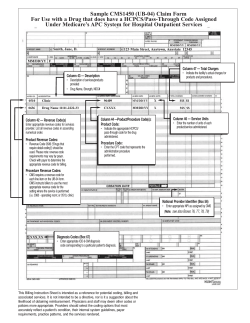
Key Republicans Question CMS’ $7 Billion Contract To Develop, Run Demos
InsideHealthPolicy.com’s Inside CMS exclusive news on the most powerful agency in health care Vol. 17, No. 41 - October 9, 2014 CMS Seeks Innovative Models It Can Test in Medicaid Managed Care CMS is seeking stakeholders’ advice on whether the agency should test Medicaid managed care reforms in the areas of pharmacy and medication management, value-based insurance design, telehealth, hospice care, long term services and supports, network design, behavioral health, and provider incentive arrangements like accountable care organizations in order to potentially reduce costs while improving quality of care. The agency’s innovation center says in a broader request for information issued Thursday (Oct. 2) that fiscal pressure and market developments have led health plans to increasingly come up with innovations in care delivery, plan design, beneficiary and provider incentives and network design. Although these innovations have proven successful, their adoption in the range of CMS stand-alone programs — such as Medicaid managed care, Medicare Advantage, Medicare Prescription Drug Plans — that use health plans as delivery systems has been limited, CMS says. “Testing models of these types of innovations will require collaboration with health plans, states, and other stakeholders,” CMS says. “These models will complement the Innovation Center’s existing portfolio of models that use innovative methods of payment to improve health care quality and reduce costs.” The RFI notes that Medicaid has continued on page 6 Key Republicans Question CMS’ $7 Billion Contract To Develop, Run Demos Key Republican lawmakers question CMS’ ability to oversee a $7 billion contract awarded by the innovation center to drive demonstrations on major delivery reforms across agency programs, raising their concerns in a letter to CMS Thursday (Oct. 2) as the innovation center was seeking stakeholders’ input on a wide array of potential innovations. The lawmakers want to know how CMS will make sure failed demonstrations aren’t repeated and that the Center for Medicare and Medicaid Innovation’s work won’t duplicate what’s done in other agencies. The letter to HHS Secretary Sylvia Burwell came as CMMI rolled out a broad “Health Plan Innovation Model Concepts” request for information laying out plans for continued on page 8 Brookings Scholars Suggest Evidence Development Policy For Specialty Rx CMS could require that makers of specialty drugs collect evidence on those products in return for reimbursement, a policy known as “coverage with evidence development,” Brookings Institution scholars said last week. CMS and commercial insurers rarely use the policy, and when they do, it’s usually for medical devices, but experts noted that the cost of analyzing data is dropping so it might be feasible for drugs. Coverage with evidence development allows provisional coverage of unproven, but promising products. CMS created the policy to get products to patients faster, but many argue it’s often used to slow access. Gregory Daniel, managing director for evidence continued on page 11 CMS, ONC Give Providers More Time To Apply For EHR Hardship Exemption CMS on Tuesday (Oct. 7) reopened the period to request hardship exemptions from so-called meaningful use requirements for electronic health records, giving some doctors and hospitals another opportunity to avoid penalties in 2015. The move follows stakeholders’ calls earlier this year for more time to submit hardship requests and lawmakers’ requests that some providers attesting to meaningful use for the first time in 2014 be allowed to avoid penalties in 2015. CMS told Inside Health Policy that there are still some issues surrounding availability and implementation of the 2014 certified EHRs, and the agency wanted to make sure that providers aren’t penalized because of those continued on page 12 Key Republicans Again Question Value Of CMS’ Fraud Prevention System A key group of Republican lawmakers is again questioning how well CMS’ Fraud Prevention System is working and wants the agency to lay out its vision for predictive analytics over the next few years. The lawmakers also ask CMS how other parts of the program integrity strategy fit together and how it intends to avoid duplication. “Given the administration’s repeated statements that FPS is the key to transitioning from the traditional claimsprocessing culture of ‘pay and chase’ to a claims-processing approach that is more forward-leaning, we write to you today to request additional information regarding the FPS,” the lawmakers say in an Oct. 2 letter to CMS Administrator Marilyn Tavenner. “We believe this information is critically important to helping inform Congress regarding the degree to which CMS may be achieving it’s goals.” Republican Senators Orrin Hatch (UT), Charles Grassley (IA) and Tom Coburn (OK), along with House Energy & Commerce Committee Chair Fred Upton (R-MI), question why the Fraud Prevention System had a return on investment of only a little more than 1 to 1 (1.34 to 1), according to savings from the program as laid out in the agency’s June report. Many of the other program integrity programs have higher ROIs, the lawmakers point out. They also note that HHS often says it gets back $5 or $7 for every dollar spent in traditional anti-fraud work. Hatch and Coburn have repeatedly expressed concerns about the program. Former CMS program integrity chief Peter Budetti previously told lawmakers that preventing fraud is not as clear cut as recovering fraudulent payments, and it’s harder to determine exactly how much money a program has saved by stopping claims or removing a provider or supplier from the program. The June report put the return on investment at about $5 for every $1 spent. The agency explained that the difference between the two savings estimates occurs because “[w]hile the identified savings amount represents the amount of fraud, waste and abuse the FPS identified, the adjustment factors apply a reduction to represent the inherent challenges, both in process and resource constraints, of successfully recovering payments and preventing fraudulent schemes from quickly migrating.” The lawmakers note that the Small Business Jobs Act of 2010 gave CMS $100 million to implement the FPS, and ask the agency to detail how much is left. It appears the agency has spent, or is about to spend, it’s initial $100 million, Hatch, Coburn, Grassley and Upton say, and they would like CMS to explain the burn rate and funding plan for the FPS moving forward. Among other questions, the lawmakers also want to know how successful CMS’ predictive models have been and the agency’s projections for how many models it plans to have up and running over the next five years. They ask if CMS has a multi-year strategic plan to help guide the FPS’ evolution. If it doesn’t, the lawmakers ask CMS to commit to developing such a plan within the next year. The lawmakers also ask why only a fraction of the providers and suppliers CMS took action against because of information from the FPS system were barred from future participation in Medicare. Plus, the lawmakers ask the agency to provide specific goals, evaluation plans and potential returns on investment from program integrity efforts as a whole over the next two years, and want to know how many people are charged with finding waste, fraud and abuse in the Medicare program. Hatch, Coburn, Grassley and Upton also note that CMS has more than a dozen separate programs or initiatives in place to combat waste, fraud and abuse, and ask if these programs overlap in scope and purpose — a long-held complaint from providers who say that multiple program integrity contractors sometimes seek to review the same claim. The Government Accountability Office has noted that there is some overlap among contractors and CMS should be more clear about how such situations are to be handled. The lawmakers ask how CMS’ myriad efforts are coordinated or interrelated, if they are at all. — Michelle M. Stein Hot Documents Now Available on InsideHealthPolicy.com The following new documents are available on InsideHealthPolicy.com, our new online health news service. Subscribers to InsideHealthPolicy.com also have access to hundreds of other health-related documents, daily news updates, and a searchable archive of back issues. CMS Seeks Input On Wide-Ranging Health Plan Innovation Ideas Grassley Urges CMS To Encourage Home Dialysis In ESRD Pay Rule Kidney Care Partners Comments On 2015 ESRD Pay Rule HHS Checklist Advises Health Community On Ebola Preparedness CMS Releases Home Health Conditions of Participation For Medicare, Medicaid CMS Reopens Submission Period For EHR Meaningful Use Hardship Exemption As Cancer Drug Costs Criticized, PhRMA Points To Drug Makers’ Investment In Clinical Trial Failures CMS Issues Guidance On Renewals For Catastrophic Coverage Not an online subscriber? Inside CMS subscribers can get a free, two-week trial to InsideHealthPolicy.com by calling 1-800-424-9068 or signing up at http://www.insidehealthpolicy.com/health_signup.asp. 2 INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 CMS Solicits Ideas On Ways To Test Care Coordination In Medigap CMS’ innovation center is seeking ideas from stakeholders on care coordination innovations that could be tested in Medigap and Retiree Supplemental health plans relating to case management and services that mange cost and improve the quality of health for beneficiaries. The move has some stakeholders worried that the agency may be trying to tun Medigap into an Medicare Advantage-like program. The call for ideas is part of a larger CMS request for information on developing innovative payment and delivery models across CMS programs including Medicare Advantage, Medicare Part D and Medicaid managed care. Medigap and Retiree Supplemental health plans are purchased or obtained by some beneficiaries in the traditional Medicare fee-for-service program to pay some of the costs — deductibles, coinsurance, and inpatient days beyond certain covered limits — that Medicare doesn’t cover. The Center for Medicare and Medicaid Innovation is concerned that Medigap and Retiree Supplemental Plans lack incentives to manage the cost and quality of care. The RFI notes that Medicare is the primary payer that “bears the lion’s share of Medicare beneficiary health costs,” and Medigap and supplemental plans don’t kick in until after fee-for-service benefits have been exhausted. “Thus, in contrast to other private health plans, some stakeholders have identified the generally limited incentives Medigap and Retiree Supplemental plans have to manage the cost and quality of care, despite the higher average total cost of care associated with their enrollees compared to beneficiaries with no supplemental coverage,” CMMI adds. CMMI asks insurers who provide Medigap or retiree Supplemental plans to describe what specific programs they already have in place for case management and care coordination and asks stakeholders in general how a test model could be designed. A health care lawyer and a beneficiary advocate — both speaking on background — question whether CMS is looking at ways to turn Medigap into something akin to Medicare Advantage. Both point out that this is not what Medigap and Retiree Supplemental plans were designed to be. Medigap and Retiree Supplemental plan coverage kicks in to cover cost sharing after fee-for-service Medicare — which is the primary payer — determines that coverage is medically necessary and paid benefits, the lawyer points out. Whereas Medicare Advantage replaces fee-for-service, with beneficiaries enrolling in insurance plans that are the primary payer for coverage. “If Medigap adopts practices to control cost and quality of care it acts more like like Medicare Advantage,” the lawyer said. In testing models for Medigap, which is regulated by states, the beneficiary advocate also says problems may arise between CMS and state regulators. In the RFI CMMI asks stakeholders what supports CMS could provide in coordinating the tests with state regulators. The lawyer said CMS could waive federal regulations on Medigap that “might prevent issuers in the ‘demo’ from coordinating care in the program, but would have a harder time with state regulators, who if they weren’t on board with the goals and methods of the test model could simply block it from taking place in their specific states.” CMMI is giving stakeholders until Nov. 3 to respond to the RFI. — Todd Allen Wilson Economist: MA Needs To Move To Exchange-Like Competitive Pricing Economist and health policy expert Paul Ginsburg said Tuesday (Oct. 7) that given the growth of Medicare Advantage to 30 percent of Medicare beneficiaries — with a tripling of enrollment in the program since 2005 — it is time to move MA from an administered pricing model to the market pricing approach used by the ACA’s insurance exchanges. Ginsburg, pointing to the current low spending growth rate in Medicare and successes with market pricing in Medicare Part D and the exchanges, said now would be an opportune time to move MA to a competitive pricing model. “Given how mainstream Medicare Advantage is, it’s time to bring policy in line with that space,” Ginsburg told attendees of the California Association of Physician Groups colloquium on physicians groups in Medicaid Advantage held in Washington. “There’s a need for a departure from administered pricing. It’s increasingly hard to do administered pricing well.” Ginsburg said the problem is that administered pricing in MA works much like policies from the late 18th century that created tariff protections for American manufacturers designed to help the “infant industries” compete with European manufacturers. This created an organized interest to maintain the tariffs once they were no longer necessary. He said MA worked well early on in areas where traditional Medicare spending was high, such as in Southern California and Florida, with generous benefits benchmarks with no premiums. Lawmakers from other parts of the country wanted to extend those same benefits to their constituents, which led to even more benefit benchmarks in the program, Ginsburg said. Ginsburg contended that under this system MA insurance plans compete by “maximizing benefits rather than by INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 3 reducing premiums and increasing quality.” It is time to move away from administered pricing because the MA program is no longer an “infant industry,” he said. Ginsburg said the best model is one based on the ACA health exchanges where plans are offered in metal tiers with government subsidies based on the second lowest silver tier plan, and where benefits are standardized on actuarial value and include geographic variation. In MA the silver plan would reflect the actuarial values of traditional Medicare. MA plans could then bid premiums for current traditional Medicare benefits, and then offer richer or lesser plans with different premiums, Ginsburg said. He added that these plans may need to be reviewed to make sure premium differentials align with the benefits being offered. He said under this proposal risk adjustment issues would have to be addressed, because inadequate risk adjustment could make traditional Medicare artificially less competitive. Ginsburg cited a June Medicare Payment Advisory Commission risk adjustment model recommendation using a beneficiary’s base year costs as a model and including a number of reinsurance mechanisms as a promising starting point. An alternative — what Ginsburg calls the “second best solution” — comes from a 2013 Bipartisan Policy Center proposal that Ginsburg worked on. In this proposal MA plans would submit two bids — one under the current administered pricing system and one under a competitive system using a standard benefits package. In the event that two plans in a region submit bids that are lower than the current system, then a competitive system would take affect in that region, he said. Ginsburg said the benchmark in this system could be an average of bids or percentiles. Ginsburg is not advocating doing away with traditional Medicare. In both proposals traditional Medicare would serve as the basis for the regulatory sphere and as competition for MA plans to reduce costs and increase quality. This would create a dynamic where if plan providers in an area boost the MA rates substantially, then beneficiaries would move back to traditional Medicare because MA plans would no longer be competitive. “The presence of traditional Medicare makes a big difference,” Ginsburg said. “For me, not having traditional Medicare is complete non-starter for this.” — Todd Allen Wilson Advocates To MCOs: Closely Watch How States Implement Medicaid HCBS HHS officials and advocates for people with disabilities are advising Medicaid managed care organizations (MCOs) to pay close attention to how states implement a new home and community-based services rule issued by CMS earlier this year. One advocate warned insurers last week that states’ failure to fully live up to the new HCBS rules could make MCOs the target of lawsuits. States have until March 17 to submit transition plans for moving Medicaid beneficiaries with disabilities out of institutional care and into home and community-based care models by March 2019 under a rule released by CMS in January. Concerns over how states will implement the rule came up during an America’s Health Insurance Plans conference on Medicaid last Thursday (Oct. 2). Patricia Nobbie, program specialist in the Office of Special Programs in the Center for Disability and Aging Policy at the HHS Administration for Community Living, said the HCBS rules are designed to make sure people with disabilities have the same access to their community that people without disabilities enjoy. In order to receive Medicaid HCBS funding, patients will not be allowed to be housed in large institutions such as hospitals, treatment centers and nursing homes, or smaller settings that essentially operate in the same manner as institutionalized care. This means, Nobbie said, clusters of group homes that serve only people with disabilities, farmsteads, residential schools and gated communities or settings that use restraints or isolation to control beneficiary behavior will be deemed by CMS to be institutional settings. Beneficiaries would also need to be able to receive their services outside of their residential setting and be able to seek jobs in competitive, integrated settings as opposed to sheltered workshops, enclaves or mobile work crews designed only for people with disabilities. Beneficiaries with disabilities will also have full tenant rights in their residences — and even in provider-owned homes and apartments — under the new HCBS rules. The rules are designed to make HCBS “person centered” and extend “supports and services beyond medically acute needs,” Nobbie said. She advised Medicaid managed care MCOs to take part in the public comment period following states’ submissions of their transition plans to CMS, as well as attending town hall meetings and talking with beneficiaries with disabilities and their support networks to make sure that contracts they enter into with states truly meet the needs of people receiving HCBS. “I encourage you to stay in touch with the public in your different states where you are trying to provide services,” Nobbie said. “Listen to what they have to say, because people who are using services are your best source of information. They are actually the experts.” Ari Ne’eman, president and co-founder of the Autistic Self Advocacy network and who also has autism, and Bruce 4 INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 Darling, president and CEO of the Center for Disability Rights, echoed Nobbie, saying the new rules come from decades of work by the civil rights movement for people with disabilities. Darling said that states and MCOs, when planning changes to state Medicaid programs to meet the new standards, should avoid just trying to meet minimum compliance with the rules. “We want you to think about this in the context of civil rights,” Darling said. “When you’re looking at these minimum compliance standards and folks are trying to figure out how they can wiggle through to make this work — if it feels hinkey, it’s probably wrong.” He warned that MCOs that sign contracts with states whose programs don’t live up to the full spirit of the new HBCS rules could be sued by beneficiaries who are pushing to live fully integrated lives. “Given the vagueness in these rules and the way they evolve there will be an effort to determine who has the deepest and broadest pocket,” Darling said. — Todd Allen Wilson CMS Gauging Interest In Adding Hospice Benefits to Medicare Advantage CMS is considering allowing Medicare Advantage plans to take responsibility for hospice care — which has traditionally been carved out of managed Medicare — and letting the plans provide both hospice and other MA benefits at the same time. A hospice trade group official said it’s unclear if MA plans would be required to meet the same hospice standards required by fee-for-service Medicare and also questioned exactly how hospice services would be financed under MA. The agency’s innovation center floated the idea as part of a broader request for information from stakeholders on myriad innovations that potentially could be instituted across CMS programs. CMS hopes to gauge the level of interest in ways to give MA plans the option to offer hospice, or palliative care benefits, alongside curative care to enrollees. At the moment, hospice is carved out of MA and beneficiaries who elect hospice have other care needs covered by FFS. Beneficiaries also must forgo curative care to access palliative care services offered by hospices. Congress’ Medicare payment advisers earlier this year recommended that Congress include the Medicare hospice benefit in the MA package in 2016. The agency also announced in March that it would test allowing beneficiaries to use both a limited hospice benefit and other Medicare services under fee-for-service Medicare. CMS said the feefor-service model will look at whether beneficiaries would use hospice earlier in their disease if they were also allowed to keep seeking curative treatments as well. Theresa Forster, vice president for hospice policy and programs at the National Association for Home Care and Hospice, said stakeholders expect the agency to announce the participants in the fee-for-service demonstration in the near future. CMS asks stakeholders what factors the agency should consider if it moves forward with a demonstration to integrate hospice with curative care in MA. Stakeholders should weigh in on quality and outcomes metrics, beneficiary protections, as well as any other important design factors, CMS says. The agency also asks how much time stakeholders would need to prepare a bid for the June bid cycle. Forster said it’s unclear if higher bid rates would be included or allowed under the demonstration, or if the same hospice treatments would be expected to be provided with less financing. A lot of hospices get very costly patients who need intense care near the end of their life, she said. It is also unclear how plans would be expected to offer supportive services that hospices normally offer but aren’t always reimbursed by CMS, Forster said. Stakeholders will also be looking at whether hospices added into the MA plans would need to meet the same standards as those in fee-for-service, Forster said. Forster did note that there would be less concerns about payer responsibility with drugs if MA plans were responsible for drugs under both hospice and Part D plans. But approval could be a concern, she said. Allowing for curative and palliative care could appeal to people who want both options, Forster said, and could encourage people to enter hospice. One beneficiary advocate said offering access to both types of care could be a positive development for beneficiaries, though the advocate said the approach could also provide incentives for beneficiaries to move to MA if fee-for-service doesn’t ultimately provide the same options. Another beneficiary advocate, however, said there is a fundamental tension between the two types of care, and there is a mismatch with providing both types of care at the same time. Forster said there could be times when there is tension between palliative care and curative care, and beneficiaries would need a dedicated care team to work through those tensions. The first advocate said there are concerns around putting plans in the driver’s seat, and the demonstration would need to make sure doctors and prescribers are making the decisions about what care a beneficiary needs. — Michelle M. Stein INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 5 MHPA: RFI A Chance For Plans To Highlight Success . . . begins on page one a long history of using managed care for large segments of beneficiaries in the state and federal health care program for low-income families and individuals. Nearly three-quarters of beneficiaries are in a managed care program, with 51 percent enrolled in comprehensive full-risk plans, and many states that have expanded Medicaid under the Affordable Care Act have chosen to use managed care as the primary delivery system, CMS notes. In the section of the RFI dealing with Medicaid managed care CMS asks stakeholders to describe successful models states are using to implement incentives for plans in promoting value-based purchasing, such as withholds and other financial incentives in their contracts with managed care plans. The National Association of Medicaid Directors has been pressing CMS to give states flexibility to customize innovation models in their contracts with managed care plans, as the agency works for the first time since 2002 to update its Medicaid managed care regulations. States often use withholding practices, such as not enrolling beneficiaries in plans or removing beneficiaries from plans, in order to ensure contract compliance on the part of Medicaid managed care plans. The RFI also asks stakeholders to describe successful state efforts to promote alignment of provider incentives and quality metrics across managed care plans. Medicaid Health Plans of America director of federal policy Amy Ingham said the RFI is a positive opportunity for managed care plans to highlight work they are doing to improve quality of care while controlling costs and innovative programs they have developed. “Some of the unique programs the health plans have in individual markets they serve in, or across states — some of the plans that serve in multiple markets or multiple states, have in place to serve the population enrolled are remarkable,” Ingham said. “This is an opportunity for us to highlight to CMS what some of those programs look like and what some of the successes have already been.” She noted that Medicaid managed care plans already work with the CMS’ innovation center on a number of test models, including the dual-eligibles demonstration. Ingham said it will also give Medicaid managed care plans the chance to let CMS know what particular areas should be focused on in innovation model tests. Ingham said she thinks one of the areas plans would like to focus on is integrating behavioral health and physical health, though she can’t speak for member plans and how they’ll individually respond. “That’s been a huge focus for our health plans for a long time,” she said. “There’s a pretty broad understanding that physical health impacts mental health, mental health effects physical health. There needs to be a more integrated approach.” The National Governors Association recently announced that it is starting a year-long initiative in Alabama, Nevada and Washington state to look at how to make broader, system-wide applications for innovations happening in those states’ Medicaid programs. Washington state and Nevada are looking at models for integrating behavioral and physical health delivery. The agency has given interested parties until Nov. 3 to submit comments and ideas on the RFI. — Todd Allen Wilson CMS Eyes Adding Flexibility To Medication Therapy Management Program CMS’ Center for Medicare and Medicaid Innovation is weighing whether adding more flexibility to the medication therapy management program would help Part D plans cut costs and improve medication adherence as the agency looks to design demonstrations across Medicare and Medicaid, including under Medicare Advantage and Part D. Some stakeholders say added flexibility on which patients would be eligible for MTM could help target those who need the service the most, though one beneficiary advocate wonders how CMS plans to innovate around a program that up to this point has not been very successful. CMS, as part of a “Health Plan Innovation Model Concepts” Request for Information issued Thursday (Oct. 2), seeks input on a variety of potential demonstrations around Medicare Advantage, Medigap plans and Part D plans. “Health plans increasingly responded to market developments and fiscal pressures with innovations in care delivery, plan design, beneficiary and provider incentives, and network design,” CMMI says. “Though evidence suggests that these innovations may reduce cost, improve quality, and enhance beneficiary satisfaction, adoption of some of these innovations has been limited” in Part D, MA, MA-PD, Medicaid managed care plans and Medigap plans, the RFI says. CMMI says that while the Part D program has successfully kept premiums stable, and some plans have shown success in increasing medication adherence, the agency wants to know if more flexibility would further control costs and improve quality. Research into private sector drug plans shows that successfully limiting costs and improving outcomes comes through evidence-based benefit design, drug comparative effectiveness research and financial incentives, CMMI says, and the agency is considering testing some new models in Part D. “These programs could involve strategies that vary the intensity and delivery of MTM services based on beneficiary risk level, and could provide reinforcing financial incentives, including beneficiary/provider incentives and performance- 6 INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 based payment to stand-alone PDPs, based on total cost of care,” the RFI says. CMMI asks plans to explain whether they offer MTM beyond what is required by CMS and whether they use services beyond program requirements. The agency currently requires that beneficiaries spend more than $3,000 on medications per year and have two or more chronic diseases to be eligible for MTM services. Earlier this year, CMS proposed to expand the MTM program to more beneficiaries as part of a controversial Part D rule, but the agency ultimately did not move forward with the expansion after some complained it would hurt plans’ profits. CMMI asks stakeholders to explain the challenges within the current program or benefit structure guidelines, and also asks if they would recommend greater flexibility for MTM targeting or service requirements to improve outcomes. The agency also asks how models that combine MTM and financial incentives should be designed, and says stakeholders should explain how proposed models would lead to savings and preserve or improve quality. The RFI asks stakeholders whether there are any other factors that CMS needs to take into account when developing a model that combines MTM risk strategies, risk stratification, differential cost sharing, and financial incentives, as well. The agency asks plans if they would be interested in participating in such a demonstration, and if MA plans should be included in such models. Joel White, president of the Council for Affordable Health Coverage and who works with the medication-adherence coalition Prescriptions for Healthy America, says added flexibility around which beneficiaries should receive MTM would be very helpful, as success for such a program would ideally mean finding those who are having trouble with medication adherence and helping them head off potential complications. The current criteria to target beneficiaries are outdated, White says. The limitations on the program were originally put in place to help contain the benefit and make sure it targeted those who didn’t have their medications under control. But based what is known now, cost may not be the best way to target that population, he says. Some beneficiaries may spend a lot on drugs, but they have their chronic conditions under control, while others may not spend as much but could be heading toward a potentially preventable event, White says. CMS’ MTM expansion proposal in the Part D rule earlier this year signaled that the MTM effort had previously been getting more prescriptive over time, rather than more flexible, White says. The National Association of Chain Drug Stores says it is pleased the RFI includes approaches to make MTM accessible to more beneficiaries, as one of NACDS’ priorities includes passage of a bill to allow for a patient with one chronic condition to receive MTM services. In a previous response to an RFI from CMS on beneficiary engagement and incentives, NACDS told the agency it should consider a copay waiver program for Medicare and Medicaid beneficiaries who successfully participate in an MTM program. Because such a program would improve adherence, it would generate savings overall, NACDS says. White also says that high costs are a barrier to adherence, and financial incentives for beneficiaries could be one way to try to address that barrier. One beneficiary advocate, however, questions why CMMI is taking a program with questionable success into a demonstration that includes financial incentives. An Avalere analysis released this summer found that only half of enrollees eligible for MTM received those benefits, though beneficiaries in MA plans were more likely to receive a comprehensive medical review. The beneficiary advocate says flexibility in targeting could help in getting more beneficiaries who could be helped by the program involved, and changes to the program could help it be more successful. But until the program is more appropriately delivered, the beneficiary advocate questions whether CMMI has an adequate base on which to innovate. — Michelle M. Stein Industry Questions Grassley’s Request To Hike Pay For Home Dialysis Training Sen. Charles Grassley (R-IA) is urging CMS to pay significantly more for home-dialysis training in the End Stage Renal Disease final rule expected to be issued within the next two weeks, but dialysis companies worry that an increase in training pay would be at the expense of payment for services provided at facilities. “Can CMS adopt a reimbursement policy that covers the actual cost of HHD training that remains within the overall reduced ESRD budget as directed by Congress earlier this year? Such a solution would not require adding money to the ESRD budget and would ensure access and good outcomes for patients,” Grassley writes in a Friday (Oct. 3) letter to CMS Administrator Marilyn Tavenner. Kidney Care Partners, which represents industry and patients, only supports an increase to home dialysis training if it’s paid for with an overall increase to the dialysis program. “KCP does not support increasing the payment amount for the training add-on, unless CMS adds new money to the system,” the group states in an Aug. 29 letter to CMS. “Any increases should not be made in a way that removes funds from the current bundled payment amount.” Grassley writes that CMS acknowledges that $50.16 per-day reimbursement for training patients on home dialysis is INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 7 insufficient. Research indicates that Medicare reimbursement covers 20 percent of the cost of training. Those studies are being updated, but Grassley says CMS should not wait for the updated results because they aren’t expected to alter the basic conclusion. Nephrologists estimate that 10 percent to 15 percent of dialysis patients could handle home dialysis, but less than 2 percent currently use the service, and less than 25 percent of dialysis facilities offer it. Although it would cost more for nurses to train patients on home dialysis, Grassley says home dialysis is cheaper overall and leads to better outcomes. “Home hemodialysis not only offers patients treatment flexibility, better clinical outcomes, and a better quality of life, but is also cost-effective,” Grassley writes. — John Wilkerson CMS Beefs Up Efforts To Compare Nursing Homes, Home Health Performance CMS on Monday (Oct. 6) unveiled updated policies to help people choose nursing homes and an agency official indicated that new measures for scoring nursing home performance will be added in the coming months. The agency also proposed conditions of participation for home health providers that are aimed at assessing performance. The changes were timed with President Obama’s signing of the Improving Medicare Post-Acute Care Transformation Act. That law gives CMS money to collect payroll data from nursing homes, and the agency will use the information to determine facility staff levels, staff mix and turnover. Nursing homes are judged in part on staffing levels, but facilities self report so many people don’t trust the information. CMS will begin verifying those reports with payroll data next year, and by 2016 CMS will report that data on its Nursing Home Compare website, an agency official said. CMS is reviewing the scores that nursing homes receive from the five-star program. If too many are achieving high scores, the agency will revamp the quality measures to make them more difficult to meet, which in turn would differentiate facilities and make it easier for consumers to compare them, the official said. CMS expects to change at least some quality measures in the coming months. CMS also proposed a regulation to change the conditions that home health organizations must meet to participate in Medicare. “Conditions of participation” are the minimum health and safety standards that home health organizations must meet to qualify for Medicare reimbursement.—John Wilkerson Republicans Want Accounting Of CMMI Contract . . . begins on page one innovations and demonstrations across Medicare and Medicaid. “The scope of the Innovation Center’s mandate, funding, and its breadth of operations raises serious concerns about the potential lack of transparency and accountability for CMMI,” the letter says. “[T]here’s a substantive concern that — without effective management and oversight — CMMI projects could be wasteful, duplicative, or ineffective, while costing taxpayers billions of dollars.” The ACA gave CMMI $10 billion for fiscal 2011-2019 plus $10 billion for following 10-year periods for selection, testing, and evaluation of new payment and delivery system models. At least $25 million needs to be available every year for the testing and evaluating new projects. But Senate Finance top Republican Orrin Hatch (UT), House Energy & Commerce Chair Fred Upton (R-MI), and Ways & Means Chair Dave Camp (R-MI) point out that CMMI’s projects are not required to achieve savings right away and the ACA did not require external review or a third-party evaluation of the quality of the projects CMS funds, and they say this could lead to problems. The letter is not the first time Republicans have brought up concerns with the innovation center. Previously lawmakers indicated they wanted more control over setting the center’s priorities. Some lawmakers also considered cutting CMMI as an option to help pay for previous patches to the flawed Medicare physician payment system. The lawmakers are particularly concerned with the Research, Measurement, Assessment, Design and Analysis (RMADA) contracts CMS signed recently with 15 companies. The contracts are for companies to design, build, run and implement and evaluate a range of research, payment, or delivery system reform models to test if they can cut costs for Medicare, Medicaid and CHIP, according to FedBizOpps.gov. “Unfortunately, CMS’ contract oversight lapses and management errors in recent years raise concerns about CMS’ ability to effectively oversee multiple contracts of this complexity and scope,” the lawmakers say. They are asking CMS to explain exactly how the up to $7 billion contract will be paid for, and whether the money will all come from CMMI’s $10 billion appropriation or from other funds. If it will all come from the $10 billion ACA appropriation, the lawmakers ask CMS to detail how much CMMI will have left over. The lawmakers also want CMS to provide a description of what CMMI has planned for the 15 organizations that were granted the RMADA contracts, and how much each task is projected to cost over the next year. They also ask how CMS will keep the new contractors from repeating previously failed CMS demos. Hatch, Upton and Camp also ask if the organizations that retained the RMADA contracts will work with other parts 8 INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 of CMS beyond CMMI. The Government Accountability Office in 2012 put out a report that found some duplication among CMMI models and other CMS offices after three GOP Senate Finance members asked GAO to look into CMMI. The lawmakers ask CMS if the agency has since taken any efforts to make sure the work under the RMADA contract doesn’t duplicate or overlap any of CMS’ other existing projects. “The statute specifically prohibits administrative or judicial review of the models selected, the model design or the and details, or even determinations regarding budget neutrality. Accordingly, what processes or policies has CMS instituted to ensure that $7 billion allocated is not wasteful, duplicative, or ineffective?” Hatch, Upton and Camp ask. The lawmakers ask Burwell to respond by the end of the month. — Michelle M. Stein Minnesota’s Basic Health Program Talks ‘On Track’ The top health official in Minnesota tells Inside Health Policy that the state and CMS are making good progress as they negotiate the terms of what will likely become the ACA’s first Basic Health Program (BHP), under which states can cover lower-income residents using 95 percent of funding that would otherwise be used for tax credits and cost-sharing reduction payments. The state is seeking flexibility to initially offer one managed care plan instead of the law’s required two, apply a minimum value test, and get around the required 30-day grace period for premium payments — as other states interested in pursuing a BHP in the future watch closely. Minnesota, the lone state to pursue the program for 2015, sent in a proposed blueprint earlier this year and Department of Health Commissioner Lucinda Jesson tells IHP the goal is to submit a final blueprint this month and gain CMS approval by Nov. 1. “Things are very much on track,” she said, adding that the state is planning for a Jan. 1, 2015 live date. The BHP — which covers people earning from 134 percent of the federal poverty level (just above the Medicaid threshold) to 200 FPL or lawfully present non-citizens earning up to 200 percent FPL — was envisioned to help address the lower-income people who often “churn” from public to private coverage. A handful of states had initially expressed interest in pursing the option. But, due to various delays and federal policy decisions, Minnesota is the only state pursuing the program for the 2015 plan year, although New York has passed legislation and is looking to have a BHP in place by 2016. While Minnesota has taken the lead on the BHP, other states are interested in pursuing the program in the future. Several states, including Arkansas, Vermont New Mexico, Idaho, and Massachusetts, have looked into the program. California’s state-based exchange explored the option early on, but it was rejected by the exchange board. Sources suggest that several factors chilled states’ momentum on the BHP. First, the federal government delayed issuing guidance, and ultimately delayed implementation of the program from 2014 to 2015. When CMS did issue final regulations in March of this year, the government stuck with its decision preventing states from using any BHP funds for administering the program. Sources suggest that while the inability to use BHP funding for operations may have played a role in state decision-making, the delay in guidance was a larger factor. States also had concerns that the program would impact their exchange risk pools, potentially increasing exchange premiums by taking younger from the pool. But an Urban Institute report from 2011 suggests that states could mitigate the impact by applying risk adjustment to both pools, and other sources also say those worries could be worked through. Minnesota has actually been covering the population that would be served by the BHP, yet mostly with state dollars, for quite some time under its MinnesotaCare program, Jesson notes. Therefore, she says, the state saw the advent of exchange coverage as a step forward in one sense, but also a step back for this particular population which had access to more comprehensive benefits and lower premiums than is available in many exchange plans. The BHP allows the state to continue providing the coverage to the MinnesotaCare population, yet with federal dollars. There are currently about SUBSCRIPTIONS: 703-416-8500 or 800-424-9068 [email protected] NEWS OFFICE: 703-416-8577 Fax: 703-416-8543 [email protected] Health Group Publisher: Chief Editor: Managing Editor: Associate Editor: Contributing Editors: Donna Haseley ([email protected]) John Wilkerson ([email protected]) Michelle M. Stein ([email protected]) Todd Allen Wilson ([email protected]) Amy Lotven ([email protected]) Rachana Dixit Pradhan([email protected]) Production Manager: Production Specialists: Lori Nicholson Daniel Arrieta, Michelle Moodhe Inside CMS is published every Thursday by Inside Washington Publishers, P.O. Box 7167, Ben Franklin Station, Washington, DC 20044. Subscription rates: $705 per year in U.S. and Canada; $755 per year elsewhere (air mail). © Inside Washington Publishers, 2014. All rights reserved. Contents of Inside CMS are protected by U.S. copyright laws. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or translated into any language in any form or by any means, electronic or mechanical, without written permission of Inside Washington Publishers. INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 9 70,000 people on MinnesotaCare, Jesson says, and that number is increasing every day. The program is only for adults because children in that income range are all on Medicaid. Under Minnesota’s plan, beneficiaries would pay from $0 to $50 for their coverage depending on family size and income. Residents under age 21, American Indians and people earning under 55 percent of the federal poverty level would pay no premiums. Members of the military who completed active duty within two years and their family members are also exempt from premiums for a year. Jesson says that the new MinnesotaCare plans will offer comprehensive coverage, including strong mental health benefits. She was also pleased that the program will allow MinnesotaCare to lift its previous $10,000 cap on hospital coverage. Another benefit is that the population will not be receiving subsidies and thus will not have to deal with the tax reconciliation issues, she said. Minnesota will also allow people to enroll in the BHP throughout the year, like in Medicaid. Jesson also says that a key to being able to implement a BHP is having the proper IT systems in place. One of the benefits of the state’s decision to establish its own exchange was that the IT infrastructure could handle the existing MinnesotaCare population, she says. It’s not that Minnesota has been free of IT challenges, but it is much easier if the systems are able to communicate, she says. The blueprint indicates there are still several functionalities on which the state still needs to work, including coordinating plan enrollment when consumers move from MinnesotaCare to a qualified health plan, processing changes in circumstances, and handling the renewal process. The states has contingency plans in place for functions that are not ready to go live by the deadline. The state is also asking for some exemptions from the rule for the first, and transitional, plan year. While the law requires the program to offer participants a choice of two managed care plans, the state is asking for a waiver in year one because 15 counties will only have one plan available. The remaining 72 counties will have at least two plans. The state intends to offer its plans through five licensed health maintenance organizations (HMOs) and three non-licensed HMOs participating in CHIP/Medicaid. The state is also asking CMS for an exemption that would allow it to apply a “minimum value test” as well as an affordability test for BHP applicants who have access to employer-sponsored coverage. This means that someone who has coverage that may be “affordable” (meaning that out-of-pocket spending is no more than 9.5 percent of income), yet does not have an actuarial value of at least 60 percent, would still have access to the program. HHS’ final BHP regulation had said an applicant is only eligible if the employer’s offer of coverage is deemed unaffordable, but the minimum value test did not apply. The state says that applying both the minimal value and the affordability test to employer-sponsored coverage is the “only workable” reading of the section. If Minnesota were to implement the BHP rule as interpreted without the minimum value test, applicants with income below 200 percent of the federal poverty level who have access to employer-sponsored coverage that does not meet the standard “would very well be barred from the program that was specifically designed to aid them,” the state writes. The state further notes that the same workers would otherwise be eligible for premium tax credits, but they would be subject to qualified health plan’s higher premiums and out of pocket spending that Minnesota is attempting to mitigate through the program. The state also points out that the federal government would save money by allowing the exemptions, since the government would be responsible for only 95 percent of subsidies and cost-sharing reduction payments if these individuals were allowed to be enrolled in the BHP rather than an exchange plan. Minnesota is also seeking a waiver from the BHP’s required 30-day “grace period” for premium payments. Instead the state seeks a 20-day “reinstatement period,” which would allow someone to catch up on payments without a gap in coverage. Jesson was unable to comment on whether CMS was amenable to the state’s request since the negotiations are ongoing. The BHP was actually started as a state-based program in Washington state and Sen. Maria Cantwell (DWA) had pushed for its expansion to other states as part of the ACA. The state was expected to take up the program and had sent in a proposal in 2012, but work was put on hold after the federal government failed to provide feedback, sources said. The state recently had to cut funding for its own BHP program and got a federal bridge waiver to keep enrollees covered until the Medicaid expansion went into effect. The state-supported BHP expired on Dec. 31, 2013 Washington state sources say that there is still a lot of interest in the BHP, but whether and when it moves forward in the state is a matter of both economics and priorities. Ensuring that the Medicaid expansion and the state-based exchange are working smoothly is the number one goal, says a source. Additionally, the sources say, the state needs to do more work to understand the new market dynamics since previous assumptions about the program came out prior to implementation of Medicaid expansion and the state-based exchange. The sources says that in order to continue with — and go beyond — the planning phase, and to answer the outstanding questions, the state will need more legislative and budget authority. The officials say they look forward to the next 10 INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 legislative session,which takes place from January to April. New York state recently secured authorization to move forward with the BHP, and has plans to implement the program in 2016, says Elizabeth Benjamin, vice president of health initiatives at the NY-based Community Service Society of New York and a member of the state’s BHP workgroup. The state still needs to submit a blueprint and get approval and Benjamin is hopeful negotiations with CMS will go smoothly. Benjamin expects the state will have “very modest” premiums of $20 or so for the plans. This is a great opportunity for lower-income New Yorkers, she said. — Amy Lotven Evidence Development Feasible For Specialty Rx . . . begins on page one development & innovation at Brookings Institution’s Engelberg Center for Health Care Reform, said if CMS and commercial plans were to apply the policy to drugs, it might be used for purposes other than temporarily covering drugs to determine how well they work — the poster child for expensive specialty drugs is Sovaldi, which cures 95 percent of people infected with the most common type of hepatitis C. “The concept for specialty drugs is interesting,” Daniel said at a Brookings Institution event on Oct. 1. “It doesn’t necessarily have to be provisional, but is there a way we can tie payment for a particular drug to ensure that providers and the health care system as a whole are contributing to the development of better evidence on how to use these products?” he asked. Daniel said Sovaldi works well so there isn’t reason to show the product works, but coverage with evidence development could be a way to identify the types of patients who should receive drugs first and to glean information on how doctors use them. Public and commercial plans could require drug makers to collect data, typically with a registry, in return for covering specialty drugs, he said. “It’s only worthwhile if the evidence that that data brings outweighs the cost for doing the research and collecting the data,” Daniel said. Mark McClellan, director of the Health Care Innovation and Value Initiative at the Brookings Institution, made similar comments while simultaneously speaking across town at an America’s Health Insurance Plans conference. He said the cost of analyzing data is falling, which makes it more feasible to apply the coverage with evidence development policy to specialty drugs. McClellan and Daniel discussed policies for specialty drugs in general and did not speak about policies specific to Sovaldi. The hepatitis C drug’s high cure rate is unique and that makes it difficult to handle the price because instead of paying for treatments over decades, such as with HIV treatments, the cost is paid upfront. McClellan said another approach might be to pay drug companies to cure people, instead of merely paying only for the drug. This approach would give drug makers the responsibility of identifying the right patients to treat and getting patients to stick to their drug regimens. Daniel said this approach could be used in either a bundled pay arrangement or in an accountable care organization or medical home, although he noted that a criticism of bundled pay is that it discourages the use of new, expensive products. Others at the Brookings event also discussed ways to finance coverage of expensive drugs. The idea of spreading out the cost of drugs with mortgages or other financial instruments has been bandied about for a while, but McClellan said he isn’t a fan of buying on credit. Likewise, Al Engelberg, of the Engelberg Foundation, said at the Brookings event that financing the cost of drugs does not deal with the underlying question of whether they should be so expensive, and there is at some point a “natural cap” on what states and taxpayers are willing to pay. Andrew Allison, of Allison Health Care Consulting, said it isn’t clear that state constitutions even allow for long-term financing. He also said it would be difficult to convince state health care officials and lawmakers to mortgage the cost of a cure because of concerns that those same patients will get sick again.—John Wilkerson Angoff: HHS Pledge To Release Rates Prior to Open Enrollment ‘Too Late’ A former administration official says HHS’ assertion that 2015 premiums will be released prior to the start of open enrollment is a step forward, but the agency is still not letting consumers review and comment upon proposed rates as it should under the law. Jay Angoff, who once headed the HHS Office of Consumer Information and Insurance Oversight and is now with law firm Mehri & Skalet, on Tuesday (Sept.30) filed a complaint in federal court against HHS on behalf of the Consumers Council of Missouri in a bid to get the administration to release rate filings before they are finalized. HHS, meanwhile, will not say exactly when the final rates will be released, with a spokesperson only telling Inside Health Policy that they will be released for all 50 states prior to the start of open enrollment on Nov. 15. At least one GOP lawmaker is demanding that the rates be released on the day that they are certified — Nov. 3, one day before the mid-term elections. The suit says the consumer groups have the right to obtain the information on rate filings and HHS has no legal basis for failing to disclose those records. Consumer groups had filed a Freedom of Information Act request on Aug. 20 to INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 11 obtain information on proposed rates and insurers, but HHS had not provided the information that was requested. In response to the lawsuit, an HHS spokesperson said: “We are readying the rate change information. The department is committed to providing consumers accurate information so they can make informed decisions, and therefore, before the beginning of open enrollment, the agency will publish final insurance rates for all 50 states.” Angoff in an emailed statement pointed out that under HHS’ rule on insurance rate review “the public is supposed to be able to comment on proposed rate increases, so that regulators can consider those comments in determining whether to approve those increases, so that HHS can consider those comments in determining whether to deem rates unreasonable, and so that the exchange — whether federal or state — can consider those comments in determining whether to allow the insurer proposing the rate to sell on the exchange.”“If HHS discloses rates and the justifications for them only after rates have already been implemented, then it is too late for any of those things to happen,” he said. “Nevertheless, anything HHS releases will be a step forward.” Republicans have charged that the administration moved the 2015 open enrollment date from Oct. 1 to Nov. 15, and later the final QHP certification date from Oct. 17 to Nov. 3, in order to avoid a public outcry before the elections should premiums increase significantly. CMS says it moved the final certification date in response to concerns from issuers who said the agency’s original three-day turnaround time for signing agreements was too tight. CMS has also said that it moved the open enrollment date to Nov. 15 in order to push out the certification process so that issuers had more time to understand and factor in information on their new enrollees. Several sources, however, have told Inside Health Policy that insurers never requested that change, and the Blue Cross and Blue Shield Association has even urged the administration to reverse that decision. Sen. Lamar Alexander (R-TN) said in a Thursday (Oct. 2) statement that the administration should release data as soon as the plans are formalized. “The administration will know exactly what health insurance plans cost on November 3, and they should release that information to the public on that day so families can start to plan,” he said. “If the Senate majority leader hadn’t sent the Senate home so he could keep his majority, we could be debating my bill to make health care premium costs available to Americans before open enrollment.” Last December, Alexander joined Wyoming Republican senators John Barrasso and Mike Enzi in introducing the Premium Disclosure Act, which would require the administration to make the rates available prior to open enrollment. (Last year, rates were not available until day one). The bill would have also set a permanent annual open enrollment period from Oct. 15 to Dec. 7. Meanwhile, the average finalized rates remain relatively low. According to the latest data from PriceWaterhouse Cooper’s Health Research Institute, among the seven states — including the District of Columbia - that have finalized rates, the average premium is $327 and the average increase from 2014 is just 2.5 percent. The states include Colorado, Maryland, New York, Ohio, Oregon, and Rhode Island. For all 38 states and DC that HRI has information on, the average premium is $382 and the average increase is 6 percent. However, HRI notes that the rates can vary significantly across states, pointing out that in Colorado, average rates range from a 22 percent decrease to a 35 percent increase. — Amy Lotven Hardship Exemption Helps Some Avoid EHR Penalties . . . begins on page one problems. American Hospital Association policy director Chantal Worzala said the move means some hospitals may be able to recoup penalties, but added that it’s unclear exactly how this will be done. “For hospitals, this announcement opens up the possibility that CMS might give some IPPS hospitals the opportunity to recoup meaningful use penalties that began on Oct. 1. This benefit is limited to hospitals that could not meet the July 1 deadline to attest to meaningful use, but might have been able to if the flexibility rule had been in place then. We’re unclear how this will be made official and operationalized but appreciate CMS’s intent.” The American Medical Association also commended CMS’ decision to accept more hardship exemption applications and said the move will help physicians avoid an unfair penalty in 2015. Robert Tennant, senior advisor with the Medical Group Management Association said the announcement is very welcome news and shows CMS is willing to be responsive the providers’ concerns. CMS and the Office of the National Coordinator for Health IT say the new deadline for submitting hardship exemptions for 2014 will be Nov. 30. More information about the application process will be shared soon, the agency says. CMS says they will address the issue with hardship exemptions in an upcoming rule, though the application is already available on the agency’s website. Previously, the hardship exception deadline was April 1 for hospitals and July 1 for physicians. The AMA and MGMA had asked CMS to push back that deadline for doctors, as the final rule providing flexibility around attesting to meaningful use in 2014 wasn’t released until the end of August. The AMA and MGMA said that without the final version of the rule, it was hard for doctors to know if they needed to request a hardship 12 INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 exemption. However, CMS originally stuck with its deadlines. The agency first said in February that it would allow hardship exemptions for providers who tried to upgrade their EHRs but didn’t have time to fully implement them in 2014. The exemption originally allowed providers to avoid an EHR penalty because the technology wasn’t available, current EHRs couldn’t be upgraded in time to attest to meaningful use, and Stage 2 requirements couldn’t be incorporated into providers’ workflow in time to meet the requirements. CMS later finalized a rule that said providers wouldn’t have to use 2014 certified systems until 2015. For 2014, the agency said providers could use the older 2011 version of EHRs or a combination of the older technology and the 2014 technology to attest to meaningful use. But physicians attesting to meaningful use for the first time in 2014 who planned to take advantage of the rule and use 2011 technology could not attest to meaningful use until after the effective date of the rule, Oct. 1, according to CMS. Because of this, some physicians who may have avoided 2015 penalties by attesting to meaningful use for the first time before Oct. 1 were left facing potential cuts — a prospect that did not sit well with some lawmakers. CMS’ decision to re-open the hardship exemption applications gives these providers another chance to avoid the meaningful use penalties — although providers who are not attesting for the first time may also apply for the hardship exemption. CMS says that in order to apply for the latest round of hardship exemptions, providers must have been unable to fully implement the 2014 EHR because the technology wasn’t available and doctors must have been unable to attest by Oct. 1 using the flexibility provided in the final rule. Hospitals that were unable to attest by July 1 using that same flexibility will also be able to apply for the latest round of hardship exemptions. AMA President Robert Wah says in a statement that “[g]iving physicians more time to file for a hardship exemption provides necessary relief as many physicians are struggling to meet a number of reporting mandates to avoid multiple penalties.” Tennant said MGMA hopes this is the first of many announcements addressing remaining issues around the 2014 meaningful use reporting period, and signals a willingness on CMS’ part to be responsive to requests for increased flexibility around the 2015 reporting period, as well. Stakeholders have repeatedly asked for CMS to allow providers to attest for three months, rather than a full year, in 2015. — Michelle M. Stein Vit als: A Vitals continued on next page Health P olicy Blog Po Excerpts of Inside Health Policy Blogs AHA, Rebuffing HHS Settlement, Asks Court To Enforce 90-Day Medicare Appeals Deadline The American Hospital Association has asked a federal court to immediately rule that HHS must comply with the 90-day limit for Medicare claims appeals, saying HHS’ “strained interpretation” of the law effectively imposes no deadlines for appeals decisions. AHA’s brief, which also rebuffs HHS’ recent settlement offer and asks the federal DC District Court to reject HHS’ motion to dismiss the suit, is the latest action in an ongoing battle between hospitals and the department over the Medicare appeals backlog. “(T)here can be no dispute that the ninety-day deadlines are statutory requirements to which HHS has failed to adhere,” AHA and three other hospital plaintiffs write in the brief filed last week. “It is only now, as a defendant in litigation, that HHS has taken the incredible position that the ninety-day deadlines are mere suggestions by Congress that the Secretary is at liberty to exceed by years,” it adds. The brief continues: “Under HHS’s strained interpretation of the Medicare Act, HHS would not be required to meet any deadlines for deciding Medicare claim appeals — a view that is at odds not only with the plain language of the statute, which should end the inquiry, but also the structure of the law and the intent behind it.” The hospitals argue that “Mandamus,” or an order that HHS immediately comply, is the only meaningful remedy for what they allege are the department’s “unlawful and egregious” delays. They say that the parties agree the delays are substantial, and that HHS’ current initiatives are not sufficient to resolve them. Further, they say, HHS has “professed a disinclination either to obtain additional funds or to rein in the (Recovery Audit Contractors), and instead is content to condemn hospitals to wait in the years-long line, or consign them to an escalation process that is neither equipped to provide the hearing to which hospitals have a right under the Medicare Act nor to handle the massive backlog of claims.” AHA and the other hospitals filed suit against HHS on May 22 due to the backlog at the third level of the Medicare appeals process, which hospitals say is due to overly aggressive Recovery Audit Contractors. In July, the plaintiffs also asked the court to mandate HHS comply with the 90-day deadline. HHS recently proposed a settlement, offering to reimburse hospitals 68 cents on the dollar for denied inpatient claims if hospitals remove them from the appeals process. But, AHA has said the settlement offer is narrow and doesn’t address problems with overzealous RACs The hospitals also wonder how the settlement would affect the RACs’ fees. The plaintiffs in Thursdays brief say that the settlement offer is “yet another acknowledgment by HHS that it has an obligation to alleviate the backlog” and is also proof that it can find the funds to do so. The brief calls the settlement inadequate for two reasons: First, it will do INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 13 nothing to stop the growth in appeals; and second, it is “remarkably” narrow. — Amy Lotven FDA Urges Device Makers To Include Cybersecurity In Design Phase The Food and Drug Administration issued the final version of its cybersecurity recommendations for pre-market medical devices, recommending manufacturers consider cybersecurity during design and development and encouraging industry to submit comments on implementing those measures. “Effective cybersecurity management is intended to reduce the risk to patients by decreasing the likelihood that device functionality is intentionally or unintentionally compromised by inadequate cybersecurity,” says FDA in its recommendations. The recommendations lay out a cybersecurity framework for medical device cybersecurity that categorizes functions into “identify,” “protect,” “detect,” “respond” and “recover.” FDA’s director of emergency preparedness and operations and medical countermeasures, Suzanne Schwartz, of the Center for Devices and Radiological Health, said the healthcare industry needs to embrace cybersecurity best practices and work with government on critical cybersecurity issues. She made those remarks at an event last week hosted by the National Institute of Standards and Technology and the Department of Health and Human Services. The governing agencies of the healthcare industry have already put forth an effort to be involved on setting cybersecurity requirements, as HHS works with industry to determine how cybersecurity falls under the Health Insurance Portability and Accountability Act. — Inside Cybersecurity Enroll America Hopes To Get 2,000 New Certified Application Counselors Enroll America said Wednesday (Oct. 1) that it hopes to recruit and train more than 2,000 certified application counselors and recruit new in-person assisters for the next open enrollment period that begins in November. The nonprofit’s president previously told Inside Health Policy that it would be taking on a more active role to recruit people and organizations to be CACs — which have smaller roles than their navigator counterparts — however Wednesday’s announcement was the first time the group quantified how many CACs it hopes to recruit. Enroll America said it will launch a number of training sessions for CAC recruits and will provide ongoing logistical support, materials and recruitment to make sure the new assisters have all that they need to successfully enroll people into coverage. Enroll America President Anne Filipic previously said she doesn’t think a dramatically different strategy is needed for the second open enrollment period to get people covered. Rather, she says, it’s about doubling down on the most effective tactics from the first cycle in order to enroll harderto-reach populations. The announcement on Wednesday also reaffirms that Enroll America will keep staff in the same 11 states that it chose ahead of the first open enrollment period. They are: Texas, Tennessee, Florida, Michigan, Ohio, New Jersey, 14 Pennsylvania, North Carolina, Illinois, Georgia and Arizona. The group will have 200 staff across all of those states. — Rachana Dixit Pradhan GAO Opinion On Risk Corridor Authority Generates Praise From Both GOP, HHS Both Republicans and HHS are spinning a Government Accountability Office opinion on the ACA’s risk corridor program as positive news, because the GAO found that fiscal year 2014 appropriations language gives HHS authority to fund the program yet also said that Congress must include certain language in its fiscal year 2015 budget for the department to be able to collect and disperse payments next year. GOP lawmakers had requested that GAO examine the administration’s legal authority to administer the program without a direct appropriation. The risk corridor program is designed to protect insurers from inaccurate rate setting for qualified health plans in the initial years of the exchanges. Specifically, the GAO found that CMS’ program management appropriations and its authority to collect user fees would have supported making risk corridor payments to insurers in 2014, but similar program management language must be included in FY2015 appropriations. An HHS spokesperson says officials are “pleased that the GAO affirmed the department’s authority to use fees it collects from issuers to fund the temporary risk corridors program, an important initiative designed to help encourage more issuers to participate in the marketplace and compete on price and quality, ultimately giving consumers more affordable coverage options.” The GAO report came in response to a query from Sen. Jeff Sessions (R-AL) and Rep. Fred Upton (R-MI), who had questioned the administration’s authority to run the program without a specific appropriation. HHS argued in a May 20 letter that it had authority under the department’s ability to collect and disperse user fees. The user fee language was included in CMS’ program management appropriations, which provides the agency funding but does not specify how it must be spent. The language also said that funds collected would be credited to the account and remain available until 2019. GAO in its opinion agrees that the FY2014 “program management” language and user fee authority makes the funding available for the program, but also says,“Appropriations acts, by their nature, are considered non-permanent legislation. Thus,similar language must be included in FY2015 appropriations. The Republicans saw this opinion as supporting their stance. “GAO has confirmed beyond dispute that the Department of Health and Human Services has no legal authority to disburse risk corridor payments under Obamacare absent a congressional appropriation,” Sessions said.“I hope this nips in the bud any ideas this overreaching administration might have of paying out money not appropriated by Congress. Such expenditures would violate bedrock separation of powers principles in the Constitution.” Upton added, “We had serious concerns with the legality of the Obama administration’s plan from the get go, and the INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 government’s watchdog confirms we were right.” GAO also notes that the terms and conditions of CMS’ program management appropriations — which allow for risk corridor payments to insurers — continue as a part of the current Continuing resolution that is set to expire Dec. 11. — Amy Lotven Congressional Marketplace Hotline Launched In Advance Of Open Enrollment CMS informed congressional offices that the Congressional Marketplace Hotline will re-open Wednesday (Oct.1), 45 days in advance of the 2015 qualified health plan open enrollment season which begins Nov. 15 and ends Feb.15, 2015. The hotline is available for DC-based congressional offices with questions regarding the Federally Facilitated Marketplace, the agency says in a notice sent to Capitol Hill Tuesday (Sept. 30). District staff can work with their established contacts in the CMS regional offices, the memo adds. The hotline will be open from 9 a.m to 6 p.m. Monday through Friday. Staff can also ask questions via a designated email. — Amy Lotven Zogenix Submits Application For Reformulated Pure Hydrocodone Drug Zogenix, Inc., the pharmaceutical company that developed the controversial hydrocodone drug without any abuse deterrent properties, submitted a supplemental new drug application (sNDA) for a modified formulation of Zohydro Wednesday (Oct. 1) that contains addition inactive ingredients that are intended to make the product more difficult to abuse by injection and snorting. “The new technology being added to Zohydro ER represents a meaningful advancement because it incorporates properties designed to deter abuse yet maintain the efficacy of the medication, a central consideration throughout the product’s development,” said Stephen Farr, president of Zogenix. The company anticipates a target action date early in 2015, and if the product is approved will transition from the currently marketed Zohydro, which does not have abusedeterrent properties, to the new formulation. This comes after Purdue Pharma’s single-entity hydrocodone bitartrate tablet that was formulated to include abuse-deterrent properties was granted priority review designation during the summer. The original non-abuse deterrent Zohydro received pushback from lawmakers and consumer advocates who are concerned about preventing opioid overdose. FDA Commissioner Margaret Hamburg responded to concerns over Zohydro in an editorial in USA Today the same day the sNDA was announced. “While we appreciate the concern surrounding our recent approval of Zohydro, it should be recognized that Zohydro is a time-released analgesic that, without the added risk of acetaminophen, fills a need for pain patients who respond best to hydrocodone,” she said, adding that Zohydro is a very small fraction of the opioid marketplace. She also said that singling out any individual opioid product detracts the nation’s focus from interventions that could make a real difference in opioid abuse. FDA at the end of October will be hosting a public meeting to discuss the development, assessment and regulation of abuse-deterrent formulations of opioid medications, focusing on scientific and technical issues related to the development and in vitro assessment of these products. — Erin Durkin CMS Looks To Medicare Advantage For Exchange Network Adequacy Rules CMS is eying Medicare Advantage as a model for future rules on exchange plan network adequacy, sources tell Inside Health Policy. Consumer and patient sources say MA concepts could be useful for developing additional exchange rules, but add that they think state insurance regulators should maintain control in setting specific thresholds and minimums. In March, CMS said it intended to use information from the 2015 federal exchange qualified health plan application process to help craft time and distance or other standards for FFE QHP provider networks in the future. Those standards will be reflected in future rules, CMS wrote in the final 2015 letter to issuers on the FFE. A patient advocate source says that message — and the fact that MA uses time and distance standards as a part of its network adequacy rules — plus informal conversations with the agency have led stakeholders to believe that CMS is looking to MA as a model for exchange network adequacy rules. The source says it’s possible the agency could implement new standards for 2016, but it’s still unclear. Provider networks in the exchanges have generated significant controversy because insurers are increasingly using narrow networks as a way to keep their premiums low. The move has led congressional Republicans to allege the ACA is preventing people from keeping their doctors. So far, the only quantitative standards CMS has put in place for exchange networks are for the inclusion of essential community providers. For 2015, CMS said an insurer had to show that at least 30 percent of available ECPs in each plan’s service area participate in the provider network, an increase from the 20 percent used for 2014. In addition, CMS expected that the issuer list the contract offers it extended to all available Indian health providers and at least one ECP in each ECP category in each county of the service area. For general network adequacy in 2015, CMS only said it is using a more uniform “reasonable access” standard to INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 15 evaluate plans’ provider networks, focusing closely on providers that historically have raised concerns because they may not be included. These include hospital systems, mental health providers, oncology providers and primary care providers, CMS said. But no quantitative standards are in place. The patient advocate said CMS would have to make some adaptations to the MA network standards, but the general feeling is that it doesn’t make sense for CMS to completely reinvent the wheel for the exchanges and many of the concepts in MA could be used. For example, MA classifies regions based on whether they are more urban or rural. The source adds that the needs of Medicare beneficiaries align with patients who have chronic disease more generally. If it’s a choice between CMS creating MA-type quantitative standards versus no quantitative standards at all, we’ll take MA, the patient advocate says. But a consumer advocate says it’s “low hanging fruit” for CMS to look at MA. Even with standards for that program, it hasn’t stopped insurers from slimming down their provider networks very suddenly. It’s not a magic bullet for addressing network problems, the source says. Gretchen Jacobson, an associate director with the Kaiser Family Foundation’s program on Medicare policy, said during a recent Alliance for Health Reform briefing that there are many differences between MA and the exchanges, such as patient demographics and the fact that MA is a county-based system. But it’s often the same companies that are offering MA plans and plans on the exchanges, so uniformity in rules could make things easier. There will also be enrollees who begin in the exchanges and then eventually transition to MA when they turn 65, Jacobson noted. During the briefing, Stephanie Mohl with the American Heart Association said her group has recognized there needs to be state flexibility as well, because what works in Montana is not going to be what works in California. Mohl, a consumer representative to the National Association of Insurance Commissioners, said the representatives took the position that there should be several quantitative standards for QHP networks but each state should set its own. Groups have heard loud and clear from state regulators that having a single national standard is not going to go over very well, she said. Mohl said such standards could touch on areas like provider-to-enrollee ratios, and time and distance standards. Another consumer advocate says wait-time standards could also be an option. The source agrees that the federal government might need to set some baseline for network adequacy — as a backstop in case the states don’t do anything — but added that the delivery of health care is local and there can’t just be arbitrary time and distance rules. State insurance regulators repeatedly have voiced concerns about the federal government drafting uniform network adequacy rules, saying the requirements should remain in the states’ control because they understand their markets better. The NAIC is in the process of revising its network adequacy model law, a process it hopes to finish in the next couple of months. — Rachana Dixit Pradhan UnitedHealth Pushes Primary Care Changes To Expand Access A top official with UnitedHealth Group says whether or not newly insured individuals complain about having adequate access to primary care services there are many problematic signs across the health care system that access could be at risk. The nation’s largest insurer released a new report on ways to expand primary care availability, which includes recommendations such as increasing reimbursement to non-physician providers and expanding the use of effective valuebased payment models. Richard Migliori, chief medical officer of UnitedHealth Group, suggests the insurer has not yet begun to see high levels of complaints about accessing primary care services from people who recently gained coverage through the exchanges or Medicaid. But a variety of evolving trends portend that access may be at risk, and when the system begins to fall apart people start visiting the emergency room for non-emergency care, he said. The report points out that an estimated 70 percent of emergency room visits by commercially insured patients in the United States are for non-emergencies. In its new report outlining recommendations for improving primary care capacity and delivery, the insurer says the ACA could spur an additional 25 million to seek primary care visits annually due to increases in insurance coverage, the requirement that insurers cover essential health benefits in the individual and small group markets, and the elimination of cost-sharing for a wide range of preventive services. Migliori said the report found that primary care is aggregated in higher income areas and more available in places where people are more likely to be insured, so physician supply is only one part of the problem. In addition to there being more people seeking services and the poor distribution of primary care physicians, “(p)eople are showing up with a higher burden of chronic disease,” Migliori said. The insurer suggests that Medicare and Medicaid should provide greater reimbursement to non-physician providers, saying that a “significant barrier” to achieving more progress in expanding primary care capacity and improving care delivery is current payment policy. Migliori also told Inside Health Policy that budgets for rural health clinics and federally qualified health centers should be examined by policymakers so that those assets can be grown. The role of retail clinics in providing primary and preventive care should be expanded, the insurer recommends in its 16 INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 report, as should the use of patient-centered medical homes and accountable care organizations. Of the 220 accountable care organizations in the Medicare Shared Savings Program, 53 ACOs earned bonuses and another 52 reduced Medicare costs but not by enough to share in savings, according to the first-year results released by CMS in September. Migliori said the insurer “absolutely” believes in the patient-centered medical home and it continues to invest in those models. If you think of the number of physicians that practice in small groups, the medical home model could be a way for them to transition to greater care coordination, he added. United operates 13 medical home programs in 10 states for commercially insured populations, and they include more than 2,000 physicians and 300,000 enrollees. The insurer says an evaluation of four programs in Arizona, Colorado, Ohio and Rhode Island for 40,000 patients between 2009 and 2012 found average gross savings of 7.4 percent of medical costs in the third year. Including the costs of any interventions, the programs on average saved roughly 6.2 percent of medical costs. The carrier says it has seen a six-to-one return on investment in care coordination activities. United adds that independent evaluations for four medical home programs in Rhode Island, Colorado and Ohio showed improvement on several quality measures — including preventive and chronic care, access, care coordination and patient satisfaction. The insurer found notable success in managing diabetes for patients, but not all measures met targets, especially those related to certain cancer screenings. — Rachana Dixit Pradhan CMS: States May Seek Exchange Grants Without Filing Blueprint CMS says states may apply for exchange establishment grants to fund consumer assistance activities, even if they have not completed the state-based exchange or partnership-exchange blueprint approval process, potentially easing the process for states to get more federal funding before HHS stops awarding the money. The information was included in a Frequently Asked Questions document from Sept. 19. States may only apply for exchange establishment grants for two more cycles — Oct. 15 and Nov. 14 — and the law says exchanges must be selfsustaining starting next year. In the FAQ, CMS writes that states may apply for the funding for consumer assistance establishment activities — including consumer assistance programs such as the in-person assister program — if governors attest that their states will complete the blueprint approval process. States that wanted to transition to a state-based exchange or a partnership exchange for 2015 had to submit blueprints to CMS by June, but an agency spokesperson previously told Inside Health Policy that none did. Arkansas and Illinois, both now having approved partnership exchanges, have expressed a desire to transition to a full state-based exchange but not until 2016. On the other hand, Nevada and Oregon — which were both conditionally certified as state-based exchanges for 2014 — are now defaulting to the federally facilitated exchange for enrollment because their own systems were dysfunctional. New Mexico was approved as a state-based exchange, but it relies on the FFE for individual enrollment next year, and Idaho wants to take itself off federal technology for 2015. In guidance from March on spending exchange grants, CMS said states with state-based or partnership exchanges that provide coverage this year may request “no-cost extensions” of their exchange establishment grants beyond Dec. 31, 2014, if they need more time to complete the design, development and implementation work. However, the funding can’t be used to cover rent, software maintenance, telecommunications, utilities and base operational personnel and contractors. CMS reiterates in the latest FAQ that 2014 state-based exchanges and partnership exchanges may not apply for establishment grant funding to pay for ongoing operations. But the agency also makes clear that for new grants awarded this year the project period can last up to one year after the money is awarded. One source says they believe this clarification means state-based and partnership exchanges could apply for grants by November and have the ability to spend that funding for most, if not all, of next year. At its September board meeting, Connecticut exchange officials indicated that CMS may provide award opportunities for outreach and education establishment funds for which the exchange would apply. Board meeting materials said CMS was moving in this direction “in acknowledgment of the need for continued community-based consumer support beyond the limited 90-day enrollment period.” Connecticut’s Department of Social Services would also submit a symmetrical funding filing, the exchange wrote, because the composition of enrollees would be predominantly Medicaid eligible. One source tracking state developments adds that Washington state has been very interested in figuring out how to fund its outreach activities next year. At the end of August, exchange staff suggested the state would apply for a $22 million supplement to an existing Level One establishment grant, $3.2 million of which would be for communications and outreach work. The Washington exchange wrote in meeting materials that the $3.2 million would be for enhanced funding for in-person assister lead organizations, additional training funds and continued tribal assister funding. — Rachana Dixit Pradhan INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 17 Bayh: Chances of Device Tax Repeal ‘Very Good’ If GOP Wins Senate CHICAGO — There is a “better than 50/50” chance that Republicans will repeal the ACA’s device tax if they win the Senate in November, Evan Bayh, former Democratic Indiana senator and governor, said Tuesday (Oct. 7) at the Advanced Medical Technology Association’s medtech conference. A Republican-controlled Congress likely would first vote to repeal the Affordable Care Act, and after that measure is vetoed would settle on other changes to the law, such as a repeal of the industry-opposed excise tax, said Bayh, now a partner at McGuireWoods. The 2.3 percent excise tax was levied on medical device firms starting in January 2013. Device company CEOs said they have delayed hiring employees and scaled back research and capital projects, but those restrictions could be reversed with a repeal of the tax. Key lawmakers view repealing the tax as a priority and the president is not tied to the policy, Bayh said. If Democrats maintain a slim majority in the Senate a device tax repeal is still possible, he added. Under that scenario, many moderate senators could press for a device tax repeal in exchange for support of other measures in the final years of the Obama presidency. However, the chances of a repeal would be “very good” if Republicans win the majority, Bayh added. “If the Republicans don’t overreach on other things, I think this is doable,” he said. GOP members would likely try to fully repeal the ACA but the president would veto such a bill. Other changes, such as eliminating the employer mandate or repealing the device tax could be considered, and the president is not likely to issue a veto solely based on the medical device tax, he said. Finding an offset for the tax has been a challenge in advancing its repeal. But Bayh described the tax as a “rounding error” in terms of the federal budget. He noted there are “half of a dozen” items that could be looked at for savings. With political alignment, the issue of finding an offset could be resolved, he said. “If there’s the political will, they’ll find the fiscal way,” he said. “So generally you get asked this question now by people who really don’t want to do it; they’re just trying to put up an obstacle that could be overcome.” — Alaina Busch McBournie Nurses Voice Concerns About Lack Of Safety Precautions Against Ebola Nurses and their supporters across the United States are voicing a variety of concerns about the U.S. health care sector’s readiness to provide them with adequate health protections as cases of Ebola infection begin sprouting up outside the disease-ravaged region of West Africa, including a patient in Dallas and most recently a nurse in Spain — with ensuring adequate protective gear a key priority of advocates. A key occupational health expert expressed hope the growing Ebola crisis and other disease exposure concerns would spur the Occupational Safety and Health Administration to speed up its infectious disease rulemaking. Of paramount concern to the nursing community, and still a largely unanswered question, is the availability of enough personal protective equipment (PPE) in hospitals, which advocates say is crucial to avoid spread of the disease to health care personnel even though Ebola is not considered an airborne virus easily transmitted throughout care settings, such as through building ventilation systems. Hospital groups have insisted that U.S. facilities are up to the challenge of preventing the spread of Ebola, however. The Centers for Disease Control and Prevention (CDC) also recently came out with sweeping guidance in the form of a checklist designed for “health care coalitions” to prevent spread of the disease. Nurses’ groups are further exhorting their members to review any available infectious disease guidelines as well as checklists with respect to preventing infections. The main goal, according to the American Nurses Association (ANA), is to ensure that members understand the disease, how it is transmitted and what precautions are necessary to protect health care providers and the public. “Now that the first travel-related case of the disease in the United States has been reported in Dallas, it is critical that all members of the health care team have appropriate knowledge, education and personal protective equipment to effectively provide care to patients,” said Pam Cipriano, ANA president, in a statement Thursday (Oct. 2). “ANA also underscores the need to practice meticulous infection control at all times.” Cipriano said containing Ebola will require inter-professional collaboration and working in teams of nurses, physicians and other providers to properly treat and stop the spread of the disease, noting that ANA regularly communicates with and provides information from CDC regarding infectious diseases and emergency preparedness. She also alluded to the mistakes revealed by the Texas case, saying: “(W)hen an error or omission occurs while treating a patient, it is time for the entire health care team to review its processes and checklists in order to learn from emerging situations, improve the flow of information and continuously improve practices that result in improved safety for everyone involved. In any emergency or infectious disease outbreak, health care teams must be constantly vigilant and 18 INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 seek out the latest information and evidence-based practices to respond to the evolving situation.” The American Hospital Association provides a range of resources to its members on its Ebola preparedness page accessible from its main website. CDC and the HHS Assistant Secretary for Preparedness and Response as part of Ebola guidance to hospitals state that the employer should “[a]ssess and ensure availability of appropriate personal protective equipment (PPE) and other infection control supplies (e.g., hand hygiene supplies) to all health care personnel.” Global media reports in the wake of the nurse’s Ebola diagnosis in Spain depicted a fear-filled environment among nurses including in the United States, particularly about whether hospital employers are equipped with PPE in the event of worldwide outbreaks. Organized labor for nurses has urged that employers provide respirators in environments of suspected Ebola, but it’s not clear if the equipment would be adequately available if more cases occur in the United States. Aaron Trippler, director of government affairs for the American Industrial Hygiene Association, tells Inside OSHA Online that the Ebola situation as well as other recent disease outbreaks underscore the urgency of the health care community to re-emphasize PPE and other safety precautions — and nurses are a main driver of that effort. “We can see the same problem occurring,” he says. “People are concerned about the lack of PPE availability.” Trippler says the situation also highlights a need for OSHA “to move a little quicker on an infectious disease rule. We need to get everybody involved in this. Nurses are just scared to death about Ebola.” “What we have noticed over the last 10 years is an increasing concern” about infectious diseases in general, he says. “It’s why CDC is overburdened in trying to address these things.” — Christopher Cole NIOSH Experts Worry Health Workers Face Exposures To Anti-Cancer Drugs National Institute for Occupational Safety and Health (NIOSH) officials express deep concern that a large contingent of U.S health care workers who deal with cancer patients face highly risky exposures to hazardous chemicals, raising red flags in a new study coming out in a major occupational health journal about issues ranging from inadequate personal protective equipment (PPE) to lack of worker training despite widely available guidance. “Chemotherapy drugs save lives of cancer patients but also can result in adverse health outcomes in workers who are exposed to these drugs, including cancer, reproductive problems, and organ damage when recommended safe handling guidelines are not followed,” NIOSH chief John Howard said. “Safeguarding healthcare workers from potential occupational hazards is an essential part of providing good jobs for these dedicated women and men, and furthering high-quality patient care.” The new study published online in the Journal of Occupational and Environmental Hygiene finds that recommended safe handling practices for workers who administer antineoplastic drugs in health care are not always followed. The American Industrial Hygiene Association (AIHA) and American Conference of Governmental Industrial Hygienists jointly publish the journal. Results are derived from the “2011 Health and Safety Practices Survey of Healthcare Workers,” the largest federally sponsored survey of health workers in the United States, which addresses safety and health practices in use of hazardous chemicals, according to AIHA. The paper presents findings on administrative and engineering control practices, PPE, and barriers to using recommended PPE during administration of antineoplastic drugs by nearly 2,100 oncology nurses and other personnel. Respondents completed a module addressing antineoplastic drug administration. Among the report’s findings are several examples of practices NIOSH says may increase exposure risk, shown as a percent of respondents: • Not always wearing two pairs of chemotherapy gloves (80 percent) or not even a single pair (15 percent). • Failure to always wear nonabsorbent gown with closed front and tight-fitting cuffs (42 percent). • Intravenous tubing primed with antineoplastic drug instead of a non-drug containing liquid by the respondent (6 percent) or by the pharmacy department (12 percent). • Potentially contaminated clothing taken home (12 percent). • Spill or leak of antineoplastic drug during administration (12 percent). • Lack of hazard awareness training (4 percent). • Skin contact with antineoplastic drug (4 percent). Several organizations or agencies including OSHA and NIOSH have long made available “authoritative safe handling guidelines,” but the survey indicates recommended exposure controls were not always used, according to an AIHA statement. That’s “highly noteworthy” considering that there is no safe level of exposure to cancer-causing agents, according to the group. “Reported barriers to using PPE suggest that there is a perception that exposures are inconsequential or so rare that employers or workers feel PPE use is not justified.” Researchers conclude that better risk communication is needed to ensure that employers and employees are fully aware of hazards and precautionary measures, AIHA says, adding that commitment from all levels in the health care INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014 19 organization is “essential to adequately protect workers from one of the most toxic classes of chemical agents” used in health care. An abstract of the report notes that many antineoplastic drugs are known or suspected human carcinogens where no safe exposure level exists. “Authoritative guidelines developed by professional practice organizations and federal agencies for the safe handling of these hazardous drugs have been available for nearly three decades.” Howard has made concerns about hazardous drugs in health care a prominent issue and has long encouraged states to leverage research data in addressing such hazards. — Christopher Cole OSHA May Send Infectious Disease Rule To Small Business Panel By Nov. The Occupational Safety and Health Administration reportedly is on the cusp of sending out, as soon as late this month or sometime in November, the necessary materials to start a small business review process on the agency’s proposed rulemaking to help prevent workplace exposures to infectious diseases, a source closely following the issue tells Inside OSHA Online. OSHA in its earlier consultations with stakeholders signaled that it was considering a vertical rule model for infectious diseases — which would address not just infectious agents but specific industry settings, such as hospitals or long-term care facilities. Regulators also were considering a program standard for the possible rule, officials said, involving a “Plan, Train, Do” model for worker protections Beginning the review process would represent a major step forward on the envisioned rule, though OSHA is still a long way off from proposing and eventually finalizing a rule, if the agency proceeds that far. OSHA previously held informal consultations with stakeholders ahead of trying to devise a rule, and the materials for review would at the very least provide budget and small business officials the broad contours of what OSHA is considering. OSHA is required for proposed rules with major impact to submit them for a review under the Small Business Regulatory Enforcement Fairness Act. The review panel comprises officials from OSHA, OMB and the Small Business Administration Office of Advocacy. Small entity representatives meet with the federal officials to review what OSHA has outlined and provide input on economic impact, which OSHA must consider as it formally proposes a rule. The agency had earlier pegged May 2014 for commencing the small business review process. That could now start by this fall, according to the source. “They’re still shooting [to get] the material out to the small business review panel by the end of October or November. … [OSHA] had hoped to provide materials by mid-November.” While the proposal might not include the precise regulatory text of OSHA’s planned rule “they have to have an outline of what they’re planning on doing,” the source adds. — Christopher Cole SUBSCRIPTION ORDER FORM Sign me up to receive Inside CMS at $705 per year in the U.S. and Canada; $755 per year elsewhere (air mail). Name __________________________________________________ Affiliation _______________________________________________ Address _________________________________________________ City/State/Zip ____________________________________________ Phone __________________________________________________ E-mail __________________________________________________ To order by mail: Send this coupon to Inside CMS, P.O. Box 7167, Ben Franklin Station, Washington, DC 20044. To order by phone, fax or e-mail: Call 800-424-9068 (in the Washington, DC area, 703-416-8500). Fax to 703-416-8543. E-mail at [email protected] Please check one: Visa MasterCard Bill me Check enclosed (DC subscribers add 6% sales tax) Card number _________________________________________ Name on the card _____________________________________ Signature _______________________________________________ 20 Exp. date ____________________________________________ INSIDE CMS — www.InsideHealthPolicy.com — October 9, 2014
© Copyright 2026