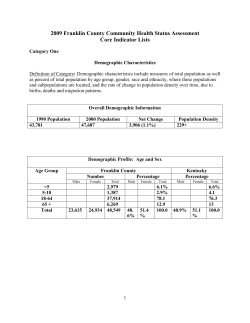
Change in the estimated glomerular filtration rate
clinical investigation http://www.kidney-international.org & 2013 International Society of Nephrology see commentary on page 550 Change in the estimated glomerular filtration rate over time and risk of all-cause mortality Tanvir C. Turin1, Josef Coresh2, Marcello Tonelli3, Paul E. Stevens4, Paul E. de Jong5, Christopher K.T. Farmer4, Kunihiro Matsushita2 and Brenda R. Hemmelgarn1,6 1 Department of Medicine, University of Calgary, Calgary, Alberta, Canada; 2Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; 3Department of Medicine, University of Alberta, Edmonton, Alberta, Canada; 4 Kent Kidney Care Centre, East Kent Hospitals University NHS Foundation Trust, Canterbury, Kent, UK; 5Department of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands and 6Department of Community Health Sciences, University of Calgary, Calgary, Alberta, Canada Using a community-based cohort we studied the association between changes in the estimated glomerular filtration rate (eGFR) over time and the risk of all-cause mortality. We identified 529,312 adults who had at least three outpatient eGFR measurements over a 4-year period from a provincial laboratory repository in Alberta, Canada. Two indices of change in eGFR were evaluated: the absolute annual rate of change (in ml/min per 1.73 m2 per year) and the annual percentage change (percent/year). The adjusted mortality risk associated with each category of change in eGFR was assessed, using stable eGFR (no change) as the reference. Over a median follow-up of 2.5 years there were 32,372 deaths. Compared to the reference participants, those with the greatest absolute annual decline less than or equal to 5 ml/min per 1.73 m2 per year had significantly increased mortality (hazard ratio of 1.52) adjusted for covariates and kidney function at baseline (last eGFR measurement). Participants with the greatest increase in eGFR of 5 ml/min per 1.73 m2 per year or more also had significantly increased mortality (adjusted hazard ratio of 2.20). A similar pattern was found when change in eGFR was quantified as an annual percentage change. Thus, both declining and increasing eGFR were independently associated with mortality and underscore the importance of identifying change in eGFR over time to improve mortality risk prediction. Kidney International (2013) 83, 684–691; doi:10.1038/ki.2012.443; published online 23 January 2013 KEYWORDS: chronic kidney disease; epidemiology and outcomes; mortality risk Correspondence: Brenda R. Hemmelgarn, Division of Nephrology, Foothills Medical Centre, 1403 29th Street NW, Calgary, Alberta, Canada T2N 2T9. E-mail: [email protected] Received 18 March 2012; revised 8 October 2012; accepted 18 October 2012; published online 23 January 2013 684 Studies have consistently demonstrated that more advanced chronic kidney disease (CKD) is associated with an increased risk of mortality across both general and high-risk populations.1–5 However, these reports have predominantly considered kidney function at baseline, without consideration of how the change in kidney function over time influences the risk of such outcomes. There has been a growing interest in the association between change in kidney function and risk of adverse outcomes. Although populationbased studies have reported an association between declining kidney function specifically and adverse clinical outcomes,6–11 kidney function can be highly variable and improve over time in some patients.10,12 Although recent studies have reported an association between improvements in kidney function (increasing estimated glomerular filtration rate (eGFR)) and risk of mortality,7,8 these studies were limited by their select study population (CKD patients only7) and small study size.7,8 Using a population-based cohort of individuals receiving routine clinical care in a single Canadian province, we investigated the association between changes in kidney function over time and risk of all-cause mortality. We explored change in kidney function using two indices: absolute annual rate of change and the annual percentage change. We hypothesized that both increasing and declining eGFR would be associated with higher mortality risk, as compared with stable kidney function. RESULTS Among the participants, 54.8% had an eGFR X90, 37.9% had an eGFR in the range of 60–89, 4.9% had an eGFR in the range of 45–59, 1.7% had an eGFR in the range of 30–44, and 0.7% had an eGFR in the range of 15–29 (all eGFR in ml/min per 1.73 m2). The median number of measurements available for the study participants was 3. The distribution of annual rate of change appeared normal and centered near the origin (Figure 1). The mean annual rate of change was 1.04 ml/min per 1.73 m2 per year (s.d.: 3.83), with a median of 0.91 ml/min per 1.73 m2 per year (interquartile Kidney International (2013) 83, 684–691 clinical investigation TC Turin et al.: Short-term change in eGFR and ESRD range (IQR): 2.98 to 1.07). The distribution of the annual percentage change in eGFR, which also appeared normal, is shown in Figure 1. The mean annual percent change in eGFR was 1.52 percent/year (s.d.: 6.05), with a median of 1.07 percent/year (IQR: 3.77 to 1.34). Compared with study participants, individuals excluded because of an inadequate number of serum creatinine measurements (less than three outpatient serum creatinine measurements spanning a time period of four calendar years—Figure 2) were younger, with fewer comorbidities and 0.15 Density 0.1 0.05 0 –20 –10 –5 0 5 10 20 Annual rate of change in eGFR 0.1 Density 0.08 0.06 0.04 0.02 0 –50 –25 –10 0 10 25 Annual percentage change in eGFR 50 Sensitivity analyses Figure 1 | Distribution of annual rate of change and annual percentage change in estimated glomerular filtration rate (eGFR). At least one measurement At least one measurement a higher level of eGFR at baseline (Supplementary Appendix Table S1 online). Among the study cohort, 135,804 (25.7%) had stable kidney function (no change in kidney function over the accrual period), 133,723 (25.6%) had a positive slope (improved kidney function), and 257,785 (48.7%) had a negative slope (declining kidney function). Participants experiencing a greater annual decline or increase in eGFR were more likely to be female and had a higher prevalence of comorbidities, in comparison with those with stable kidney function (Table 1). Over a median follow-up of 2.5 years, there were 32,372 (6.1%) deaths. Adjusted mortality rates were higher, with both declining and increasing eGFR (Table 2), as compared with those with stable kidney function: the greater the change in eGFR, the higher the mortality risk. Mortality rates (per 1000 person-years) were highest for participants with an increase in eGFR of 5 ml/min per 1.73 m2 per year or more (rate 16.52; 95% confidence interval (CI): 15.78–17.25) and participants with a decline in eGFR of 5 ml/min per 1.73 m2 per year or more (rate 11.27; 95% CI: 10.90–11.65). Similarly, higher mortality rates were observed for increasing as well as declining percentage change in eGFR (Table 3). The mortality rate was highest (rate 15.15; 95% CI: 14.52–15.78) for participants with an increase in eGFR of X7 percent/year or more, followed by participants with a decline in eGFR of X7 percent/year (rate 11.60; 95% CI: 11.20–11.99). Compared with those with stable eGFR, the adjusted risk of death was almost two-fold higher in participants with an increase in eGFR of X5 ml/min per 1.73 m2 per year (hazard ratio (HR) 2.20; 95% CI 2.10–2.31), whereas those with a decline in eGFR of X5 ml/min per 1.73 m2 per year also had 2-fold increased risk (HR 1.52; 95% CI: 1.46–1.57) (Figure 3). Similarly, we observed a U-shaped relation between percentage change in eGFR per year and all-cause mortality (Figure 4). The risk of mortality was 2.02 times higher (95% CI: 1.92–2.11) for the participants with an increase in eGFR of X7 percent/year or more, and the mortality risk was 1.56 times higher (95% CI: 1.49–1.62) for the participants with a decrease in eGFR of 7 percent/year or more. When stratified by category of baseline kidney function (eGFR X90, 60–89, 45–59, 30–44, and 15–29 ml/min/ 1.73 m2), increasing as well as declining eGFR was associated At least one measurement End of study Year 1 Year 2 Year 3 Year 4 Follow-up for outcome ascertainment after last measurement 31 March 2009 eGFR accrual during 1 May 2002 to 31 December 2007 Figure 2 | Overview of cohort creation. eGFR, estimated glomerular filtration rate. Kidney International (2013) 83, 684–691 685 clinical investigation TC Turin et al.: Short-term change in eGFR and ESRD Table 1 | Baseline characteristics of study participants by annual absolute rate of change in eGFR Annual absolute rate of change in eGFR (ml/min per 1.73 m2 per year) p5 4 3 2 1 0 1 2 3 4 X5 62,402 (11.8) 28,457 (5.4) 40,676 (7.7) 55,649 (10.5) 70,601 (13.3) 135,804 (25.7) 44,542 (8.4) 31,612 (6.0) 20,861 (3.9) 13,857 (2.6) 24,851 (4.7) Female gender Aboriginal Diabetes Hypertension 58.6 (17.6) 63.2 2.8 23.2 54.8 59.2 (16.3) 62.0 2.2 18.7 50.3 59.6 (16.0) 60.4 2.1 18.1 59.8 59.6 (15.7) 59.8 1.9 17.3 48.8 59.7 (15.3) 59.3 1.8 17.5 48.1 60.0 (15.1) 57.9 1.9 18.0 48.7 60.1 (15.3) 56.3 2.0 18.4 50.0 59.5 (15.5) 57.7 1.9 18.8 49.4 58.8 (15.6) 58.7 2.0 19.0 48.9 58.3 (16.2) 60.5 2.3 19.6 49.0 55.9 (17.0) 63.1 3.0 19.3 46.3 Proteinuria Normal Mild Heavy Unmeasured 53.3 8.7 3.6 34.4 60.0 7.1 2.0 30.9 61.8 6.9 1.7 29.6 64.0 6.5 1.5 28.1 65.5 6.2 1.2 27.1 65.8 6.0 1.2 27.0 64.2 6.4 1.1 28.3 63.8 6.3 1.1 28.8 61.8 6.7 1.2 30.3 61.7 6.8 1.3 30.2 58.6 7.0 1.4 33.0 Kidney function at baseline eGFR X90 eGFR 60–89 eGFR 45–59 eGFR 30–44 eGFR 15–29 14.9 51.2 18.1 10.3 4.1 21.4 56.4 13.5 6.1 2.0 25.4 54.8 12.4 5.2 1.7 32.4 50.4 11.1 4.4 1.4 39.2 46.3 9.4 3.7 1.3 41.7 45.9 8.3 3.1 0.9 39.1 50.0 7.8 2.7 0.4 40.8 50.4 6.5 2.1 0.2 44.1 48.5 5.8 1.5 0.2 47.8 45.6 5.0 1.5 0.1 57.0 38.9 3.5 0.6 0.0 Cerebrovascular disease Peripheral vascular disease CHF COPD Cancer Myocardial infarction Peptic ulcer disease 7.2 5.6 12.2 22.7 12.6 7.6 3.9 5.4 4.0 7.7 19.7 9.7 5.4 3.2 5.1 3.6 6.5 18.6 9.6 4.9 2.9 4.5 3.2 5.7 17.9 9.1 4.4 2.8 4.5 3.0 4.9 17.3 8.8 4.0 2.6 4.4 2.9 4.6 17.4 8.8 3.9 2.7 4.9 3.1 5.2 18.0 9.2 4.3 2.7 4.7 3.2 5.1 18.3 9.2 4.4 2.9 5.3 3.2 5.7 18.8 9.5 4.6 2.8 5.8 3.7 6.2 19.9 9.8 4.5 3.1 6.4 3.7 6.8 21.5 11.0 5.0 3.3 Socioeconomic status Pensioner Low With subsidy 32.3 4.6 7.6 31.3 3.4 7.2 31.9 3.2 7.4 31.2 3.0 7.6 30.9 3.0 7.6 31.3 2.9 7.5 31.5 3.1 7.3 29.9 2.4 7.3 28.9 3.6 7.3 28.2 4.1 7.2 24.3 4.9 8.2 N (%) Age, mean(s.d.), years Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate. Socioeconomic status was categorized as high income (annual adjusted taxable family income XCAD39250), low income (annual adjusted taxable family income oCAD 39250), low income with receiving social assistance, and pensioners (age X65 years). Table 2 | Adjusted all-cause mortality rates, per 1000 person-years, by annual absolute rate of change in eGFR Annual absolute rate of change in eGFR (ml/min per 1.73 m2 per year) Events, n Patients, n p5 4 3 2 1 0 1 2 3 4 X5 6462 62,402 1808 28,457 2292 40,676 1853 55,649 3221 70,601 6426 135,804 2600 44,542 1793 31,612 1385 20,861 1088 13,857 2444 24,851 Adjusted rate 11.27 8.47 7.77 7.50 7.07 7.41 9.00 9.08 10.65 12.54 16.52 (95% CI) (10.90–11.65) (8.05–8.90) (7.41–8.12) (7.19–7.81) (6.79–7.34) (7.19–7.64) (8.62–9.39) (8.62–9.53) (10.05–11.24) (11.76–13.33) (15.78–17.25) Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate. Rates are adjusted for age, sex, diabetes, hypertension, socioeconomic status, kidney function, proteinuria, and history of cancer, cerebrovascular disease, congestive heart failure, chronic obstructive pulmonary disease, myocardial infarction, peptic ulcer disease, and peripheral vascular disease at baseline (last measurement). with an increased risk of death across all categories of baseline kidney function (Supplementary Appendix Table S2 online and Supplementary Appendix Figure S1 online). Similar results were obtained when the change in eGFR was defined as percentage change in eGFR per year (Supplementary Appendix Table S3 online and Supplementary Appendix 686 Figure S2 online). Results were similar when baseline was defined as the first serum creatinine measurement during the accrual period. In addition, similar results were observed when excluding participants with acute kidney injury–related hospitalization during the eGFR accrual period, when stratified analysis was carried out by baseline socioeconomic Kidney International (2013) 83, 684–691 clinical investigation TC Turin et al.: Short-term change in eGFR and ESRD Table 3 | Adjusted all-cause mortality rates, per 1000 person-years, by annual percentage change in eGFR Annual percentage change in eGFR (percent/year) Events, n Patients, n Adjusted rate (95% CI) p7 6 to 5 4 to 3 2 to 1 0 1 to 2 3 to 4 5 to 6 X7 8665 57,111 2297 38,375 2932 65,837 4009 107,786 4187 113,847 3359 67,327 2319 37,845 1535 19,456 3069 21,728 11.60 (11.20–11.99) 8.70 (8.30–9.10) 7.74 (7.43–8.06) 7.41 (7.15–7.67) 7.43 (7.17–7.68) 9.03 (8.68–9.38) 9.87 (9.43–10.31) 11.26 (10.66–11.87) 15.15 (14.52–15.78) Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate. Rates are adjusted for age, sex, diabetes, hypertension, socioeconomic status, kidney function, proteinuria, and history of cancer, cerebrovascular disease, congestive heart failure, chronic obstructive pulmonary disease, myocardial infarction, peptic ulcer disease, and peripheral vascular disease at baseline (last measurement). 10.00 Hazard ratio (95% CI) 2.20 1.68 1.52 1.14 1.05 1.01 1.00 1.22 1.22 1.43 0.95 1.00 (Reference) 0.10 –4 –3 –2 –1 0 1 2 3 4 .5 Proportion - –5 of patients (11.8%) (5.4%) (7.7%) (10.5%) (13.3%) (25.7%) (8.4%) (6.0%) (3.9%) (2.6%) (4.7%) Annual rate of change in eGFR Figure 3 | Risk of all-cause mortality by annual rate of change in estimated glomerular filtration rate (eGFR) adjusted for covariates at the baseline (last measurement). Models were adjusted for age, sex, diabetes, hypertension, socioeconomic status, kidney function, proteinuria, and history of comorbidities. CI, confidence interval. Hazard ratio (95% CI) 10.0 2.02 1.56 1.17 1.04 1.22 1.32 1 to 2 (12.7%) 3 to 4 (7.1%) 1.51 1.00 1.0 0.1 Proportion of patients 1.00 (Reference) - –7 (10.8%) –5 to –6 –3 to –4 –1 to –2 (7.2%) (12.4%) (20.4%) 0 (21.5%) 5 to 6 (3.7%) .7 (4.1%) Annual percentage change in eGFR Figure 4 | Risk of all-cause mortality by annual percentage change in estimated glomerular filtration rate (eGFR) adjusted for covariates at the baseline (last measurement). Models were adjusted for age, sex, diabetes, hypertension, socioeconomic status, kidney function, proteinuria, and history of comorbidities. CI, confidence interval. Kidney International (2013) 83, 684–691 687 clinical investigation status, as well as when analysis was performed including participants with an eGFR o15 ml/min/1.73 m2 (data not shown). DISCUSSION In this community-based cohort, change in eGFR over a period of up to 4 years (and requiring at least three eGFR measurements) was associated with an independent and graded increase in the risk of death. Compared with participants with stable kidney function, both declining and increasing eGFR was associated with a higher risk of death. The risk was consistent across subgroups of kidney function categories. Our observations regarding declining eGFR and mortality risk are consistent with other reports. The Cardiovascular Health Study included 4380 community-dwelling older adults with normal kidney function6 and reported a rate of change of eGFR over a 7-year follow-up period. Sixteen percent of participants experienced rapid decline in eGFR (rate 43 ml/min per 1.73 m2 per year). Rapid decline in kidney function was associated with a two-fold increased risk of all-cause mortality. It is interesting to note that even in the group of patients who had rapid decline in eGFR, the average eGFR at the end of the follow-up period was 69 ml/min per 1.73 m2 (whereas average baseline eGFR for participants was 79 ml/min per 1.73 m2). Similarly, in our study, a change in eGFR among higher levels of baseline kidney function was also associated with increased risk of death, suggesting that even with preserved kidney function the rate of change has prognostic information for future mortality risk. The Atherosclerosis Risk In Communities (ARIC) cohort8 also examined the association between 3- and 9-year changes in eGFR and the risk of death in 13,029 participants by dividing the patients into quartiles on the basis of percentage annual change in eGFR. The authors reported that the quartile of patients with the greatest annual decline in eGFR over 3 years was at a 22% greater risk of death compared with the patients who experienced minimal annual decline in eGFR. Similarly, patients with the greatest annual decline in eGFR over 9 years were at 41% greater risk of death compared with the patients who experienced minimal annual decline in eGFR. Data from the Department of Veterans Affairs has provided similar results9 where, during a median of 2.6 years, 10%, 28%, and 24% of participants experienced mild, moderate, and severe CKD progression, respectively (defined as eGFR loss of 0–1, 1–4, 44 ml/min/year), with an increased risk of death for those with moderate or severe CKD progression. Finally, a recent study of 15,465 patients with stage 3 and 4 CKD receiving primary care at a single institution7 reported an 84% increase in mortality for those with decreasing eGFR (median –4.8 ml/min per 1.73 m2 per year, IQR: –8.2 to –3.2), compared with those with stable eGFR. Apart from eGFR, changes in serum albumin and C-reactive protein (CRP) over time have also been studied and were reported to be associated with adverse outcome risk. Among adult hemodialysis patients, a decrease in serum 688 TC Turin et al.: Short-term change in eGFR and ESRD albumin was associated with increased mortality risk.13 Although there was no association between change in albumin and all-cause mortality in the Longitudinal Aging Study Amsterdam,14 an increase in CRP levels15 was associated with an increased mortality risk among the elderly. Why is declining kidney function associated with an increased risk for death? Declining kidney function may contribute to increased risk by aggravating cardiovascular risk factors, endothelial dysfunction, oxidative stress, and vascular damage, as well as through activation of the renin–angiotensin system induced by renal impairment.6,8,16,17 Further, worsening kidney function among patients with relatively severe impaired kidney function status may result in decreased appetite, decreased physical function, and overall frailty,6,18,19 thus indirectly contributing to a higher mortality risk among this subgroup. Our finding that increasing eGFR is associated with excess mortality is also similar to previous studies. Perkins et al.7 reported that, in comparison with patients with stable eGFR, increasing eGFR was associated with a 42% increased risk of death. Among ARIC study participants with stage 3 CKD,8 the group with minimal decline or an increase in eGFR (annual change: –0.33 to 42.94%) also experienced a two-fold increased risk of death. Recently Al-Aly et al.10 reported that (compared with patients with mild CKD progression) those who experienced no decline in kidney function exhibited a trend toward increased risk of death (HR 1.15; 95% CI: 0.99–1.24). The explanation for the association between increasing eGFR over time and increased risk of death is not apparent, but this finding might be attributable to lower serum creatinine generation as a result of reduced muscle mass associated with chronic debilitating conditions.10,20 Although our analysis included only outpatient serum creatinine measurements and further focused on those who had measurements available over longer time horizons (median eGFR accrual period 3.0 years) to minimize the effect of acute kidney injury, the residual confounding from resolving acute kidney injury may also have contributed to the observed increased mortality risk associated with improvement in kidney function. However, exclusion of patients with acute kidney injury–related hospitalizations during the eGFR accrual window did not qualitatively affect our study results. These findings indicate toward the possibility that increasing eGFR could be a marker of illness rather than an independent risk factor for death. Our study is strengthened by its large sample size, which allowed us to study participants with a broad range of baseline kidney function. Our study also has limitations. The study cohort was limited to individuals who had outpatient serum creatinine measurements as part of routine care, and therefore does not include individuals who did not access medical services. This might have resulted in inclusion of patients with comorbid conditions associated with a more rapid change in eGFR and increased risk of the adverse outcomes. However, as we studied mortality among subjects with an estimate of kidney function, this limitation does not Kidney International (2013) 83, 684–691 TC Turin et al.: Short-term change in eGFR and ESRD invalidate our findings. Given that we have used multiple serum creatinine measurements over time, laboratory drift over time may have influenced the study results. However, the impact of this potential laboratory drift is expected to be minimal, as we calibrated measurements across time periods against a subset of healthy participants. Further, we have previously reported on the increased mortality risk associated with short-term changes in kidney function (adults with at least two outpatient eGFR measurements during a 1-year accrual period).21 The categorization of baseline kidney function categories was based on eGFR values alone, which may have led to misclassification of kidney function. In addition, although we have adjusted for the presence and severity of proteinuria, the majority of these measurements were based on urinary dipstick, limiting our ability to assess change in proteinuria levels over time. Finally, although we adjusted for demographic factors, measured comorbidities, and proteinuria, we were unable to adjust for covariates such as body mass index, blood pressure control, cause of kidney disease, and smoking status, introducing the possibility of residual confounding. We also could not adjust for drug use, as this information is available for a subsection of the population in Alberta aged 65 years and older. However, given the magnitude of the observed associations, this limitation is unlikely to invalidate our conclusions. In conclusion, we found that both declining and increasing eGFR over time were independently associated with mortality risk. These results suggest that monitoring change in eGFR over time may enhance future mortality risk prognostication in addition to the baseline kidney function. MATERIALS AND METHODS Study population and data source The study cohort consisted of adults, aged 18 years or older, in Alberta, Canada who had at least three outpatient serum creatinine measurements spanning a time period of four calendar years (Figure 2). We used the data repository of the Alberta Kidney Disease Network22 to create the study cohort. The cohort accrual period was from 1 May 2002 to 31 December 2007, with follow-up extending to 31 March 2009 (the date up to which outcome data were available). Patients receiving chronic dialysis or a kidney transplant on or before cohort entry were identified from the databases of Northern Alberta and Southern Alberta Renal Programs and administrative data using a validated algorithm and were excluded from the current analysis.23,24 Patients who developed endstage renal disease during the follow-up period were retained in the analysis. Among 1,818,451 patients with at least one outpatient serum creatinine measurement, there were 529,954 participants with three or more measurements over four calendar years. After exclusion of 642 participants with a first eGFR o15 ml/min per 1.73 m2, a total of 529,312 participants were included. Magnitude of change in kidney function The CKD-EPI equation25 was used to estimate the glomerular filtration rate using outpatient serum creatinine measurements from the accrual period. Serum creatinine measurements during the study period were standardized to a central laboratory. This reference laboratory (Capital Health Region, year 2009) used an isotope Kidney International (2013) 83, 684–691 clinical investigation dilution mass spectrometry reference standard. Gender-specific correction factors were used to ensure province-wide standardization of serum creatinine values over time. Change in eGFR over time was estimated using all available outpatient eGFR measurements for each patient during the accrual period. We used two indices to describe the magnitude of change in eGFR: (a) the absolute annual rate of change and (b) the annual percentage change. The absolute annual rate of change in eGFR was calculated by fitting a least-squares regression6 to all measurements for each patient, where the slope of the regression line describes the absolute rate of change for eGFR over time. The percentage change in eGFR was calculated assuming a linear change on the log scale, consistent with prior work.8 Given the size of the cohort, we were able to define change in eGFR using a number of categories. The absolute annual rate of change in eGFR was categorized as p 5, 4, 3, 2, 1, 0, 1, 2, 3, 4, andX5 ml/min per 1.73 m2 per year. The annual percentage change in eGFR was categorized as p 7, 6 to 5, 4 to 3, 2 to 1, 0, 1–2, 3–4, 5–6, and X7 percent/year. Assessment of covariates Baseline was defined as the date of the last eGFR measurement during the 4-year accrual period, and was the point at which followup for outcome ascertainment (all-cause mortality) commenced (Figure 2). The date of the last eGFR measurement was chosen for the baseline, as this is the point when the patient is seen by the clinician and the time at which previous changes in kidney function will be taken into consideration and extrapolated for prediction of future risk. All covariates were assessed at the baseline. On the basis of Government of Alberta health-care insurance records,26 socioeconomic status was characterized as high income (annual adjusted taxable family income X$39,250 CAD), low income (annual adjusted taxable family income o$39,250 CAD), low income with subsidy (receiving social assistance), and pensioners (65 years of age and older).3,22 Using validated algorithms27,28 from hospital discharge records and physician claims, diabetes mellitus and hypertension were identified. The Deyo classification of Charlson comorbidities were identified from the physician claims and hospitalization records using validated ICD-9-CM and ICD-10 coding algorithms.29 Kidney function at baseline was divided into categories of eGFR X90, 60–89, 45–59, 30–44, and 15–29 ml/min per 1.73 m2, respectively. Baseline proteinuria was estimated by urine albumin:creatinine ratio (ACR) or urine dipstick based on outpatient random spot urine measurements, and was categorized as normal, mild, heavy, or unmeasured based on ACR (normal: o30 mg/g, mild: 30–300 mg/g, or heavy: 4300 mg/g) or urine dipstick (negative: no trace, mild: trace or 1 þ , or heavy: 2 þ ).3,30 Serum albumin or CRP levels were not available for the study participants. Assessment of outcome The primary outcome of interest was all-cause mortality, as determined from Vital Statistics data of the Alberta Health and Wellness Registry file. Outcome ascertainment was prospectively performed from the date of the last outpatient serum creatinine measurement in the accrual period (baseline) to the end of the study (31 March 2009). Statistical analyses Poisson regression was used to estimate all-cause mortality rates, expressed per 1000 person-years of follow-up, for each group of 689 clinical investigation TC Turin et al.: Short-term change in eGFR and ESRD change in eGFR, after adjustment for sociodemographic variables, baseline kidney function, proteinuria, and covariates. If the Poisson assumption was not met, a quasi-Poisson model was used.31 Cox proportional hazards models were used to estimate the adjusted risk of all-cause mortality associated with each group of change in kidney function, with stable kidney function (0 ml/min per 1.73 m2 per year for the absolute rate of change and 0 percent/year for percentage change) used as the reference. The proportional hazards assumption was tested and met. Participants were censored at study end (31 March 2009) if they were still at risk or at an earlier date if they experienced the event of interest or if they left the province. We performed several sensitivity analyses to verify the robustness of our study findings. We repeated analyses stratified by baseline eGFR category for rate of change by both absolute rate of change and percentage change. We also repeated all analyses in which ‘baseline’ was defined as the first eGFR measurement during the 4year accrual period. We also repeated analyses excluding participants who had an acute kidney injury–related hospitalization32 during the eGFR accrual period. Analysis was also undertaken stratified by socioeconomic status categories. Finally, we also repeated analyses including participants with an eGFR o15 ml/min/1.73 m2. Statistical analyses were performed using SAS version 9.2 (SAS Institute, NC) and STATA version 11.2 (STATA, College Station, TX). The institutional review board of the University of Calgary approved the study. 14. DISCLOSURE 15. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. All the authors declared no competing interests. 16. ACKNOWLEDGMENTS TCT is supported by Fellowship Awards from the Canadian Institutes of Health Research, Canadian Diabetes Association, and the Interdisciplinary Chronic Disease Collaboration (ICDC) team grant funded by Alberta Innovates—Health Solutions (AI-HS). BRH and MT are supported by AI-HS Salary Awards. BRH is supported by the Roy and Vi Baay Chair in Kidney Research, and MT is supported by a Canada Research Chair. JC and KM are supported by grants to the CKD Prognosis Consortium from the National Kidney Foundation and its sponsors. 17. 18. 19. 20. SUPPLEMENTARY MATERIAL Appendix Figure S1. Risk of all-cause mortality by annual rate of change in eGFR across baseline levels of kidney function. Appendix Figure S2. Risk of all-cause mortality by annual percentage change in eGFR across baseline levels of kidney function. Appendix Table S1. Characteristics of patients included and excluded in the study cohort. Appendix Table S2. All-cause mortality risk by annual rate of change in eGFR, stratified by baseline eGFR category. Appendix Table S3. All-cause mortality risk by annual percentage change in eGFR, stratified by baseline (last measurement) eGFR category. Supplementary material is linked to the online version of the paper at http://www.nature.com/ki 21. 22. 23. 24. 25. 26. REFERENCES 1. 2. 690 Fried LF, Katz R, Sarnak MJ et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 2005; 16: 3728–3735. Go AS, Chertow GM, Fan D et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305. 27. 28. Hemmelgarn BR, Manns BJ, Lloyd A et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303: 423–429. Turin TC, Tonelli M, Manns BJ et al. Chronic kidney disease and life expectancy. Nephrol Dial Transplant 2012; 27: 3182–3186. Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073–2081. Rifkin DE, Shlipak MG, Katz R et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 2008; 168: 2212–2218. Perkins R, Bucaloiu I, Kirchner H et al. GFR decline and mortality risk among patients with chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 1879–1886. Matsushita K, Selvin E, Bash LD et al. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol 2009; 20: 2617–2624. Cheng TYD, Wen SF, Astor BC et al. Mortality risks for all causes and cardiovascular diseases and reduced GFR in a middle-aged working population in Taiwan. Am J Kidney Dis 2008; 52: 1051–1060. Al-Aly Z, Zeringue A, Fu J et al. Rate of kidney function decline associates with mortality. J Am Soc Nephrol 2010; 21: 1961–1069. Shlipak MG, Katz R, Kestenbaum B et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol 2009; 20: 2625–2630. Shlipak MG, Katz R, Kestenbaum B et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol 2009; 30: 171–178. Pifer TB, Mccullough KP, Port FK et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int 2002; 62: 2238–2245. Schalk B, Visser M, Bremmer M et al. Change of serum albumin and risk of cardiovascular disease and all-cause mortality. Am J Epidemiol 2006; 164: 969. Alley DE, Crimmins E, Bandeen-Roche K et al. Three-year change in inflammatory markers in elderly people and mortality: The Invecchiare in Chianti Study. J Am Geriatr Soc 2007; 55: 1801–1807. Manjunath G, Tighiouart H, Ibrahim H et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community* 1. J Am Coll Cardiol 2003; 41: 47–55. Weiner DE, Tighiouart H, Amin MG et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15: 1307–1315. Shlipak MG, Stehman-Breen C, Fried LF et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis 2004; 43: 861–867. Odden MC, Chertow GM, Fried LF et al. Cystatin C and measures of physical function in elderly adults. Am J Epidemiol 2006; 164: 1180–1189. Kovesdy CP, George SM, Anderson JE et al. Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr 2009; 90: 407–414. Turin TC, Coresh J, Tonelli M et al. One-year change in kidney function is associated with an increased mortality risk. Am J Nephrol 2012; 36: 41–49. Hemmelgarn BR, Clement F, Manns BJ et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol 2009; 10: 30. Clement F, James M, Chin R et al. Validation of a case definition to define chronic dialysis using outpatient administrative data. BMC Med Res Methodol 2011; 11: 25. Manns BJ, Mortis GP, Taub KJ et al. The Southern Alberta Renal Program database: a prototype for patient management and research initiatives. Clin Invest Med 2001; 24: 164–170. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. Premium assistance program: Premium subsidy 2010; http:// www.health.alberta.ca/services/premium-subsidy.html (accessed 14 January 2013). Hux JE, Ivis F, Flintoft V et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002; 25: 512–516. Quan H, Khan N, Hemmelgarn BR et al. Validation of a case definition to define hypertension using administrative data. Hypertension 2009; 54: 1423–1428. Kidney International (2013) 83, 684–691 clinical investigation TC Turin et al.: Short-term change in eGFR and ESRD 29. 30. Quan H, Sundararajan V, Halfon P et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–1139. Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46: 205–217. Kidney International (2013) 83, 684–691 31. 32. Gardner W, Mulvey E, Shaw E. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol Bull 1995; 118: 392–404. Waikar SS, Wald R, Chertow GM et al. Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol 2006; 17: 1688–1694. 691
© Copyright 2026