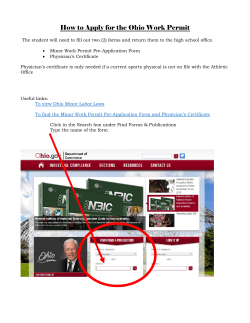
Patient Name
SPECIMEN INFORMATION
Ordering Physician Name
The Cancer Center
1234 Main Street
Dallas, TX 12345
(123) 456-7890
IC
AL
Primary Tumor Site: Ovary
Specimen Site: Ovary
Specimen Collected: XX/XX/2013
Specimen Received: XX/XX/2013
Initiation of Testing: 1/16/2013
Completion of Testing:
Specimen Id: XYZ-123
Case Number: TN13-111111
Date Of Birth: XX/XX/1960
Sex: Female
SE
.
Patient Name
ORDERED BY
U
PATIENT
Agents Associated with
Potential BENEFIT
FO
R
C
LI
N
Molecular Intelligence Ovarian Surface Epithelial Carcinoma
Comprehensive PLUS NGS Panel Summary
O
N
LY
.
docetaxel, paclitaxel
TM
ON NCCN COMPENDIUM
cisplatin, carboplatin
N
irinotecan, topotecan
Potential Targets
Associated
with CLINICAL TRIALS
T
Agents Associated With
Potential LACK OF BENEFIT
BRAF
KRAS
liposomal-doxorubicin
everolimus, temsirolimus
GNA11
leuprolide, megestrol acetate
nab-paclitaxel
Her2/Neu
capecitabine, pemetrexed
temozolomide, dacarbazine
PU
imatinib
cMET
R
PO
SE
S
O
gemcitabine
tamoxifen, fulvestrant,
letrozole, anastrozole
TM
sunitinib
E
OFF NCCN COMPENDIUM
MI-2013-01-26.0
doxorubicin, epirubicin
IV
vemurafenib
AT
fluorouracil
IL
LU
ST
R
trastuzumab
SA
M
PL
E
R
EP
O
R
T.
The selection of any, all or none of the matched agents resides solely with the discretion of the treating physician. Decisions on patient care and treatment must
be based on the independent medical judgment of the treating physician, taking into consideration all applicable information concerning the patient’s condition,
such as patient and family history, physical examinations, information from other diagnostic tests, and patient preferences, in accordance with the applicable
standard of care. Decisions regarding care and treatment should not be based on a single test such as this test or the information contained in this report.
** FINAL REPORT **
Patient: Patient Report
TN13-111111
Physician: Ordering Physician Name
4610 South 44th Place / Phoenix, AZ 85040 / (888) 979-8669 / Fax: (866) 479-4925 / CLIA 03D1019490 / Zoran Gatalica, M.D., DSc, Medical Director
Caris MPI, Inc. d/b/a Caris Life Sciences
Clinical History
SE
.
53 year old woman with ovarian carcinoma
IC
AL
U
Submitted Pathologic Diagnosis
Serous Carcinoma
LI
N
Specimens Received (Gross Description)
The specimens consist of:
FO
R
C
1 (A) Tissue Biopsy Formalin Vial - Client ID (XYZ-123).
Interpretation (Caris Life Sciences Microscopic Diagnosis):
N
O
T
Ovarian carcinoma
LY
.
Electronic Signature
PO
SE
S
O
N
By my electronic signature, I as the attending pathologist affirm that I have personally reviewed and examined microscopically the prepared slide(s)
and that the above diagnosis has been made or confirmed by me.
R
Disclaimer
IV
E
PU
All of the individual assays that are available through Caris Life Sciences® Molecular Intelligence™ Services (Caris Molecular Intelligence) were developed and validated by Caris
MPI, Inc. d/b/a Caris Life Sciences and their test performance characteristics were determined and validated by Caris Life Sciences pursuant to the Clinical Laboratory Improvements
Amendments and accompanying regulations ("CLIA"). Some of the assays that are part of Caris Molecular Intelligence have been cleared or approved by the U.S. Food and Drug
Administration (FDA). The clinical reference laboratory of Caris MPI, Inc. is certified under CLIA to perform high complexity testing, including all of the assays that are part of the
Caris Molecular Intelligence.
LU
ST
R
AT
The CLIA certification number of each Caris MPI, Inc. laboratory performing testing in connection with Caris Molecular Intelligence can be found at the bottom of each page. This
Report includes information about therapeutic agents that appear to be associated with clinical benefit based on NCCN Compendium guidelines, relevance of tumor lineage, level of
published evidence and strength of biomarker expression, as available, reviewed and assessed by Caris Life Sciences. The agents are not ranked in order of potential or predicted
efficacy. The finding of a biomarker expression does not necessarily indicate pharmacologic effectiveness or lack thereof. The agents identified may or may not be suitable for use
with a particular patient and the report does not guarantee or suggest that any particular agent will be effective with the treatment of any particular condition. Caris Life Sciences
expressly disclaims and makes no representation or warranty whatsoever relating, directly or indirectly, to this review of evidence or identified scientific literature, the conclusions
drawn from it or any of the information set forth in this Report that is derived from such review, including information and conclusions relating to therapeutic agents that are included
or omitted from this Report.
T.
IL
The decision to select any, all or none of the matched agents resides solely with the discretion of the treating physician. Decisions on patient care and treatment must be based on
the independent medical judgment of the treating physician, taking into consideration all applicable information concerning the patient’s condition, such as patient and family history,
physical examinations, information from other diagnostic tests, and patient preferences, in accordance with the applicable standard of care. Decisions regarding care and treatment
should not be based on a single test such as this test or the information contained in this report.
R
EP
O
R
The information presented in the Clinical Trials Connector™ section of the Report is compiled from sources believed to be reliable and current. We have used our best efforts to make
this information as accurate as possible. However, the accuracy and completeness of this information cannot be guaranteed. The contents are to be used for clinical trial guidance
and may not include all relevant trials. Current enrollment status for these trials is unknown. The clinical trials information present in the biomarker description was compiled from
www.clinicaltrials.gov. The contents are to be used only as a guide, and health care providers should employ clinical judgment in interpreting this information for individual patients.
Specific entrance criteria for each clinical trial should be reviewed as additional inclusion criteria may apply. Caris Life Sciences makes no promises or guarantees that a healthcare
provider, insurer or other third party payor, whether private or governmental, will provide reimbursement (instead of coverage) for any of the tests performed.
SA
M
PL
E
The next generation sequencing assay performed by Caris Life Sciences examines tumor tissue only and does not examine normal tissues such as tumor adjacent tissue or whole/
peripheral blood. As such, the origin of any mutation detected by our assay may either be a somatic (not inherited) or a germline mutation (inherited) and will not be distinguishable
by this assay. It is recommended that results be considered within the clinical context and history of the patient. If a germline inheritance pattern is suspected then counseling by
a board certified genetic counselor is recommended.
** FINAL REPORT **
Patient: Patient Report
TN13-111111
Physician: Ordering Physician Name
4610 South 44th Place / Phoenix, AZ 85040 / (888) 979-8669 / Fax: (866) 479-4925 / CLIA 03D1019490 / Zoran Gatalica, M.D., DSc, Medical Director
Caris MPI, Inc. d/b/a Caris Life Sciences
SE
.
Agents Associated with Potential BENEFIT
Data
Reference
Level*
Method
Result
Value
cisplatin,
carboplatin
ERCC1
IHC
Negative
2+ 20%
✔
gemcitabine
RRM1
IHC
Negative
1+ 30%
✔
Her2/Neu
CISH
Amplified
2.40
✔
PGP
IHC
Positive
1+ 30%
TOP2A
FISH
Not Amplified
1.33
ER
IHC
Negative
1+ 20%
PR
IHC
Positive
1+ 20%
✔
22, 23
TS
IHC
Negative
1+ 5%
✔
25, 26, 27
Her2/Neu
CISH
Amplified
2.40
✔
33, 34
Her2/Neu
IHC
3+ 20%
✔
35
Decreased
Potential
Benefit
Lack of
Potential
Benefit
LI
N
Potential
Benefit
IC
✝
AL
Test
1, 2
10
11, 12
13, 14
✔
11, 15,
16, 17
✔
22, 23, 24
S
O
N
LY
.
N
✔
E
IV
Positive
AT
trastuzumab
PU
R
fluorouracil,
capecitabine,
pemetrexed
PO
SE
leuprolide,
megestrol acetate
O
T
doxorubicin,
liposomaldoxorubicin,
epirubicin
FO
R
C
Agents
U
Clinical Association
= Greater level of evidence
LU
ST
R
*The level of evidence for all references is assigned according to the Literature Level of Evidence Framework consistent with the US Preventive
Services Task Force described further in the Appendix of this report. The data level of each biomarker-drug interaction is the average level
of evidence based on the body of evidence, overall clinical utility, competing biomarker interactions and tumor type from which the evidence
was gathered.
IL
= Intermediate level of evidence
R
T.
= Lower level of evidence
SA
M
PL
E
R
EP
O
✝ Refer to Appendix for detailed Result and Value information for each biomarker, including appropriate cutoffs, unit of measure, etc.
** FINAL REPORT **
Patient: Patient Report
TN13-111111
Physician: Ordering Physician Name
4610 South 44th Place / Phoenix, AZ 85040 / (888) 979-8669 / Fax: (866) 479-4925 / CLIA 03D1019490 / Zoran Gatalica, M.D., DSc, Medical Director
Caris MPI, Inc. d/b/a Caris Life Sciences
SE
.
Agents Associated with Potential LACK OF BENEFIT
Result
Value
PGP
IHC
Positive
1+ 30%
TUBB3
IHC
Positive
2+ 30%
irinotecan,
topotecan
TOPO1
IHC
Negative
2+ 20%
tamoxifen,
fulvestrant,
letrozole,
anastrozole
ER
IHC
Negative
1+ 20%
PIK3CA
Next
Gen SEQ
Presumed
Pathogenic
G1007R
PTEN
IHC
Positive
SPARC
Monoclonal
IHC
Negative
SPARC
Polyclonal
IHC
Negative
MGMT
IHC
c-KIT
Next
Gen SEQ
Decreased
Potential
Benefit
Lack of
Potential
Benefit
Data
Reference
Level*
LI
N
Potential
Benefit
C
✔
3, 4
✔
5, 6
✔
7, 8, 9
✔
18, 19,
20, 21
2+ 60%
✔
28, 29, 30
0+ 100%
✔
31, 32
1+ 20%
✔
31, 32
1+ 50%
✔
36, 37
Wild Type
✔
41, 42, 43
Next
Gen SEQ
Wild Type
✔
38, 39, 40
Next
Gen SEQ
Wild Type
✔
41, 42, 43
imatinib
IL
c-KIT
E
IV
LY
.
N
O
S
PO
SE
SA
M
PL
E
R
EP
O
R
T.
sunitinib
PU
R
LU
PDGFRA
Positive
AT
temozolomide,
dacarbazine
R
nab-paclitaxel
ST
everolimus,
temsirolimus
N
O
T
docetaxel, paclitaxel
✝
AL
Method
IC
Test
FO
R
Agents
U
Clinical Association
** FINAL REPORT **
Patient: Patient Report
TN13-111111
Physician: Ordering Physician Name
4610 South 44th Place / Phoenix, AZ 85040 / (888) 979-8669 / Fax: (866) 479-4925 / CLIA 03D1019490 / Zoran Gatalica, M.D., DSc, Medical Director
Caris MPI, Inc. d/b/a Caris Life Sciences
U
SE
.
Expanded Mutational Analysis by Next Generation Sequencing
Frequency (%)
Exon
APC
E1353X
59
16
Result
LI
N
Alteration
Pathogenic
C
Gene
IC
AL
Genes Tested With Alterations
FO
R
Interpretation: A nonsense variant was detected in this sample.
S
O
N
LY
.
N
O
T
APC or adenomatous polyposis coli is a key tumor suppressor gene that encodes for a large multi-domain protein. This protein exerts
its tumor suppressor function in the Wnt/b-catenin cascade mainly by controlling the degradation of b-catenin, the central activator of
transcription in the Wnt signaling pathway. The Wnt signaling pathway mediates important cellular functions including intercellular adhesion,
stabilization of the cytoskeleton, and cell cycle regulation and apoptosis, and it is important in embryonic development and oncogenesis.
Mutation in APC results in a truncated protein product with abnormal function, lacking the domains involved in b-catenin degradation.
Somatic mutation in the APC gene can be detected in the majority of colorectal tumors (80%) and it is an early event in colorectal
tumorigenesis. APC wild type patients have shown better disease control rate in the metastatic setting when treated with oxaliplatin, while
when treated with fluoropyrimidine regimens, APC wild type patients experience more hematological toxicities. APC mutation has also
been identified in oral squamous cell carcinoma, gastric cancer as well as hepatoblastoma and may contribute to cancer formation. Various
clinical trials (on www.clinicaltrials.gov) investigating agents which target this gene and/or its downstream or upstream effectors maybe
available, which include the following: NCT01351103.
BRAF
PU
R
PO
SE
Germline mutation in APC causes familial adenomatous polyposis, which is an autosomal dominant inherited disease that will inevitably
develop to colorectal cancer if left untreated. COX-2 inhibitors including celecoxib may reduce the recurrence of adenomas and incidence of
advanced adenomas in individuals with an increased risk of CRC. Turcot syndrome and Gardner's syndrome have also been associated with
germline APC defects. Germline mutations of the APC have also been associated with an increased risk of developing desmoid disease,
papillary thyroid carcinoma and hepatoblastoma.
G464E
55
33
Pathogenic
E
Interpretation: A pathogenic variant was detected in this sample.
LU
ST
R
AT
IV
BRAF encodes a protein belonging to the raf/mil family of serine/threonine protein kinases. This protein plays a role in regulating the
MAP kinase/ERK signaling pathway initiated by EGFR activation, which affects cell division, differentiation, and secretion. BRAF somatic
mutations have been found in melanoma (43%), thyroid (39%), biliary tree (14%), colon (12%), and ovarian tumors (12%). Patients with
mutated BRAF genes have a reduced likelihood of response to EGFR targeted monoclonal antibodies, such as cetuximab in colorectal
cancer. A BRAF enzyme inhibitor, vemurafenib, was approved by FDA to treat unresectable or metastatic melanoma patients harboring
BRAF V600E mutations. Various clinical trials (on www.clinicaltrials.gov) investigating agents which target this gene may be available, which
include the following: NCT01543698, NCT01352273, NCT01709292.
R
GNA11
T.
IL
BRAF inherited mutations are associated with Noonan/Cardio-Facio-Cutaneous (CFC) syndrome, syndromes associated with short stature,
distinct facial features, and potential heart/skeletal abnormalities.
Q209L
43
4
Pathogenic
EP
O
Interpretation: A pathogenic variant was detected in this sample.
SA
M
PL
E
R
GNA11 is a proto-oncogene that belongs to the Gq family of the G alpha family of G protein coupled receptors. Known downstream signaling
partners of GNA11 are phospholipase C beta and RhoA and activation of GNA11 induces MAPK activity. Over half of uveal melanoma
patients lacking a mutation in GNAQ exhibit somatic mutations in GNA11. Activating mutations of GNA11 have not been found in other
malignancies. Various clinical trials (on www.clinicaltrials.gov) investigating agents which target this gene may be available, which include
the following: NCT01587352, NCT01390818, NCT01143402.
** FINAL REPORT **
Patient: Patient Report
TN13-111111
Physician: Ordering Physician Name
4610 South 44th Place / Phoenix, AZ 85040 / (888) 979-8669 / Fax: (866) 479-4925 / CLIA 03D1019490 / Zoran Gatalica, M.D., DSc, Medical Director
Caris MPI, Inc. d/b/a Caris Life Sciences
Frequency (%)
Exon
Result
G12D
50
18
Pathogenic
SE
.
Alteration
KRAS
AL
Gene
U
Genes Tested With Alterations
IC
Interpretation: A pathogenic variant was detected in this sample.
FO
R
C
LI
N
KRAS or V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog encodes a signaling intermediate involved in many signaling cascades
including the EGFR pathway. KRAS somatic mutations have been found in pancreatic (57%), colon (35%), lung (16%), biliary tract (28%),
and endometrial (15%) cancers. Mutations at activating hotspots are associated with resistance to EGFR tyrosine kinase inhibitors (erlotinib,
gefitinib) and monoclonal antibodies (cetuximab, panitumumab). Various clinical trials (on www.clinicaltrials.gov) investigating agents which
target this gene may be available, which include the following: NCT01248247, NCT01229150.
Several germline mutations of KRAS (V14I, T58I, and D153V amino acid substitutions) are associated with Noonan syndrome.
25
2
T
G1007R
O
PIK3CA
Presumed Pathogenic
LY
.
N
Interpretation: A variant of unknown clinical significance was detected in this sample. However, this variant has been detected in more
than 5 cancer samples increasing the likelihood that this is a pathogenic mutation.
PO
SE
S
O
N
PIK3CA or phosphoinositide-3-kinase catalytic alpha polypeptide encodes a protein in the PI3 kinase pathway. This pathway is an active
target for drug development. PIK3CA somatic mutations have been found in breast (26%), endometrial (23%), urinary tract (19%), colon
(13%), and ovarian (11%) cancers. Somatic mosaic activating mutations in PIK3CA are said to cause CLOVES syndrome. PIK3CA mutations
have been associated with benefit from mTOR inhibitors (everolimus, temsirolimus). Evidence suggests that breast cancer patients with
activation of the PI3K pathway due to PTEN loss or PIK3CA mutation have a significantly shorter survival following trastuzumab treatment.
PIK3CA mutated colorectal cancer patients are less likely to respond to EGFR targeted monoclonal antibody therapy. Various clinical
trials (on www.clinicaltrials.gov) investigating agents which target this gene may be available, which include the following: NCT00877773,
NCT01277757, NCT01219699.
PU
ALK
CTNNB1
FGFR2
JAK2
NPM1
RET
VHL
R
AT
IV
E
AKT1
CSF1R
FGFR1
IDH1
NOTCH1
RB1
TP53
ATM
EGFR
FLT3
JAK3
NRAS
SMAD4
c-KIT
ERBB2
GNAS
KDR
PDGFRA
SMARCB1
CDH1
ERBB4
HNF1A
MLH1
PTEN
SMO
SA
M
PL
E
R
EP
O
R
T.
IL
LU
ST
ABL1
cMET
FBXW7
HRAS
MPL
PTPN11
STK11
R
Genes Tested Without Alterations
** FINAL REPORT **
Patient: Patient Report
TN13-111111
Physician: Ordering Physician Name
4610 South 44th Place / Phoenix, AZ 85040 / (888) 979-8669 / Fax: (866) 479-4925 / CLIA 03D1019490 / Zoran Gatalica, M.D., DSc, Medical Director
Caris MPI, Inc. d/b/a Caris Life Sciences
Title
Phase
Potential
Target
Drug(s)
NCT01543698
A Phase Ib/II Study of LGX818 in Combination
With MEK162 in Adult Patients With BRAF
Dependent Advanced Solid Tumors
1
BRAF , KRAS
MEK162
NCT01531361
Vemurafenib and Sorafenib in Advanced
Cancer
1
BRAF
sorafenib ,
vemurafenib
C
NCT01517399
Drug-drug Interaction Study of Tivantinib
(ARQ 197) With Omeprazole, S-warfarin,
Caffeine, Midazolam, and Digoxin in Cancer
Subjects
1
cMET
ARQ 197
TX
NCT01468922
Pazopanib and ARQ 197 for Advanced Solid
Tumors
1
cMET
NCT01450384
Pemetrexed Disodium and Sorafenib Tosylate
in Treating Patients With Advanced Solid
Tumors
1
BRAF
NCT01449058
A Phase Ib Study of MEK162 Plus BYL719 in
Adult Patients With Selected Advanced Solid
Tumors
1|2
NCT01392521
Phase Ib Study of PI3(Phosphoinositol 3)Kinase Inhibitor BAY80-6946 With MEK
(Mitogen-activated Protein Kinase) Inhibitor
BAY86-9766 in Patients With Advanced
Cancer
NCT01390818
Trial of MEK Inhibitor and PI3K/mTOR
Inhibitor in Subjects With Locally Advanced or
Metastatic Solid Tumors
NCT01376310
NCT01363232
IC
AL
U
Locations
LI
N
MA, TX
T
FO
R
TX
O
N
Protocol
SE
.
TM
Clinical Trials Connector
MD
sorafenib
VA
BRAF , KRAS
BYL719
MA, UT
1
BRAF , KRAS
Bay 86-9766
AZ, TX
1
GNA11
GSK1120212 Rollover Study
2
BRAF , KRAS
GSK1120212
Safety, Pharmacokinetics and
Pharmacodynamics of BKM120 Plus MEK162
in Selected Advanced Solid Tumor Patients
1
BRAF , KRAS
MEK162
MA, MI, NY, SC
MEK162 and RAF265 in Adult Patients With
Advanced Solid Tumors Harboring RAS or
BRAFV600E Mutations
1
KRAS
MEK162
FL, OR, UT
Safety, Pharmacokinetics and
Pharmacodynamics of BEZ235 Plus MEK162
in Selected Advanced Solid Tumor Patients
1
BRAF , KRAS
MEK162
MA, TX, WI
Sorafenib Tosylate and Stereotactic
Radiosurgery in Treating Patients With Brain
Metastases
1
BRAF
sorafenib
TN
MSC1936369B ,
SAR245409
MA, TN
(XL-765)
AZ, CO, MI, NH, PA, TN,
TX, WA
EP
O
R
T.
IL
LU
ST
R
AT
IV
E
PU
R
PO
SE
S
O
N
LY
.
ARQ 197
E
R
NCT01352273
SA
M
PL
NCT01337765
NCT01276210
** FINAL REPORT **
Patient: Patient Report
TN13-111111
Physician: Ordering Physician Name
4610 South 44th Place / Phoenix, AZ 85040 / (888) 979-8669 / Fax: (866) 479-4925 / CLIA 03D1019490 / Zoran Gatalica, M.D., DSc, Medical Director
Caris MPI, Inc. d/b/a Caris Life Sciences
To view the rest of the report, contact a
Caris Molecular Intelligence representative today.
(888) 979-8669
[email protected]
** FINAL REPORT **
Patient: Patient Report
TN13-111111
Physician: Ordering Physician Name
4610 South 44th Place / Phoenix, AZ 85040 / (888) 979-8669 / Fax: (866) 479-4925 / CLIA 03D1019490 / Zoran Gatalica, M.D., DSc, Medical Director
Caris MPI, Inc. d/b/a Caris Life Sciences
© Copyright 2026