid-filled cysts may all interfere with the accuracy of the BladderScan instrument. The BladderScan bladder volume instrument should not be used on these patients. Urinary catheters, scar tissue, incisions, sutures, surgical staples, and fluid-filled cysts may interfere with the accuracy of the BladderScan instrument, but in many cases, the BladderScan instrument can still be used. These patients should be assessed on an individual basis. If possible, scan above, beside, or below scar tissue or bandages. 9. __T__ If the Probe needs to be aimed further downward, but is too close to the symphysis pubis, the operator should tilt the Probe so it points down into the pelvic cavity. This is because ultrasound cannot be transmitted through bone. 10.__F__You must hold down the scan button until the scan is complete. It is not necessary to hold down the scan button during scanning; simply press and release the scan button to initiate the bladder volume measurement. Verathon Inc. 800.331.2313 www.verathon.com 133 Urology for Primary Care Appendices APPENDICES Appendix A – AUA Guideline Flow Diagram for Diagnosis and Treatment of BPH Appendix B – The American Urology Association Symptom Index for BPH Appendix C – Incontinence Questionnaire (UDI-6) Appendix D – Flow Rate Nomograms Appendix E – Categories of Prostatitis Appendix F – Voiding Diary Appendix G – Frequency Volume Chart Verathon Inc. 800.331.2313 www.verathon.com 134 Urology for Primary Care From the American Urological Association (AUA) Guideline Flow Diagram for Diagnosis and Treatment of BPH – Appendix A Appendix A Verathon Inc. 800.331.2313 www.verathon.com Urology for Primary Care Appendix B SYMPTOM INDEX FOR BPH Not at all Less than 1 time in 5 Less than half the time About half the time More than half the time Almost always 1. Over the past month, how often have you had the sensation that your bladder was not completely empty after you finished urinating? 0 1 2 3 4 5 2. Over the past month, how often have you had to urinate again less than two hours after you last finished urinating? 0 1 2 3 4 5 3. Over the past month, how often have you found you stopped and started again several times while urinating? 0 1 2 3 4 5 4. Over the past month, how often have you found it difficult to postpone urination? 0 1 2 3 4 5 5. Over the past month, how often have you had a weak urinary stream? 0 1 2 3 4 5 6. Over the past month, how often have you had to push or strain to begin urinating? 0 1 2 3 4 5 None 1 time 2 times 3 times 4 times 5 or more times 0 1 2 3 4 5 Urinary Symptoms 7. Over the last month, how many times did you typically get up to urinate each night, from the time you went to bed until the time you got up in the morning? TOTAL AUA Symptom Score = Sum of questions 1 – 7 a) 0-7 b) 8-19 c) 20-35 __________ Mild __________ Moderate __________ Severe Quality of Life Bother Score 1. If you had to spend the rest of your life with your urinary condition just the way it is now, how would you feel about that? Delighted Pleased Mostly satisfied Mixed (about equally satisfied and dissatisfied) Mostly dissatisfied Unhappy Terrible 0 1 2 3 4 5 6 From the American Urological Association (AUA) Symptom Index for BPH – Appendix A with the International Prostate Symptom Score (IPSS) Verathon Inc. 800.331.2313 www.verathon.com Urology for Primary Care Appendix C INCONTINENCE QUESTIONNAIRE (UDI-6) Patient Name:_________________________________________ Date of Visit:_____________________ Please circle the number that best describes what you are feeling. Not at all Slightly Moderately Greatly 1. Frequent urination 0 1 2 3 2. Urine leakage related to the feeling of urgency 0 1 2 3 3. Urine leakage related to physical activity, coughing, or sneezing 0 1 2 3 4. Small amounts of urine leakage 0 1 2 3 5. Difficulty emptying your bladder 0 1 2 3 6. Pain or discomfort in the lower abdomen or genital area 0 1 2 3 TOTAL Score = Sum of questions 1 – 6 __________ Do you experience, and if so, how much are you bothered by: Verathon Inc. 800.331.2313 www.verathon.com Urology for Primary Care Appendix D Flow Rate: Max Flow__________________cc/sec Average Flow Rate Avg. Flow__________________cc/sec Maximum Flow Rate 30 30 +1 0 Mean 25 Voiding Time:__________________________sec 25 0 Mean -1 20 Residual:______________________________cc 20 15 -2 10 -3 cc/sec -1 cc/sec Volume:_______________________________cc -2 15 10 -3 Is this a usual voiding?_____________________ 5 Time to peak:____________________________ 0 5 0 100 200 300 400 100 500 200 300 400 500 Volume (cc) Volume (cc) Interpretation:____________________________ ________________________________________ ___________________________________________ From the Krane-Siroky flow rate nomograms. Verathon Inc. 800.331.2313 www.verathon.com Urology for Primary Care Appendix E Prostatistis Localization: Interpretation of the Meares-Stamey Four-Glass Test, and the Pre- and Post Prostatic Massage I. Meares-Stamey Test: Category Acute Bacterial Prostatitis – Category I: Urine Culture: VB1 (Initial) +/- VB2 (Mid-Stream) + VB3 (Post Prostatic Massage) + Expressed Prostatic Secretions (EPS) + - +/- + + Inflammatory Type: Category IIIA - - - + Non-inflammatory Type: Category IIIB - - - - Chronic Bacterial Prostatitis – Category II: Chronic Abacterial Prostatitis – Category III: Chronic Pelvic Pain Syndrome II. Pre- and Post Massage Test: • Obtain a Mid-Stream Clean Voided specimen. • Perform Prostate Massage. • Collect an initial stream urine specimen after the massage. • Review both the pre- and post urine sediment for any increase in leukocytes, or bacterial. (Urine is reviewd on microscopy for WBSs and also sent for culture.) Category Pre-Massage Post Massage WBC Culture WBC Culture Acute Bacterial Prostatitis: Category I + + + + Chronic Baterial Prostatitis Category II +/- +/- + + Inflammatory Type: Category IIIA - - + - Non-inflammatory Type: Category IIIB - - - - Chronic Abaterial Prostatitis Category III Chronic Prostaic Pelvic Pain Syndrome Adapted from Nickel, J.C. and Schaeffer, A.J.; Prostatitis: Evolving Management Strategies. Urologic Clinics of North America Vol. 26, No. 4, November 1999, P. 741 Verathon Inc. 800.331.2313 www.verathon.com Urology for Primary Care Appendix F Your Daily Bladder Diary Your Name: Date: This diary will help you and your health care team. Bladder diaries help show the causes of bladder control trouble. The “sample” line (below) will show you how to use the diary TIME DRINKS What kind? Sample Coffee How much? 2 cups URINE How many times? ACCIDENTAL LEAKS How much? (circle one) ACCIDENTS DID YOU FEEL A STRONG URGE TO GO? (circle one) (circle one) Yes No 6-7 am Yes No 7-8 am Yes No 8-9 am Yes No 9-10 am Yes No 10-11 am Yes No 11-12 noon Yes No 12-1 pm Yes No 1-2 pm Yes No 2-3 pm Yes No 3-4 pm Yes No 4-5 pm Yes No 5-6 pm Yes No 6-7 pm Yes No From the National Kidney and Urologic Information Clearinghouse sm med lg sm med lg Verathon Inc. 800.331.2313 WHAT WERE YOU DOING AT THE TIME? Sneezing, exercising, having sex, lifting, etc. Running www.verathon.com Urology for Primary Care TIME Appendix F DRINKS What kind? How much? URINE How many times? How much? (Circle one) ACCIDENTAL LEAKS ACCIDENTS DID YOU FEEL A STRONG URGE TO GO? (Circle one) (Circle one) 7-8 pm Yes No 8-9 pm Yes No 9-10 pm Yes No 10-11 pm Yes No 11-12 midnight Yes No 12-1 am Yes No 1-2 am Yes No 2-3 am Yes No 3-4 am Yes No 4-5 am Yes No 5-6 am Yes No WHAT WERE YOU DOING AT THE TIME? Sneezing, exercising, having sex, lifting, etc. I used ______pads. I used _____diapers today (write number). Questions to ask my health care team: ______________________________________________________________________________________ _____________________________________________________________________________________________________________________ _________________________________________________________________________________________________________________________________ From the National Kidney and Urologic Information Clearinghouse Verathon Inc. 800.331.2313 www.verathon.com Urology for Primary Care Appendix G Frequency Volume Chart Name_______________________________________________________________ Date______________________________ Record TIME, VOLUME and LEAKAGE episodes. (Code W for drops/wet pants, WW for soaked clothes/pads) If urgency occurs, Code + for moderate, ++ for severe. TIME VOLUME URGENCY LEAKAGE ACTIVITY/COMMENTS Record number of drinks ______________ Record number of pads_______________ Please note activity, which might be linked to leakage episode (e.g., coughing) or urgency. From Diary A (Baily et al 1989), showing a page for single day use Verathon Inc. 800.331.2313 www.verathon.com Urology for Primary Care Bibliography BIBLIOGRAPHY Introduction Abrams, P., editorial, “LUTS, BPH, BPE,BPO: A Plea for the Logical Use of Correct Terms,” Reviews in Urology, Spring, 65, 1999. Fryman, R.J., Abrams, P., Campbell’s Urology Update, vol. 1, no. 4, 2000. Bladder Outlet Obstruction 5th International Consultation on BPH, Paris, France, 2000. AUA Guideline on Management of Benign Prostatic Hyperplasia (2003). “Diagnosis and Treatment Recommendations.” Journal of Urology August 2003, Vol 170 No. 2: Chapter 1. McConnell, J., Bueschen, A., Bruskewitz, R., (faculty) “Urological Topic Showcases BPH Current Diagnosis and Treatment Recommendations,” AUA annual meeting, San Antonio, TX, May, 1993. Lepor H., Williford, W.O., Barry, M.J., Brawer, M.K., et al., “The Efficacy of Terazosin, Finasteride, or Both in Benign Prostatic Hyperplasia,” NEJM, 335(8): 533-9, 1996. McConnell, J.D., Bruskewitz R., Walsh, P., et al., PLESS Study Group: “The Effect of Finasteride on the Risk of Acute Urinary Retention and the Need for Surgical Treatment Among Men with Benign Prostatic Hyperplasia,” NEJM, 338:9, 557-563, 1998. McConnell, J.D., Barry, M.J., Bruskewitz, R.,C., et al., “Benign Prostatic Hyperplasia: Diagnosis and Treatment, Clinical Practice Guideline No. 8,” U.S. Department of Health and Human Services, Public Health Agency for Health Care Policy and Research, AHCPR Publication No. 94-0582, Rockville, MD, Feb., 1994. McConnell, J.D., “Current Medical Therapy for Benign Prostatic Hyperplasia: The Scientific Basis and Clinical Efficacy of Finasteride and Alpha-Blockers,” Campbell’s Urology Update, sixth ed., no. 3, 1-12, 1992. McConnell, J.D., Bruskewitz, R., Walsh, P., et al. “The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia.” N Engl J Med. 1998; 338:557-563. McConnell, J.D., Roehrborn, C.G., Oliver, O.M., et al. “The long term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia.” For the MTOPS Research Group. Barry MJ., Fowler, Jr., F.J., O’Leary, M.P., et al, “The American Urological Symptom Index for Benign Prostatic Hyperplasia,” J Urol, 148: 1549-1557, 1992. Verathon Inc. 800.331.2313 www.verathon.com i Urology for Primary Care Bibliography Lloyd, LK, “The Rationale for Alpha Blockade in the Treatment of BPH,” Urology Advisory Council Syllabus, Abbot Park, ILL, 1993. Siroky, M.B., Olsson, C.A., Krane, R.J., “The Flow Rate Nomogram. II. Clinical Correlation,” J Urol, 123:208-210, 1980. NIH news release, “Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), March 8, 2000. Prostatitis Meares, E.M., and Stanley, T.A., “Bacteriologic Localization Patterns In Bacterial Prostatitis, Investigative Urology, 5:492, 1968. Meares, E.M., “Prostatitis and Related Disorders,” Campbell’s Urology, sixth ed., 807-822. Nickel, J.C., “Prostatitis: Evolving Management Strategies, The Urologic Clinics N.A.,” Infections In Urology, vol. 26, no. 4, 1999. Executive Summary, Chronic Prostatitis Workshop, National Institutes of Diabetes and Digestive and Kidney Diseases, NIH, Rockville, MD, Dec. 7-8, 1995. Krieger, J.N., Nyberg, L., Nickel, J.C., “NIH Consensus Definition and Classification of Prostatitis,” JAMA, 282:236-237, 1999. Overactive Bladder P. Abrams, et al., “The Standardization of Terminology of Lower Urinary Tract Function: Report from the Standardization Subcommittee of the International Continence Society,” Neurourology and Urodynamics 21: 167-178 (2002). Klutke, C.G., Raz, S., “Evaluation and Treatment of the Incontinent Female Patient,” The Urologic Clinics N.A., vol. 22, no. 3, August, 1995. Raz S., Little N.A., Saad J., “Female Urology,” Campbell’s Urology, sixth ed., vol. 3, chapter 75, 7282-2828, (Walsh, Retik, Stamey, Vaugham, editors). Wein, A.J., Levin, R.B., Barrett, D.M., “Voiding Function and Dysfunction,” Adult and Pediatric Urology, second ed., vol. 1, chapter 28 A&B, 933-1091. Wein, A.J., “Neuromuscular Dysfunction of the Lower Urinary Tract,” Campbell’s Urology, sixth ed., vol. 1, chapter 12, 573-658, (Walsh, Retik, Stamey, Vaugham, editors). Verathon Inc. 800.331.2313 www.verathon.com ii Urology for Primary Care Bibliography Blaivas, J.G., “Intrinsic Sphincter Deficiency,” Campbell’s Urology Update No. 17, 1-11, 1996, (Walsh, Retik, Stamey, Vaughan, editors). Resnick, N.M., “An 89-Year-Old Woman With Urinary Incontinence,” JAMA, vol. 276, 18321840, 1996. “Clinical Practice Guideline for Urinary Incontinence in Adults,” Agency for Health Care Policy and Research, 1996. “Let’s Talk About Bladder Control for Women,” National Kidney and Urologic Diseases Information Clearing House, National Institute of Diabetes and Digestive Kidney Diseases, National Institutes of Health. Fryman, R.J., Abrams, P., “Current Diagnosis in the Management of Men With Lower Urinary Tract Symptoms,” Campbell’s Urology Update Vol. 1, no. 4., 2000 (Walsh, Retik, Vaughan, Wein, editors). Abams, P., Klvemark, B., “Frequency Volume Charts: An Indispensable Part of Lower Urinary Tract Assessment,” Scand J Urol Nephrol (suppl 179) 30: 47-53, 1996. Ditropan XL, prescribing information, Alza Corporation, Palo Alto, CA., 1999. Detrol LA, prescribing information, Pharmacia Corporation, Peapack, NJ. The Aging Male Tenover J.L., “Testosterone and the aging male,” J Androl 1997 18(2):103-106. Hajjar R.R., Kaiser F.E., Morley J.E., “Outcomes of long-term testosterone replacement in older hypogonadal males: A retrospective analysis,” J Clin Endocrinol Metab 1997; 82: 3793-6. Silber S.J., “Effects of age on male fertility,” Semin Reprod Endocrinol 1991; 9: 241-8. Schwartz D., Mayaux M.J., Spira A., Moscato M.L., Jouannet P., Czyglik F., David G., “Semen characteristics as a function of age in 833 fertile men,” Fertil Steril 1983; 39: 530-5. Haidl G., Jung A., Schill W.B., “Aging and sperm function,” Hum. Reprod. 1996; 11: 558-60. Bremner W.J., Vitiello M.V., Prinz P.N., “Loss of circadian rhythmicity in blood testosterone levels with aging in normal men,” J Clin Endocrinol Metab 1983; 56: 1278-81. Wermer A.A., “The male climacteri,” JAMA 1939; 119: 1441-3. Feldman H.A., Goldstein I., Hatzichristou D.G., Kranc R.J., McKinlay J.B., “Impotence and its medical and psychosocial correlates: Results of the Massachusetts Male Aging Study,” J Urol 1994; 151: 54-61. Verathon Inc. 800.331.2313 www.verathon.com iii Urology for Primary Care Bibliography Muller J.F., Mittleman A., Maclure M., Sherwood J.B., Tofler G.H., “Triggering myocardial infarction by sexual activity. Low absolute risk and prevention by regular physical exertion. Determinants of Myocardial Infarction Onset Study Investigators,” JAMA 1996; 275: 1405-9. Hajjar R.R., Kaiser F.E., Morley J.E., “Outcomes of long-term testosterone replacement in older hypogonadal males: A retrospective analysis,” J Clin Endocrinol Metab 1997; 82: 3793-6. Tremblay R.R., Morales A., “Canadian practice recommendations for screening, monitoring and treating men affected by andropause or partial androgen deficiency,” The Aging Male 1998; 1: 213-8. Anderson R.A., Wallace E.M., Wu F.C.W., “Effect of testosterone enanthate on serum lipoproteins in man,” Contraception 1995; 52: 115-9. Crook D., “Androgen therapy in the aging male: assessing the effect on heart disease,” The Aging Male 1999; 2: 151-6. Initial Evaluation of Hematuria for Primary Care Physicians & Initial Management of Urinary Calculus Disease for Primary Care Physicians Campbell, Meredith F., Patrick C. Walsh, Alan B. Retik, eds. Campbell's Urology. W.B. Saunders Company; 8th edition (June 15, 2002). Gillenwater, Jay Y., Stuart S. Howards, John T. Grayhack, Michael Mitchell, eds. Adult and Pediatric Urology. Lippincott Williams & Wilkins; 4th Bk&Cdr edition (January 15, 2002). Siroky, Mike B., Robert D. Oates, Richard K. Babayan, eds. Handbook of Urology: Diagnosis and Therapy. Lippincott Williams & Wilkins; 3rd edition (April 1, 2004). Sexual Medicine for Men: Male Erectile Dysfunction See endnotes in chapter for references. Sexual Medicine for Women: Management of Women with Desire, Arousal, and Orgasm Sexual Health Concerns The American College of Obstetricians and Gynecologists. New diagnosis codes available October 1 2003. Available at: http://www.acog.org/departments/dept_notice.cfm?recno=6&bulletin=2701. Accessed June16, 2005. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSMIV-TR (Text Revision). 4th ed.Washington, DC: American Psychiatric Association; 2000. Verathon Inc. 800.331.2313 www.verathon.com iv Urology for Primary Care Bibliography Basson R. “Female sexual response: the role of drugs in the management of sexual dysfunction.” Obstet Gynecol. 2001; 98: 350-353. Basson, R., J. Berman, A. Burnett, et al. “Report of the international consensus development conference on female sexual dysfunction: definitions and classifications.” J Urol. 2000; 163: 888-893. Baulieu, E.E., G. Thomas, S. Legrain, et al. “Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue.” Proc Natl Acad Sci U S A. 2000; 97: 4279-4284. Braunstein, G.D., D.A. Sundwall, M. Katz, et al. “Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo controlled trial.” Arch Intern Med. 2005; 165: 1582-1589. Dennerstein, L., J. Randolph, J. Taffe, et al. “Hormones, mood, sexuality, and the menopausal transition.” Fertil Steril. 2002; 77 Suppl 4: S42-S48. Dog, T.L. “Menopause: Review of Botanical Dietary Supplement Research.” Presented at: NIH State-of-the-Science Conference on Management of Menopause-Related Symptoms; March 2123, 2005; Bethesda, MD. Ettinger, B., D. Grady, A.N. Tosteson, et al. “Effect of the Women’s Health Initiative on women’s decisions to discontinue postmenopausal hormone therapy.” Obstet Gynecol. 2003; 102: 1225-1232. Evidence-based medicine: a new approach to teaching the practice of medicine. Evidence-Based Medicine Working Group. JAMA. 1992; 268: 2420-2425. Guyatt, G.H., R.B. Haynes, R.Z. Jaeschke, et al. Users’ Guides to the Medical Literature: XXV. Evidence-based Medicine: Principles for Applying the Users’ Guides to Patient Care. Evidence-Based Medicine Working Group. JAMA. 2000; 284: 1290-1296. Heim, L.J. “Evaluation and differential diagnosis of dyspareunia.” Am Fam Physician. 2001; 63: 1535-1544. Hersh, A.L., M.L. Stefanick, and R.S. Stafford. “National use of postmenopausal hormone therapy: annual trends and response to recent evidence.” JAMA. 2004; 291: 47-53. Institute of Medicine. Guidelines for Clinical Practice: From Development to Use. Washington, DC: National Academy Press, 1992. Jacoby S. Special Report: The 1999 AARP/Modern Maturity survey on sexual attitudes and behavior. Available at: http://www.aarpmagazine.org/lifestyle/relationships/great_sex.html. Accessed June 16, 2005. Verathon Inc. 800.331.2313 www.verathon.com v Urology for Primary Care Bibliography Kelly, M.P., D.S. Strassberg, and C.M. Turner. “Communication and associated relationship issues in female anorgasmia.” J Sex Marital Ther. 2004; 30: 263-276. Keshavarz, H., S.D. Hillis, B.A. Kieke, and P.A. Marchbanks. “Hysterectomy surveillance? United States, 1994-1999.” MMWR Surveill Summ. 2002; 51 (SS05): 1-8. Laumann, E.O., A. Paik, and R.C. Rosen. “Sexual dysfunction in the United States: prevalence and predictors.” JAMA. 1999; 281: 537-544. National Institutes of Health. “National Institutes of Health State-of-the-Science Conference statement: management of menopause-related symptoms.” Ann Intern Med. 2005; 142 (12 Pt1): 1003-1013. Nelson, H.D. “Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review.” JAMA. 2004; 291: 1610-1620. Nicolosi, A., E.O. Laumann, D.B. Glasser, E.D. Moreira Jr., A. Paik, and C. Gingell for the Global Study of Sexual Attitudes and Behaviors Investigators’Group. “Sexual behavior and sexual dysfunctions after age 40: the global study of sexual attitudes and behaviors.” Urology. 2004; 64: 991-997. Pauls, R.N., S.D. Kleeman, and M.M. Karram. “Female sexual dysfunction: principles of diagnosis and therapy.” Obstet Gynecol Survey. 2005; 60: 196-205. Sarrel, P.M. “Sexuality and menopause.” Obstet Gynecol. 1990; 75 (4 Suppl): 26S-30S. Segraves, R.T., A. Clayton, H. Croft, et al. “Bupropion sustained release for the treatment of hypoactive sexual desire disorder in premenopausal women.” J Clin Psychopharmacol. 2004; 24: 339-342. Suckling, J., A. Lethaby, and R. Kennedy. “Local oestrogen for vaginal atrophy in postmenopausal women.” Cochrane Database Syst Rev. 2003; (4): CD001500. World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems. Geneva, Switzerland: World Health Organization; 1992. Management of Uncomplicated Urinary Tract Infections Recent relevant publications by the author: Nickel, J.C. “Management of Urinary Tract Infections: Historical Perspective and Current Strategies. Part I: Before Antibiotics.” J Urol 173(1): 21-26, 2005. Nickel, J.C. “Management of Urinary Tract Infections: Historical Perspective and Current Strategies. Part II: Modern Management.” J Urol 173(1): 27-32, 2005. Verathon Inc. 800.331.2313 www.verathon.com vi Urology for Primary Care Bibliography Nickel, J.C. “Practical Management of Recurrent Urinary Tract Infections in Premenopausal Women.” Rev Urol. 7: 11-17, 2005. Nickel, J.C. “Amoxicillin/clavulanate for the treatment of uncomplicated cystitis in women: A historical perspective.” Nature Clinical Practice Urology. 2(8): 2-3, 2005. Nickel, J.C., J.C. Lee, J.E. Grantmyre, and D. Polygenis. “Natural history of urinary tract infection in a primary care environment in Canada.” Can J Urol 12(4): 1918, 2005. Nicolle, L., P.A.M. Anderson, J. Conly, T.C. Mainprize, J. Meuser, J.C. Nickel, V.M. Senikas, and G.G. Zhanel. “Uncomplicated Urinary Tract Infection: Current Practice and Impact of Antibiotic Resistance on Empiric Treatment.” Canadian Family Physician (in press, 2005). Other relevant publications: Foxman, B. and R.R. Frerichs. “Epidemiology of urinary tract infection: 1. Diaphragm use and sexual intercourse.” Am J Public Health 1985; 75(11): 1308-1313. Gupta, K., S.L. Hillier, T.M. Hooton, et al. “Effects of contraceptive method on vaginal microbial flora: A prospective evaluation.” J Inf Dis 2000; 181(2): 595-601. Gupta, K., T.M. Hooton, P.L. Roberts, and W.E. Stamm. “Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women.” Annals of Internal Medicine in young women. Ann Intern Med. 2001; 135(1): 9-16. Harrison, W.O., K.K. Holmes, M.E. Belding, et al. “A prospective evaluation of recurrent urinary tract infections in women.” Clinical Research 1974; 22:125A. Hooton, T.M., D. Scholes, J.P. Hughes, et al. “A prospective study of risk factors for symptomatic urinary tract infection in young women.” N Engl J Med 1996; 335(7): 468-474. Hooton, T.M. and W.E. Stamm. “Diagnosis and treatment of uncomplicated urinary tract infection.” Infect Dis Clin North Am. 1997; 11(3): 551-581. Mabeck, C.E. “Treatment of uncomplicated urinary tract infection in non-pregnant Women.” Postgraduate Med J. 1972; 48(556): 69-75. Nickel, J.C., J. Wilson, A. Morales, and J. Heaton. “Value of urological investigation in a targeted group of women with recurrent urinary tract infections.” Canadian J Surg 34: 591-594, 1992. Nicolle, L.E. and A.R. Ronald. “Recurrent urinary tract infection in adult women: Diagnosis and treatment.” Infect Dis Clin North Am 1987; 1(4): 793-806. Verathon Inc. 800.331.2313 www.verathon.com vii Urology for Primary Care Bibliography Pfau, A., T. Sacks, and D. Engelstein. “Recurrent urinary tract infections in pre-menopausal women: prophylaxis based on an understanding of the pathogenesis.” J Urol. 1983; 129(6):1153-1157. Reid, G. and J. Burton. “Use of Lactobacillus to prevent infection by pathogenic bacteria.” Microbes and Infec 2002; 4(3): 319-324. Rosenberg, M. “Pharmacoeconomics of treating uncomplicated urinary tract infections.” Int J Antimicrob Agents. 1999; 11(3-4): 247-251 discussion 261-264. Patton, J.P., D.B. Nash, and E.Abrutyn. “Urinary tract infections: economic considerations.” Med Clin North Am. 1991; 75(2): 495-513. Schaeffer, A.J. and B.A. Stuppy. “Efficacy and safety of self-start therapy in women with recurrent urinary tract infections.” J Urol. 1999; 161(1): 207-211. Schappert, S.M. “Ambulatory care visits to physician offices, hospital out patient departments, and emergency departments: United States, 1996.” Vital Health Stat. 1999; 13 (143): I-IV, 1-39. Stamm, W.E., G.W. Counts, and K.F. Wagner, et al. “Antimicrobial prophylaxis of recurrent urinary tract infections: A double-blind, placebo controlled control.” Ann Intern Med 1980; 92(6): 770-775. Stapleton, A, R.H. Latham, C. Johnson, and W.E. Stamm. “Post coital antimicrobial prophylaxis for recurrent urinary tract infection. A randomized double-blind placebo controlled trial.” JAMA. 1990; 264(6): 703-706. Uehling, D.T., W.J. Hopkins, E. Balish, et al. “Vaginal mucosal immunization for recurrent urinary tract infection: phase II clinical trial.” J Urol 1997;157: 2049-2052. Vosti, K.L. “Recurrent urinary tract infections: Prevention by prophylactic antibiotics after sexual intercourse.” JAMA. 1975; 231(9): 934-940. Walker, E.B., D.P. Barney, J.N. Mickelsen, et al. “Cranberry concentrate: UTI prophylaxis.” J Fam Pract 1997; 45:167-168. Warren, J.W., E. Abrutyn, J.R. Hebel, J.R. Johnson, A.J. Schaeffer, and W.E. Stamm. “Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women.” Infectious Diseases Society of America (IDSA). Clin Infect Dis 1999; 29: 45-58. Wong, E.S., M. McKevitt, K. Running, et al. “Management of recurrent urinary tract infections with patient-administered single-dose therapy.” Ann Intern Med 1985; 102(3): 302-307. Neonatal Circumcision: An American Enigma Verathon Inc. 800.331.2313 www.verathon.com viii Urology for Primary Care Bibliography American Academy of Pediatrics, Committee on Fetus and Newborn: Standards and Recommendations for Hospital Care of Newborn and Infants, ed 5. Evanston, Il., American Academy of Pediatrics, 1971. American Academy of Pediatrics, Committee on Fetus and Newborn: report of the ad hoc task force on circumcision. Pediatrics 56:610-611, 1975. American Academy of Pediatrics: Report of the task force on circumcision. Pediatrics 84:388391, 1989. American Academy of Pediatrics: Report of the task force on circumcision. Pediatrics 103:686693, 1999. Benini, F., C.C. Johnston, D. Faucher, et al. Topical anesthesia during circumcision in newborn infants. JAMA 270: 850-853, 1993. Blass, E.M. and L.B. Hoffmeyer. Sucrose as an analgesic for newborn infants. Pediatrics 87:215218, 1991. Christakis, D.A., E. Harvey, Dm Zerr, et al. A trade-off analysis of routine newborn circumcision. Pediatrics 105:246-249, 2000. Cook, L.S., L.A. Koutsky, and K.K. Holmes. Circumcision and sexually transmitted diseases. Am J Public Health 84:197-201, 1994. Dunsmuir, W.D. and E.M. Gordon. The history of circumcision. Br J Urol 83 (suppl): 1-12, 1999. Frisch, M., S. Friis, Sk Kjaer, et al. Falling incidence of penile cancer in an uncircumcised population. BMJ 311:1471, 1995. Gairdner, D. The fate of the foreskin. BMJ 2:200-203, 1949. Gee, W.F. and J.S. Ansell. Neonatal circumcision: A ten year review with comparison of the Gomco clamp and Plastibell device. Pediatrics 58: 824-827, 1976. Gunnar, M.R., R.O. Fischer, S. Korsvik, et al. The effects of circumcision on serum cortisol and behavior. Psychoneuroendocrinology. 6: 269-275, 1981. Kaplan, G.W. Circumcision: An overview. Curr Probl Pediatr 7: 1-33, 1983. Kaplan, H.W. Complications of circumcision. Urol Clin North Am 10:543-549, 1983. Lander, J., B. Brady-Fryer, J.B. Metcalfe, et al. Comparison of ring block, dorsal penile nerve block, and topical anesthesia for neonatal circumcision: A randomized clinical trial. JAMA 278: 2157-2162, 1997. Verathon Inc. 800.331.2313 www.verathon.com ix Urology for Primary Care Bibliography Laumann, E.O., C.M. Massi, and E.W. Zuckerman. Circumcision in the United States. JAMA 277: 1052- 1057, 1997. Larsen, G.L. and S.D. Williams. 85:808-812, 1990. Postneonatal circumcision: Population profile. Pediatrics Lerman, S.E. and J.C. Liao. Neonatal circumcision. Pediatr Clin North Am 48 (6): 1539-1557, 2001. Magoha, G.A. and R.F. Kaale. Epidemiological and clinical aspects of carcinoma of the penis at Kenyatta National Hospital. East Afr Med J 72:359-361, 1995. Moses, S., F.A. Plummer, J.E. Bradley, et al. The association between lack of male circumcision and risk for HIV infection: A review of the epidemiological data. Sex Transm Dis 21:201-210, 1994. Oster, J. Further fate of the foreskin: Incidence of Preputial adhesions, phimosis, and smegma amongst Danish schoolboys. Arch Dis Child 43: 200-203, 1968. Remondino, P.C. History of circumcision from earliest times to the present. Philadelphia, PA Davis, 1891. Schoen, E.J. The status of circumcision in newborns. N Engl J Med 322:1308-1312, 1990. Schoen, E.J., C.J. Colby, and G.T. Ray. Newborn circumcision decreases the incidence and costs of urinary tract infections in the first year of life. Pediatrics 105:789-793, 2000. Weiss, R., T. Tamminen-Mobius, O. Koskimies, et al. Characteristics at entry of children with sever primary vesicoureteral reflux recruited for a multicenter, international therapeutic trial comparing medical and surgical management. The International Reflux Study in Children. J Urol 148: 1644-1649, 1992. Williamson. P.S. and M.L. Williamson. Physiologic stress reduction by a local anesthetic during newborn circumcision. Pediatrics 71: 36-40, 1983. Wiswell, T.E., F.R. Smith, and J.W. Bass. Decreased incidence of urinary tract infections in circumcised male infants. Pedaitrics 75:901-903, 1983. Wiswell, T.E., H. Tencer, C.A. Welch, et al. Circumcision in childhood beyond the neonatal period. Pediatrics 92:791-793, 1993. Verathon Inc. 800.331.2313 www.verathon.com x 0900-0608-10-86
© Copyright 2026