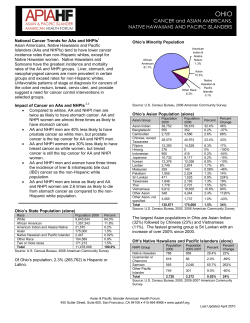
Document 8577
Ta b l e o f C o n t e n t s Letter from Leadership . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1 Biomedical Research at Ohio State . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 Noted Research Advocate’s Address . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 Major Research Programs of 2006 . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13 Current NIH Program Grants and Center Grants . . . . . . . . . . . . . . . .36 Major Research Centers Affiliated With OSUMC . . . . . . . . . . . . . . .40 School and Department-Based Research Programs and 2006 Highlights . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .61 Technology Commercialization & Partnerships . . . . . . . . . . . . . . . . .110 Medical Center Information Warehouse . . . . . . . . . . . . . . . . . . . . . . .113 Translational Research . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .118 Clinical Trials Research . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .120 Faculty Serving on National Study Sections and Review Panels . . .131 Research Core Facilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .141 University Resources for Biomedical Research . . . . . . . . . . . . . . . . .146 Research Partnerships . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .148 Educational Initiatives Promoting Biomedical Research . . . . . . . . .156 2006 Sponsored Research Grants by Department . . . . . . . . . . . . . .159 Selected High-Impact Publications by Department . . . . . . . . . . . . .187 Statistical Year in Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .205 Contact Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .207 Acknowledgments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .210 Commentary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .211 Letter from Leadership 2006 was a pivotal year for biomedical research at Ohio State University Medical Center and may have set the course of our research for years to come. All of our 2006 research activities culminated in a three-month span at the end of the year when we: opened a 10-story biomedical research tower (BRT); hosted a major industry collaboration symposium that attracted leaders from the worlds of science, industry, law and government; and conducted branding exercises that gave us a new logo and affirmed our commitment to improving lives through personalized health care. The BRT, which doubles the amount of biomedical research space at Ohio State, houses faculty from multiple programs and will hasten the pace of scientific discovery that translates to innovative treatments for patients. The symposium, held in the newly opened BRT, allowed participants to explore opportunities for university/industry collaboration and to showcase emerging biomedical technologies needing commercial partnerships. This daylong event included a “technology marketplace” where corporate, angel and venture investors could take the first steps toward meeting directly with the inventors behind some of the Medical Center’s newest products and discoveries. After months of planning, we conducted highly visible branding exercises in a 24-hour celebration that symbolized our around-the-clock dedication to personalized health care – the integrative practice of medicine and individual support based on a person’s unique biologic, behavioral and environmental characteristics. The celebration emphasized that personalized health care is the next step in the Medical Center’s strategic plan and is being incorporated in our mission areas of research, education and patient care. We hope that message is apparent throughout our fourth annual Research Report, which chronicles some of the many ways during 2006 that we guided scientific discovery toward medical advancement. Fred Sanfilippo, MD, PhD Senior Vice President and Executive Dean for Health Sciences CEO, Ohio State University Medical Center Wiley “Chip” Souba, MD, ScD Dean, Ohio State University College of Medicine Caroline Whitacre, PhD Associate Vice President for Health Sciences Research Vice Dean for Research Director, School of Biomedical Science, Ohio State University College of Medicine 2007 Research Report 1 B i o m e d i c a l R e s e a r c h AT O H I O S TAT E 2006 saw continued growth in biomedical research that enhances personalized health care at Ohio State University Medical Center (OSUMC), which in November took a giant step forward by opening an eagerly awaited Biomedical Research Tower (BRT) on West 12th Avenue – the largest research facility on the Ohio State campus. The year was also marked by increased extramural funding for medical researchers and publication of their significant findings in high-profile scientific journals. The next several pages spotlight the top biomedical research stories of 2006 at the Medical Center, starting with the BRT opening and what it means to both the University and the community. 2 Ohio State University Medical Center The tower has 10 stories and 264,000 square feet of dedicated research space designed in open-lab layouts to promote interdisciplinary interaction among research teams. BIOMEDICAL RESEARCH TOWER HELPS ADVANCE PERSONALIZED HEALTH CARE Hundreds of people attended the Nov. 3, 2006, grand opening celebration for Ohio State University Medical Center’s $160 million Biomedical Research Tower (BRT), a symbol of the institution’s commitment to research that will benefit personalized health care. The BRT, under construction since 2003 on West 12th Avenue, opened its doors in December 2006 to researchers pursuing discoveries that will dramatically advance patient care in many medical disciplines. The celebration featured remarks from Mary Woolley, a nationally recognized advocate for science and president of Research!America, a nonprofit alliance working to elevate health research among the nation’s priorities (see related story, page 5). “This structure allows for productive interactions among faculty, students and postdoctoral researchers. It’s a very powerful model,” says Caroline Whitacre, PhD, vice dean and associate vice president for Health Sciences Research at the Medical Center. “The design allows for faster progression of research. We’re constantly looking to progress faster, publish faster and be more successful at securing external grant funding. This will only help that process along.” About 60 faculty scientists and their teams, totaling approximately 500 research staff, moved into the five research floors that opened initially. The first occupiers included research teams in cancer, cardiovascular disease, neuroscience and infectious diseases. Three floors of the building remain shelled and will be built out in a second phase of development over the next several years. “More scientists and students want to be here than we have been able to accommodate,” says Fred Sanfilippo, MD, PhD, senior vice president and executive dean of Health Sciences, and CEO of Ohio State’s Medical Center. “Just having this building under construction was a powerful recruitment tool because it demonstrated our vision for the Medical Center that resonates with biomedical scientists. 2007 Research Report 3 “More importantly,” he adds, “this building represents a major investment in biomedical research that holds promise for identifying biological mechanisms of disease, which is at the crux of personalized health care. These scientific advances will aid us in delivering preventive and diagnostic care and treatments that match individuals’ unique characteristics and needs.” Eventually, the tower will house more than 100 faculty scientists and their teams – a total of 800 researchers – specializing in cancer and cancer genetics, cardiovascular and lung disease, and highfield imaging, as well as biology, biotechnology and biomedical informatics programs. It will also expand programs in such emerging fields as neurological disorders, heart failure and heart imaging, pharmacogenomics and targeted molecular therapies, microbial pathogenesis and biodefense, and tissue engineering. The tower is expected to bolster the state’s economy by generating approximately 17,000 new jobs in 4 Ohio State University Medical Center biomedical technology-related fields and bringing an estimated $3.7 billion to Ohio over the next decade. Among the building’s special features are linear equipment corridors in which to locate large pieces of equipment – such as freezers, refrigerators and centrifuges – that generate noise and heat as well as cause vibrations. The tower also features “green” construction efforts, including locating offices on one side of the building and labs on the other so recirculated air in offices is separate from lab air that is exhausted to the outside. Lights throughout the building have auto-shutoff mechanisms and motion activation. Bonds were sold to finance most of the building and will be repaid in large part by grant revenue earned by researchers. Additional funding is provided through private fundraising, federal grants, state capital appropriations and University support. Noted Research Advocate The keynote speaker at the Nov. 3, 2006, dedication of Ohio State’s new Biomedical Research Tower (BRT) was Mary Woolley, a nationally recognized advocate for research and president of Research!America, a nonprofit alliance working to elevate health research among the nation’s priorities. Her address appears below. Thank you for having me here today on this historic occasion to talk about the importance of research to Ohio and to our nation. It’s a fact that research changes history, including the history of health and well-being. Many people alive today do not know what an iron lung is; many physicians have never seen a case of smallpox. Life saving and quality of life-enhancing aspects of daily living that we regard as common sense today were not always so: consider childhood vaccinations, screening for breast and prostate cancer, effective antihypertensive medications, the use of seat belts, putting babies on their backs to sleep, practicing safe sex, screening the blood supply for toxic agents, getting flu shots – the list goes on. The fact is that today’s common sense is based on yesterday’s research. Research will lead us to tomorrow’s common sense and will in the process help us save lives and money as we advance toward the era of personalized medicine and put the products of research to work in evidence-based fashion. SPEAKS AT BRT DEDICATION Thinking back about things we didn’t used to take for granted is a useful way to capture the ongoing progress of medical research. At Research!America we call this the “Then – Now – Imagine” framework – the perfect theme to capture the excitement and promise of medical research. The accomplishments of medical research over the past 60 years have taught us to think big about what will come next, and then to take the “imagine” step aggressively. For example, and in keeping with some of the research that’s happening here at Ohio State, think back: Then … to the 1970s, when only one child in 10 survived cancer. Now … seven of 10 children who develop cancer are alive five years after diagnosis. Imagine … eliminating suffering and death due to childhood cancer by the year 2015. Then … heart disease killed quickly and without warning. Now … deaths from heart disease have dropped by 60 percent; it is no longer the No. 1 killer of Americans under the age of 85. Imagine … eliminating preventable deaths due to heart disease. “Then – Now – Imagine” has long been the driving spirit of science. This theme expresses a commitment to discovery and its translation to better health. 2007 Research Report 5 The United States, founded by leaders of the Enlightenment, has always had high aspirations realized through a commitment to science and innovation. Americans today – including the people of Ohio – continue to be intrigued by the challenge to innovate. As we’ve heard, this new building promises to bolster the state’s economy by creating 17,000 new jobs and bringing $3.7 billion to Ohio in the next 10 years. The combination of societal aspiration and the record of return on investment in science would seem to make investment in science almost irresistible. Several years ago we held focus group sessions here in Columbus, asking citizens what they thought about research. In response to a question about whether and why it is important to invest in basic research, the proprietor of a dry-cleaning establishment memorably said, “I’ll tell you why basic research is important to me – it’s because I believe in possibilities.” Yet, there is surprising resistance to increased investment in research at the federal level to match the aspirations of the people of Ohio and all across the nation. You may be aware that funding for the National Institutes of Health, which not too long ago was doubled in anticipation of capturing the enormous promise of research at the beginning of this century, has now been cut for the first time in 30 years. “I believe in possibilities.” Doesn’t that say it all about American values, the American spirit, American determination and accomplishment? The possibilities offered by research have never been greater. We have never had as many researchers at work unraveling the mysteries of disease and disability, and their cure, treatment and prevention. This is an exciting moment of possibility – possibilities we are on the cusp of realizing – leading to lives that will be saved and enhanced because of research that will be conducted here in this magnificent new building. The contributions to research and health that those who will work here will make are worthy of our admiration. These are the people who will be making groundbreaking discoveries to fight cancer and to better understand neurological disorders, heart failure and heart imaging, pharmacogenomics, targeted molecular therapies, microbial pathogenesis and biodefense, and tissue engineering. These scientists will deliver for you on your investment – Ohio’s investment – and these are the people who will be on the front lines of realizing the aspirations of Americans to put research to work – faster – to produce new cures, treatments and preventions. Public opinion data confirms the value citizens place on innovation, as does everyday experience. Research promises and delivers better products, better jobs, better health and better quality of life. 6 Ohio State University Medical Center Ohio State University has earned double-digit percentage increases in support from the NIH in the past two years and has more than doubled its annual NIH funding over the past six years. But cuts and a flat-funded NIH budget do not bode well for sustained robust federal support for the cutting-edge science under way here at Ohio State. What can be done to address and redress the declining NIH budget? Now, especially just a few days before the election, all of us who are committed to putting research to work must think and act politically. I don’t mean in a partisan manner. Research is not a partisan issue. There are and always have been champions for research on both sides of the aisle. From public opinion polls we know that seven in 10 potential voters say they would be more likely to vote for a candidate who is a strong supporter of federal spending for medical, health and scientific research; 55 percent say that investing more in medical research is important now even in the face of competing budget demands; and 67 percent of Americans say support for embryonic stem cell research is an important issue in deciding how they will vote in November. These are potential voters’ views – but what about the positions of candidates running for office? Unless the people of Ohio are different from the rest of the nation, most of us don’t know the positions on medical research issues of most incumbents running for re-election, not to mention the positions of their challengers. Research!America and the Albert and Mary Lasker Foundation have created a new voter guide, Your Candidates–Your Health, to provide stakeholders in research and the public at large with educational information on candidates’ positions. The novelty and value of this resource has earned attention from PARADE magazine, which has alerted its enormous national readership – 75 million strong – about the importance of checking this guide before voting. Let me challenge you to answer whether your federal representatives are strongly identified with medical research as a national priority? Congresswoman Deborah Pryce most certainly is; we salute you, Congresswoman, for your leadership on this issue so critical to us all. Unfortunately, the vast majority of Ohio’s Congressional candidates have not said “yes” to making medical research a much higher national priority. You and people you know all over the state can change that! If candidates for Congress heard from their constituents about the importance of spending more than 1 percent of the federal budget on medical research that seeks to gain an understanding and eventual conquest of every disease you can name, then they would certainly take heed and act accordingly. Our voter guide makes it easy not only for you to find out where your candidates stand but also provides links to contact your candidates and urge them to make medical research a higher priority in their campaigns and, for the winners, in their legislative work on the Hill. You can find our voter guide on the Web at: www.YourCandidatesYourHealth.org. While I am speaking of the importance of contacting elected officials, I want to applaud the leaders of Ohio State for the development of your Medical Advocacy Program with a stated goal to have at least one medical advocate representing each of the 88 counties in Ohio, as well as the officials Ohio sends to Washington. These advocates are empowered to make contact with their elected representatives at the local, state and federal levels to make the case for improving the level of quality in health care and medical education, and for growth in investment in medical and health research. To the best of my knowledge, this is a unique program in the nation, and one I hope will soon be emulated from coast to coast. I urge you to join me and Research!America in making the future bright for research and researchers, and for the American public that wants research to succeed. We must elect candidates who will heed the call of Americans who not only imagine a healthier future powered by research, but who are increasingly demanding that their elected officials heed that clarion call. Imagine a Congress composed of a strong majority who support investing more in medical research and will assure a policy environment that enhances rather than inhibits medical research. Imagine that research is given a chance to succeed at the rate of scientific opportunity, not stifled in short-sighted, ill-advised budget cutting. Imagine that significantly enhanced support for research becomes the No. 1 priority of Congress. And imagine that strengthened federal investment in research is the first step in assuring nonpartisan commitment to addressing other ills facing our nation. Daring to imagine is a hallmark of great science, great leadership and great progress. As Ohio Pulitzer Prizewinning poet Rita Dove has said, “Without imagination we can go nowhere.” Dove is, like you who will work in this research tower, unafraid to imagine the possibilities. I salute her spirit, and I salute the spirit of the research community. I am proud to be an advocate for medical research and privileged to be here today on this historic occasion for the future of health, based on research. I wish all of those who will be working in this glorious new home for research the greatest success. I look forward to celebrating your accomplishments. (A commentary by Mary Woolley appears on the inside back cover of this report.) 2007 Research Report 7 13 ELECTED AS AAAS FELLOWS The American Association for the Advancement of Science (AAAS) in 2006 elected 18 more Ohio State University faculty members as AAAS Fellows, including 13 who are in Health Sciences colleges or research institutes. Ohio State led the nation in new AAAS fellows each year from 2003-05, and the University’s 2006 total was the nation’s second highest. The AAAS is the largest federation of scientists worldwide and among the most renowned. In 2006 it elevated 449 members to Fellow for their distinguished efforts to advance science and its applications. The 13 new AAAS Fellows from Health Sciences colleges or research institutes at Ohio State are: Sanford Barsky, MD, Pathology; Charles Capen, DVM, PhD, Veterinary Biosciences; Patrick Green, PhD, Veterinary Biosciences; Joanna Groden, PhD, Molecular Virology, Immunology and Medical Genetics; Nyla Heerema, PhD, Pathology; Tim HuiMing Huang, PhD, Molecular Virology, Immunology and Medical Genetics; Pravin Kaumaya, PhD, Obstetrics and Gynecology; A. Douglas Kinghorn, PhD, Medicinal Chemistry and Pharmacognosy; Nina Mayr, MD, Radiation Medicine; Stephen Osmani, PhD, Molecular Genetics; Deborah Parris, PhD, Molecular Virology, Immunology and Medical Genetics; Christopher Walker, PhD, Pediatrics, and Molecular Virology, Immunology and Medical Genetics; Mary Ellen Wewers, PhD, MPH, RN, Health Behavior and Health Promotion. SCIENTISTS TARGET EPIGENETIC SILENCING OF GENES IN LEUKEMIA WITH $11.84 MILLION GRANT The National Cancer Institute (NCI) awarded researchers at OSUMC a five-year, $11.84 million grant to study chronic lymphocytic leukemia (CLL) and translate basic research findings into clinical trials for patients with CLL, one of the most common forms of leukemia among adults in the Western 8 Ohio State University Medical Center Hemisphere. The grant, titled “DNA methylation & chromatin modifications: mechanisms & applications in cancer therapy,” was awarded to a team led by Program Director and Principal Investigator Samson Jacob, PhD, a professor of Molecular and Cellular Biochemistry and co-leader of the Experimental Therapeutics Program in Ohio State’s Comprehensive Cancer Center. Jacob says the longterm objective of the grant is to advance knowledge of the epigenetic regulation of gene expression in cancer cells and apply it to treatment (epigenetic therapy). Epigenetic changes are alterations in gene function that occur without a change in the genetic sequence of a cell’s DNA. CALCIUM + VITAMIN D SUPPLEMENTATION DOES AN OLDER BODY GOOD The older the woman, the more likely that consistent use of calcium and vitamin D supplements will help reduce her risk for osteoporosis, according to results of a national clinical trial conducted as part of the Women’s Health Initiative (WHI). In final results answering the initiative’s questions about women’s health, study leaders say that even the slight benefits demonstrated by the trial, which involved more than 36,000 participants, suggest calcium and vitamin D supplementation provides a health benefit to postmenopausal women. These findings were published in the Feb. 16, 2006, issue of the New England Journal of Medicine. “The value of a study this large is that it shows, even if only on a small scale, that this intervention can lower the risk of osteoporosis within two to three years,” says Rebecca Jackson, MD, lead author of the article and principal investigator for the WHI at OSUMC. STUDY SHOWS CELLS HAVE NATURAL DEFENSE AGAINST HIV Scientists at OSUMC discovered a mechanism that cells use to fight off the human immunodeficiency virus (HIV), the cause of AIDS. Their findings indicate that two proteins that normally help repair cellular DNA can also destroy the DNA made by HIV after it enters a cell. This HIV DNA is essential for the virus to survive and reproduce. The study, led by Richard Fishel, PhD, professor of Molecular Virology, Immunology and Medical Genetics, was published in Proceedings of the National Academy of Sciences. These findings could lead to a new strategy for treating HIV infection and AIDS, one that might complement current therapies and would probably be less susceptible to viral drug resistance – an increasingly urgent dilemma for patients and doctors. “Our findings identify a new potential drug target, one that involves a natural host defense,” Fishel says. “HIV treatments that target cellular components should be far less likely to develop resistance.” MICRO RNA FINGERPRINT IDENTIFIED IN PLATELET FORMATION identified the miRNAs also say some of them, when acting abnormally, may contribute to certain leukemias. “We found that a specific set of miRNA genes is turned off in normal platelet development, but turned on in certain platelet-related leukemia cells,” says lead author Ramiro Garzon, MD, clinical instructor in the College of Medicine. The study was published in Proceedings of the National Academy of Sciences. MiRNA has only recently been acknowledged as an important force in biology. Carlo Croce, MD, director of Ohio State’s Human Cancer Genetics Program, was the first to discover a link between miRNA and cancer. In this study, Croce and Garzon, along with colleagues from M.D. Anderson Cancer Center, examined miRNA activity in the earliest phases of platelet development. DIURETICS MAY NOT BE BEST WAY TO REDUCE CHF WATER RETENTION Researchers at OSUMC believe they may have identified a new agent to reduce excess fluid build-up in patients with congestive heart failure (CHF). The agent avoids the sodium-depleting effects of diuretics, now the most commonly used drugs for this purpose. Though diuretics reduce excess fluid buildup that is characteristic of CHF, they also can cause the kidneys to excrete more sodium than water, which can have damaging effects. The drug under investigation, called lixivaptan, appears as effective as a diuretic in helping patients get rid of excess water and also retains proper sodium levels in the body, says William Abraham, MD, director of Cardiovascular Medicine and lead author of a study published in the Journal of the American College of Cardiology. Abraham also will serve as international principal investigator of a multi-center phase III trial to further evaluate the drug. A few newly identified microRNAs (miRNAs) appear to play a significant role in the development of platelets – blood cells critical to the body’s ability to form clots following an injury. The scientists who 2007 Research Report 9 MUTATED GENE PREDICTS RETURN OF COMMON LEUKEMIA A new study at OSUMC shows that mutations in a cancer-related gene may help predict whether a common subtype of acute myeloid leukemia (AML) is more likely to return in patients. Researchers found that patients with mutations in a gene called KIT – which is already linked to this form of AML – were associated with significantly higher recurrence rates. The findings were presented at a plenary session during the annual meeting of the American Society of Clinical Oncology by Peter Paschka, MD. Paschka was completing a research fellowship under the guidance of Clara D. Bloomfield, MD, a Distinguished University Professor who also serves as cancer scholar and senior adviser to Ohio State’s Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute. “These findings could represent a significant advance in the therapy of AML,” says Paschka, lead author for the study. “They could lead to more effective targeted therapies and improved cure rates for certain AML patients.” CPR DEVICE DOES NOT IMPROVE SURVIVAL Researchers seeking ways to improve survival from cardiac arrest were surprised by results from a study comparing manual cardiopulmonary resuscitation (CPR) compressions with those given by a Food and Drug Administration (FDA)-approved mechanical device. The study, conducted in five cities including Columbus, showed that victims of sudden cardiac arrest were more likely to be discharged alive from the hospital if they received manual CPR rather than CPR administered by the mechanical device. Study results were published in the Journal of the American Medical Association. Michael Sayre, MD, of Emergency Medicine at OSUMC, was principal investigator for the Columbus study site. Lynn White, clinical 10 Ohio State University Medical Center research manager in Emergency Medicine, coordinated the 34 medic crews involved in the Columbus study. “Everyone thought the device was a great idea and that its ability to provide compressions of much higher quality than those administered by humans would be lifesaving,” White says. “The results are not what we anticipated.” DEVICE ZAPS THE PAIN OUT OF MIGRAINES An electronic device designed to “zap” migraine pain before it starts may be the next form of relief for millions who suffer from the debilitating disease. Results from a study led by researchers at OSUMC found that the experimental device appears effective in eliminating headache when administered during the onset of the migraine. The device, called TMS, interrupts the aura phase of the migraine, often described as electrical storms in the brain. Auras are neural disturbances that signal the onset of migraines. Yousef Mohammad, MD, principal investigator for the study at Ohio State, says patients reported a significant reduction in nausea, noise and light sensitivity post-treatment. “Perhaps the most significant effect of using the TMS device was on the two-hour symptom assessment, with 84 percent of the episodes in patients using the TMS occurring without noise sensitivity,” he says, noting that their work functioning also improved and they reported no side effects. NEW MOUSE STRAIN MIMICS CHRONIC LEUKEMIA An OSUMC study shows that a new mouse strain offers the first animal model for an incurable leukemia and should aid drug development. The TCL-1 transgenic mouse develops a malignancy that closely mimics chronic lymphocytic leukemia (CLL). Lack of an animal model has hampered development of treatments for CLL as well as research into its causes and the changes that drive drug resistance. “This strain shares many of the molecular and genetic features of human CLL, responds to drugs used to treat the disease and develops drug resistance that renders treatment ineffective, as often happens in CLL patients,” says John Byrd, MD, professor of Internal Medicine and a specialist in CLL at Ohio State’s Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute. The study was published in the journal Blood. The TCL-1 strain was originally developed by Ohio State cancer researcher Carlo Croce, MD, and his lab team. NEW BIOMARKER OF LIVER DYSFUNCTION DURING SEPSIS IDENTIFIED OSUMC researchers have found that high levels of a certain protein in the bloodstream during severe infection is a biomarker for damage and metabolic derangement in the liver. Their study, published in the journal Critical Care Medicine, provides insight into the mechanisms of sepsis-induced organ failure, the leading cause of death in medical intensive care units. These findings indicate that changes in organ function are explained by events in mitochondria, the primary source of cell energy. Led by Elliott Crouser, MD, a pulmonologist and critical care researcher, this study is the first to show how mitochondrial populations change during sepsis. “This sheds light on the mechanism by which the function of vital organs, such as the liver, becomes compromised during sepsis,” Crouser says. “We observed an inverse relationship between blood levels of the liver mitochondrial enzyme, carbamoyl phosphate sythetase-1 (CPS-1), and mitochondrial respiratory capacity in the liver during the subacute stages of sepsis.” VAGINAL BIRTH A SAFE OPTION AFTER MULTIPLE C-SECTIONS Mark Landon, MD, vice chair of Obstetrics and Gynecology at OSUMC, evaluated the risk of complication and success of vaginal birth after cesarean section (VBAC) in women who had had more than one prior cesarean delivery. Published in the journal Obstetrics and Gynecology, the study was conducted through the National Institutes of Health. Data included more than 45,000 patients with previous cesarean section, including almost 18,000 undergoing a trial of labor or an attempt at VBAC. “This was the first large study of VBAC in which certain outcomes, such as uterine rupture, were studied prospectively,” says Landon. The study showed that uterine rupture risk is not significantly increased in women with multiple prior cesarean deliveries compared with those who have had a single prior operation. VAMPIRE BAT SALIVA HELPS BATTLE STROKES Stroke victims will gain an improved drug treatment if research involving a vampire bat saliva derivative shows the results some health professionals are anticipating. The study is testing the effectiveness of a compound derived from bat saliva in reducing the risk of brain damage after the onset of an acute stroke. If approved, the drug will triple the window of time for initiating emergency stroke treatment and offer other advantages over medications currently in use, including T-PA (recombinant tissue plasminogen activator), the only FDA-approved drug for treating ischemic stroke. Andrew Slivka, MD, a neurologist and principal investigator for the study at OSUMC, says doctors have only a threehour period to initiate effective stroke treatment with T-PA. But he says the new medication, called rotundus salivary plasminogen activator, or desmoteplase, extends the treatment window to nine hours and appears to be much more effective against clots than T-PA. A study analyzing multi-center data collected over four years confirmed that most women who have had multiple prior cesarean deliveries can expect to achieve a successful vaginal birth. The study, led by 2007 Research Report 11 FRONT-LINE IMMUNE CELLS MATURE IN FOUR STAGES, STUDY SHOWS STUDY EVALUATES EFFECTS OF ANTIOXIDANTS, FISH OIL ON AMD Researchers at OSUMC found that natural killer (NK) cells, one of the body’s front-line defenses against cancer and infections, mature from progenitor stem cells in four discrete stages and that this happens in secondary lymphoid tissue, such as tonsils and lymph glands. Scientists have long known that the other two major types of immune cells in the body, T cells and B cells, develop respectively in the thymus and bone marrow, but the site and stages of human NK cell development had eluded investigators. These new findings, published in the Journal of Experimental Medicine, advance the understanding of NK cells, which help trigger broader immune responses such as the body’s permanent protection following vaccination. Principal Investigator Michael Caligiuri, MD, director of Ohio State’s Comprehensive Cancer Center, says understanding the secrets of NK cell development in humans could lead to new therapies for cancer, infection and for patients with immune deficiencies. OSUMC researchers are among scientists nationally trying to determine if certain nutrients can decrease risk of vision loss. This multicenter, randomized clinical trial builds on an earlier study that found that antioxidant vitamins and minerals, including vitamins C and E, beta-carotene, zinc and copper, when taken orally, reduced progression of advanced age-related macular degeneration by 25 percent and moderate vision loss by 19 percent. Approximately 4,000 participants will be recruited to determine if a modified combination of vitamins, minerals and fish oil can further slow vision loss from age-related macular degeneration. “Previous studies suggest other nutrients may protect vision as well,” says Robert Chambers, MD, an ophthalmologist and the study’s principal investigator at Ohio State. The trial will add lutein and zeaxanthin – yellow pigments found in the macula, the area responsible for central vision – along with the omega-3 fatty acids DHA and EPA, which are derived from fish and vegetable oils. ‘GENE SCREEN’ MAY SHED LIGHT ON WOUND HEALING Emerging technology could help OSUMC researchers learn why some wounds heal quickly and others last months or years. Scientists are using laser capture microdissection to pluck a single cell from wound samples for use in examining the genetics of healing. “Traditionally there’s been no way to tell what’s going on in a wound except by visualization and what a biopsy says – whether it’s infected or cancerous. We’re advancing this knowledge,” says Gayle Gordillo, MD, director of the Plastic Surgery Research Lab and principal investigator for the study, informally called the “Gene Screen.” This analysis may demonstrate which genes predict healing and which predict failure to heal. Researchers hope to improve diagnostic screening and identify genetic targets for new drugs to stimulate healing. Seven U.S. centers are involved with the study. “This is the first time screening is being done like this in a wound clinic,” says Chandan Sen, PhD, executive director of Ohio State’s Comprehensive Wound Center. 12 Ohio State University Medical Center UROLOGY DEPARTMENT ESTABLISHED In November 2006, the Ohio State University Board of Trustees changed the Division of Urology to a Department of Urology in the College of Medicine to improve the unit’s visibility and increase its ability to attract academic urologists and physician scientists. Urology had been a division within the Department of Surgery. Division Director Robert Bahnson, MD, was chosen to chair the new department. Bahnson holds the Dave Longaberger Chair in Urology at Ohio State, where he has been on the medical faculty since 1996. A urologist at Ohio State’s James Cancer Hospital and Solove Research Institute, Bahnson is a fellow of the American College of Surgeons and a member of the American Urologic Association. Last year he was elected to the American Association of Genitourinary Surgeons, a group of leading academic urologists from around the world who study diseases of the genitourinary system. Membership is limited to 75 physicians. Major Research Programs OF 2006 In 2005, Ohio State University Medical Center identified six “Signature Programs” to drive future success in three mission areas: research, education and patient care. The Signature Programs – Cancer, Critical Care, Heart, Imaging, Neurosciences and Transplantation – are characterized by demonstrated leadership, national reputation and potential for growth that can help the Medical Center become a top-tier academic institution. The Medical Center also identified Behavioral Medicine, Biomedical Informatics and Genetics as “Support Programs” that are instrumental to continued success of the Signature Programs and to the Medical Center overall. This section of the Research Report highlights 2006 accomplishments in each of the Signature and Support programs, as well as in two other “Programs of Interest”: Electrophysiology and Microbial Pathogenesis/Biodefense. 2007 Research Report 13 The overall goal of the Cancer Signature Program, led by Michael Caligiuri, MD, is to reduce cancer morbidity and mortality through basic, clinical, prevention and population scientific research that translates to improved patient Samson Jacob, PhD, co-leader of the Experimental Therapeutics Progam in Ohio State’s Comprehensive Cancer Center (OSUCCC), is principal investigator for an $11.84 million program project grant awarded by the National Cancer Institute to help OSUCCC scientists study chronic lymphocytc leukemia and translate basic research findings into clinical trials for patients with this disease. care, thus advancing the mission of becoming a world-class healthcare enterprise focused on improving quality of life for patients with cancer in Ohio and beyond. S I G N AT U R E P RO G R A M Cancer Cancer Signature Program Steering Team - (seated from left): David Schuller, MD; Michael Caligiuri, MD; Clara D. Bloomfield, MD; and (standing from left): Julian Bell; Dennis Smith; Chris Scarcello; William Carson III, MD; Michael Lairmore, DVM, PhD. (not shown): Steven Clinton, MD, PhD; Carlo Croce, MD; Electra Paskett, PhD, MSPH; David Williams, MD. 14 Ohio State University Medical Center The National Cancer Institute has awarded $3 million to help Ohio State’s Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute (OSUCCCJames) conduct phase II clinical trials on the effectiveness of cancer drugs under development. Principal investigator is Miguel Villalona, MD (left), shown here with Catherine Balint of the OSUCCC-James’ Clinical Trials Office, and Gregory Otterson, MD, of the OSUCCC’s Molecular Biology and Cancer Genetics Program. Led by Michael Caligiuri, MD, the Cancer Signature Program is embodied in the Ohio State University Comprehensive Cancer Center (OSUCCC), one of only 39 National Cancer Institute (NCI)-designated cancer centers in the nation. The patientcare component of the OSUCCC is the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute (The James). The OSUCCC, also directed by Caligiuri, is a network of seven interdisciplinary cancer-related research programs that collectively comprise more than 265 faculty investigators representing 14 colleges at Ohio State. Their overall goal is to reduce cancer morbidity and mortality through basic, clinical, prevention and population scientific research that translates to improved patient care, thus advancing the Cancer Signature Program’s mission of becoming a world-class healthcare enterprise focused on improving quality of life for patients with cancer in Ohio and beyond. Cancer Signature Program highlights of 2006: • The OSUCCC realized an increase in NCI funding for cancer research to $39.6 million, up 12.5 percent from $35.2 million in 2005. Total external grant funding for cancer research exceeds $100 million. • Twenty cancer researchers were recruited to the OSUCCCJames. • The NCI awarded a five-year, $11.84 million program project grant to principal investigator Samson Jacob, PhD, co-leader of the OSUCCC’s Experimental Therapeutics Program, to study chronic lymphocytic leukemia and translate basic research findings into clinical trials. • The NCI awarded the OSUCCC a $3 million contract to conduct phase II clinical trials to determine the effectiveness of anticancer drugs under development. The contract adds to the range of novel cancer therapies available to patients at the OSUCCC-James, which is one of only five institutions in the nation with NCI contracts for conducting both phase I and phase II clinical trials. Miguel Villalona, MD, is principal investigator for the new phase II contract. • The American Cancer Society awarded a $960,000 grant for a four-year study to determine whether freeze-dried black raspberry lozenges can slow or stop the return of oral cancer, which has one of the highest recurrence rates. Christopher Weghorst, PhD, of the OSUCCC’s Molecular Carcinogenesis and Chemoprevention Program, is principal investigator. • In conjunction with Ohio State’s Department of Radiology, the OSUCCC received a three-year, $738,813 NCI grant to develop noninvasive imaging assessment methodologies that reveal early biologic response to cancer treatment. Michael Knopp, MD, PhD, chair of Radiology and a member of the OSUCCC’s Experimental Therapeutics Program, is project leader. The OSUCCC is one of only eight cancer centers nationwide to receive this grant. • Nearly 240 clinical trials are open on new cancer therapies and prevention strategies, some of which are available nowhere else. • U.S.News & World Report ranked The James Cancer Hospital and Solove Research Institute 21st among “America’s Best Hospitals” for cancer care in 2006. 2007 Research Report 15 Researchers in the Critical Care Signature Program, led by Clay Marsh, MD, focus on the biological variability of each patient to establish links between genetic abnormalities and outcomes of patients in the Intensive Care Unit (ICU). They want to understand how From left are Anasuya Sarkar, PhD, and Mark Wewers, MD, researchers in the Critical Care Signature Program. approaches in the ICU affect the long-term outcomes of patients who survive their critical care illness or injury. S I G N AT U R E P R O G R A M Critical Care Critical Care Signature Program Steering Team – (from left): Elliott Crouser, MD; Shiva Rahmanian, MD; Stephen Hoffmann, MD; and Clay Marsh, MD. (not shown): Steven Steinberg, MD; Roy Essig, MD; Thomas Reilley, DO; Charles Cook, MD; Naeem Ali, MD; Randy Smith, RN; Terri Gillenwater; and Kam Sigafoos. 16 Ohio State University Medical Center Members of the Critical Care Signature Program’s ICU research team include: (standing from left) Scott Aberegg, MD; Naeem Ali, MD; Dave Jarjoura, PhD; and (seated) Jyoti Kamal, PhD. By studying and applying new paradigms of pathogenesis, the Critical Care Signature Program provides novel multidisciplinary treatments for patients with acute, life-threatening illnesses or injuries. Program leader Clay Marsh, MD, says a team of experts is needed to manage the most seriously ill or injured patients. “We are building a unified program in research, education, and clinical care that brings new solutions to these patients through innovation and discovery,” says Marsh, who also directs the Division of Pulmonary, Allergy, Critical Care and Sleep Medicine. Marsh says researchers in this program focus on the biological variability of each patient to establish links between genetic abnormalities and outcomes of patients in the Intensive Care Unit (ICU). “We want to understand how approaches in the ICU affect the long-term outcomes of patients who survive their critical care illness or injury,” he explains. “Through this platform of personalized and predictive medicine, we can not only transform ICU care, but also the way medicine is practiced in other areas.” Critical Care Signature Program highlights of 2006: • The program recruited seven new faculty and acquired $1.3 million in new grants. • Program members developed the first Fundamentals of Critical Care Support course approved by the Society of Critical Care Medicine, an international multidisciplinary organization, for all intensive care unit healthcare workers. • James O’Brien, MD, Naeem Ali, MD, Scott Aberegg, MD, MPH, Stephen Hoffmann, MD, John Mastronarde, MD, MPH, Jyoti Kamal, PhD, and David Jarjoura, PhD, focused on improving the quality and consistency of daily clinical care on a number of fronts. With the Information Warehouse (IW), they have built an Intensive Care Unit (ICU) Minimum Dataset that collects information about all ICU patients and the care they receive. They also focused on the care of patients with sepsis, and they have another IW application that is collecting information about patients enrolled in a septic registry and blood bank. • Susheela Tridandapani, PhD, and her group investigated mechanisms of host response to infectious agents using a combination of genomewide analyses of mRNA and miRNA expression along with immunological, molecular and biochemical approaches. Their goal is to identify molecular targets for therapeutic intervention in inflammation-associated disease states such as sepsis. • Mark Wewers, MD, and Anasuya Sarkar, PhD, worked on the role of caspase-1 in sepsis and outcomes. Sepsis is characterized by activation of a host of inflammatory pathways. Recent studies by Sarkar and Wewers have revealed a strong tie between the enzyme caspase-1 and the sepsis immune response. Caspase-1 deficient mice are protected from death caused by intraperitoneal bacteria. Importantly, the caspase1 effect appears to be due to its role in causing the programmed cell death of splenic lymphocytes. Thus, future studies directed at inhibiting caspase-1 may provide novel approaches to treating sepsis. • The Division of Pulmonary, Allergy, Critical Care and Sleep Medicine was ranked 24th in the nation in 2006 by U.S.News & World Report. 2007 Research Report 17 Research within the Heart Signature Program, which was led in 2006 by William Abraham, MD, director of the Division of Cardiovascular Medicine, focuses on many areas, including heart failure, ischemic disease, transplantation, In the prep room of the MR/CT Lab in Ohio State’s Ross Heart Hospital are: (from left) research assistant Tam Tran; Subha Raman, MD, medical director of Cardiovascular Magnetic Resonance/Computed Tomography (CMR/CT); radiologic technologist Anne Garcia; clinical research specialist Beth McCarthy; Sarah Neff, RN; and research nurse Michelle Ballinger, RN. arrhythmias/electrophysiology and vascular disease. S I G N AT U R E P RO G R A M Heart Heart Signature Program Steering Team – (seated from left): William Abraham, MD; Benjamin Sun, MD; Larry Anstine; (standing from left): Rich Davis, PhD; Charles Bush, MD; Karen Jackson; Randy Allen; Judy Gilliam, RN; Randy Homan; and Jay Zweier, MD. (not shown): Donna Cruz-Huffmaster; Michael Knopp, MD, PhD; Vanessa Moses; Muthu Periasamy, PhD; Patrick Vaccaro, MD; and the program’s newest member, Thomas Ryan, MD. 18 Ohio State University Medical Center Among researchers in the Heart Signature Program are members of the lab team of Sanjay Rajagopalan, MD (third from right), including: (from left) Sutha Prasad, PhD; visiting scholar Thomas Kampfrath; Qinghua Sun, MD, PhD; Ryan Williams; Britten Farrar, MD; and medical student Theodore Chang. Translational research, or converting basic science discoveries to clinical care, occurs across all aspects of the Heart Signature Program, which was led in 2006 by William Abraham, MD, director of the Division of Cardiovascular Medicine and associate director for clinical and translational research at Ohio State’s Davis Heart and Lung Research Institute. Research within this Signature Program focuses on heart failure, ischemic disease, transplantation, arrhythmias/electrophysiology and vascular disease. Heart care is provided at Ohio State University Medical Center’s Ross Heart Hospital, University Hospital, University Hospital East, and at local and regional outpatient clinics associated with Ohio State. Educational opportunities within this Signature Program include fellowship training in cardiovascular medicine, cardiac surgery and vascular surgery, along with doctoral, postdoctoral and continuing medical education programs. Heart Signature Program highlights of 2006: • The program recruited 13 faculty members in cardiology and two in cardiac surgery. • The program continued work toward recruiting a nationally renowned medical scientist to direct the Ohio State University Heart Center, which includes cardiovascular medicine, vascular surgery and cardiothoracic surgery services across the research, education and patient care mission areas. The Heart Center primarily involves Ohio State’s Ross Heart Hospital and the University’s Davis Heart and Lung Research Institute. In 2007 the program recruited Thomas Ryan, MD, formerly of the Duke Heart Center, to this position. • The program increased its total National Institutes of Health (NIH) research awards to $12.73 million in 2006. • The Division of Cardiovascular Medicine has approximately 90 clinical research studies, including investigator-initiated single-site studies, NIH-sponsored multisite trials, and industry-sponsored trials. Deanna Golden-Kreutz, PhD, clinical research manager, says studies span heart failure, interventional cardiology, electrophysiology, imaging, genotyping, sports/prevention, sleep disorders, transplant/cardiothoracic surgery, emergency medicine and pulmonary hypertension. Of note is the Division’s national leadership in the investigational use of hemodynamic monitors, ventricular support devices and cardiac CT/MR. • The research team of Subha Raman, MD, seeks discoveries afforded by high-resolution, noninvasive imaging to improve cardiovascular care. The group’s close integration with the clinical mission ensures that successful research efforts can be seamlessly translated into prevention, early diagnosis and personalized treatment to improve cardiovascular health. Results have delivered more accurate, less invasive detection of cardiovascular disease that has improved outcomes. • The lab team of Sanjay Rajagopalan, MD, furthered its study of artherosclerosis-related complications and the role of risk factors, including environmental determinants on the initiation and progression of atherosclerotic plaque. The lab, funded by four grants from the National Institutes of Health, has focused on MRI imaging of plaque and high-resolution intravital techniques to understand early leukocyte endothelial interactions in atherosclerosis. • Periannan Kuppusamy, PhD, focuses on imaging research, having published a landmark paper in the American Journal of Physiology – Heart and Circulatory Physiology on the labeling of stem cells with oxygen-sensing nanoprobes. Based largely on this pioneering work, Kuppusamy has received a research grant of $1.35 million for four years from the National Institutes of Health for his study on stem cell therapy in the heart. This will help him develop probes and imaging methods for noninvasive tracking and assessment of the stem cell engraftment, repair and angiogenesis in damaged heart tissue. The technology will help physicians monitor cell therapy and could open avenues for treating heart disease. 2007 Research Report 19 The research backbone of the Imaging Signature Program, led by Michael Knopp, MD, PhD, professor and chair of the Department of Radiology, is the Wright Center of Innovation in Biomedical Imaging, also known as the Shown in the MRI control room for the 3 Tesla Magnet at the Wright Center of Innovation are: (from left) Robert McKenney, PhD, director of imaging research and administrative services in the Department of Radiology; research scientist Amir Abduljalil, PhD (seated); and David Beversdorf, MD, assistant professor of Neurology. Biomedical Structural, Functional and Molecular Imaging Enterprise at Ohio State. S I G N AT U R E P RO G R A M Imaging Imaging Signature Program Steering Team - (seated from left): Rich Davis, PhD; Donna Cruz-Huffmaster; William Yuh, MD; Jean McCabe; Joseph Yu, MD; and (standing from left): Philip Skinner; Marc Conselyea; Michael Knopp, MD, PhD; Nathan Hall, MD, PhD; Robert McKenney, PhD; Steve Tumblin; Bruce Lauer. (not shown): Kent Hess. 20 Ohio State University Medical Center Shown in the treatment/preparation area of the animal facility at the Wright Center of Innovation are: (from left) Yukihisa Takayama, MD, visiting scholar; Jonda Leser, research nurse; PhD student Ananth Narayanan; and Petra Schmalbrovk, PhD, associate professor of Radiology. Over the past 30 years, medical imaging has advanced beyond mere projection to cross-sectional views at ever-higher speeds and resolutions that enhance observation of medical problems and provide more opportunities for minimally invasive therapeutics. Ohio State University Medical Center’s Department of Radiology has been a pacesetter in biomedical imaging research that translates to innovative clinical applications. The research backbone of the Imaging Signature Program – led by Michael Knopp, MD, PhD, professor and chair of the Department of Radiology – is the Wright Center of Innovation in Biomedical Imaging, also known as the Biomedical Structural, Functional and Molecular Imaging Enterprise at Ohio State. The Wright Center was launched in May 2003 with state funding from a $9.1 million Third Frontier Grant and an $8 million Biomedical Research and Technology (BRTT) award. Knopp is principal investigator for both. The Center is designed to advance magnetic resonance imaging (MRI), positron emission tomography (PET) and mobile imaging technologies while creating an extensive imaging and bioinformatics structure. It also supports other biomedical research endeavors at Ohio State. Imaging Signature Program highlights of 2006: • The state of Ohio announced continued funding for the Wright Center of nearly $8 million for three years starting in May 2006. This initiative provides the foundation for the next level of hybrid imaging at Ohio State. • The National Cancer Institute awarded Ohio State’s Comprehensive Cancer Center (OSUCCC), in conjunction with the Department of Radiology, a three-year grant of $738,813 to continue developing noninvasive imaging assessment methodologies to reveal early biologic response to cancer treatment. Michael Knopp, MD, PhD, a member of the OSUCCC, is project leader. The OSUCCC is one of only eight cancer centers nationwide to receive this grant. • As a result of a state grant application involving several community partners for a large project proposal for imaging research, the Department of Radiology realized an award of $700,000 from one of the partners, Pfizer Inc., and gained wider opportunities with state and industry partners for imaging endeavors. • The Department of Radiology’s budgeted research portfolio exceeds $40 million, up from $1.2 million in 2002. • Five faculty were recruited in 2006. • Collaboration continues with Novartis Pharmaceuticals in advanced imaging methodologies for clinical trials; 22 trials are under way. • The Department of Radiology is in its fourth year as a core lab for the national Cancer and Leukemia Group B (CALGB) in studying mechanisms of DNA damage and repair at the molecular level. • Molecular-level research is keeping pace with National Institutes of Health awards in: characterizing mechanisms of chemopreventive agents, cancer therapy in combination with chemical and radiological agents, modulation of genes and proteins regulating cellular proliferation and apoptosis, and genetic damage and repair; studying anticancer topoisomerase poisons, including analysis of new drugs, proteomic analysis of post-translational modifications of topoisomerases associated with drug exposure, and disruption of cancer cell metabolism by anticancer drugs; and investigating genomic instability in cancer pathogenesis with a focus on the regulation of DNA damage in normal and cancerous cells and on mechanisms of cross-talk among molecular pathways that control cellular homeostasis. 2007 Research Report 21 Technological advancement, innovative research and an aging population are driving an upswing in demand of treatment for neuroscience conditions. Research from multiple disciplines benefits the Neurosciences Signature Researchers in the Neurosciences Signature Program include: (from left) Amy Lovett-Racke, PhD; Michael Racke, MD; Dana McTigue, PhD; and Phillip Popovich, PhD. Program, which is led by E. Antonio Chiocca, MD, PhD, director of the Department of Neurological Surgery. S I G N AT U R E P R O G R A M Neurosciences Neurosciences Signature Program Steering Team - (seated from left): Anthony Young, PhD; Kyle Sharp; E. Antonio Chiocca, MD, PhD; Anand Satiani. (standing from left): Allan Yates, MD, PhD; William Pease, MD; John Kissell, MD; Michael Racke, MD; Wolfgang Sadée, PhD; David Sheppard; Sara Widing; Caroline Whitacre, PhD. (not shown): Michael Beattie, PhD; Jacqueline Bresnahan, PhD; Debbie Buonaiuto; Norton Neff, PhD; Radu Saveanu, MD; D. Bradly Welling, MD, PhD; Todd Wheeler; William Yuh, MD. 22 Ohio State University Medical Center Members of the Dardinger Laboratory for Neuro-Oncology and Neuroscience Research include: (seated from left) Kazuhiko Kurozumi, MD, PhD; Nina Dmitrieva, PhD; Shigeru Tanaka, MD, PhD; and: (standing from left) Balveen Kaur, PhD; Michal Oskar Nowicki, PhD; E. Antonio Chiocca, MD, PhD, director of the Department of Neurological Surgery; Sean Lawler, PhD; Mariano Viapiano, PhD; and Suzanne Camilli, MSPH, lab manager. Not shown are: Laboratory Chief Yoshinaga Saeki, MD, PhD; Masatake Suzuki, PhD; Akihiro Otsuki, MD, PhD; Kazue Kasai, PhD; Hiroshi Nakashima, PhD; Jakub Godlewski, PhD; and Bin Hu, PhD. Led by E. Antonio Chiocca, MD, PhD, the Neurosciences Signature Program involves research from multiple disciplines, including neurological surgery, neurology, neurosciences, molecular neurobiology, pharmacology, psychiatry, psychology, ENT (ear, nose and throat), the Center for Brain and Spine Injury, and others. The Neurosciences clinical program encompasses neuro-oncology/skull base, spine trauma, stroke/cerebrovascular, neuromuscular/multiple sclerosis, neurodegenerative disorders/dementia, and neuromodulation. Clinical research within this program occurs in the Cognitive Neuroscience Laboratory, the Dardinger Laboratory for NeuroOncology and Neuroscience Research, and in the departments of Neurology, Neurological Surgery, and Physical Medicine and Rehabilitation, as well as in the School of Allied Medical Professions. Basic research is conducted in the Neurobiology of Disease Institute, the Center for Molecular Neurobiology, the Department of Neuroscience, the Department of Pharmacology and in the Division of Neuropathology. Neurosciences Signature Program highlights of 2006: • The program recruited 10 acclaimed faculty in Neurology, Neurological Surgery and Psychiatry. • The Department of Neurological Surgery and the Department of Neurology were recognized by U.S.News & World Report on the magazine’s annual listing of top-ranked clinical programs for the third consecutive year. • Department of Neurological Surgery faculty performed 1,439 neurosurgical procedures, including gamma knife radiosurgery, fractionated stereotactic radiosurgery (FSRS), endovascular obliteration of brain aneurysms and deep brain stimulation. • Inpatient volume throughout the Neurosciences Signature Program increased from 2,925 in 2005 to 3,278 in 2006 (10.5 percent), while outpatient visits rose from 25,220 in 2005 to 29,480 in 2006 (16.9 percent). • The Department of Neurological Surgery received outside research funding exceeding $1.6 million. Total research awards for all neuroscience PIs was $21.09 million in 2006 (this number is by principal investigator, or PI, not by department). Individual PIs include some from the departments of Molecular and Cellular Biochemistry, Psychiatry and Pharmacology, as well as the departments of Neurological Surgery, Neurology and Neuroscience . • The Dardinger Neuro-Oncology Center, co-directed by E. Antonio Chiocca, MD, PhD, and Herbert Newton, MD, participated in nine clinical trials and saw its outside research funding – including grants from the National Institutes of Health – top $1.75 million. • Scientists within this Signature Program published several articles in high-impact scientific journals such as Proceedings of the National Academy of Sciences and Cancer Research. • In one of the program’s largest collaborative research projects, medical scientists Herbert Newton, MD, Robert Cavaliere, MD, Sean Lawler, PhD, E. Antonio Chiocca, MD, PhD, and Yoshinaga Saeki, MD, PhD, are working with Abhik Ray-Chaudhury, MD, of Neuropathology, and the laboratory team of Carlo Croce, MD, chair of the Department of Molecular Virology, Immunology and Medical Genetics, to investigate microRNA and its role in brain tumor transformation and pathogenesis. 2007 Research Report 23 Transplantation expertise at Ohio State University Medical Center is consolidated in the Comprehensive Transplant Center, which is led by Ronald Ferguson, MD, PhD, and combines the Medical Center’s more than 35 years of experience in solid organ Amer Rajab, MD, PhD, transplant surgeon and director of Ohio State's Pancreas and Islet Transplant Program, is shown with Elizabeth Diakoff, MD, an endocrinologist who collaborated with Rajab and his team to develop clinical protocols for islet cell transplant. S I G N AT U R E P R O G R A M transplantation with research advances that translate to innovative patient care. Tr a n s p l a n t a t i o n Transplantation Signature Program Steering Team - (seated from left): Todd Pesavento, MD; Amy Pope-Harman, MD; Susan Moffatt-Bruce, MD, PhD; Gregg Hadley, PhD; (standing from left): Ronald Ferguson, MD, PhD; Mitchell Henry, MD. 24 Ohio State University Medical Center Gregg Hadley, PhD, recently joined Ohio State's Comprehensive Transplant Center team as deputy director of research. He focuses on the mechanisms of organ allograft rejection and pancreatic islet allograft rejection in hopes of developing therapeutics to prevent such rejection. The Transplantation Signature Program, led by Ronald Ferguson, MD, PhD, coordinates the clinical care of patients needing kidney, liver, pancreas, heart or lung transplants. This program also interfaces with the Medical Center’s Cancer Signature Program (blood and marrow transplantation) and the Cornea Transplant Program. Transplantation expertise at the Medical Center is consolidated in Ohio State’s Comprehensive Transplant Center (CTC), which was established in 2005 as the only comprehensive adult transplant program in central Ohio. The CTC combines the Medical Center’s more than 35 years of experience in solid organ transplantation with research advances that translate to innovative patient care. By creating a forum for collaboration among experts from multiple transplantation disciplines, the CTC team is able to transfer best clinical practices and research expertise across specialties to enhance - and ultimately advance - patient care. Transplantation Signature Program highlights of 2006: • The CTC established a medical council to oversee patient treatment strategies. • The CTC developed multidisciplinary organ-specific patient care teams for sophisticated pre- and post-transplant clinical management. • The CTC recruited five new faculty members, including Gregg Hadley, PhD, as deputy director of research. Hadley is a professor of Surgery whose research program is focused on defining immunologic mechanisms underlying: 1) rejection of transplanted tissue and organs; and 2) graft-versushost disease elicited following bone marrow transplantation. • In collaboration with other disciplines, the CTC increased National Institutes of Health research funding to more than $500,000 in total direct costs. • The CTC hosted Ohio State’s first National Symposium on Transplant Critical Care. • CTC investigators initiated or maintained 16 separate clinical trials of innovative immunosuppressive strategies in organ transplant recipients. • 346 patients received solid organ transplants at Ohio State’s Medical Center. Based on volume, the Medical Center’s kidney transplant program is one of the top 10 in the nation, and its kidney-pancreas transplant program ranks among the top four. 2007 Research Report 25 Led by Ronald Glaser, PhD, the Institute for Behavioral Medicine was established to stimulate and expand interdisciplinary collaboration through experiments involving social and behavioral influences, stress hormones and the The Behavioral Medicine Support Program includes the General Clinical Research Center Core Laboratory led by William Malarkey, MD, who is shown here with: (from left) lab manager Susan Mosely and clinical lab technologists Marilyn Welt and Trina Wemlinger. Not shown is clinical lab technologist Nancy Hughey. S U P P O RT P RO G R A M immune response on the health of human subjects and animal models. B e h av i o ra l M e d i c i n e Behavioral Medicine Support Program Steering Team – (from left): John Sheridan, PhD; William Malarkey, MD; Ronald Glaser, PhD. 26 Ohio State University Medical Center The Behavioral Medicine Support Program includes the OSU Stress and Health Study team led by Janice Kiecolt-Glaser, PhD (third from right). Shown with her from left are: Lindsay Mays; Nathan Deichert, PhD; Jean-Philippe Govin (seated); Cathie Atkinson, PhD; and Alessa Smyth. The Institute for Behavioral Medicine Research (IBMR) is the unit in which Ohio State’s multidisciplinary program in psychoneuroimmunology is housed. Led by Ronald Glaser, PhD, the IBMR was established to stimulate and expand interdisciplinary collaboration through experiments involving social and behavioral influences, stress hormones and the immune response on the health of human subjects and animal models. • Psychoneuroimmunology is an interdisciplinary program involving four colleges (Medicine; Public Health; Dentistry; and Social and Behavioral Sciences), including six academic departments (Molecular Virology, Immunology & Medical Genetics; Psychology; Psychiatry; Internal Medicine; Oral Biology; and Biostatistics) and two other Health Sciences centers (the Davis Heart and Lung Research Institute and the Comprehensive Cancer Center). This rapidly developing field examines interactions among the nervous, endocrine and immune systems, as well as implications of these connections that will translate to healthcare practices. • Behavioral Medicine Support Program highlights of 2006: • • The laboratory of Virginia Sanders, PhD, was the first to report on how CD86 (B7-2), a molecule that provides a costimulatory signal necessary for T-cell activation and survival, signals intracellularly. • Daniel Ankeny, PhD, Virginia Sanders, PhD, Phillip Popovich, PhD, and co-workers showed that experimental spinal cord injury elicits chronic activation of a B cell- • • dependent autoimmune response. In this novel study, high levels of anti-DNA antibodies were detected in spinal cordinjured rats with a pattern that is similar to that seen in systemic lupus erythematosus. Studies were published by Eric Yang, PhD, Clay Marsh, MD, Ronald Glaser, PhD, and colleagues that show how stress may affect tumor progression independent of the immune response to a tumor. The laboratory team of Ronald Glaser, PhD, explored the role that psychological stress plays in modulating the expression of latent Epstein-Barr virus, a human tumor virus. Scientists in this program are addressing the interplay between psychological factors and immune function as it relates to basal cell carcinoma, the most common form of human cancer. They also performed studies addressing how interactions between polyunsaturated fatty acid levels and depressive symptoms were related to proinflammatory cytokine synthesis in older adults. A further study in progress in the IBMR addresses the immune and endocrine consequences of a yoga session. Preliminary data from that study provided the basis for a National Cancer Institute grant that will address fatigue and inflammation in breast cancer survivors and show how a yoga intervention may impact fatigue and inflammation. 2007 Research Report 27 The Department of Biomedical Informatics (BMI), led by Joel Saltz, MD, PhD, is playing a critical role in initiatives to translate, integrate, share and analyze information that will improve medical diagnosis, treatment and patient outcomes. Daniel Janies, PhD, of Biomedical Informatics, reviews image data with student Victoria Best. SUPPORT PROGRAM Biomedical Informatics Biomedical Informatics Steering Team – (standing from left): Tahsin Kurc, PhD; Herb Smaltz, PhD; Metin Gurcan, PhD; Umit Catalyurek, PhD; and (seated from left): Jeffrey Parvin, MD, PhD; Joel Saltz, MD, PhD; Philip Payne, PhD. Inset: Jyoti Kamal, PhD 28 Ohio State University Medical Center Members of the Biomedical Informatics Support Program lab team include: (standing from left) Scott Oster; Tahsin Kurc, PhD; Justin Permar; Berkant Barla Cambazoglu, PhD; David Ervin; and (seated from left): Stephen Langella; Joel Saltz, MD, PhD; Tony Pan; Shannon Hastings. Not shown are Metin Gurcan, PhD, and Ashish Sharma, PhD. In 21st-century medicine, decisions are data-driven. With scientists and clinicians generating data in unprecedented amounts, health professionals must manage information to make it meaningful. The Department of Biomedical Informatics (BMI), led by Joel Saltz, MD, PhD, is playing a critical role in initiatives to translate, integrate, share and analyze information that will improve medical diagnosis, treatment and patient outcomes. • Biomedical Informatics Support Program highlights of 2006: • • BMI is shaping the development of cancer informatics technologies at the national and local levels. The goal of the National Cancer Institute’s Cancer Biomedical Informatics Grid (caBIG™) is to develop applications and the underlying systems architecture that connect data, tools, scientists and organizations in an open federated environment. The BMI middleware group developed caGrid, middleware that allows sharing of data and analytical tools among cancer researchers. caGrid has been adopted for imaging, clinical trials, tissue banking and high-throughput molecular studies. The in vivo imaging group has developed software for reviewing and analyzing radiology and pathology images. • BMI’s medical informatics team developed Quest, a database-independent application to support ad hoc query development for Ohio State’s Integrated Cancer Biology Program (ICBP) group. This application is integratable with other software tools, such as Bioconductor, R, and GenePattern. BMI’s middleware group will deploy caGrid to the national ICBP group to coordinate research among ICBP centers. • BMI is a leader in imaging informatics – creating tools, algorithms and technologies to share and analyze biomedical image data. One emphasis is on algorithm development for clinical image analysis and automated classification of different cancers. A second focus is on using image analysis to • • quantitatively characterize the phenotypic features at molecular, cellular and tissue levels in 2-D and 3-D spaces in breast cancer and the tumor microenvironment. BMI’s work in systems biology/bioinformatics ranges from mapping the spread of the avian flu virus to analyzing gene regulation. Research to plot the course of H5N1, a dangerous form of avian flu, used the online mapping program Google Earth and has received international attention. Researchers hope this information will help predict where the next outbreak of the virus may occur. BMI is expanding the systems biology area through the joint recruitment (with Ohio State’s Comprehensive Cancer Center) of Jeffrey Parvin, MD, PhD, from Harvard University. This expansion will focus on using many data sources to discover and analyze biochemical processes in such areas as breast cancer. Computational and lab-based scientists will collaborate to identify critical steps in carcinogenesis. BMI initiated a program in Clinical and Translational Research Informatics. Philip Payne, PhD, was recruited in a joint appointment with Ohio State’s MedCenter IS group to serve as both a BMI faculty member and in a new position as translational research informatics architect for the Medical Center. He will research knowledge engineeringbased approaches to the design of informatics platforms supporting the discovery, integration and analysis of phenotypic, bio-molecular and research operations data. He will also shape translational research information systems in the context of his Medical Center duties. BMI’s high-end computing group extended its work in combinatorial algorithms and parallel computing. Combinatorial algorithms are an enabling technology for scientific computing, especially for large-scale problems and high performance. These techniques are improving computational performance in pathology image analysis and will be applied to statistical genetics and detection of genetic interactions to predict genetic risk. 2007 Research Report 29 The Human Cancer Genetics Program is aligned with the Medical Center’s Department of Molecular Virology, Immunology and Medical Genetics (MVIMG), which has been chaired by Carlo Croce, MD, since he arrived Researchers in the Genetics Support Program include: (from left) Carlo Croce, MD; Huiling He, MD; Albert de la Chapelle, MD, PhD; and Krystian Jazdzewski, MD, PhD. at Ohio State in 2004. Researchers in this Department study the molecular genetics of human disease and disease-causing organisms. SUPPORT PROGRAM Genetics Genetics Support Program Steering Team – (from left): Chang-Gong Liu, PhD; Kay Huebner, PhD; Hansjuerg Alder, PhD; Carlo Croce, MD. 30 Ohio State University Medical Center The Genetics Support Program includes the lab team of Joanna Groden, PhD (standing, third from right), who is shown here with: (seated from left) Kaylan Haizlip, graduate researcher; Patrick Grierson, graduate researcher; Sakmitri Bhattacharyya, PhD; and: (standing from left) Kiran Nadella, PhD; Cathy Ebert, research consultant; Kyle Osterbrock, undergraduate researcher; Jeremy Keirsey, graduate researcher; Qi Wang, research associate; Jiang Qian, PhD; Groden; Poorvi Dalal, student assistant; and Betty Russell, graduate researcher. Ohio State’s Human Cancer Genetics Program was already world renowned when Carlo Croce, MD, was recruited in 2004 to assume leadership from program founder Albert de la Chapelle, MD, PhD, who wanted to devote more time to research. Since then, these two internationally acclaimed geneticists and their colleagues have worked in tandem to guide the program to even greater heights in the study of genetics, which may hold the keys to curing cancer and other diseases. The Human Cancer Genetics Program is aligned with the Medical Center’s Department of Molecular Virology, Immunology and Medical Genetics (MVIMG), which has been chaired by Croce since he arrived at Ohio State. Researchers in this Department study the molecular genetics of human disease and disease-causing organisms. Their expertise ranges from basic biophysical analysis to clinical translation in molecular genetics of cancer, immunology and immunogenetics, and bacterial and viral pathogenesis. • Faculty were awarded more than $8.9 million in research funds; 46 percent of Department faculty salaries are covered by research awards. • In 2006, the Department recruited researcher Matthew During, MD, PhD, an expert in neurobiology, gene therapy and vector development. • Carlo Croce, MD, Albert de la Chapelle, MD, PhD, and Kay Huebner, PhD, identified specific microRNAs as causative for solid and hematopoietic tumors. • Richard Fishel, PhD, vice chair of research for MVIMG, discovered novel DNA repair-based cellular mechanisms for defense against retroviral infection. • Tim Huang, PhD, Christoph Plass, PhD, and Ramana Davuluri, PhD, devised new combined bioinformatics methods for systems biology analysis. • Christoph Plass, PhD, described epigenetic regulation of tumor-suppressor genes in lung and head and neck tumors. Genetics Support Program highlights of 2006: • MVIMG has 34 tenure track faculty and 13 research track faculty. More than 65 percent of faculty garner independent research funds and nearly 65 percent maintain multiple grant awards. 2007 Research Report 31 Investigators in Ohio State’s Center for Microbial Interface Biology (CMIB) are leading the way to new diagnostic strategies, therapies and vaccines against a number of microscopic killers. Internationally recognized scientists are per- The Microbial Pathogenesis/Biodefense Program includes the research lab of Bradford McGuire, MD, PhD, shown here with research associate Manjusha Kulkarni, PhD. forming innovative bench and translational science designed to improve the health of the nation. PROGRAM OF INTEREST Microbial Pathogenesis/ Biodefense Microbial Pathogenesis/Biodefense Steering Team – (from left): Robert Munson, PhD; Joanne Turner, PhD; John Gunn, PhD; and Larry Schlesinger, MD. 32 Ohio State University Medical Center Investigators in the Microbial Pathogenesis/Biodefense Program include the lab team of Amal Amer, MD, PhD (left), shown here with research assistant Anwari Akhter (center) and graduate research associate Laura Frantz. The United States and other nations face a growing crisis related to suffering and death from a variety of infections. These include: ongoing pandemics of tuberculosis, AIDS and malaria; emerging and re-emerging infectious agents such as influenza and food-borne illnesses; multidrug-resistant pathogens, recently highlighted by the case of XDR tuberculosis; community-associated staph infections; and infections in immunocompromised individuals such as those with cancer, transplants or illnesses requiring time in hospital critical care units. Investigators in Ohio State’s Center for Microbial Interface Biology (CMIB) are leading the way to new diagnostic strategies, therapies and vaccines against a number of these invisible killers. Internationally recognized scientists are performing innovative bench and translational science designed to improve the health of the nation. A focus on broad-based interdisciplinary scientific platforms is enhancing the effectiveness and efficiency of discoveries. This is exemplified by a significant growth in funded programs, publications and international visibility related to the Center’s activities. The CMIB also is partnering with several Ohio State colleges, centers and institutes as well as the Medical Center’s Signature Programs. Program highlights of 2006: • The CMIB was awarded University “Center” status by the Ohio State Board of Trustees in December 2006 (http://cmib.osu.edu). The CMIB has seven core faculty members. Five of them, along with their lab personnel and the Center’s administrative leadership, have moved to the 10th floor of the Biomedical Research Tower. Membership has grown to 58 faculty, representing units across the Columbus and Wooster campuses and Columbus Children’s Research Institute. Grant support for core faculty members in 2006 totaled $11.16 million. • A campus-wide Biosafety Level 3 (BSL 3) core facilities program was established under the governance of the CMIB. All Ohio State investigators and off-campus partners have access to these state-of-the-art facilities. This is one of the most developed university biocontainment programs in the country. • Ohio State became a member of the National Institutes of Health (NIH)-funded Great Lakes Regional Centers of Excellence in Biodefense and Emerging Infectious Diseases Research Program. CMIB faculty members have been awarded a program grant and a development project totaling $1.15 million. • The CMIB is part of a team of investigators from six colleges (Medicine, Veterinary Medicine, Biological Sciences, Pharmacy, Public Health, and Food, Agricultural and Environmental Sciences) that received a Provost Targeted Investment in Excellence program totaling $4.79 million and entitled “Public Health Preparedness for Infectious Diseases.” • Two faculty members were recruited in a creative partnership with the Department of Microbiology in the College of Biological Sciences: Chad Rappleye, PhD, from Washington University (fungal pathogenesis), and Stephanie Seveau, PhD, from the University of Michigan (Listeria pathogenesis). • The CMIB “Host-Pathogen Seminar Series” brought six internationally recognized experts in microbe-host interactions to campus during 2006. 2006 grant highlights include: • Lung innate immune responses to Francisella tularensis: a central role for the macrophage (National Institutes of Health/National Institute of Allergy and Infectious Diseases Region V Great Lakes Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research) Larry Schlesinger, MD, PI. This project studies the lung innate immune response to Francisella tularensis, the causative bacterium of tularemia and a targeted agent of bioterrorism. This $1.05 million program project grant includes investigators in both the CMIB and the Davis Heart and Lung Research Institute. • Salmonella antimicrobial peptide resistance (National Institutes of Health/National Institute of Allergy and Infectious Diseases) - John Gunn, PhD, PI. This study, funded at $1.16 million, focuses on the PmrA-PmrB regulon, including the identification and characterization of PmrAPmrB-regulated genes necessary for antimicrobial peptide resistance and lipopolysaccharide (LPS) modification. 2007 Research Report 33 Electrophysiologists (EPs) – cardiologists who treat electrical malfunctions of the heart – use sophisticated technology and equipment to identify and destroy, or ablate, heart tissue responsible for arrhythmias. Ohio State’s Ross Heart Hospital team has nine EPs who are experts in the various arrhythmia treatment options, including the next generation of Electrophysiologists at Ohio State's Ross Heart Hospital use the new Stereotaxis Magnetic Navigation System technology to perform cardiac ablations. The system's computer-guided functionality allows the physician to better control the motion of the catheter as it is navigated to the heart. catheter-based care: Stereotaxis Magnetic Navigation System, which uses magnet-guided catheters to provide precise control not found in other equipment for treating arrhythmias. PROGRAM OF INTEREST Electrophysiology Electrophysiology Program Steering Team - (from left): Ashish Gangasani, MD; Ralph Augostini, MD; Emile Daoud, MD; Mahmoud Houmsse, MD; Steven Kalbfleisch, MD; Macy Smith, MD; Raul Weiss, MD; David Hart, MD; Doron Menachemi, MD. 34 Ohio State University Medical Center Shown in one of the state-of-the-art electrophysiology labs at Ohio State's Ross Heart Hospital are electrophysiologists Emile Daoud, MD (left), and Steven Kalbfleisch, MD (right). The Electrophysiology Program at Ohio State’s Ross Heart Hospital offers a full range of medical and catheter-based treatments for cardiac arrhythmia, an abnormal rhythm of the heart that can cause it to pump less effectively and lead to problems with heart chamber contractions. An arrhythmia occurs when the heart beats too fast, beats too slowly or skips beats because its electrical impulses become disjointed or disorganized. Some arrhythmias may barely cause symptoms, but others can result in such complications as fainting, stroke or cardiac arrest. Electrophysiologists (EPs) – cardiologists who treat electrical malfunctions of the heart – use sophisticated technology and equipment to identify and destroy, or ablate, heart tissue responsible for arrhythmias. The Ross Heart Hospital team has nine EPs who are experts in the various arrhythmia treatment options, including the next generation of catheter-based care: Stereotaxis Magnetic Navigation System, which uses magnet-guided catheters to provide precise control not found in other equipment for treating arrhythmias. Electrophysiologist Emile Daoud, MD, says patients benefit from this technique by having a quicker, safer procedure. And afterward, he adds, many patients no longer require medication for heart rhythm problems. Program highlights of 2006: • Five electrophysiologists were recruited to bolster arrhythmia treatment capabilities. • Approximately 120 new staff – including nurses, technicians, researchers and administrators – were hired to support the expanded team of electrophysiology physicians. • Multidisciplinary specialty clinics were established for Atrial Fibrillation and Genetic Arrhythmias. • An electrophysiology research section was established in Ohio State’s Davis Heart and Lung Research Institute with the addition of five electrophysiology research nurses. • The Electrophysiology Program has more than 25 research protocols. • The program structured an electrophysiology floor with specially trained staff to tend to the treatment of patients with arrhythmias. It also designed a 24-hour consultative service. • Two additional invasive electrophysiology labs were established along with the Stereotaxis Magnetic Navigation System, thus providing a total of five invasive electrophysiology laboratories. • The program extended its electrophysiology fellowship from one to two years and from one to two trainees per year. 2007 Research Report 35 esearc Current NIH PROGRAM PROJECT GRANTS, CENTER GRANTS AND LARGE MULTIPROJECT GRANTS IN 2006 Program Project Grants (PPGs) and Center Grants from the National Institutes of Health are among the largest and most prestigious funding awards in biomedical research. Headed by a lead investigator, these multimillion dollar grants comprise several individual projects, each equivalent to an NIH R01 grant and led by a co-investigator. This enables several investigators to work together toward a common mission. This section of the Research Report reviews active PPGs, CGs and other large-scale grants at Ohio State University Medical Center. 36 Ohio State University Medical Center NIH Program Project Grants, Center Grants and Large MultiProject Grants in 2006 Project 3: Chemokine regulation in human SLE nephritis – Brad Rovin, MD Project 4: Pathogenesis of SLE relapse in humans – Lee Hebert, MD PROGRAM PROJECT GRANTS INNATE IMMUNITY: ELUCIDATION/ MODULATION CANCER THERAPY Michael Caligiuri, MD, lead investigator – Department of Internal Medicine, Division of Hematology and Oncology. This PPG stresses innovative clinical cancer immunotherapy trials based on current understanding of innate immunology, as well as basic investigation into innate immune effector cell function in preparation for subsequent, more refined clinical cancer immunotherapy trials. DNA METHYLATION AND CHROMATIN MODIFICATIONS: MECHANISMS AND APPLICATIONS IN CANCER Samson Jacob, PhD, program director – Department of Molecular and Cellular Biochemistry. The longterm objective of this PPG is to advance basic understanding of the epigenetic regulation of gene expression in cancer cells and to rapidly translate the basic discovery of the molecular mechanisms into clinical trials in patients with chronic lymphocytic leukemia (CLL). Project 1: Innate immunity: elucidation/modulation cancer therapy – John Byrd, MD, and Pierluigi Porcu, MD Project 1: Clinical investigation of epigenetic therapy – John Byrd, MD Project 2: Homeostatic control of FcyR-triggered macrophage function – Susheela Tridandapani, PhD Project 2: Identification of methylated genes in chronic lymphocytic leukemia – Christoph Plass, PhD Project 3: Immune regulation of natural killer (NK) cell subsets – Michael Caligiuri, MD, Sherif Farag, MD, PhD, and Ramana Davuluri, PhD Project 3: Altered expression of protein tyrosine phosphatase by methylation: potential role in tumor suppression – Samson Jacob, PhD Project 4: Two signal requirements for NK immunity – William Carson III, MD Project 4: Histone modification and changes in chromatin: silencing of tumor-suppressor genes – Mark Parthun, PhD GENETIC AND CLINICAL RISK FOR HUMAN SLE NEPHRITIS Project 5: Brg1 and hBrm-associated histone methyltransferase: target genes in cancer therapy – Saïd Sif, PhD Lee Hebert, MD, program director – Department of Internal Medicine, Division of Nephrology. This project is studying risk factors for systemic lupus erythematosis (SLE) nephritis and its relapse in humans. GENETIC ANALYSIS OF THE BREAST TUMOR MICROENVIRONMENT Project 1: Genetic variants of CR1 and FcR in human SLE nephritis – Daniel Birmingham, PhD Michael Ostrowski, PhD, program director – Department of Molecular and Cellular Biochemistry. This PPG uses human genetic approaches and mouse genetic models to study how non-tumor cells in direct contact with tumor Project 2: Complement C4 and HLA class III genes in human genes in human SLE – Chack-Yung Yu, PhD 2007 Research Report 37 cells help cancer progress. Research focuses on the tumor microenvironment in breast cancer progression. Project findings may apply to any cancer of epithelial tissue, including prostate, lung, colon and liver. Project 1: Genetic alterations in the epithelial and stromal compartments of breast adenocarcinomas – sub-grant with Cleveland Clinic Foundation – Charis Eng, MD, PhD and structural changes throughout the human genome that appear linked to many types of cancer. Primary goals are to: increase understanding of complex epigenetic interactions in neoplasms; and use high-end information for improved prognosis, intervention and treatment of human female cancers. Project 1: Dissecting hierarchies of epigenetic control in gene silencing – Tim Hui-Ming Huang, PhD Project 2: Role of Rb and E2Fs in regulating stromal/epithelial interactions during mammary carcinogenesis – Gustavo Leone, PhD Project 2: Integrating genomic and epigenomic alterations in cancer and its microenvironment – Shili Lin, PhD Project 3: Role of the Ras/ets-2 pathway in breast tumor progression – Michael Ostrowski, PhD Project 3: Chromatin landscaping of TGF-P/SMAD signaling targets – Ramana Davuluri, PhD MECHANISMS OF CHRONIC PATHOBIOLOGY IN ALLOGRAFTS Project 4: Predicting drug resistance in cancer genomes by DNA methylation profiling – Tim HuiMing Huang, PhD Arthur Strauch III, PhD, program director – Department of Physiology and Cell Biology. This project studies the alloimmune processes that generate TGFbeta and the mechanisms by which TGFbeta promotes pathogenesis in allografts. Project 1: Role of alloantibodies in allograft pathobiology – Ronald Pelletier, MD Project 2: Role of macrophages in remodeling and rejection of solid organ transplants – Clay Marsh, MD Project 3: Myofibroblasts and fibrositis after cardiac transplant – Arthur Strauch, PhD INTEGRATED CANCER BIOLOGY PROGRAM (ICBP) COLLABORATIVE AGREEMENT INTERROGATING EPIGENETIC CHANGES IN CANCER GENOMES Tim Hui-Ming Huang, PhD, program director – Department of Molecular Virology, Immunology and Medical Genetics. This project studies two chemical 38 Ohio State University Medical Center SPECIALIZED CENTER GRANTS SPECIALIZED CENTER OF RESEARCH: EXPERIMENTAL THERAPEUTICS IN CLL John Byrd, MD, program director – Division of Hemotology and Oncology. This SCOR focuses on chronic lymphocytic leukemia (CLL), especially in patients at high risk due to the presence of a genetic abnormality. The central theme is the pursuit of preclinical (basic science) and clinical investigations of multi-targeted therapies. The team consists of four collaborating groups at Ohio State. The likely outcomes of the studies are: a better understanding of the effectiveness of an existing drug (flavopiridol) in treating CLL; development of better methods to use the drugs depsipeptide and HDAC-42 in conjunction with immunotherapy to treat CLL; development of more potent forms of depsipeptide and HDAC-42; and identification of targets (proteins) that may form the basis for the discovery of drugs to treat CLL. One notable aspect of this SCOR team is the inclusion of a chemistry team capable of changing existing drugs to improve their effectiveness and creating new drugs based on the results of the research projects pursed by the SCOR. Project 1: Pre-clinical and clinical development of flavopiridol in CLL – Michael Grever, MD Project 2: Chromatin remodeling as a therapeutic target to enhance biolic therapies in CLL – Michael Freitas, PhD Project 3: Therapeutic targeting of novel kinases in CLL – John Byrd, MD through continued basic, translational and clinical research. This grant finances the OSUCCC leadership and administration. It also funds several shared resources that facilitate collaboration among researchers. The OSUCCC comprises seven research programs, including Cancer Control, Experimental Therapeutics, Immunology, Molecular Biology and Cancer Genetics, Molecular Carcinogenesis and Chemoprevention, Pediatric Oncology, and Viral Oncogenesis. Project 4: Molecular target-based therapeutics for CLL – Ching-Shih Chen, PhD OSU NEUROSCIENCE CORE REDUCING CERVICAL CANCER IN APPALACHIA Electra Paskett, PhD, MSPH, program director – College of Public Health, Epidemiology Division and Comprehensive Cancer Center member. The objective of this Center Grant is to conduct two interventions aimed at reducing cervical cancer rates and also to examine strains of human papillomavirus (HPV) among women in Appalachia. Project 1: Cervical cancer screening among Appalachian populations – Electra Paskett, PhD Project 2: Tobacco use and cessation among Appalachian women – Mary Ellen Wewers, PhD Project 3: Correlates of abnormal pap smears in Appalachia – Electra Paskett, PhD John Oberdick, PhD, program director – Department of Neuroscience – This core funding enhances investigator access to a variety of animal model systems at Ohio State. The aims are: to support current National Institute of Neurological Disorders and Stroke (NINDS)-funded research projects; to facilitate interactive projects among principal investigators; and to facilitate interactions among basic and translational researchers at Ohio State. Core A: Administration – PI, John Oberdick, PhD Core B: Genetics (transgenic mouse & zebra fish) – PI, Anthony Young, PhD Core C: Rodent behavioral phenotyping – PI, Randy Nelson, PhD Core D: Electrophysiology – PI, Mike Xi Zhu, PhD Reducing cervical cancer in Appalachia, diversity training supplement – Kimberly Kelly, PhD Core E: Confocal microscopy – PI, Anthony Brown, PhD CORE GRANTS OSU COMPREHENSIVE CANCER CENTER SUPPORT GRANT (OSUCCC) Michael Caligiuri, MD, program director – Department of Internal Medicine, Division of Hematology and Oncology. The overall goal of the OSUCCC is to reduce cancer morbidity and mortality 2007 Research Report 39 esearc Major Research Centers AFFILIATED WITH OHIO STATE UNIVERSITY MEDICAL CENTER Several interdisciplinary health sciences programs at The Ohio State University have become so large and prominent that they attract significant external funding and merit their own administrative structure. These major research centers, which also benefit from their proximity to the main Ohio State campus with its equally renowned colleges in other disciplines, are highlighted in this section. 40 Ohio State University Medical Center COMPREHENSIVE CANCER CENTER (OSUCCC) Michael Caligiuri, MD, Director The Cancer Centers Program of the National Cancer Institute supports specific academic and research institutions throughout the United States to sustain broad-based, coordinated, interdisciplinary programs in cancer research. In 1976 the National Cancer Institute (NCI) designated The Ohio State University as one of the nation’s first Comprehensive Cancer Centers. The OSUCCC focuses on all aspects of cancer care: prevention, diagnosis, treatment, control, rehabilitation and education. As one of only 39 NCI-designated Comprehensive Cancer Centers, the OSUCCC’s top priority is translating basic research findings into clinical applications. The OSUCCC has approximately 215 cancer investigators representing 14 of the 18 colleges at Ohio State, as well as another 60 cancer investigators at affiliated institutions, including Columbus Children’s Hospital (CCH) and the Cincinnati Children’s Hospital Medical Center (CCHMC). The OSUCCC comprises seven research programs whose member investigators collectively generate nearly $125 million annually in cancer-relevant research funding, more than three quarters of which is peer-reviewed funding. Ongoing Research Programs • Cancer Control – Focuses on early detection, survivorship and behavioral strategies related to cancer prevention and control. Co-Leaders: Electra Paskett, PhD, MsPH, and Mary Ellen Wewers, PhD, MPH, RN • Experimental Therapeutics – Evaluates novel therapeutics, imaging, cell therapeutics and drug targeting. Co-Leaders: Michael Grever, MD, and Samson Jacob, PhD • Immunology – Focuses on basic T-cell biology, with applications in vaccine development, and on cellular innate immunity, with applications in antibody- dependent cellular cytotoxicity. Leader: William Carson III, MD • Molecular Biology and Cancer Genetics – Seeks to understand the control of gene expression as it relates to cell proliferation, DNA replication, differentiation, developmental regulation and the molecular basis of cancer. Co-Leaders: Albert de la Chapelle, MD, PhD, and Michael Ostrowski, PhD • Molecular Carcinogenesis and Chemoprevention – Investigates effects of genetic alterations induced by chemical toxins and infectious agents, identifies tumor-suppressor genes and studies other aspects of multi-stage carcinogenesis. Leader: Steven Clinton, MD, PhD • Pediatric Oncology – Enhances the care of children with blood diseases and cancer through innovative research and facilitation of translational research studies, and improves care for cancer survivors. Co-Leaders: John Perentesis, MD (at CCHMC) and Stephen Qualman, MD (at CCH) • Viral Oncogenesis – Seeks to discover which retroviruses contribute to human cancer and to develop and implement gene-delivery strategies using retroviral vectors. Co-Leaders: E. Antonio Chiocca, MD, PhD, and Patrick Green, PhD Research Accomplishments of 2006 • The OSUCCC – James Cancer Hospital and Solove Research Institute received a five-year, $1.25 million Livestrong Survivorship Center of Excellence grant from the Lance Armstrong Foundation (LAF). The grant will provide operational support for research, educational and patient support services for cancer survivors. Charles Shapiro, MD, is principal investigator of the grant and director of the survivorship center, which is one of only seven such centers funded by the LAF in the United States. • Led by principal investigator Samson Jacob, PhD, co-leader of the Experimental Therapeutics Program, the OSUCCC received a five-year, $11.84 million program project grant from the National Cancer Institute (NCI) entitled “DNA Methylation and Chromatin Modifications: Mechanisms & 2007 Research Report 41 Applications in Cancer Therapy.” This is the sixth NCI programmatic grant awarded to OSUCCC investigators in the past five years. • The OSUCCC increased its level of research funding from the NCI by 12.5 percent, from $35.2 million in 2005 to $39.6 million in 2006. • Through collaborations with multiple Ohio State University colleges and departments, the OSUCCC recruited 20 cancer faculty, physicians and scientists. • Former OSUCCC Director Clara Bloomfield, MD, who now serves as cancer scholar and senior adviser to the OSUCCC – James Cancer Hospital and Solove Research Institute, was selected as a Distinguished University Professor at Ohio State, the University’s highest faculty award. Bloomfield also received the prestigious Distinguished Service Award for Scientific Achievement from the American Society of Clinical Oncology (ASCO). • Another 12 OSUCCC investigators were named Fellows in the American Association for the Advancement of Science (AAAS). In all, 45 OSUCCC members are now AAAS Fellows. • Three OSUCCC members – Manisha Shah, MD; Kimberly Kelly, PhD; and Vipul Patel, MD, were recognized for their achievements by Business First’s “Forty Under Forty” program. • Carlo Croce, MD, who leads the Human Cancer Genetics Program at Ohio State and is a member of the OSUCCC’s Molecular Biology and Cancer Genetics Program, led a team of investigators from three colleges and five departments to a successful bid for a five-year, $6.1 million Targeted Investment in Excellence (TIE) award from Ohio State. The grant will focus on developing, validating and commercializing tests and microRNA drugs for the diagnosis, monitoring, prognosis and treatment of human malignancies. • The OSUCCC – James Cancer Hospital and Solove Research Institute moved from 29th in 2005 to 21st in 2006 in U.S.News & World Report’s annual rankings of “America’s Best Hospitals” for cancer care. 42 Ohio State University Medical Center COMPREHENSIVE TRANSPLANT CENTER Ronald Ferguson, MD, PhD, Director Established in February 2005, Ohio State’s Comprehensive Transplant Center encompasses all solid organ and cellular transplantation programs at OSUMC and involves faculty from a wide cross-section of clinical and research programs. Thoracic, abdominal and cellular transplant programs at Ohio State formerly operated independently of one another within their respective academic units. These programs enjoyed many recognized successes, especially in the kidney, pancreas and bone marrow transplant programs – which are among the best in the country – but University officials determined that even more could be accomplished by working more closely together, particularly on research projects. “Ohio State and other top transplant programs around the country have on-site research and education programs that serve as an engine to clinical care,” says Ronald Ferguson, MD, PhD, director of the Comprehensive Transplant Center. “The Center is a common thread that weaves through each of the programs and is a driver for enhancing collaboration.” Each transplant program maintains direct links to its respective academic department, but the Comprehensive Transplant Center is closely involved in support and operation of the programs, including grant acquisition and faculty recruitment. Within the Center, teams of researchers and clinicians from each of the transplant areas pursue projects of common interest in transplantation, including immunopharmacology, immune assessment monitoring and engineering, building information systems and biostatistic databases, and public policy and ethics. Among projects the Center is pursuing are an islet cell transplantation program and expansion of the lung and liver transplantation programs. DOROTHY M. DAVIS HEART AND LUNG RESEARCH INSTITUTE Jay Zweier, MD, Director The Ohio State University’s Dorothy M. Davis Heart and Lung Research Institute (OSU DHLRI) is one of the nation’s few free-standing facilities devoted entirely to the research of diseases affecting the heart, lungs and blood vessels. Its mission is to develop novel strategies to prevent and cure heart and lung diseases. The Institute is an internationally recognized center of excellence for medical research that leads to findings with broadbased applications to other diseases and conditions. Ongoing Research Programs Four major thematic areas: • Myocardial ischemia and metabolism • Myocyte biology and disease • Inflammation, fibrosis and immune function • Regenerative medicine Sub-areas of specialized focus: • Cardiovascular and molecular imaging • Cardiovascular genomics and pharmacogenomics • Myocardial salvage and regeneration post myocardial infarction • Molecular therapies and devices for the treatment of heart failure • Fibrosis, remodeling and lung injury • Innate immune system function • Mitochondria biology and critical care disease • Control of lung inflammation to prevent lung fibrosis • Environmental and smoking-induced heart and lung disease • Tissue engineering and stem cell biology • Nitric oxide biology and signaling • Redox biology and free-radical research • Molecular and cellular therapies of tissue repair and wound healing Research Accomplishments of 2006 • Demonstrated that sustained reverse left ventricular structural remodeling, at one year, can be achieved with cardiac resynchronization, and that this efficacy is a function of the etiology of heart failure. • Demonstrated that monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. • Identified an aspirin derivative that donates nitric oxide, is highly effective in myocardial protection and shows potential to enhance cancer therapy. • Determined the effects of platelet antigen polymorphism on platelet inhibition by aspirin, clopidogrel or their combination. • Demonstrated that microRNA-155 regulates human angiotensin II type 1 receptor expression. • First identified that abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel are linked to exercise-induced sudden cardiac death. • Demonstrated that mitochondrial iPLA2 activity modulates apoptosis through the release of cytochrome c from mitochondria and influences the permeability transition. • Demonstrated that proteasome inhibition downregulates endothelial nitric oxide synthase phosphorylation and function. • Characterized the mechanism by which nitric oxide and nitrosothiol generation occurs from organic nitrates. • Performed the first transcriptome analysis of the ischemia-reperfused remodeling myocardium, which identified the temporal changes in inflammation and extracellular matrix. • Collaboratively recruited key assistant professors in the Department of Internal Medicine’s Division of Cardiovascular Medicine and in the School of Public Health. • Collaboratively recruited key professors in the Department of Internal Medicine’s Division of Cardiovascular Medicine and in the Department of Surgery’s Division of Cardiothoracic Surgery. 2007 Research Report 43 • Established an Intramural Thematic Grants Program to encourage and support DHLRI investigators in developing programs and careers in the four major thematic research areas. In autumn 2006, the program funded 12 investigators for a total of $831,855. • Identified three genetic mutations that predispose young individuals to sudden cardiac death. • Received two National Institutes of Health programmatic grants (PPGs) in ischemic heart disease and myocardial protection in partnership with other institutions. • Received approximately $4 million from the National Institutes of Health and the National Institute of Biomedical Imaging and Bioengineering for establishing the nation’s first program for proton electron hyperpolarization imaging. • Received funding for the first redox proteomics grant. • Created the Ohio State University Vascular Research and Imaging Center and recruited a director. • Continued growth of the Cardiac MRI and CT program. • Created the Clinical and Research Center for Echocardiography and recruited a director. • The Comprehensive Wound Center, established by DHLRI investigators, became a University-recognized center. GENERAL CLINICAL RESEARCH CENTER (GCRC) William Malarkey, MD, Director 2006 was an exciting year for Ohio State University’s General Clinical Research Center, which has approximately 100 active protocols from 39 departments in 10 colleges. Much of the research in this Center is interdisciplinary, and GCRC investigators continue to be supported via national funding agencies such as the National Institutes of Health. 44 Ohio State University Medical Center Ongoing Research Programs • Agriculture – Testing the validity of a simulated environment with dangerous farm equipment using endocrine measures to validate stressful simulations • AIDS/HIV – Phase I clinical trials for HIV-infected subjects requiring hemodialysis • Autism – Attention Deficit Hyperactivity Disorder symptoms in autism; Risperidone and behavior therapy in children with pervasive developmental disorders; oral human immunoglobulin in autism • Behavioral Medicine – Olfactory sensitivity and physiological responses; caregiver stress in parents of children with mood disorders; chronic stress and immune function • Cancer – Stress and immunity project for women with breast cancer; oral cancer studies; prostate cancer and lycopene supplementation • Cardiology – Management of patients with heart failure; chronic heart failure and survivors of an acute myocardial infarction • Community – Resource and education projects in Appalachian areas involving smoking cessation; cancer and health behavior; nutrition, cancer and the Amish • Critical Care Medicine – Obese critically ill: outcome and process disparities • Diabetes – Prevention trials; hyperglycemia and hyperinsulinemia; type 2 diabetes mellitus; natural history in African-Americans; management of type 2 diabetes mellitus by conjugated linoleic acid (CLA) • Economics – Understanding human response to economic risk with MRI and genetic polymorphisms • Endocrinology – Metabolic energy requirements for normal menstrual function; insulin resistance; adiponectin in people of West African ancestry • Exercise – Exercise as therapy for asthma; yoga, exercise, immune function and health; insulin resistance in football players • Neurology – New therapies for amyotrophic lateral sclerosis; multiple sclerosis; spinal muscular atrophy; myasthenia gravis; muscular dystrophy; effects of psychosocial stress and genetics on cognition • Nutrition – Lifestyle and body weight changes in young females; breast cancer risk in overweight and obese premenopausal women • Obesity – Aging, stress and chronic inflammation • Obstetrics and Gynecology – Gestational diabetes and inflammatory markers; vaginal ultrasound cerclage trial; psychosocial factors associated with inflammatory and antibody responses to influenza vaccination in pregnancy • Ophthalmology – Idiopathic intracranial hypertension • Pediatrics – Hypothalamic pituitary adrenal axis function in adolescent antisocial females; growth hormone and endothelial function; natural history study of the development of type 1 diabetes; childhood obesity; hypoglycemia and endothelial function; health consequences of sleep apnea in obese children and its association with gastroesophageal reflux • Pulmonary – Alveolar macrophage proteomics in HIV-associated emphysema; COPD in patients with AIDS; pulmonary rehabilitation exercise on biobehavioral outcomes in COPD • Renal – Genetic and clinical risk factors for human SLE nephritis; African-American study of kidney disease and hypertension; influence of fenofibrate therapy on kidney function in CKD patients • Smoking Cessation Studies – Nicotine dependence; race and nicotine metabolism • Stress – Stress and wound healing; chronic stress and immune function; caregiver stress and chronic inflammation; Omega-3 dietary supplementation, immune function, and mood • Surgery – Gene expression profiles in healing and non-healing wounds • Transplantation – Bone architecture in patients with renal transplant; living unrelated kidney donor and sibling follow-up • Education – The GCRC Mentoring Program, directed by Philip Diaz, MD, over the past year has recruited young physicians interested in clinical research and is preparing them to implement research proposals. In 2006, eight protocols were submitted by GCRC mentees. A K23 application prepared by Naeem Ali, MD, was funded. In addition, Dara Schuster, MD, associate program direc- tor, has established an introductory course on clinical research. Its objectives are to introduce undergraduates to clinical research as a career opportunity, to teach basic skills with didactic lectures and hands-on training, and to provide a mentored experience in clinical research. Research Accomplishments of 2006 • The GCRC is an important partner of a 2005awarded T32 grant entitled “Eliminating Barriers to Effective Training in Clinical Investigation,” a grant directed by Philip Binkley, MD. Only 10 of these grants were funded nationally. Funded through August 2010, this training proposal in predoctoral clinical research will propose solutions to major barriers to effective training and present a strategy for determining whether the curriculum addresses a critical need for increasing numbers of clinician scientists. Specifically, this program provides predoctoral nursing, dental and medical students with an innovative curriculum of biostatistics, study design and ethical conduct of research in an interactive team-learning approach, delivered as a core curriculum to all students in these respective programs at Ohio State and at outside institutions for whom videoconference participation will be offered. These students can enter a short course in clinical research and an intensive year-long curriculum culminating in a master of public health degree with emphasis in clinical investigation. • Nursing – Approximately 30 percent of GCRC protocols involve pediatric participants. As of this year, 100 percent of RNs on the unit have completed or maintained their Pediatric Advanced Life Support training. Also, the majority of nurses are certified in Advanced Cardiac Life Support as an increasing number of protocols require exercise testing. 2007 Research Report 45 • Bionutrition – The resources (diet assessment, body composition and metabolism, and cardiovascular testing and human performance) of the GCRC’s Bionutrition Core have been increasingly used by investigators. The Center has developed an ability to enter body composition data directly into databases from a bedside tablet computer. Bionutrition research training programs for nurses, technicians and students are being developed. • Core Laboratory – The Laboratory Core, composed of processing and analytical laboratories, provides analytical support for investigators. The present menu, which is expanding, contains research assay panels in: endocrine and neuroendocrine; metabolism, diabetes, obesity; inflammatory; and bone assays. • Informatics – The Informatics Core offers services in database management, administrative computing and project management. A major piece of software written in 2006 by Informatics personnel makes research patient scheduling more efficient. This application is written in mod_perl using a PostgreSQL database and is released under the GNU General Public License. Consequently, this open-source program will be freely available to GCRCs nationally. • Research Subject Advocacy (RSA) – The Center’s Research Subject Advocacy program, which began in 2002, ensures that GCRC studies are designed, implemented and conducted safely and ethically, affording the highest priority to the protection of human subjects. The RSA is a resource and adviser to GCRC personnel, prospective and current researchers, human subjects/research participants, and the GCRC Advisory Committee (GAC). • Emerging Clinical Programs – The GCRC is strengthening its connection with new clinical programs, such as the Center in Chronic Wound Healing. The GCRC is participating in the growth of this Center, which will be a coordinating center for 16 university wound-healing programs across the country. One protocol has already been received for evaluating microarrays and proteomics from wound biopsies obtained from chronic wound patients to better understand the pathogenesis of wounds. And one paper published by this group and supported by GCRC resources is 46 Ohio State University Medical Center entitled, “Wound site neutrophil transcriptome in response to psychological stress in young men” (Gene Expression, 2005). • CTSA Development and Opportunities – GCRC staff have been involved in the CTSA process, which led to a grant submission in January 2007. As part of this process, the GCRC will be incorporated into the Participant and Clinical Interactions Resources (PCIR) Core, which provides a highquality, ethical, safe and cost-effective environment that fosters education and training while encouraging participation by the community and investigators in clinical and translational lifespan research. To transform the GCRC structure, the Center incorporated various Health Sciences and community clinical and translational resources into the PCIR, which will link to the other key functions of the CTSA. Other areas to be integrated into the PCIR include: - Columbus Children’s Hospital Clinical Studies Center – This is a 3,000-square-foot facility on the sixth floor of the Outpatient Care Center on the Children’s Hospital campus. The facility is staffed by eight clinical research nurse coordinators and a psychometrician trained in psychometric assessments. It is equipped to perform: specimen sample collection, processing and storage; research subject recruitment; and data collection and management. - Comprehensive Cancer Center Clinical Treatment Unit (CTU) – Housed in the Medical Center’s James Cancer Hospital and Solove Research Institute, the CTU has 1,700 square feet for outpatient studies involving phase I and II chemotherapy protocols. To enhance its community translational component, the GCRC has incorporated two additional units as “laboratories” to create a “research unit without walls”: • Primary Care Practice-Based Research Network for Pediatric and Adult Patients (PBRN) – This is a network of 24 primary care sites throughout central Ohio that serves as a community-based translational research laboratory for a research faculty with members from nine colleges and 22 departments at Ohio State, including Pediatrics. • Ohio Extension Service – The Ohio Extension Service is part of the College of Food, Agriculture and Environmental Sciences at Ohio State. Extension supports operations in all 88 counties of Ohio. A major focus involves linking local community needs with Ohio State researchers. Community-based health-related research is a major thrust of Extension. CENTER FOR BIOSTATISTICS Stanley Lemeshow, PhD, Director The Center for Biostatistics provides expertise with which biomedical investigators within and outside The Ohio State University can collaborate in study design, data management and statistical analysis of clinical, epidemiological, public health and laboratory research data. As a central resource, the Center has more than 20 full-time staff who respond to requests for support of biomedical investigations. The Center also involves many faculty in the Division of Biostatistics and the Department of Statistics in this collaborative effort. David Jarjoura, PhD, is the Center’s managing director. Ongoing Research Programs • Current grant activity includes eight Biostatistics Core-type grants funded by the National Institutes of Health. • The Center is also supported by 10 R01s, 11 R21s and various other grants. • The grants bring in more than $26 million per year to Ohio State. • The grants involve collaborations with the colleges of Medicine, Public Health, Veterinary Medicine and Nursing, and with Columbus Children’s Hospital. Research Accomplishments of 2006 • Center staff collaborated in writing more than 90 grant proposals and had 48 publications, with another 45 in submission. The Center promotes the development of long-term collaborative relationships between its staff and other investigators to increase efficiency, optimize productivity and enhance responsiveness. Center personnel include a director, a managing director, an administrator, a human resource professional, five PhD biostatistical scientists, nine MS biostatisticians, a systems programmer, a systems specialist, four PhD graduate students and three undergraduate students. CENTER FOR CRITICAL CARE Clay Marsh, MD, Director In the spring of 2006, Clay Marsh, MD, professor of Internal Medicine, was named director of a newly established Center for Critical Care at Ohio State University Medical Center. Marsh also directs the Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine and is associate director for lung research at Ohio State’s Dorothy M. Davis Heart and Lung Research Institute. Medical Center CEO Fred Sanfilippo, MD, PhD, says Marsh is an excellent choice to advance the mission of critical care medicine, which also has been identified as one of six Signature Programs at the Medical Center. “Through his translational research leadership and national stature, we will be able to more fully integrate critical and clinical care, research and education between medicine, surgery and anesthesiology,” Sanfilippo says. “Research has shown that implementing evidence-based practices improves outcomes for patients in critical care centers.” 2007 Research Report 47 Ongoing Research Programs • The Center for Critical Care provides innovative solutions for patients undergoing critical care and for those with lung disease. It focuses on personalized health care that defines illness and treatment for each patient and transitions care from disease management to prevention. • The Center has begun training professionals who care for patients in the Intensive Care Unit (ICU) environment with the Society of Critical Care Medicine-sponsored Fundamentals of Critical Care Support course – the first effort for global training in using this tool in the ICU. • The Center’s focus on sepsis (organ failure from infection) and personalized health care for patients is moving forward with the establishment of a registry of patients diagnosed with sepsis. This resource will allow caregivers to define risk factors leading to sepsis and identify new targets for patients with this life-threatening disease. • Clinically, the Center is leveraging the Medical Center’s Information Warehouse to ensure that the Center is executing evidence-based medicine for all patients in the ICU environment. Caregivers are tracking outcomes to define new pathways that will more effectively treat patients in the ICU. CENTER FOR KNOWLEDGE MANAGEMENT JOHN A. PRIOR HEALTH SCIENCES LIBRARY Susan Kroll, MLS, Director Now in its second phase of development, the Ohio State University Center for Knowledge Management (CKM), housed in the John A. Prior Health Sciences Library, will become one of the nation’s most comprehensive repositories for global biomedical knowledge and intellectual capital. The library provides cost-effective access to biomedical knowledge, identifies and makes available knowledge and key research findings, expedites packaging of information content as 48 Ohio State University Medical Center reusable and sharable resources, facilitates understanding and helps incorporate information resources into work processes. The CKM offers: upto-date information and tools to support instructional technology; graphic, photographic and print production; interactive multimedia; e-learning management; and an ability to meet the customized Web needs of its customers. Ongoing Research Programs Center for Knowledge Management • Tim Cain, PhD, is principal investigator for OSU:pro, a phase 1 implementation of a campuswide data-integration tool for tracking faculty and staff expertise at Ohio State. Funded by a $480,000 grant, the project is sponsored by the offices of Academic Affairs, Health Sciences University CIO and University Libraries. • In May 2006, Tim Cain, PhD, Chris Fish and Elizabeth Sabatino filed a provisional patent application with the U.S. Patent and Trademark Office for a data-integration tool for synthesizing the tacit and explicit knowledge of University personnel. • In July 2006, Daniel Sabatino, Dave Dipietro, Pam Pierron and Tammy Thompson filed an invention report with the Office of Technology Licensing and Commercialization at Ohio State. T. MedSTAR: Leveraging data services to manage, track and contextualize the academic performance, records and curricular activities of professional students. Prior Health Sciences Library • Pamela Bradigan, principal investigator, and Fern Cheek, co-investigator, are working to determine what type of academic medical center library support is provided to biomedical researchers conducting clinical trials. • Jane Case-Smith, principal investigator, and Carol Powell, co-investigator, are analyzing trends in occupational therapy research for the past five years by reviewing articles published in journals in that field. • Lynda Hartel, principal investigator, and Fern Cheek, co-investigator, are examining electronic book technology to assess how OSUMC faculty, staff and students use this format for their information needs. • After implementation of the National Institutes of Health Public Access Policy in 2005, Lynda Hartel, principal investigator, and Jan Maxwell, co-investigator, are examining libraries’ and publishers’ commitments to this forum, assessing the different mix of models, education of grant recipients to the policy and promotion of open access publishing to faculty members. • Eric Schnell, principal investigator, is researching these questions related to library technology: Are libraries structured or organized to innovate? Which technologies will soon affect libraries? How can Web site mashups and Web services be used in libraries? • The John A. Prior Health Sciences Library was designated as an Outreach Library and awarded a $2,000 grant (May 2006-April 2007) by the Greater Midwest Region of the National Networks of Libraries of Medicine. Marguerite Weibel is principal investigator. CENTER FOR MICROBIAL INTERFACE BIOLOGY (CMIB) Larry Schlesinger, MD, Director The Center for Microbial Interface Biology (CMIB), created by Larry Schlesinger, MD, in 2002, was awarded official University center status by the Ohio State University Board of Trustees in December 2006. The CMIB is a multidisciplinary research center focused on microbe-host interactions that promotes and coordinates interdisciplinary research and training opportunities in infectious diseases, microbial pathogenesis and biodefense. The CMIB also manages the Columbus campus Biosafety Level III core research facilities, which are available to the University research community, University collaborating researchers and non-University researchers. In 2006, the CMIB had six core faculty members, and general membership grew to 50 faculty, representing units across the Columbus and Wooster campuses. Research funding for core faculty totaled $5.55 million ($10.55 million in review). These faculty had 20 papers published or in press along with numerous published abstracts. They also had several invited lectureships and review panel appointments, both nationally and internationally. Ongoing Research Programs • Salmonella antimicrobial peptide resistance (National Institutes of Health/National Institute of Allergy and Infectious Diseases) – John Gunn, PhD. The objective of this award is to further characterize the role of the Salmonella PmrAB regulon, specifically the PmrAB-regulated pmrHFIJKLM operon, in LPS modification, resistance to antimicrobial peptides, and in virulence. • CD8 T cells and immunity to tuberculosis in old mice (National Institutes of Health/National Institute on Aging) – Joanne Turner, PhD. The objective of this award is to understand how CD8 T cells contribute to protective immunity in the elderly during infection with M. tuberculosis. • TB and innate immune regulation of lung macrophages (National Institutes of Health/ National Institute of Allergy and Infectious Diseases) – Larry Schlesinger, MD. The objective of this award is to determine the role of surfactant components in regulating the early interactions between Mycobacterium tuberculosis and macrophages, the host cell niche for this intracellular pathogen. • Characterization of the surface metalloproteases of Trypanosoma cruzi (American Heart Association) – Brad McGwire, MD, PhD. The objective of this award is to better define the role of a major surface metalloprotease, gp63, of Trypanosoma cruzi, the agent of South American trypanosomiasis, in the pathogenesis of disease and its contribution to heart failure. 2007 Research Report 49 Research Accomplishments of 2006 • Lung innate immune responses to Francisella tularensis: a central role for the macrophage (National Institutes of Health/National Institute of Allergy and Infectious Diseases Region V Great Lakes Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research) – Larry Schlesinger, MD. This project studies lung innate immune response to Francisella tularensis, the causative bacterium of tularemia and a targeted agent of bioterrorism. The most worrisome infectious agents of bioterrorism are those that would be artificially disseminated as aerosols to the lungs. Thus, a clearer understanding of lung immune response to these bacteria, especially as they relate to interactions with macrophages, is essential for identifying molecular targets for diagnostic strategies, as well as targeted immune therapies to enhance host immunity. This program project grant includes investigators in both the CMIB and Ohio State’s Davis Heart and Lung Research Institute. • Salmonella antimicrobial peptide resistance (National Institutes of Health/National Institute of Allergy and Infectious Diseases) – John Gunn, PhD. Salmonellae encounter numerous anatomic sites during infection, including the inhospitable environment of the macrophage phagosome, where they are able to survive and replicate. Within host phagocytes and at mucosal surfaces is a potent group of cytotoxic agents (i.e., antimicrobial peptides, or AP). The PmrA-PmrB twocomponent regulatory system is activated when Salmonellae are within host macrophages, and this system is necessary for resistance to AP, which involves modifications of the lipopolysaccharide (LPS) that decrease peptide binding. This study focuses on the PmrA-PmrB regulon, including the identification and characterization of PmrA-PmrBregulated genes necessary for AP resistance and LPS modification, and the determination of the role of PmrA-PmrB-mediated LPS modifications in Salmonella virulence. • In vitro predictors of susceptibility for reactivation tuberculosis (American Lung Association) – Joanne Turner, PhD. Approximately one third of 50 Ohio State University Medical Center the world’s population is infected with M. tuberculosis; however, only a small portion will develop active tuberculosis. Identifying individuals who are predisposed to reactivate an infection with M. tuberculosis would result in timely intervention and reduce the opportunity for disease transmission. Identifying immunological markers of susceptibility to reactivating an infection would provide such a tool. This grant uses several well-characterized mouse models that are resistant or susceptible to reactivation of a chronic infection with M. tuberculosis to study immunity in the lung and peripheral blood. Studies focus on the relationship between increased IL-10 and a loss of IFN-α production, and on changes that occur at a period of infection that precedes reactivation. Predicting when reactivation is likely to occur would help identify individuals who are progressing toward disease, resulting in timely intervention and reduced disease transmission. • After being awarded official University center status by the OSU Board of Trustees in December 2006, the CMIB moved into the 10th floor of the recently completed Biomedical Research Tower. • CMIB core faculty members had 20 papers published or in press in 2006. • Total research funding for 2006 for CMIB core faculty members was $5.55 million ($10.55 million in review). • Ohio State was established as a member of the National Institutes of Health-funded Great Lakes Regional Centers of Excellence (RCE) in Biodefense and Emerging Infectious Diseases Research Program. CMIB faculty members were awarded a program grant and a development project totaling $1.15 million. • CMIB core faculty received the following awards: - Larry Schlesinger, MD – Donald V. Unverferth Department of Medicine Research Award, Department of Medicine, Ohio State University - Joanne Turner, PhD - American Association of Immunologists Pfizer-Showell travel award • CMIB 2006 awards for research grants included: - Lung Innate Immune Responses to Francisella tularensis: A Central Role For The Macrophage $1.05 million (includes investigators in both the CMIB and DHLRI) to study lung innate immune responses to Francisella tularensis, the causative bacterium of tularemia and a targeted agent of bioterrorism - Altered M. tuberculosis Mannosylation and the Macrophage - $1.8 million to study the surface carbohydrates of M. tuberculosis that play a central role in pathogenesis CENTER FOR MINIMALLY INVASIVE SURGERY W. Scott Melvin, MD, Director The Center for Minimally Invasive Surgery (CMIS) is a multidisciplinary center dedicated to excellence in patient care, clinical training, research and outcomes studies pertaining to the techniques and technology of minimally invasive surgery. The Center strives to expand the ever changing field of surgery. 2006 brought further development of the Center’s research endeavors. Research Accomplishments of 2006 • The Center has 21 active research protocols, 11 of which were added during 2006; secured research funding totals over $300,000 annually. Projects include 17 human and four animal protocols. CMIS faculty collaborated with other Ohio State departments and investigators on eight research protocols. • Through collaboration between the CMIS and the Department of Anesthesiology, the National Institutes of Health awarded a grant entitled “Neural control of large intestinal mucosal.” Additionally, the National Institute of Diabetes and Digestive and Kidney Disease awarded funding to investigate “Purinergic regulation in enteric neural reflexes (in IBD).” Tissue samples taken during minimally invasive bariatric and colon surgery provide materials for these endeavors. Continued work between the physicians is planned and additional NIH funding opportunities are being explored. • The CMIS remains committed to cutting-edge technology. One of the newest research topics is the possibility of surgery using no external incisions. The expanding world of natural orifice translumenal endoscopic surgery (NOTES) requires a combination of endoscopic and laparoscopic surgical skills. The CMIS is working with industry to develop safe and effective methods of performing NOTES. It is the only institution in the United States with approval for a human clinical trial to evaluate the safety and feasibility of this type of surgery. NOTES is being used at the Medical Center as a diagnostic procedure for cancer patients prior to surgery. Upcoming phases of NOTES research at Ohio State include developing a standard of care for these procedures, continuing to identify instrumentation needs, and improving safety and efficiency. From basic data collection on bacterial contamination in transgastric surgery to the development of necessary instrumentation, the CMIS is on the cutting edge of the surgical future. CENTER FOR ROBOTIC AND COMPUTERASSISTED SURGERY Vipul Patel, MD, Director An ever-expanding component of minimally invasive surgery is the use of robotic technology for procedures that involve only minor disruption to the body compared with traditional open surgery. The Center for Robotic and Computer-Assisted Surgery at Ohio State is widening the Medical Center's international reputation in robotic surgery from both a clinical and educational standpoint - providing outstanding patient care and training surgeons from around the world in this burgeoning discipline. The Center is directed by Vipul Patel, MD, associate clinical professor in the Department of Urology. 2007 Research Report 51 Patel, a specialist in prostate and kidney cancer, also directs the Medical Center's Robotics and Minimally Invasive Urologic Surgery Program. He was recruited to the Medical Center in 2005 to use the da Vinci® Surgical System for radical prostatectomy, or surgical removal of the prostate gland. He has performed more than 1,500 robotic prostatectomies in his career and is one of only two physicians in the world who have performed more than 1,000 robotic surgical procedures overall. Patel also plays a lead role in teaching this discipline to surgeons from around the globe who come to the new Center at Ohio State for robotics training. CENTER FOR MOLECULAR NEUROBIOLOGY Anthony Young, PhD, Director The mission of the Center for Molecular Neurobiology is to reach the highest echelons of scholarship by performing quality basic research and providing superior training in developmental, cellular and molecular neuroscience. Research strengths include the use of three molecular genetic model systems (mice, zebra fish and Drosophila) as well as research directly applicable to humans. This is an intercollegiate program whose faculty have joint appointments in the Center as well as in departmental tenure-initiating units. They can thus contribute to the missions of their departmental units and form bridges between the Center and departments within separate colleges. Ongoing Research Programs • Christine Beattie, PhD, studies molecular mechanisms controlling vertebrate axon guidance during development and motor neuron degeneration in spinal muscular atrophy and amyotrophic lateral sclerosis using zebra fish as a model system. • Anthony Brown, PhD, uses molecular, biochemical and imaging approaches to study the assembly and axonal transport of neurofilaments. • Tsonwin Hai, PhD, studies the role of the stressinducible transcription factor ATF3 in diabetes and cancer. 52 Ohio State University Medical Center • Paul Henion, PhD, studies molecular regulation of embryonic cell diversification and cell fate specification, proliferation, survival and differentiation in the nervous system. • Jeff Kuret, PhD, employs molecular, cellular and pharmacological methods to investigate Alzheimer disease pathogenesis. His work focuses on biochemical mechanisms in the formation of lesions that are the hallmarks of the disease, and on development of inhibitors of lesion formation as potential therapeutics. • Sung Ok Yoon, PhD, studies molecular mechanisms of growth factor-mediated action in the nervous system, with a focus on regulation of cell survival and apoptosis under pathological conditions. Research Accomplishments of 2006 • During cancer development, stress signals designed to eliminate tumorigenic cells are encountered. Cancer cells that survive this process have managed to foil the hardwired stress response. Studies by Tsonwin Hai, PhD, and associates have shed light on this process by showing that ATF3, a gene that normally eliminates cells as part of the stress response, is co-opted to become an oncogene, and that this phenomenon may play an important role in breast cancer progression. In addition, they have found that ATF3 acts as a mediator for cancer cells to respond to stromal signals. Thus, ATF3 transmits the signals to the transcriptional networks and elicits dichotomous responses. • James Jontes, PhD, has developed a novel cell model to explain how connections among the trillions of synapses in the central nervous system are formed. The model involves formation of nonspecific prosynaptic interactions followed by specific synapses through recruitment of bona fide specific adhesion molecules via changes in intracellular trafficking to generate reproducible patterns of synaptic connectivity. His laboratory is testing this hypothesis and the role of the cadherin family of adhesion molecules through analysis of the developing zebra fish nervous system. • Through behavioral studies using “knock-out” mice, John Oberdick, PhD, has found that L7/PcP2, a protein expressed in cerebellar Purkinje cells, plays an essential role in sensorimotor function. At the biochemical and physiological levels, Oberdick and Michael Zhu, PhD, have found that L7/PcP2 functions as a modulator of G protein signaling, and that L7 fine-tunes the activity of voltage-gated calcium channels (Cav 2.1) through G proteins. Oberdick and Zhu postulate that disruption of L7 function may play a role in certain behavioral disorders in humans. DARDINGER NEURO-ONCOLOGY CENTER E. Antonio Chiocca, MD, PhD, Co-Director (top) Herbert Newton, MD, Co-Director (bottom) The Dardinger Neuro-Oncology Center is co-directed by E. Antonio Chiocca, MD, PhD, chair of the Department of Neurological Surgery, and Herbert Newton, MD, director of the Division of NeuroOncology in the Department of Neurology. Dardinger Center researchers and physicians come from the Department of Neurological Surgery and the Division of Neuro-Oncology. Research faculty include Yoshinaga Saeki, MD, PhD, administrative chief of the Dardinger Laboratory for Neuro-Oncology and Neurosciences, along with Balveen Kaur, PhD, Sean Lawler, PhD, and Mariano Viapiano, PhD. Three neurosurgeons – Mario Ammirati, MD, MBA, Ehud Mendel, MD, FACS, and Atom Sarkar, MD, PhD – joined the clinical and research staff in 2006. Ammirati directs the Dardinger Skull Base Microneurosurgery Laboratory, Mendel co-directs the Spinal Biodynamics and Ergonomics Laboratory, and Sarkar directs the Dardinger Nanotechnology in Neuroscience Laboratory. Newton, Robert Cavaliere, MD, Carol Volpi, RN, Myrna Bowler, RN, and Jill Brown, MS, make up the Dardinger Center’s neuro-oncology team. In 2006, the Center participated in nine clinical trials. Research funding, including grants from the National Institutes of Health, topped $1.75 million. Ongoing Research Programs • E. Antonio Chiocca, MD, PhD, oversees research conducted within the Dardinger Laboratory for Neuro-Oncology and Neurosciences by the various laboratory teams: • Yoshinaga Saeki, MD, PhD, develops therapeutic strategies for neurological, neoplastic and genetic disorders. • The laboratory group of Balveen Kaur, PhD, is studying changes that occur in the microenvironment of gliomas in response to treatment so as to learn how treatment strategies can be exploited to maximum potential. • Sean Lawler, PhD, and his laboratory team are studying cell-signaling mechanisms in disorders of the central nervous system (cancer and neurodegeneration) to develop novel therapies. • Mariano Viapiano, PhD, and his lab team study the composition of the extracellular matrix (ECM) of gliomas. • The microneurosurgical skull base laboratory of Mario Ammirati, MD, MBA, develops surgical approaches to tumors at the base of the brain, educates residents in these techniques, and partners with private and non-private organizations to develop technology for clinical use. • The spine and spine cancer laboratory of Ehud Mendel, MD, FACS, evaluates physiologic forces that impact spinal health and methods to optimize surgical therapy of spinal disorders. • Atom Sarkar, MD, PhD, and his nanotechnology laboratory team study the relationship between single-molecule mechanics and disease states, particularly the micromechanical mechanisms that underlie the formation of pathologic fiber in Parkinson’s disease and that regulate the migration and spread of glioblastoma multiforme tumors. • Herbert Newton, MD, Robert Cavaliere, MD, Sean Lawler, PhD, E. Antonio Chiocca, MD, PhD, and Yoshinaga Saeki, MD, PhD, are working with Abhik Ray-Chaudhury, MD, Neuropathology, and the laboratory team of Carlo Croce, MD, chair of the Department of Molecular Virology, Immunology and Medical Genetics, to investigate microRNA and its role in brain tumor transformation and pathogenesis. 2007 Research Report 53 • Dardinger Center researchers are also teaming with Rolf Barth, MD, Pathology, in a study of carboranyl nucleosides as delivery agents for neutron capture therapy of gliomas. • Phillip Popovich, PhD, of the Department of Molecular Virology, Immunology and Medical Genetics, has been working on a spine model of cancer and will be collaborating with Ehud Mendel, MD, FACS, who directs the Department of Neurological Surgery’s Spine and Spine Cancer Program. • Herbert Newton, MD, co-director of the Dardinger Center, edited the Handbook of Brain Tumor Chemotherapy, a guide for physicians, clinicians and basic researchers seeking better ways to treat primary and metastatic brain tumors. Newton also wrote or co-wrote eight of the book’s 35 chapters. Other contributing authors from Ohio State include Dardinger Center Co-Director E. Antonio Chiocca, MD, PhD; Kaveh AsadiMoghaddam, MD, PhD, Neurological Surgery; and Abhik Ray-Chaudhury, MD, Pathology. For more detailed information about laboratory team research within the Dardinger Center, as well as a list of research accomplishments, see the Department of Neurological Surgery section of this annual report (page 84). Ongoing Research Programs • The Nisonger Center’s dual diagnosis program studies the co-occurrence of psychiatric disorders and mental retardation, especially methods of assessment and motivational systems. Research Accomplishments of 2006 • The Social Security Administration awarded the Association of University Centers on Disability a five-year, annually renewable contract to further the Social Security commissioner’s proposal to improve timeliness and accuracy of childhood disability adjudications. In the second year of the project expansion, The Nisonger Center has been selected to participate in this initiative. The Center will develop, test and operate prototype pediatric medical units to provide clinical expertise for state and federal adjudicators who make initial decisions or review claims on appeal regarding eligibility for Supplemental Security Income. PRIMARY CARE RESEARCH INSTITUTE Larry Gabel, PhD, Director THE NISONGER CENTER FOR MENTAL RETARDATION AND DEVELOPMENTAL DISABILITIES Steven Reiss, PhD, Director The Nisonger Center is a member of the national network of University Centers for Excellence in Developmental Disabilities. In operation since 1972, the Center offers: services that fill gaps in Ohio programs; interdisciplinary training; technical assistance and consultation to Ohio mental retardation and developmental disability agencies; and applied research. 54 Ohio State University Medical Center In September 2000, faculty from the Department of Family Medicine, the Division of General Internal Medicine, the Division of Ambulatory Pediatrics and other academic disciplines at Ohio State established the Ohio State Primary Care Research Institute (PCRI). Funded initially by the Health Resources and Services Administration of the U.S. Department of Health and Human Services, the PCRI completed its second three-year period of infrastructure funding during 2006. Continuing on a self-sustaining basis, the PCRI fosters, facilitates and reports collaborative interdisciplinary research to optimize health. It aims to be recognized as a center of excellence for the quality, quantity and impact of its research on the professional literature, the development of public policy and health outcomes. Ongoing Research Programs • The PCRI’s collective laboratory is the 24-site Ohio State Primary Care Practice-Based Research Network (OSUPC-PBRN), which covers all of Franklin County and serves some 107,000 patients annually. The OSUPC-PBRN consists of 10 clinical practice sites of the Ohio State Primary Care Network, nine Close-to-Home Health Centers of Children’s Hospital and five practices of the Columbus Neighborhood Health Center, Inc. With 107 primary care physicians, these 24 practices serve a diverse patient population and provide care through nearly 308,000 patient visits a year. Research Accomplishments of 2006 • Douglas Post, PhD, is principal investigator for a clinical trial on Efficacy of Web-Based Training in Skin Cancer. Melanoma is a major health problem that affects more than 50,000 Americans each year and continues to increase in incidence, unlike most cancers. Although it is almost always visible on the skin and almost always curable when caught at an early stage, many still die from this disease. Primary care physicians commonly fail to look at key areas of the skin during examinations and hence miss opportunities to save lives. There is evidence that teaching basic skills related to skin cancer may improve physician examination and counseling. This project is testing efficacy of the Skin Continuing Education Course (compared to a course on weight control) via a randomized trial in a sample of primary care physicians. Methods include first developing a Basic Skin Cancer Triage (BSCT) curriculum for the Webbased skin course and then developing a comparable Web-based course on obesity/overweight assessment and counseling as a comparison intervention. Physicians will be randomly allocated to one of the two courses. The primary endpoint will be physician performance of skin examination during routine visits at 12 months after the course, and physician counseling around skin cancer issues. This will be assessed by patient exit interviews and physician self-assessment. Efficacy of the course for improving physician skin cancer triage skills and changing physician attitudes and knowledge regarding skin cancer issues will be assessed. • Kelly Kelleher, MD, MPH, is principal investigator for a Trial of Automated Risk Appraisal in Adolescents (TARAA). A partnership among Columbus Children’s Hospital, the Close to Home Primary Care Centers and www.flipsidemedia.com, this project aims to improve services for problem drug use/abuse and other risk-taking behavior for youth in primary care settings through research on early identification and monitoring. The study compares an intervention that combines computerized risk assessment and telephone support to usual care plus mailed screening results. Investigators want to: compare the frequency of problem drug use and abuse identified in the intervention group with use and abuse among youths in the usual-care group; examine the frequency of counseling, referral, psychotropic medication or other interventions for youth who screen positive for problem drug use and abuse on risk assessment; and evaluate the effect of the telephone support program on return to primary care, likelihood of completing referrals, number of primary care visits, and satisfaction with services after four months. • Mira Katz, PhD, is principal investigator for Patient Activation to Increase Colon Cancer Screening, a randomized, controlled clinical trial that focuses on improving colorectal cancer screening rates by “patient activation.” The study will test the effectiveness of patient communication skills training coupled with colorectal cancer screening information and barriers to counseling to improve screening rates. Part of a partnership with the Columbus Neighborhood Health Center, Inc., this research has far-reaching potential because, if shown to be effective, this colorectal cancer-screening program can be shared to improve screening among medically underserved populations. • Judy Groner, MD, is principal investigator for a clinical trial titled Can Changing How Mom Eats Prevent Obesity in Toddlers? This project aims to reduce the rising rate of obesity in very young children by helping mothers adopt focused eating patterns. Lowincome urban infants and mothers starting well-child care in Children’s Hospital Primary Care Network constitute the study population. The project contains a controlled study of two interventions: an addition to the nutritional anticipatory guidance offered during well-child visits, which focuses on structuring maternal-eating behavior; and an augmentation of the 2007 Research Report 55 advice given in physician practices by providing access to a six-week transition to parenting group, which focuses on the eating advice given in the clinics. Participating mothers are randomized to either receive an invitation to join a group or not. Anticipated outcomes of the project include: achieving a 10-percent reduction in the percentage of overweight toddlers in the intervention/support group; improving infant and toddler eating patterns in relation to accepted standards; increasing mothers’ readiness and recognition of a need to change family eating habits; and increasing the structure of maternal eating patterns. If effective, this low-cost intervention would fit well into current pediatric practices. • Doug Post, PhD, is principal investigator for a $240,000 project funded by the National Cancer Institute and titled Patient-Centered Communication During Chemotherapy. Studies have indicated that communication problems between cancer patients and clinicians are a major barrier to managing patients’ pain, depression and fatigue. This project addresses this problem by developing and evaluating a personal digital assistant (PDA)-based patient communication intervention for breast cancer patients undergoing chemotherapy. Patients are asked to complete fatigue, depression and pain inventories on a PDA at the beginning of chemotherapy and once a week through completion of treatment. On the day before each treatment, a summary of fatigue, depression and pain scores is integrated with a tailored patient communication skills training program and displayed on the PDA. Patients are taught through role modeling how to effectively communicate their symptoms and are encouraged to bring the PDA with their symptom summaries to each chemotherapy treatment so they can share the information with their clinician. Effects of the intervention are assessed over the course of treatment. Focus groups are then used with study participants to assess their responses to the intervention and their perceptions of the system’s value. • Electra Paskett, PhD, MSPH, is principal investigator for a $1.4 million Ohio Patient Navigator Research Project funded by the American Cancer 56 Ohio State University Medical Center Society. This project, which aims to alleviate disparities in timely diagnosis and treatment of breast, cervical and colorectal cancer, represents a partnership among the Ohio State University Comprehensive Cancer Center, the Ohio State Primary Care Practice-Based Research Network, and community partners (e.g., the Ohio American Cancer Society Division and the Ohio Commission on Minority Health). Specific aims include: assessing baseline rates and barriers, as well as strategies to overcome barriers to receiving timely diagnostic and treatment services; developing the Ohio Patient Navigator Research Program through a consortium of institutions in Ohio; implementing and evaluating the patient navigator program in 12 clinics/health centers using a group-randomized, controlled design to assess the efficacy of this intervention in reducing time to delivery of cancer care and noncancer resolution, or reducing time to cancer diagnosis and treatment after an abnormal finding from a detection procedure for breast, cervical or colorectal cancer; assessing barriers to implementing the intervention program; conducting a cost-effectiveness evaluation of the program; and assessing the impact of the program on community-level indicators. Study results are expected to reduce the burden of cancer in underserved populations. COMPREHENSIVE WOUND CENTER Chandan Sen, PhD, FACN, FACSM, Executive Director Every month roughly 1,000 patients with chronic, non-healing wounds receive advanced, research-based care at Ohio State's Comprehensive Wound Center (CWC), which opened in 2005 at the Martha Morehouse Medical Plaza on Kenny Road in Columbus. Led by Executive Director Chandan Sen, PhD, the CWC is a hub for wound sciences and care, a place where National Institutes of Health (NIH)-funded basic research meets clinical application as the fundamental principles of wound healing — starting at the genetic level — are translated from the lab to the bedside. The program is co-led by highly recognized wound care physicians such as Richard Schlanger, MD, PhD, clinical scientists such as Gayle Gordillo, MD, and genomic experts such as Sashwati Roy, PhD. Ongoing Research Programs • The CWC is an integral component of Ohio State’s proposed National Institutes of Health Center for Clinical and Translational Sciences (CCTS). Chandan Sen, PhD, directs the Novel Clinical and Translational Methodologies program of the CCTS. Sen, a professor of Surgery and of Molecular and Cellular Biochemistry, is a member of the board of directors of the national Wound Healing Society. In 2007, the CWC started its first placebo-controlled, randomized clinical trial addressing wound-healing therapeutics. Through partnership with the Floridabased wound care management company National Healing Corporation, the CWC is conducting multicenter clinical studies. These efforts are supported by Ohio State's unique bioinformatics infrastructure, the Information Warehouse. • CWC patient care is based on comprehensive treatment protocols that stem from research and employ such techniques as hyperbaric oxygen therapy, topical oxygen therapy, transcutaneous oxygen monitoring to determine vascular problems and promote spontaneous healing, negative pressure therapy for accelerated wound healing, and the use of synthetic growth factors and tissue coverings. On average, more than 50 wound care physicians from throughout the country come to Ohio State every month for advanced training and education. In 2009, the Mathematical Biosciences Institute of Ohio State will host a conference on Mathematical Modeling of Wound Healing. Supported by the National Science Foundation, this conference will be chaired by Sen and Professor Philip Maini of Oxford University in London, United Kingdom. INSTITUTE FOR BEHAVIORAL MEDICINE RESEARCH (IBMR) Ronald Glaser, PhD, Director The Institute for Behavioral Medicine Research (IBMR) was established to stimulate and expand interdisciplinary collaboration through experiments involving social and behavioral influences on the immune response and health of human subjects and animal models. Ongoing Research Programs • Human Psychoneuroimmunology (PNI) Program – This program involves: Barbara Andersen, PhD, Psychology; Charles Emery, PhD, Psychology; Ronald Glaser, PhD, Molecular Virology, Immunology and Medical Genetics; Janice Kiecolt-Glaser, PhD, Psychiatry; Stanley Lemeshow, PhD, College of Public Health; William Malarkey, MD, Internal Medicine; and Eric Yang, PhD, Molecular Virology, Immunology and Medical Genetics. The program focuses on psychological stressors and behavioral questions that could affect health by modulating the immune and endocrine systems. One recent focus is on how chronic stressors, such as caregiving, and acute stressors, such as marital conflict, substantially enhance production of proinflammatory cytokines linked with age-associated diseases, providing a window on how stress contributes to morbidity and mortality. A program exploring the role that stress may play in skin cancer has developed into a well-funded research program. These studies will expand to include breast cancer survivors in collaboration with the Cancer Control Program in Ohio State’s Comprehensive Cancer Center. 2007 Research Report 57 • Biobehavioral Aspects of Stress and Cancer Program – Cancer survivors are the focus of work spearheaded by Janice Kiecolt-Glaser, PhD, Ronald Glaser, PhD, Electra Paskett, PhD, MSPH, and William Malarkey, MD. One project funded by the National Cancer Institute addresses the role that proinflammatory cytokines play in combination with depression among breast cancer survivors who experience debilitating fatigue, and the ability of a yoga intervention to modulate endocrine and immune responses. Barbara Andersen, PhD, Psychology, has examined biobehavioral aspects of cancer to learn whether reducing stress and changing health habits have a significant impact on cancer recurrence and survival. • Comparative Medicine/Animal Model Program – This group involves Michael Bailey, PhD, David Padgett, PhD, John Sheridan, PhD, Ning Quan, PhD, all in the Oral Biology, as well as Jon Godbout, PhD, Molecular Virology, Immunology and Medical Genetics. They focus on developing animal models for psychoneuroimmunology research that models the studies with human subjects. • Behavioral Immunology and Stress Program – The research group of Courtney DeVries, PhD, and Randy Nelson, PhD, Psychology, studies the effects of stressors on inflammation after stroke or cardiac arrest, as well as seasonality in immune function, disease and mortality. • Neuroimmunology Program – Jon Godbout, PhD, Phillip Popovich, PhD, Virginia Sanders, PhD, and Caroline Whitacre, PhD, are investigators in Molecular Virology, Immunology and Medical Genetics whose research focuses on diverse yet interrelated areas of neuroimmunology. • Neuroendocrinology Program – William Malarkey, MD, and Jeanette Webster Marketon, PhD, are investigators in Internal Medicine who focus on neuroendocrinology in a clinical setting and at the molecular level. 58 Ohio State University Medical Center Research Highlights of 2006 • Comparative Medicine/Animal Model Program – To study the effects of stress on the immune system, Michael Bailey, PhD, Jon Godbout, PhD, David Padgett, PhD, John Sheridan, PhD, and Ning Quan, PhD, develop animal models and use them to examine mechanisms by which activation of neuroendocrine pathways intersect and regulate inflammatory and immune responses. Rodent models have been developed to examine the effects of stress in three research areas: susceptibility to microbial infections; tissue repair/wound healing; and immune system/cytokine signaling to the central nervous system across the blood brain barrier. These models are used in parallel with human studies performed by other members of the IBMR. • Neuroimmunology Program – Phillip Popovich, PhD, and Caroline Whitacre, PhD, use cellular and molecular approaches to determine how spinal cord injury (SCI) influences the ability of immune cells to function both at the site of injury and in tissues throughout the body. The work of Virginia Sanders, PhD, has shown that lymphocyte activity is dramatically influenced by catecholamines – the chemical messengers of the sympathetic nervous system (SNS). For example, efficient production of antibodies by B cells requires that catecholamines bind to the beta2adrenergic receptor found on B cells and a subset of T cells. Studies between Sanders and Popovich are determining whether antibody production in SCI individuals is negatively affected due to disruption of SNS function. Sanders’ and Whitacre’s work also has relevance for diseases such as multiple sclerosis, an autoimmune disease of the brain and spinal cord caused by overactive T and B lymphocytes. Recent work in Whitacre’s lab, which focuses on multiple sclerosis, examines how gender and pregnancy influence the neurodestructive capacity of autoreactive T cells. Sanders, Popovich, Daniel Ankeny, PhD, and co-workers have shown that experimental spinal cord injury elicits chronic activation of a B cell-dependent autoimmune response. In this study, high levels of anti-DNA antibodies were detected in spinalcord-injured rats with a pattern that is similar to that seen in systemic lupus erythematosus. This is the first report that spinal cord injury can cause a clear dysregulation of B-cell function. • Behavioral Immunology and Stress Program – Courtney DeVries, PhD, developed a mouse model of cardiac arrest and cardiopulmonary resuscitation (CPR) and showed that cardiac arrest with CPR increases subsequent anxiety-like behavior and decreases social interaction. Her group also reported sex differences in recovery from focal ischemia, as well as social facilitation of wound healing. The research group of Randy Nelson, PhD, showed that prenatal day length influences immune function in adulthood. These findings might explain season of birth as a risk factor in several diseases. His group also showed the existence of trade-offs between cutaneous immune responses. In addition, Nelson reported that social environment modulates seasonal immune responses in mice. • Neuroendocrinology Program – William Malarkey, MD, investigates toxic stress and the biology of chronic inflammation, the precursor to chronic pain and most of the diseases of aging, including arthritis, cancer and cardiovascular disease. Work in progress or soon to begin includes studies on obesity, chronic stress and inflammation, and stress-reduction strategies for treating chronic inflammation. Jeanette Webster Marketon, PhD, uses molecular approaches to determine how factors such as disease status or infection modulate glucocorticoid receptor signaling. Glucocorticoid receptors are essential for life, and impaired signaling through this receptor is involved in many autoimmune/inflammatory diseases. • Human Psychoneuroimmunology Program – A recent study by Janice Kiecolt-Glaser, PhD, Ronald Glaser, PhD, Stanley Lemeshow, PhD, and others showed that dietary intake of omega-3 fatty acids may interact with depression to fuel inflammation. The cross-sectional study has provided the basis for two new funded omega-3 randomized controlled trials. CENTER FOR INTEGRATIVE MEDICINE Glen Aukerman, MD, Director The OSU Center for Integrative Medicine incorporates the art and science of caring for the whole person – mind, body and spirit – to treat and prevent disease while encouraging patients to create a condition of optimal health. The Center, located at 2000 Kenny Road, combines mainstream medicine with complementary therapies for which there is quality scientific evidence of safety and effectiveness. By offering complementary therapies ranging from acupuncture and chiropractic to massage and yoga in family medicine practice, the Center embraces a growing and increasingly recognized component of medicine. Services at the Center, which is part of the OSU Primary Care Network, include: • Traditional Western Medicine • Whole Alternative Medical Systems • Mind-Body Medicine SPORTS MEDICINE CENTER Thomas Best, MD, PhD, Co-Director (top) Christopher Kaeding, MD, Co-Director (bottom) Medical and athletics experts at The Ohio State University have joined forces in a comprehensive sports medicine initiative that unites clinical care for athletes with research and education programs to benefit the entire Ohio State community. This intensified emphasis goes far beyond clinical treatment for Ohio State athletes. Multidisciplinary research and education programs integrate many surgical and medical specialties to aid students and trainees in all segments of sports medicine. The program continues to expand and engage multiple disciplines. 2007 Research Report 59 Ongoing Research Programs • Multicenter Orthopaedic Outcomes Network/Knee – Supported by funding from the National Institutes of Health, this multicenter collaborative program evaluates outcomes and predictors of outcomes following knee ligament surgery using validated clinical outcomes instruments. Members include Ohio State, Vanderbilt University, the University of Iowa, the Cleveland Clinic and the University of Colorado. • Multicenter Orthopaedic Outcomes Network/Shoulder – This multicenter collaborative program evaluates outcomes and predictors of outcomes following shoulder surgery using validated clinical outcomes instruments. Members include Ohio State, Vanderbilt University, the Cleveland Clinic and University of Colorado. • Sports Medicine/Imaging Research Group – This collaborative group investigates innovative techniques to evaluate health and structure of articular cartilage and ligaments. • Biomechanics and Motion Capture Lab – This new program resulted from the recruitment of its leader, Ajit Chaudhari, PhD, from Stanford University. Construction of his lab is under way. Areas of initial study include identifying kinematic and anatomic predictors of injuries to the knee and shoulder. • Sports Medicine Muscle Injury Lab – Led by Thomas Best, MD, PhD, this multidisciplinary lab receives extramural funding to study skeletal muscle injury and repair. Best’s lab collaborates with other labs on the Ohio State campus, including Sudha Agarwal, PhD, Oral Biology, and Denis Guttridge, PhD, Molecular Virology, Immunology and Medical Genetics. • Sports Pulmonology Program – Led by John Mastronarde, MD, and Jonathan Parsons, MD, this program conducts research in asthma, sleep apnea and vocal cord dysfunction issues in athletes. Their work has resulted in one funded proposal and two manuscripts. 60 Ohio State University Medical Center OFFICE OF GERIATRICS AND GERONTOLOGY Linda Mauger, Interim Director and Program Manager The Office of Geriatrics and Gerontology at the OSU Health Sciences Center is a focus for aging-related activities throughout the University and is the only formally recognized unit on campus that is devoted to aging. The mission of the Office is to foster – through teaching, research and consultation – the costeffective delivery of high-quality health and social services to meet the needs of Ohio's older citizens. Through the Transdisciplinary Program in Aging, the Office provides coordinated education, research, practice and service opportunities in geriatrics and gerontology while enhancing and supporting the initiatives of individual departments, schools and colleges. Through this centralized unit for clinical gerontology, the University's initiatives in aging are being reshaped to meet the demographic imperatives of an aging society. School and Department-Based RESEARCH PROGRAMS AND 2006 HIGHLIGHTS In addition to Ohio State University Medical Center’s interdisciplinary research centers (described in the previous section), programs of specialized medical research are conducted and supervised within schools and departments in Ohio State’s College of Medicine and in facilities of the Ohio State University Health System. This section highlights notable research programs in these departments and schools. 2007 Research Report 61 DEPARTMENT OF ANESTHESIOLOGY David Zvara, MD, Chair Anesthesiology has 42 faculty members with basic and patient-oriented research interests aligned with the Medical Center’s Neurosciences, Heart, Imaging and Transplantation Signature Programs. Investigators use animal or genomic models to study mechanisms of ischemic spinal cord injury, neural plasticity, postoperative ileus, mechanosensitivity, inflammatory bowel diseases (IBD), irritable bowel syndrome, chronic heart failure, hypertension, wound healing, cell-signaling and obesity surgery. These efforts are complemented by clinical studies for ischemic spinal cord injury in thoracoabdominal aortic aneurysm patients, IBD, fMRI imaging of pain pathways and anesthesia, neuroanesthesia, heart failure with 3-D echocardiography and finite element analysis, cardio/bioimpedance, computer modeling, transplant survival and morbid obesity, weight loss and resolution of type 2 diabetes. Studies are funded through the National Institutes of Health (NIH), the National Heart Foundation or industry grants. The Department has 35 active and mostly investigatorinitiated clinical research studies. Its studies resulted in 15 peer-reviewed publications in 2006. Ongoing Research Programs • Cardiovascular Diseases: – Heart failure: Mark Gerhardt, MD, PhD, is developing a program on chronic ischemic heart failure (CHF) with a focus on β-adrenergic receptor dysfunction, molecular signaling and remodeling in a microinfarction-induced ovine model of CHF with collaborations among investigators at Ohio State and Children’s Heart Institute. Studies were funded in part by a recent National Heart Foundation (NHF) grant, a Foundation of Anesthesia and Education (FAER) development 62 Ohio State University Medical Center grant, and an earlier American Heart Association (AHA) grant. – Hypertension and vascular remodeling: Genomic modeling is a powerful approach to studying mechanisms of disease. Hamdy Hassanain, PhD, in collaboration with Neurosciences investigators and Ohio State’s Davis Heart and Lung Research Institute, is using transgenic mouse models to study heart failure, hypertension, physiological regulation of hypertension, vascular remodeling, wound healing, receptor function or dysfunction, and diabetic vascular disease. – 3-D echocardiography and finite element analysis: Nadia Nathan, MD, in collaboration with Cardiovascular Medicine, is developing a system for fully automated import of cardiac data (e.g., pressure, elasticity, geometry components) to a finite element modeling tool (Abaqus) that incorporates 3-D echocardiography geometry data and provides cardiac data quantification for potential use in managing cardiac interventions. Individual simulation of interventions may be suitable for surgical planning, training and education. • Brain and Little-Brain Imaging – Anesthesiology is developing expertise in state-of-the-art imaging techniques for clinical (whole-brain imaging) and cellular (neural networks) studies. In addition to Dr. Nathan’s 3-D echocardiography, Robert Small, MD, and interdisciplinary collaborators are applying functional magnetic resonance imaging (fMRI) to study brain activation caused by pain and in response to anesthesia. They first published this in Anesthesiology in 2004. At the cellular level, Jacqueline Wunderlich, MD, PhD, in collaboration with GI surgery (Scott Melvin, MD, Brad Needleman, MD, and Fievos Christofi, PhD), is using Ca2+ imaging to study activation and function of the “little brain in the gut,” the human enteric nervous system, in health and disease. • Ischemic Spinal Cord Injury – Hamdy ElsayedAwad, MD, and an interdisciplinary team are developing a program in ischemic spinal cord injury for patients undergoing surgery to repair thoracoabdominal aortic aneurysms (TAAA). Spinal cord injury leading to paraplegia is a devas- tating complication in TAAA. Studies in human, large animal and genomic models are focused on the cellular and molecular mechanisms of ischemic spinal cord injury. The potential exists for testing new therapeutic interventions to protect the spinal cord. • Neuroanesthesia – Clinical studies by Sergio Bergese, MD, and his investigative team – including residents and medical students – involve both investigator-initiated trials and multi-center trials, such as the National Awake Intubation Trial. Studies include testing the noise in a new MEDRAD Monitor during neurosurgery, pharmacologic influence of anesthetic agents on somatosensory-evoked potential and MEPs, monitoring variations in cerebral state index, a new drug for postoperative nausea and vomiting, and clinical evaluation of a device to speed emergence from inhaled anesthesia. The latter is in collaboration with Bachar Haschwa, MD, and Roger Dzwonczyk. • Enteric Neuroscience and Neurogastroenterology – Studies supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (PIs: Fievos Christofi, PhD, and Yun Xia, MD, PhD) are investigating the function, neuroimmune communication and dysfunction of the enteric nervous system or “little brain of the gut.” It regulates motility, secretion and vasomotor functions of the gut and is strongly influenced by immune/inflammatory responses. In disease, dysfunction can cause or contribute to diarrhea, constipation, dysmotility, obstruction, postoperative ileus, pain or irritable bowel syndrome. Christofi’s research is focused on: gut purinergic neural circuits and reflexes involved in the physiological regulation of motility and secretion; cell signaling and neuroplasticity in inflammatory bowel diseases, irritable bowel syndrome and post-operative ileus; molecular mechanisms of mechanosensitivity in gut sensory enterochromaffin cells; and the role of adenosine and nucleotide receptors as therapeutic targets in gut inflammatory diseases. Research Accomplishments of 2006 • New Grants – Mark Gerhardt, MD, PhD, received a grant from the National Heart Foundation on PKA-Phosphorylation of B2-Adrenergic Receptors in CHF. Fievos Christofi, PhD, received a five-year competing-renewal grant from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, on Adenosine Receptors as Therapeutic Targets in Inflammatory Bowel Diseases. He also was awarded an institutional RIF Award for related research. Sergio Bergese, MD, received industry grants for clinical studies or multicenter trials from Hospira, Baxter HealthCare Corp., Medrad Inc. A number of other grants are in preparation. • Genomic Models of Hypertension – Studies led by Hamdy Hassanain, PhD, and collaborators at Ohio State’s Davis Heart and Lung Research Institute, (directed by Jay Zweier, MD) and other institutions resulted in a publication in Antioxidant and Redox Signaling characterizing hypertension caused by transgenic overexpression of the small GTP-ase Rac 1 in a transgenic mouse model developed by Hassanain. Continuous Rac 1/NADPH oxidase activation leads to superoxide radical production and hypertension. An NIH grant is pending. • Heart Failure – Mark Gerhardt, MD, PhD, in collaboration with Gretel Monreal (PhD student), characterized the acute changes in myocardial electrolytes induced by left ventricular assist device (LVAD) support in heart failure (ASAIO J 2006 Nov 28, e-pub ahead of print). Other studies, in collaboration with John Bauer, PhD, and colleagues at the Children’s Heart Institute, are providing insight into intracellular remodeling in myocytes, β-receptor function and acute stress protein levels in an ovine model of chronic heart failure. Nadia Nathan, MD, is using finite element modeling software to quantify pre- and post-interventional cardiac reconstruction data based on modeling data sets from surgical ventricular repair therapy and cardiac resynchronization therapy. This was published in BioMedical Engineering OnLine. 2007 Research Report 63 • Neurogastroenterology/Neuroscience – Studies in the lab of Fievos Christofi, PhD, in collaboration with investigators in Neuroscience (Helen Cooke, PhD), at Ohio State’s Davis Heart and Lung Research Institute (Arturo Cardounel, PhD) and at Lakehead University in Canada (Zacharias Suntres, PhD), showed that a drug targeting ADOA3R is a potential therapy for experimental inflammatory bowel disease. The findings were published in IBD as “Mechanistic hypotheses generated in animal IBD models are being tested first in ex vivo human surgical IBD gut neural tissues, first-time analysis of data base gene abnormalities in IBD patients.” These studies could pave the way for human clinical trials. A second study published in the Internat J Parasitology proved that hyperexcitability and neuroplasticity in gut sensory neurons are caused by amplification of the Gs/AC/cAMP/PKA/pCREB signaling pathway in gut infected with the human pathogen Trichinella spiralis. The pathway is a general target for most neuropeptide transmitters, a major gateway to gut reflexes and a potential therapeutic target in irritable bowel syndrome. Yun Xia, MD, PhD, and collaborators in the Department of Physiology and Cell Biology at Ohio State characterized the effects of platelet-activating factor in the enteric nervous system and published their findings in the Am J Physiology. Xia is funded by a National Institute of Diabetes and Digestive and Kidney Disease Clinical Investigator Training Grant. • Ischemic spinal cord injury – Hamdy ElsayedAwad, MD, is examining biochemical changes in central and peripheral compartments of patients undergoing surgical repair of an aortic aneurysm. This clinical paradigm in a large number of patients is expected to unravel mechanisms of ischemic spinal cord injury and paraplegia in thoracoabdominal aortic aneurysm (TAAA) patients. Together, the TAAA human model, a large animal/clinical model of paraplegia and a mouse model of paraplegia suitable for genomic/mechanistic studies should provide a path from lab bench to bedside in testing new interventions to protect against paraplegia in TAAA patients. 64 Ohio State University Medical Center • Brain-Imaging – Functional magnetic resonance imaging studies (fMRI) on pain stimulation are contaminated with physiologic noise (e.g., cardiopulmonary parameters change significantly in response to pain). Robert Small, MD, in collaboration with Biomedical Engineering, Radiology and other departments at Ohio State, is identifying and subtracting physiologic noise in fMRI imaging of the brain from the response to a painful stimulation. They presented their data at the national meeting of the International Society for Magnetic Resonance in Medicine. DEPARTMENT OF BIOMEDICAL INFORMATICS Joel Saltz, MD, PhD, Chair The National Library of Medicine defines biomedical informatics as the intersection of basic informational and computing sciences with an application domain in health care and biomedicine. The Department of Biomedical Informatics (BMI) is a collaboration of computer scientists, image analysis/computer vision specialists, systems biologists/bioinformaticians and clinical informaticians who apply their skills and interests to problems at the interface between the physical/computational sciences and the biological sciences. Departmental researchers apply distributed and parallel computing techniques to data querying, retrieval and integration, imaging, simulation, medical informatics and computational biology, and they develop middleware and optimizations to grid-enable projects in the biological, medical and physical sciences. Over the past year, the BMI received 15 awards from federal sponsors with total funding exceeding $2.2 million in addition to ongoing support of over $1 million. Ongoing Research Programs: Research Accomplishments of 2006 • High End, Data Intensive and Grid Computing Research – Faculty and staff researchers in the multiscale computing area work to develop middleware technology and techniques to enable management, sharing and manipulation of data at multiple scales across heterogeneous, dynamic collections of storage and computation systems. Some application areas include: - Large-scale collaborative biomedical clinical studies - Analysis of gene expression and functional imaging information - Imaging, analysis and simulation of oil reservoirs and data-driven control of oil production - Analysis of satellite data - Analysis of multiresolution, multiple-grid simulation datasets • Image Analysis/Computer Vision – The imaging research group focuses on in vivo imaging middleware, microscopic and radiologic image registration and analysis, computer vision, machine learning, medical imaging, generalized principal component analysis, geometric theories of computer vision, and symmetry-based recognition and matching. • Systems Biology/Bioinformatics – The systems biology/bioinformatics group focuses on bioinformatic analysis of gene regulation involving chromatin, transcription factor interactions with DNA, promoter analysis and miRNA. Another line of investigation is the development of computational and evolutionary sciences in a comparative genomics context, including the development of novel phylogenetic methods to correlate genotypes and phenotypes, and to find diagnostic polymorphisms among organisms. This group has begun to study molecular changes associated with zoonoses and pandemics from a wholegenome perspective with emphasis on corona viruses (SARS) and influenza (avian and other influenza strains). • Grid Computing/Middleware Development (caGrid 1.0 Release) – A national team of researchers (led by Scott Oster, MS; Shannon Hastings, MS; Steve Langella, MS; Tahsin Kurc, PhD; and Joel Saltz, MD, PhD) from the Multiscale Computing Laboratory in the BMI released the second distribution of caGrid 1.0, a suite of tools, resources and computer software that enables researchers around the world to tap into libraries of data and genetic information that, until now, have been largely inaccessible. The BMI group is the lead developer site for caGrid 1.0, an integral part of the cancer Biomedical Informatics Grid (caBIG) announced by the National Cancer Institute (NCI) in 2004. The caBIG network will allow scientists at cancer centers, medical centers and research laboratories worldwide to share information and analytic capabilities efficiently and securely. To accomplish this, the caBIG program has developed common applications, tools, data and analytical resources, information standards and grid software infrastructure to enable programs and databases at remote institutions to quickly interact. caGrid 1.0 is the unifying architecture and operating environment for systems and applications in caBIG. The caGrid 1.0 release contains such new features as: a tool for rapidly developing caBIG-compatible data and analytical grid services; tools for administering a security infrastructure; and a portal providing a dynamic view of services running on caGrid, along with information about research institutions and service providers participating in caBIG. Other collaborators on the caGrid 1.0 project include the National Cancer Institute Center for Bioinformatics, University of Chicago/Argonne National Laboratory, Duke Comprehensive Cancer Center, ScenPro Inc., SemanticBits, LLC, Science Application International Corp. and Booz Allen Hamilton. 2007 Research Report 65 • In Vivo Imaging (IVI) Middleware Development (GridImage) – The IVI Middleware group, led by Joel Saltz, MD, PhD; Metin Gurcan, PhD; Tony Pan, MS; and Ashish Sharma, PhD, developed a Grid-aware image reviewing system (GridIMAGE) that allows practitioners to: select images from multiple geographically distributed DICOM servers; send those images to a group of human readers and computer-assisted detection (CAD) algorithms; and compare interpretations from human readers and CAD algorithms. GridImage was developed leveraging the National Cancer Institute caGrid infrastructure and is designed to help identify lung nodules on thoracic computed tomography. GridIMAGE enables researchers and clinicians to share datasets and CAD analytical resources. It also allows human readers to view and specify regions of interest. The infrastructure can support any type of distributed review. caGrid data and analytical services are used to link DICOM image databases and CAD systems, and to interact with human readers. Moreover, the service-oriented and distributed structure of the GridIMAGE framework enables a flexible system that can be deployed in an institution (linking multiple DICOM servers and CAD algorithms) as well as in a Grid environment (linking the resources of collaborating research groups). GridIMAGE allows practitioners to obtain interpretations from human readers or CAD algorithms. It also enables cooperative imaging groups to perform image-interpretation tasks associated with research. The system can be deployed within an institution as an Enterprise system to facilitate access to multiple DICOM servers and CAD systems. The current implementation of GridIMAGE carries out pulmonary nodule detection for thoracic computerized tomography (CT) images. The practitioner queries a set of image databases and selects studies, series or images to be interpreted. For each image, the practitioner can specify one or more radiologists and the CAD algorithms to be used to identify candidate nodules. Pulmonary nodule candidates are identified as three-dimensional structures and are defined using parameters that specify a boundary and/or centroid. A 66 Ohio State University Medical Center graphical user interface (GUI) has been developed to display CAD findings on CT images as overlays. Using the GUI, the practitioner can: query, select and preview subsets of distributed lung CT databases to be included in a study; select CAD algorithms and human readers for the analysis; and visualize human annotations and markups along with those produced by CAD algorithms. • Bioinformatics/Systems Biology (Adapting Google Earth to Track the Spread of Avian Influenza) – Daniel Janies, PhD, and his group have designed an interactive map of the spread of the avian flu virus (H5N1) that for the first time incorporates information from viral genomes, geography and evolution to track the spread of the virus among various hosts and will help predict where the next outbreak is likely to occur. As part of the process, the group tested hypotheses about key strains, or genotypes, of the virus that appear to be heading west and have the ability to infect humans. The team used software to build an evolutionary map of the virus’s mutations. They projected their evolutionary map onto the globe using Keyhole Markup Language (KML) available in GoogleTM Earth. Similar to a legend found on a road map, the evolutionary map uses colors and symbols to indicate which types of hosts carry the virus and the distribution of dangerous genotypes that could infect humans. Designers used TimeSpan, another function of GoogleTM Earth, to animate the westward spread of the virus from Asia to Europe and Africa over the past decade. Clicking on a specific viral isolate or junction in the interactive map generates a window revealing diagnostic mutations in the virus that distinguish one strain from another. All data in the map is linked to other resources at the National Institute of Health’s GenBank. This enables the comparison of findings about viruses in the real world with pre-existing laboratory hypotheses. With the evolutionary tree visualized in the globe, questions can be asked about the virus’s geographics, host and mutations that enable transmission from birds to mammals. The team studied genomic sequence data from 351 isolates of the virus. They were especially interested in discovering if certain hosts were carrying discrete forms of the virus and which viruses carried mutations enabling transmission to humans. The visualization was useful for generating hypotheses that were subsequently tested by applying statistical tests to the evolutionary tree. This allowed the investigators to ask whether mutations were associated with hosts or certain geographic regions by chance, or whether adaptations of the virus to new hosts and in new regions were being tracked. Janies and colleagues found no genotypes associated with mutations in hemagglutinin (HA), nor in neuraminidase (NA) that were significantly associated with any specific type of host. They did, however, find a strong association between genotype Lysine-627 in an internal protein, polymerase basic 2, that is linked to increased replication and virulence of H5N1 in laboratory mice. • Nucleosome Positions Predicted Through Comparative Genomics – DNA sequence has long been recognized as an important contributor to nucleosome positioning, which has the potential to regulate access to genes. The extent to which the nucleosomal architecture at promoters is delineated by the underlying sequence is being worked out. In collaboration with a group of investigators led by Dr. Franklin Pugh at Penn State, Ilya Ioschikes, PhD, used comparative genomics to report a genome-wide map of nucleosome positioning sequences (NPSs) located in the vicinity of all Saccharomyces cerevisiae genes (Letters, Nature Genetics 38(10)1210-1215). The group found that the underlying DNA sequence provides a very good predictor of nucleosome locations that have been experimentally mapped to a small fraction of the genome. Notably, distinct classes of genes possess characteristic arrangements of NPSs that may be important for their regulation. In particular, genes that have a relatively compact NPS arrangement over the promoter region tend to have a TATA box buried in an NPS and tend to be highly regulated by chromatin modifying and remodeling factors. Ioschikhes conducted the computational correlation searches. • High Performance Computing – Umit Catalyurek, PhD, extended his work in combinatorial algorithms and parallel computing. Combinatorial algorithms are an enabling technology for scientific computing, especially for large-scale problems and high-performance computing. Partitioning and load balancing are important issues in parallel scientific computing relating to performance and efficiency of large-scale parallel computing clusters. His work involves developing computational hypergraph models for applications with irregular data dependencies. Hypergraphs provide more generalized abstractions than graphs; hence, they are more flexible in modeling complex problems. Following Catalyurek’s early works, hypergraphs are used today for workload partitioning in parallel processing. The explosion of data in all scientific fields, especially biomedical areas, necessitates parallel computational systems with tens or hundreds of processors whose efficiency is vastly improved by the types of models and algorithms developed by Catalyurek and collaborators. This work laid the foundation for Catalyurek’s collaborative DoE SciDAC application, CSCAPES (Combinatorial Scientific Computing and Petascale Simulations) Institute. Only four SciDAC institutes were funded nationwide. Led by Old Dominion University (ODU), the CSCAPES Institute is a collaboration among researchers at ODU, Sandia National Laboratories, Argonne National Laboratory, The Ohio State University and Colorado State University. The era of petascale computing is looming and has enormous potential for scientific simulation, but it also presents challenges. Petascale machines are likely to have hundreds of thousands of processors, complex memory hierarchies and relatively poor network performance. Scientific applications that will run on these machines will involve complex multiscale or multiphase physics, adaptive meshes and sophisticated numerical methods. Harnessing the potential of high-end computers to solve such complex problems is a challenge that the CSCAPES Institute is addressing. The Institute aims to develop and deploy fundamental technologies in high-performance computing. It will work with other SciDAC research groups to integrate software tools into other codes. 2007 Research Report 67 Division of Anatomy Kenneth Jones, PhD, Director The Division of Anatomy has nine full-time faculty who served more than 2,300 undergraduates, graduate and professional students in 2005-06. Required and elective courses are offered in human anatomy, embryology, histology, neuroanatomy and radiological anatomy. The Division also provides Anatomy labs that are required in both the Med 3 Surgical rotation and the Clinical Skills Immersion Experience. The faculty’s scholarly interests lie in three areas: biomechanical effects of trauma; applied neuroanatomy; and the development and evaluation of medical education technologies. Ongoing Research Programs • John Bolte, PhD, and Kenneth Jones, PhD, expanded the Division’s portfolio in biotrauma with contracts from federal funding sources that were awarded to support studies in the Division’s Injury Biomechanics Research Laboratory. • John Bolte, PhD, organized the second annual Injury Biomechanics Symposium. Held in May 2006, it was attended by more than 85 representatives from 17 universities and two foreign countries. The third annual Biomechanics of Injury Symposium was held in May 2007. trauma that will help improve the biofidelity of anthropomorphic test devices. - A $19,959 contract from the U.S. Air Force to John Bolte, PhD, for imaging that will accurately measure human head and neck inertial properties and lead to better-designed pilot helmets. • Anatomy’s major research accomplishment in 2006 was the growth of its Injury Biomechanics Research Laboratory, which is gaining international notice. The lab’s overarching goal is to improve the safety of pedestrians, as well as occupants of motor vehicles and aircraft, by studying the biomechanical effects of trauma in controlled conditions to determine the type and magnitude of forces that correlate with specific injuries. Safety devices or protocols can then be designed to reduce or eliminate injuries. The lab’s funded projects have increased consistently over the past few years. Working there in 2006 were two PhD scientists, one MS scientist, six undergraduates, one engineering co-op and one medical student. Bolte and graduate students have been invited to present their work at national and international meetings. • The IBRL also has been the driving force behind Ohio State’s Injury Biomechanics Symposium. The third annual symposium, held in May 2007, drew participants from universities, government agencies and industry from the United States, Canada and several foreign countries. DEPARTMENT OF EMERGENCY MEDICINE Research Accomplishments of 2006 Douglas Rund, MD, Chair • Research support for the Division of Anatomy totaled approximately $1.29 million in 2006. Projects included: - Completion of a National Institutes of Health grant awarded to David Clark, PhD, for trials on stimulation of the vestibulocochlear nerve for treatment of attention deficit hyperactivity disorder. - Five contracts from the National Highway and Transportation Safety Administration that were awarded to Kenneth Jones, PhD, and John Bolte, PhD, for basic research in the biomechanics of The Department of Emergency Medicine includes 32 faculty, 37 residents, one research fellow, a research nurse coordinator, a research technician and administrative support staff. The Department’s threefold mission includes: providing 24-hour clinical care for approximately 100,000 patients per year who present to the Emergency Departments of Ohio State University Hospital and 68 Ohio State University Medical Center Ohio State University Hospital East; educating Emergency Medicine residents, medical students, fellows and other residents/fellows learning emergency medicine; and developing research programs relevant to Emergency Medicine. Department research has focused on the laboratory and clinicalbased studies. Although a fairly new academic department (established in 1990) with relatively young faculty, Emergency Medicine’s research efforts have shown steady growth. Leadership positions include chair, vice chair for clinical affairs, vice chair for research, vice chair for development, residency directors and student education director. Ongoing Research Programs • The Laboratory Research Program, under the direction of Mark Angelos, MD, is based in Ohio State’s Davis Heart and Lung Research Institute and focuses on cardiac reperfusion. • Michael Sayre, MD’s Resuscitation Research Program mission is to increase survival from sudden cardiac arrest in central Ohio and beyond by researching and developing diagnostic treatment strategies. • Robert Guthrie, MD, operates a Clinical Trials Research Program evaluating new drugs for the long-term care of patients with hypertension, cholesterol disorders and diabetes. Research Accomplishments of 2006 • The Department presented six abstracts at the Society of Academic Emergency Medicine’s national scientific meeting May 18-21, 2006, in San Francisco. At the American College of Emergency Physicians national meeting held Oct. 15-16, 2006, in New Orleans, the Department presented five abstracts, three of which were presented by residents. In addition, one abstract was presented at the American Heart Association’s Scientific Sessions held November 12-15, 2006, in Chicago, and one abstract was presented at the Resuscitation Research Symposium on Nov 11, 2006. • The Undergraduate Research Associate Program, established in January 2005 in collaboration with Volunteer Services, continues to thrive. Directed by Jeff Caterino, MD, and coordinated by Carol Schneider, RN, the program has increased the numbers of enrolled subjects and given students interested in medicine some experience interacting with patients, nurses and physicians. The program has boosted collaboration between Emergency Medicine and other departments that use volunteers’ services to screen patients for their studies. • At Emergency Medicine’s 3rd Annual Spring Research Day on May 23, 2006, invited speaker Carl Leier, MD, a professor in Cardiovascular Medicine at Ohio State, presented “Inotropic Therapy for Acute Heart Failure.” Department senior residents also made presentations. DEPARTMENT OF FAMILY MEDICINE Mary Jo Welker, MD, Chair The Department of Family Medicine provides quality health care based on a family practice model, teaching and modeling family medicine principles and values, pursuing cutting-edge research and scholarship, and providing service through personal, professional and political efforts. The Department fosters, facilitates and reports collaborative, interdisciplinary research directed toward optimizing peoples’ health. Central to these efforts is the Ohio State Primary Care Research Institute, a collaboration including faculty from the Division of General Internal Medicine and the Division of Ambulatory Pediatrics plus numerous other departments at Ohio State. Family Medicine’s collective laboratory is the 24-site Ohio State Primary Care Practice-Based Research Network, which includes all of Franklin County and serves some 107,000 patients. Numbers and funding amounts of research projects are increasing annually. 2007 Research Report 69 Ongoing Research Programs • Academic Administrative Units in Primary Care – This three-year, federally funded grant project supports the Ohio State Primary Care Research Institute in expanding research in cancer, diabetes, genetics and cardiopulmonary conditions. • Patient-Centered Communication During Chemotherapy – Funded by the National Institutes of Health, this two-year project will develop a patient-centered communication intervention for breast cancer patients undergoing chemotherapy treatment, examine patient reactions to the intervention and evaluate its effects on pain, depression and fatigue symptoms. • Teaching to the CORE: Using Core Competencies Without Losing Core Values – This three-year, federally funded grant project will enhance medical school curriculum at Ohio State, focusing on core competencies identified by the Accreditation Council for Graduate Medical Education and creating a medical school environment allowing students to retain the values of altruism and service values drawing students to primary care. • Cognitive Behavioral Therapy - Techniques and Lifestyle Changes: Reducing Systolic Blood Pressure – This study tests the hypothesis that patients can be taught to implement Lifestyle Behavioral Changes (LBCs - restricting dietary sodium, engaging in aerobic physical activities and moderating alcohol intake) augmented by Cognitive Behavioral Therapy-Techniques (CBT-T) in the time frame of a routine primary care office visit. Research Accomplishments of 2006 • Tom Best, MD, PhD, joined the Department in 2005-06 with four funded projects: Biomechanical Effects of Eccentric Exertions in the Workplace, a three-year project funded for $387,000 by the National Institute for Occupational Safety and Health; Modeling of Muscle Strains and Prediction of Injury Risk Factors, a two-year study funded for $100,000 by the AIRCAST Foundation; Effects of a Previous Strain Injury on Hamstring Muscle Mechanics During Springtime: Implications for Preventing Re-Injury, a six-month project funded 70 Ohio State University Medical Center for $125,000 by NFL Charities; and The Use of Botulinum A Toxin to Reduce Muscle Contracture and to Promote Myogenesis in a Rabbit Distraction Osteogenesis Model, a two-year project funded for $100,000 by the Orthopaedic Research and Education Foundation. Best focuses on the pathophysiology of muscle-tendon injuries. Through federal and industrial support, he has assembled a team of post-docs, graduate students and medical students who use animal models to examine the biomechanics and pathophysiology of injury and repair, with interest in the role of leukocytes. They use immunological and molecular techniques to dissect the role of NF-KB and other transcription factors in the repair of stretch-injured skeletal muscle. Best has collaborated with other scientists at Ohio State to understand more about the role of exercise in cancer-induced cachexia. They are using an animal model to explore optimal methods of attenuating muscle wasting. • Pat Fahey, MD, along with Mary Jo Welker, MD, Tom Blincoe, Donna Cruz-Huffmaster and Chris Welker, investigated downstream revenue that the Ohio State University Primary Care Network (OSU PCN) generates for Ohio State University Medical Center (OSUMC). They assessed total billings and collections in fiscal 2003-04 from five revenue streams at OSUMC from the OSU PCN. These streams – hospital billings, tests and procedures from network and specialty attending physicians, and specialist physician billings – were compared with billings and collections from the OSU PCN. The researchers used a novel weighting system to capture the concept that not all admissions or referrals of OSU PCN patients were ordered by OSU PCN physicians. Total downstream net revenue of nearly $115 million was more than six times the net revenue to the network. A downstream direct contribution margin of $52 million was 6.3 times the network loss. The researchers concluded that a primary care network can generate significant financial support for an academic medical center. Their manuscript, “Analysis of Downstream Revenue to an Academic Medical Center from a Primary Care Network,” was published in the August 2006 issue of Academic Medicine. • Larry Gabel, PhD, with support from Pat Fahey, MD, Linda Stone, MD, and others from outside the Department, completed two funded projects investigating “telehealth”: Telehealth: A Unique Solution for Health Needs of the Columbus Empowerment Zone was a three-year project funded for $108,000 by the Columbus Compact Corporation; and Ohio Telemedicine, Education, and Linkage Program (Ohio TeleHelp): A Proof-ofConcept Study, a one-year project funded for $150,000 by the Ohio Board of Regents. These projects suggested that medical outcomes via telemedicine are the same or better than outcomes of similar face-to-face medical services, and that concomitant costs of service are the same or less. A university-commercial partnership was established to create and maintain a statewide broadband network to link facilities interested in participating in a proof-of-concept study. Each facility was configured with at least a video-teleconference unit connected to all others through wired or wireless means. Networked facilities included: four nursing homes (two in Columbus, one in Cleveland and one in Waverly); a non-profit community mental health center, a non-profit community primary healthcare center, and a non-profit community refugee center, all in Columbus; a non-profit county health department in Portsmouth; a university-based hospital in Columbus; and two university-based emergency departments in Columbus and Chillicothe. A pilot study of this network revealed satisfaction by consulting physicians at the Distant Site (university hospital) and staff and patients at the Originating Sites (nursing homes). Especially apparent were savings in time, travel and staff resources that are normally part of person-to-person healthcare delivery. A later analysis suggested that using telemedicine could save Medicaid about $98.5 million annually. But Ohio Medicaid does not reimburse for care provided via telemedicine, and nor does Medicare in metropolitan areas, so there is reason to consider changing restrictive regulations. DEPARTMENT OF INTERNAL MEDICINE Michael Grever, MD, Chair The Department of Internal Medicine comprises 12 divisions, each dedicated to innovations in research, education and patient care. Following are division descriptions and research highlights for 2006: Division of Cardiovascular Medicine William Abraham, MD, Director In partnership with patients, researchers and healthcare professionals, Cardiovascular Medicine conducts clinical trials on new cardiovascular drugs, devices and therapies. The Division’s Cardiovascular Clinical Research Unit (CCRU) enables clinical researchers and basic scientists to work together to improve patient care and outcomes. The CCRU manages more than 80 clinical research projects, including investigator-initiated single-site studies, multi-site trials sponsored by the National Institutes of Health, and industry-sponsored trials. Studies span heart failure, interventional cardiology, electrophysiology, pulmonary hypertension, sleep disorders, emergency medicine, and cardiac imaging and genotyping. By linking Ohio State’s Ross Heart Hospital with the University’s Davis Heart and Lung Research Institute, faculty members have advanced patient care through research. Investigators and research staff identify patients who may be candidates for specific protocols, then follow them closely throughout the studies, usually for several years. Results from these studies form the basis for standard-of-care practices and identify additional questions for translational and basic research. 2007 Research Report 71 Ongoing Research Programs • Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION) PI: William Abraham, MD • Multicenter automatic defibrillator implantation trial II (MADIT II) PI: Charles Love, MD • Implantable cardioverter defibrillator (Chronicle ICD) PI: Garrie Haas, MD • The role of diagnosis and treatment of sleep apnea in the acute exacerbation of heart failure PIs: Rami Khayat, MD, and William Abraham, MD • Trial to assess chelation therapy (TACT) PI: Raymond Magorien, MD Research Accomplishments of 2006 • One major accomplishment has been the development of the Cardiovascular Clinical Research Unit (CCRU) as a model for clinical research at OSUMC and as a national leader in investigating new cardiovascular drug and device therapies. CCRU leaders are working with hospital revenue cycle management to formalize a billing process for clinical research. They also are working with the Medical Center’s Information Warehouse and the Department of Biomedical Informatics to design computer-based research-screening processes. • Physicians at Ohio State’s Ross Heart Hospital use a minimally invasive cardiac catheterization procedure to implant the nation’s first permanent device for monitoring and treating congestive heart failure. This investigational device, called the HeartPod (Savacor, Inc.), allows patients to monitor and change their treatment regimen, if needed, based on specifications pre-set by their physician. Ohio State was the first site in the United States to implant the HeartPod, and it continues to be 72 Ohio State University Medical Center the highest enrolling site with seven patients. Garrie Haas, MD, is the local principal investigator; Charles Bush, MD, medical director of the Ross Heart Hospital, and Charles Love, MD, director of CV Device Services, conduct the implants; and William Abraham, MD, is the national principal investigator. In 2006, Love was selected as the first electrophysiologist in the world to implant the device using a subclavian approach that allows for easier access through the chest as opposed to implanting through the leg. • The Sleep-Heart Program, a multidisciplinary clinical and research program led by William Abraham, MD, was initiated. Leadership is also provided by Philip Binkley, MD, and Garrie Haas, MD, from Cardiovascular Medicine, and Rami Khayat, MD, from the Division of Pulmonary, Allergy, Critical and Sleep Medicine. The program spans Ohio State’s Ross Heart Hospital, the Sleep Disorders Center at University Hospital East, Ohio State’s School of Public Health, and Ohio State’s Davis Heart and Lung Research Institute. The program examines translational and clinical questions that focus on the relation between respiratory disorders of sleep and cardiovascular diseases. Research tracks include: sleep apnea and autonomic control in patients with heart failure; effects of intermittent hypoxia on the heart; clinical outcomes of treatment of sleep apnea and heart failure; vascular consequences of oxidative stress in sleep apnea; and lipid metabolism in sleep apnea. The program is positioned to study questions about cardiovascular consequences of sleep disorders. • The Division’s Cardiovascular Clinical Research Unit (CCRU) continued to grow through the development of clinical research core lab facilities in cardiac stress testing, cardiac MR/CT and noninvasive imaging. Additionally, the Division and the CCRU experienced unprecedented growth in electrophysiology by adding five physicians who transferred approximately 20 clinical research studies with hundreds of active research subjects. This gives the Division one of the largest clinical research groups at OSUMC. Division of Dermatology Division of Digestive Health Mark Bechtel, MD, Director Nicholas Verne, MD, Director The Division of Dermatology collaborates with the Division of Hematology and Oncology in clinical management, clinical trials and oncologic genetic research focusing on cutaneous lymphoma and cutaneous oncology. Dermatology is also collaborating in a new cutaneous oncology focus group involving dermatology, hematology and oncology, surgical oncology, dermatopathology and cutaneous oncology researchers at Ohio State. A new cutaneous oncology dermatologic surgical facility provides more space for micrographic surgery on cutaneous malignancies. A complex medical dermatology clinic enables dermatology residents to evaluate and manage patients with autoimmune bullous diseases, collagen vascular disease and severe psoriasis. Matthew Zirwas, MD, has joined Dermatology and established the Contact and Occupational Dermatitis Center. Mark Bechtel, MD, was recognized in the 2005-2006 “Best Doctors in America.” He also serves on the American Academy of Dermatology’s Guidelines and Standards of Care Task Force. Zirwas was elected to the board of directors of the American Contact Dermatitis Society. After a major recruiting effort throughout 2006, the Department of Internal Medicine selected Nicholas Verne, MD, to direct the Division of Digestive Health. Verne is a nationally known, grant-funded physician scientist who will be responsible for building a comprehensive research program in gastroenterology. Also in this Division during 2006, Sumei Liu, PhD, continued her work with Jackie Wood, PhD, of the Department of Physiology and Cell Biology, to advance understanding of neurophysiologic control of mammalian gastrointestinal functions in health and disease states. John Fromkes, MD, continued his research in conjunction with Gary Stoner, PhD, of the Division of Hematology and Oncology, to examine the cancer-inhibiting properties of black raspberries in people at high risk for esophageal cancer. Ongoing Research Programs • David Lambert, MD, is collaborating with Ronald Glaser, PhD, director of the Institute for Behavioral Medicine Research at Ohio State, in a study funded by the National Institutes of Health on the effects of stress on basal cell carcinoma. • David Lambert, MD, is collaborating with Amanda Tolland, PhD, of Molecular Virology, Immunology and Medical Genetics, in the study of molecular genetics of squamous cell carcinoma. Division Accomplishments in 2006 • The most significant accomplishment was completing planning and starting construction of office facilities and a state-of-the-art endoscopy unit, both targeted for completion by the end of fiscal year 2007. Additional planning was completed for construction of an outpatient clinic within the new Digestive Health space. These facilities will be a key to faculty recruitment and the growth of a comprehensive research program in gastroenterology. 2007 Research Report 73 Division of Endocrinology, Diabetes and Metabolism Kwame Osei, MD, Director The Division of Endocrinology, Diabetes and Metabolism continues to excel in research, teaching and patient care. Endocrinology at Ohio State University Hospitals was cited for the 13th time among the Best Divisions in Hormonal Disorders by U.S.News & World Report. The Division will expand its accomplishments through research in diabetes, islet cell transplantation, osteoporosis and bone diseases, thyroid cancer and benign thyroidpituitary disorders. Ongoing Research Programs • Non-Human Primate Experimental Model of Islet Cell Transplantation (ICTP) (Elizabeth Diakoff, MD) • Women’s Health Initiative (WHI) and Osteoarthritis Initiative (OAI) (Rebecca Jackson, MD) • Diabetes Research Center (DRC) (Kwame Osei, MD) • Thyroid Cancer Program at the Martha Morehouse Medical Plaza (Matthew Ringel, MD) Research Accomplishments of 2006 • In conjunction with the Department of Surgery’s Division of Transplant Surgery, the Division of Endocrinology, Diabetes and Metabolism has established a Non–Human Primate Experimental Model of Islet Cell Transplantation (ICTP). The success of this phase of the program has become the backbone of the human ICTP initiative. Objectives are to develop therapies to ensure long-standing islet cell functional survival in humans by immunoprotection and to promote islet cell growth and anti-apoptosis. • The Division supports two prestigious NIH Centers: the Women’s Health Initiative (WHI) and Osteoarthritis Initiative (OAI). Given the strengths of these centers, the Division has recruited new faculty in bone epidemiology. 74 Ohio State University Medical Center • The College of Medicine’s Executive Committee approved a business plan to establish a Diabetes Research Center, which will develop treatment strategies for sustaining functional islet cell transplantation (ICTP) and pancreas allograft transplantation (PAT), diabetes and cardiovascular disease, and prevention of type 2 diabetes. A Community Diabetes Program also will be developed. • In conjunction with Ohio State’s Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute, the Division has established a Thyroid Cancer Program at the Martha Morehouse Medical Plaza. This program aims to: define the molecular basis of thyroid cancer growth and metastasis; develop chemotherapies as an adjunct for radioiodine and thyroxine therapy; and develop a translational research program in thyroid cancer. Division of General Internal Medicine Catherine Lucey, MD, Director The Division of General Internal Medicine provides comprehensive patient care to an increasingly complex community of patients while educating the next generation of physicians. Division members have achieved local and national recognition for patient care and educational leadership roles. They are also involved in numerous research projects. The Division is strongly focused on medical education. Numerous faculty are recognized as educational leaders in department and college activities. Leadership positions assumed by Division members include directorship of the new Office for Scholarship in Medical Education, chair of the College of Medicine Professionalism Education and Evaluation Committee, co-directorship of the Med 3/4 curriculum, and directorship of the new Meaning in Medicine course for medical students. Division physicians have authored multiple book chapters on topics ranging from physical diagnosis to clinical decision-making, perioperative issues and the educational approach to dealing with problem residents. Ongoing Research Programs Division of Hematology and Oncology Michael Caligiuri, MD, Director • Point of service testing in diabetes and anti-coagulation management – Mark Wurster, MD • Faculty development of community-based primary care preceptors in underserved communities – Cynthia Ledford, MD • Comparison of medical student and volunteer senior partner expectation for a regional senior partner experience in medical school – Robert Murden, MD Research Accomplishments of 2006 • Cynthia Kreger, MD, continued production of history-taking and physical exam cases for educational CDs. These are used in classes at Ohio State and sold nationwide. • The faculty development proposal by Cynthia Ledford, MD, was funded for more than $200,000, the largest grant ever obtained by a principal investigator in the Division of General Internal Medicine. • Almost half of the faculty was involved in research projects. • Cynthia Ledford, MD, received a Health Resources and Services Administration grant as principal investigator for developing a virtual classroom to help community-based faculty enhance their teaching skills. • Mitchell Medow, MD, was a core participant in a T32 training grant to increase the number of health professionals with formal education in clinical investigation. He also continued his work on the use of decision-making aids by physicians. • Harrison Weed, MD, co-edited a textbook on perioperative medicine. • Mark Wurster, MD, was involved with several projects on point-of-service testing for management of chronic medical conditions. • Cynthia Kreger, MD, Robert Murden, MD, and Mark Wurster, MD, continued to participate in Health Resources and Services Administration grants with colleagues in the Department of Family Medicine. Research in the Division of Hematology and Oncology focuses on developing drug therapies for treating solidtumor and hematologic malignancies, and on cancer prevention through nutrition and natural products. 2006 was productive for the Division as evidenced by the growth in direct research funding. Most notably, Miguel Villalona, MD, was awarded a $3 million phase II clinical trials contract from the National Cancer Institute (NCI), placing Ohio State in a group of only five other cancer institutions in America that have received NCI contracts for both phase I and phase II clinical trials. In addition, Charles Shapiro, MD, was awarded a $1.25 million grant from The Lance Armstrong Foundation to establish a cancer survivorship center at Ohio State that will work to improve the care and quality of life of cancer survivors. These and other grant awards are highlighted below in the Division’s 2006 research accomplishments. Ongoing Research Programs • Phase I Trials of Anticancer Agents – This program seeks to perform early drug development in solid and hematologic tumors with performance of detailed pharmacokinetic and pharmacodynamic studies – Michael Grever, MD • Early Therapeutics Development with Phase II Emphasis – This program studies National Cancer Institute-sponsored novel anticancer agents in phase II clinical trials – Miguel Villalona, MD • Comprehensive Program for the Prevention, Detection and Treatment of Lung Cancer – This program investigates inhalation programs for the prevention and detection of cancer. Several novel biomarkers for easy detection and innovative imaging modalities have been tested and developed – Gregory Otterson, MD 2007 Research Report 75 • Experimental Therapeutics in Chronic Lymphocytic Leukemia – This Specialized Center of Research (SCOR) grant is being used to create new and improve current therapies for chronic lymphocytic leukemia – John Byrd, MD • Epigenetic Targeted Therapy for Chronic Lymphocytic Leukemia – This program is developing targeted therapies for chronic lymphocytic leukemia and lymphoproliferative disorders – John Byrd, MD Research Accomplishments of 2006 • Miguel Villalona, MD, received a $3 million phase II contract in early therapeutics development from the National Cancer Institute. • Charles Shapiro, MD, was awarded a $1.25 million grant from The Lance Armstrong Foundation to establish a cancer survivors center at Ohio State that will improve the care and quality of life of cancer survivors. • Michael Grever, MD, is co-principal investigator (PI) for an $11.84 million program project grant awarded by the National Cancer Institute for “DNA methylation & chromatin modifications: mechanisms & applications in cancer therapy.” PI for this grant is Samson Jacob, PhD, of Molecular and Cellular Biochemistry. • Robert Baiocchi, MD, PhD, received a V Foundation Scholar Award to study Epstein-Barr virus-associated malignancies. • Kristie Blum, MD, was awarded a National Cancer Institute grant targeting transcriptional repression in chronic lymphocytic leukemia. • William Blum, MD, received a National Cancer Institute grant to help develop experimental therapeutics in adult leukemia. • Steven Clinton, MD, PhD, landed a National Cancer Institute grant to study the effects of tomato-soy juice on prostate cancer. • Thomas Lin, MD, PhD, was awarded a National Cancer Institute grant to study the efficacy of Flavopiridol in treating B-cell non-Hodgkin lymphoma. 76 Ohio State University Medical Center • Guido Marcucci, MD, received a National Cancer Institute grant to research the pharmacologic modulation of chromatin remodeling in leukemia. • Manisha Shah, MD, was awarded a National Cancer Institute grant for focusing on targeting RAF and VEGF signaling in thyroid cancer. • Clara D. Bloomfield, MD, received the Distinguished Service Award for Scientific Achievement from the American Society of Clinical Oncology. She also was elected president of the Association for Patient Oriented Research and was named a Distinguished University Professor, Ohio State’s top faculty award. • John Byrd, MD, was one of five researchers selected to receive the Leukemia & Lymphoma Society’s Stohlman Scholar Award for outstanding contributions in blood cancer research. Division of Human Genetics Albert de la Chapelle, MD, PhD, Interim Director The Division of Human Genetics (HG) serves as a single platform for translational and clinical research, clinical activities, and education and outreach. Its goals are to enhance Ohio State University Medical Center as a leader in gene discovery/characterization and molecular epidemiology, to set standards for the clinical management of patients with genetic predisposition, and to set guidelines for society regarding the research and clinical usage of genetic information. Its clinical responsibilities are to provide genetic counseling to patients and families, and expert consultations to physicians and other professionals. HG not only provides adult medical genetics care to residents of central Ohio and beyond, but it also gives patients and physicians in the community free access to HG research protocols. HG coordinates clinical-genomic databases, specimen repositories and the use of both inhouse and referral diagnostic facilities to support clinical research in human genetics. Ongoing Research Programs Division of Immunology Ronald Whisler, MD, Director • Columbus-area HNPCC (hereditary nonpolyposis colon cancer) study – Albert de la Chapelle, MD, PhD • Variants in high-risk breast cancer genes and contribution to cancer risk – Amanda Toland, PhD • Frequency and clinical spectrum of germline PTEN mutations in a population-based series of incident breast cancer cases in central Ohio – Charles Shapiro, MD • Study to identify multiple human low penetrance genes that control genetic susceptibility and resistance to cancer – Kevin Sweet, MS, CGC, and Amanda Toland, PhD Research Accomplishments of 2006 • Heather Hampel, MS, CGC, and Albert de la Chapelle, MD, PhD, along with other members of the Human Genetics (HG) staff, published the findings of the Columbus-area HNPCC study with regard to the screening of 543 newly diagnosed endometrial cancer patients in the journal Cancer Research in August 2006. As a result of this study, the Ohio State University Pathology Department and Gynecologic Oncology Division, in conjunction with the Division of HG, has begun performing immunohistochemistry staining for the four mismatch repair proteins on all newly diagnosed endometrial cancer patients at Ohio State University Medical Center as a clinical billable service. • Rebecca Nagy, MS, CGC, is collaborating with Gail Herman, MD, PhD, (Columbus Children’s Hospital) on her Ohio Department of Health Services grant titled Regional Genetics Center at Children’s Hospital. Nagy participates in a statewide directory and speaker’s bureau giving presentations on cancer genetics. • Kimberly Kelly, PhD, received funding from the Tzagournis Endowment Trust Fund to support a Cancer Family History Public Health Campaign ($99,996). Judith Westman, MD, along with Amy Sturm, MS, CGC, and Kevin Sweet, MS, CGC, are also involved in this project. This campaign promotes awareness of the importance of family history of cancer and gives the public tools to gather, evaluate and use family history to learn more about their cancer risk. The Division of Immunology makes advances in academic excellence and research while serving the University and the community in the clinical setting. The Division’s publications and peer-reviewed grants demonstrate considerable strength, with Kevin Hackshaw, MD, and Clark Anderson, MD, participating as co-investigators on program project grants. Anderson furthered his National Institutes of Health (NIH)-supported studies of Fc receptors. Kevin Hackshaw and Ronald Whisler, MD, serve as co-principal investigators with Rebecca Jackson, MD, on the Osteoarthritis Initiative funded by NIH. Division members also have been conducting clinical investigations into more efficacious treatment regimens for rheumatoid arthritis, systemic lupus erythematous, fibromyalgia, osteoporosis and osteoarthritis. Ongoing Research Programs and Research Accomplishments of 2006 • FcRn Binds and Transports Albumin – Clark Anderson, MD • Clinical Centers for the Osteoarthritis Initiative – Kevin Hackshaw, MD and Ronald Whisler, MD • Phase III randomized, double-blind, placebo-controlled, multicenter study of retreatment with rituximab in subjects with rheumatoid arthritis receiving background methotrexate – Kevin Hackshaw, MD • A randomized, phase 3, controlled, double-blind, parallel group, multicenter study to evaluate the safety and efficacy of rituximab in combination with methotrexate (mtx) compared to mtx alone in methotrexate-naive patients with active rheumatoid arthritis - Ronald Whisler, MD and Kevin Hackshaw, MD 2007 Research Report 77 Division of Infectious Diseases Larry Schlesinger, MD, Director Faculty in the Division of Infectious Diseases (ID) continued their commitment to research in 2006 by submitting 27 grants. Active funding included $13.97 million from the National Institutes of Health (NIH), the Health Resources and Services Administration (HRSA), and the Department of Health and Human Services to support research for patients with HIV/AIDS. The Division’s NIH-awarded AIDS Clinical Trials Unit (ACTU) ranked highly among research awards across the University in 2006, and its HRSA AIDS Education and Training Center continued to thrive. The Center for Microbial Interface Biology (CMIB) was awarded Center status by the Ohio State University Board of Trustees. The CMIB’s multidisciplinary educational and research programs explore fundamental questions about infectious diseases, microbial pathogenesis, bioterrorism and disaster preparedness. Research awards for core CMIB members totaled $5.55 million. Major research areas within the Division include HIV/AIDS, fungal infections, infections in the immunocompromised host, tuberculosis, parasitic infections, epidemiology and food-borne infections. Ongoing Research Programs • Adult AIDS Clinical Trials Unit (ACTU) – Led by Susan Koletar, MD, this unit is part of a national and international multicenter group that conducts clinical trials to increase knowledge about the pathogenesis, prevention, course and treatment of HIV infection and associated complications. • Epi-Centers for Prevention of Healthcare Related Infections, Ohio State Health Network Infection Control Collaborative – Under the leadership of Kurt Stevenson, MD, MPH, this collaborative includes Ohio State and 14 outreach sites that optimize electronic health information systems to improve surveillance for healthcare-associated 78 Ohio State University Medical Center infections, antimicrobial resistance and other adverse biological events. • Great Lakes Regional Center of Excellence in Biodefense and Emerging Infectious Disease Research – This program, funded by the National Institutes of Health and the National Institute of Allergy and Infectious Diseases, awarded Ohio State a grant titled “Lung innate immune responses to Francisella tularensis: a central role for the macrophage.” The grant, led by Larry Schlesinger, MD, includes four projects conducted by investigators in the Center for Microbial Interface Biology at Ohio State and by Ohio State’s Davis Heart and Lung Research Institute. The researchers are exploring lung immune responses to these bacteria – especially as they relate to interactions with macrophages – to identify molecular targets for new diagnostic strategies as well as targeted immune therapies aimed at enhancing host immunity. • CD8 T Cells and Immunity to Tuberculosis in Old Mice – Joanne Turner, PhD. This study, funded by the National Institute of Allergy and Infectious Diseases, explores how the aging immune system response differs from that of younger individuals when it encounters a pathogen. This knowledge will help design a vaccine or post-exposure therapy to protect the elderly from infectious disease. Research Accomplishments of 2006 • The AIDS Clinical Trials Unit (ACTU) at Ohio State advances AIDS care via research on the pathogenesis, prevention, course and treatment of HIV infection and associated complications through affiliation with the national and international AIDS Clinical Trials Group (ACTG). The ACTU was refunded in December 2006 for $11.9 million over seven years. By playing leadership roles for protocol development and administration of the ACTG, Ohio State participates in the design and implementation of clinical trials that seek to optimize clinical management of HIV and related complications, evaluate agents with novel mechanisms or improved toxicity profiles for treating HIV and/or major co-pathogens (e.g., tuberculosis and hepatitis), evaluate the safety, immunogenicity and efficacy of multiple candidate HIV vaccines and adjuvants, develop means of reducing HIV transmission, and minimize the risk of vertical transmission by maximizing care of HIV-infected women during child-bearing years. • The national Centers for Disease Control designated Ohio State as one of five (in the country) Epi-Centers for Prevention of Healthcare-Related Infections, a five-year project funded at $1.97 million. This Ohio State Health Network Infection Control Collaborative includes the University and 14 outreach sites that improve surveillance for healthcare-associated infections, antimicrobial resistance and other adverse biological events by optimizing available electronic health information systems. This enables facilities to focus less on data collection and more on improving processes and outcomes, particularly in small community hospitals where staffing and other resources are limited. Through the Ohio State University Health System, the Ohio State Health Network and the Ohio State University Information Warehouse, health information will be surveyed for targeted events, retrospective comparison of electronic with traditional surveillance, and prospective validation of surveillance methodology for accuracy and cost efficiency. • OSU Center for Microbial Interface Biology (CMIB) – In 2006 the CMIB (which includes leadership from the Division of Infectious Diseases) grew to 55 faculty campuswide and nearly 50 core personnel in the Biomedical Research Tower (BRT), and was awarded University Center status by the Board of Trustees. Research funding for core CMIB members in the BRT totaled $5.55 million, with $10.55 million in review. The CMIB is a member of the Great Lakes Regional Center of Excellence in Biodefense and Emerging Infectious Disease Research. It also serves as a centerpiece for collaborations in research and education with units/departments across the University. A Provost Targeted Investment in Excellence program was awarded in Public Health Preparedness for Infectious Diseases to Larry Schlesinger, MD, College of Medicine, in conjunction with the colleges of Public Health, Veterinary Medicine, Biological Sciences, Pharmacy, and Food, Agriculture and Environmental Sciences. The award totals $979,068 to the College of Medicine/Division of Infectious Diseases. Also in 2006, CMIB faculty in the BRT had 20 papers published or in press, along with numerous published abstracts, invited lectureships and review panel appointments, both nationally and internationally. • Great Lakes Regional Center of Excellence (GLRCE) in Biodefense and Emerging Infectious Diseases Research – Investigators in Ohio State’s Center for Microbial Interface Biology (CMIB) and the Division of Infectious Diseases received a fiveyear grant totaling $546,877 from the GLRCE to study lung innate immune responses to Francisella tularensis, the causative bacterium of tularemia and a targeted agent of bioterrorism. The most worrisome infectious agents of bioterrorism would be artificially disseminated as aerosols to the lungs. Thus, a clearer understanding of lung immune responses to these bacteria, especially as they relate to interactions with macrophages, is essential for identifying molecular targets for new diagnostic strategies and targeted immune therapies aimed at enhancing host immunity. This project involves investigators in both the CMIB and Ohio State’s Davis Heart and Lung Research Institute. • CD8 T Cells and Immunity to Tuberculosis in Old Mice – The elderly are more susceptible to infectious diseases, but vaccinating them is less effective when using vaccines designed for young individuals. To design a vaccine or post-exposure therapy that can protect the elderly, it is necessary to understand how the aging immune response reacts to pathogens. Using an aging mouse model of tuberculosis, researchers have found that old mice express a transient early resistance to infection that correlates with the presence of CD8 T cells within the lungs – a previously unrecognized immune mechanism that is absent from the lungs of young mice. Joanne Turner, PhD, will use a low-dose aerosol infection model of tuberculosis to further characterize this CD8 T cell population and determine when CD8 T cells become more active in the lungs of old mice, as well as the mechanism by which CD8 T cells mediate early resistance. 2007 Research Report 79 Division of Nephrology Brad Rovin, MD, Director Investigators collaborating on the Nephrology Program Project on SLE nephritis – Lee Hebert, MD, Brad Rovin, MD, Dan Birmingham, PhD, and ChackYung Yu, PhD – were invited to resubmit a competitive renewal to the National Institute of Diabetes and Digestive and Kidney Diseases. They have now published more than 40 original papers and review articles on lupus nephritis and placed four abstracts at the 8th International Congress on Systemic Lupus Erythematosus (SLE) in Shanghai, China. Rovin and Hebert are participating in four clinical trials of novel therapeutic agents for the treatment of SLE and are active members of the Lupus Clinical Trials Consortium. Clinical trials run by Anil Agarwal, MD, Nabil Haddad, MD, Hebert, Dan Spetie, MD, Ganesh Shidham, MD, Rovin, Todd Pesavento, MD, and Jon Von Visger, MD, PhD, brought more than $1.5 million in research funding to the Division. Ongoing Research Programs • SLE Program Project in Human Lupus Nephritis – This work examines clinical, genetic and novel biochemical risk factors for the onset, severity and prognosis of lupus nephritis flare. • The African-American Study of Kidney Disease and Hypertension (AASK) – This is an NIH-sponsored clinical trial of risk factors for kidney disease and hypertension in African-Americans. • The Lupus Clinical Trials Consortium (LCTC): Through this consortium, the Division of Nephrology participates in multicenter, international trials of new therapeutics for lupus and lupus nephritis. • Clinical Research in Transplantation – The Transplant Center has initiated two large prospective clinical trials. One looks at reducing complications of steroid therapy in renal transplant recipients, and the other examines homocysteine as a cardiovascular risk factor for renal transplant patients. 80 Ohio State University Medical Center • Clinical Anemia Program: The Nephrology clinical trials unit is involved in studies to define the best approaches to anemia management in patients with chronic kidney disease. Research Accomplishments of 2006 • The Program Project in Lupus Nephritis has provided novel insights into human SLE. Studies on the role of the complement system in the pathogenesis of lupus have revealed four important findings. First, variability in gene copy number of the fourth complement component (C4) is an important risk factor for onset of SLE. Specifically, low C4 gene copy number predisposes to lupus onset, while high copy number provides protection. Second, genetically determined low copy number of erythrocyte complement receptor-1 (ECR1), which interacts with complement-coated immune complexes in circulation, is associated with onset of renal manifestations of lupus. Third, E-CR1 function during SLE flare protects the kidney from damage mediated by immune complexes and complement. Fourth, the pattern of complement activation prior to and at the onset of SLE flare suggests the involvement of two complement activation pathways: the classical pathway during renal flare initiation, and the alternative pathway driving actual renal tissue damage. These data underscore the importance of the complement system, both in protecting against the onset of SLE and its manifestations, and in driving the tissue damage that defines the clinical presentation of SLE nephritis. • Adiponectin, an adipocyte-derived cytokine thought to play a role in metabolic disorders such as obesity and diabetes, has been identified as a potential biomarker of lupus nephritis. To understand the relationship between this novel cytokine and lupus, scientists launched studies to determine its potential as a modulator of inflammation. Although existing literature suggests that adiponectin may have anti-inflammatory properties, Ohio State scientists have shown that this adipokine can induce the expression of proinflammatory chemokines by endothelial cells and monocytes. They also have shown that adiponectin is present in the kidneys of patients with lupus nephritis, and that its RNA expression is upregulated compared with control renal tissue. In addition, a known adiponectin receptor is present on glomerular podocytes. These findings demonstrate that adiponectin can be made in organs other than adipose tissue, and that the expression of ligand and receptor in the glomerulus suggests a role for adiponectin in modulating glomerular function. A potential effect of adiponectin in lupus may be to augment inflammation through induction of proinflammatory cytokines. This remains to be demonstrated and is part of an ongoing effort to define the role of adiponectin in lupus nephritis. • Clinical studies of extended dosing of darbopoietin-α showed that it is possible to increase the dosing interval of this drug to once a month. This finding is likely to impact the care and quality of life of patients with chronic kidney disease and anemia who are not yet on dialysis. Darbopoietin-α was effective at extended dosing intervals in adults and older patients with anemia of chronic kidney disease. Less frequent dosing has the potential to decrease resource utilization and medication errors while enhancing patient comfort and convenience. • The Transplant Center has initiated a prospective trial to eliminate steroids as a chronic maintenance agent for renal transplant recipients. This study could help minimize steroid complications, which are a major source of morbidity in the renal transplant population. The Transplant Center is also one of the largest enrolling centers in a National Institutes of Health-sponsored clinical trial to determine if homocysteine reduction will attenuate cardiovascular events and improve the survival of transplant recipients. • The Interventional Nephrology Group examined central vein stenosis in association with internal jugular dialysis catheters and demonstrated a high incidence of this problem. This finding has generally gone unrecognized but could change the approach to temporary dialysis catheters in patients with chronic kidney disease, because central vein stenosis impairs the ability to successfully create permanent dialysis access. Division of Pulmonary, Allergy, Critical Care and Sleep Medicine Clay Marsh, MD, Director The Division has joined the Center for Critical Care and continues building along specialtybased product lines and analytical programs to “create the future of medicine to improve people’s lives.” The goal is to be a leader in personalized health care focusing on translational research. Progress is evidenced by recognition in U.S.News & World Report as the 24th best respiratory program in the country, and by having 10 faculty recognized among the Best Doctors in America.® New initiatives, current research and ongoing projects include: • Sepsis registry in the ICU with biobanking capability • Stress and depression in advanced lung disease • Pulmonary interventional procedures • Growth of the Asthma Research Center • Mechanistic work in defining biomarkers in patients with sepsis • Mechanistic programs in antibody-directed therapies in cancer, sepsis, cell life and cell death, oxidant imaging and function, cell signaling, angiogenesis, mitochondrial biology, regulation of cystic fibrosis transmembrane regulator protein (CFTR), glucocorticoid receptor biology, infectious disease and inflammation Ongoing Research Programs • Translational research in critical care, asthma, ILD, emphysema, lung cancer, sleep medicine, pulmonary hypertension, and allergy • Translational research in acute lung injury in the ICU that focuses on quality improvement, execution of evidence-based pathways and structure/function relationships in clinical delivery 2007 Research Report 81 • Translational research in pulmonary hypertension • Collaboration with Ohio State’s Davis Heart and Lung Research Institute in lung remodeling and repair, sepsis and mitochondrial biology • Studying the role of inflammatory cells in lung complications of bone marrow transplantation • Studying tissue microenvironment and human disease, along with the role of genetics in lung and critical care disease • Fibrosis, remodeling, lung injury and cancer – Clay Marsh, MD, Philip Diaz, MD, Jeanette Marketon, PhD, Estelle Boyaka, PhD, John Mastronarde, MD, Patrick Nana-Sinkam, MD, Karen Wood, MD • Innate immune system function, sepsis and infectious disease – Mark Wewers, MD, Susheela Tridandapani, PhD, Andrea Doseff, PhD, Daren Knoell, PharmD, Karen Wood, MD, Amal Amer, PhD • Mitochondria biology and critical care disease – Elliott Crouser, MD, Douglas Pfeiffer, PhD, Ruairi Fahy, MD • Process-based research and decision making – James O’Brien, MD, Naeem Ali, MD, Scott Aberegg, MD, Stephen Hoffmann, MD, Virginia Nivar, PhD • Redox biology of the lung and muscle and sleep medicine – Thomas Clanton, PhD, Ulysses Magalang, MD, Valery Khramtsov, PhD, Narasimham Parinandi, PhD, Rami Khayat, MD Research Accomplishments of 2006 • Creation of a critical care and lung genetics program focusing on microRNA expression in health and disease • Creation of a sepsis registry and biobanking program • Named as a center for the National Institutes of Health-sponsored LOTT (Long-term oxygen treatment trial) • Creation of a chronic stress and depression program in advanced lung disease 82 Ohio State University Medical Center DEPARTMENT OF MOLECULAR AND CELLULAR BIOCHEMISTRY Michael Ostrowski, PhD, Chair Research in the Department of Molecular and Cellular Biochemistry advances basic understanding of the biochemical and molecular mechanisms underlying both normal cellular processes and disease states in humans. Through multidisciplinary collaborative work, scientists can translate these basic findings into studies that will benefit patients. This research focus is exemplified by two translational National Cancer Institute program project grants for which Department faculty members serve as principal investigators. These projects include faculty members in both clinical and basic science departments across the Medical Center. In addition, all faculty in the Department of Molecular and Cellular Biochemistry are associated with Medical Center Signature Programs, including cancer, heart and neurosciences – representing the highest level of faculty participation in these programs within the School of Biomedical Sciences. Ongoing Research Programs • Genetic and epigenetic mechanisms governing the transcriptional regulation of gene expression • Molecular mechanisms underlying neuromuscular diseases • Molecular genetics of the regulation of RNA processing, transport and stability • Structural biology of homologous recombination and cytokine signaling • Molecular and cell biology of cardiovascular disease Research Accomplishments of 2006 • Samson Jacob, PhD, leads a multidisciplinary team that received a five-year, $12 million program project grant from the National Cancer Institute to study basic mechanisms and translational applications of epigenetic modifications of DNA and chromatin in human leukemia. The project team includes Department faculty members Mark Parthun, PhD, and Saïd Sif, PhD. This team covers all aspects of epigenetic modifications, including DNA methylation, histone modification and chromatin remodeling. • Tsonwin Hai, PhD, participated in a collaborative study that used a systems biology approach to identify an unexpected role for the transcription factor ATF-4 in innate immunity (Nature 441:173178). Hai discovered ATF-4 in 1989 and has used a variety of approaches, including mouse genetic models, to study the role of this stress-activated transcription factor in several biological processes, including diabetes. In this study, her mouse genetic model demonstrated that ATF-4 can also repress expression of proinflammatory genes in immune cells, a necessary step for a normal immune response. This work implicates ATF-4 as an important factor to study in inflammatory diseases, including rheumatoid arthritis and cancer. • Dan Schoenberg, PhD, director of the campuswide RNA Group, was on a team of Ohio State investigators that identified a novel mechanism that retroviruses use to increase translation of viral genes. The virus uses a unique RNA structure to recruit a cellular protein, RNA helicase A, to increase translation of viral RNA to protein. This group found that the same RNA structural element is found in many cellular RNAs, and that RNA helicase A can also increase translation of these cellular RNAs, including RNA that encode for genes involved in cancer cell growth. The work may help improve the design of vectors for gene therapy and enhance understanding of how genes are aberrantly expressed in cancer cells. • Kamal Mehta, PhD, and his group made the unexpected finding that the small molecule inhibitor of the JNK family of stress-inducible kinases, SP600125, can also inhibit phosphorylation of histone 3 at position ser10 (H2Ser10). Through studies with this compound, researchers discovered that the low-density lipoprotein receptor, involved in transporting cholesterol across cell membranes, is negatively regulated by histone H3-Ser10 phosphorylation. These studies will guide development of more effective agents for treating hypercholesterolemia and cancer. • Charles Bell, PhD, led a study that used NMR spectroscopy and X-ray crystallography to determine the three-dimensional structure of PF1378 (Pfu Pop5), one of four protein subunits of archaeal RNase P that shares a homolog in the eukaryotic enzyme. RNase P is a ubiquitous and essential enzyme in all domains of life. It is responsible for cleaving the single-stranded 5’ leader sequence of precursor tRNA, a vital reaction in the maturation of tRNA. With the elucidation of the Pfu Pop5 structure, a functional role for the protein can be hypothesized. These data provide clues about the role of Pop5 in the archaeal and eukaryotic RNase P enzymes and will lend insight into the structural and functional connections between the three domains of life of this conserved yet compositionally variable enzyme. DEPARTMENT OF MOLECULAR VIROLOGY, IMMUNOLOGY AND MEDICAL GENETICS Carlo Croce, MD, Chair Scientists in the Molecular Virology, Immunology and Medical Genetics (MVIMG) Department study molecular genetics of human disease. MVIMG was reorganized in 2004 with Carlo Croce, MD, as chair. The Department then recruited professors Richard Fishel, Joanna Groden and Kay Huebner, all PhDs, and assistant professors Samir Acharya, Louise Fong, Jonathon Godbout, Helen Pace, Yuri Pekarski, Christoph Schmutte and Amanda Toland, all PhDs. Fishel, Groden and Huebner are internationally recognized leaders in DNA repair, cancer genetics and mouse models of human cancer. The others are experts on DNA repair and genomic instability in carcinogenesis, mouse models of cancer, neuroimmunology, structural biology, tumor-suppressor gene identification and function, and functional genomics and proteomics. In 2006, the Department added Matthew During, MD, PhD, an expert in neurobiology, gene therapy and vector development. More than 65 percent of MVIMG faculty garner independent research funds; nearly 65 percent maintain multiple grant awards. MVIMG is aligned with Ohio State’s Human Cancer Genetics Program, also directed by Croce. 2007 Research Report 83 Ongoing Research Programs • Research programs within MVIMG are focused on the molecular genetics of human disease and disease-causing organisms. Expertise ranges from basic biophysical analysis to clinical translation in: - Molecular genetics of cancer (Samir Acharya, PhD; Carlo Croce, MD; Ramana Davuluri, PhD; Albert de la Chapelle, MD, PhD; Matthew During, MD, PhD; Richard Fishel, PhD; Joanna Groden, PhD; Denis Guttridge, PhD; Tim Huang, PhD; Kay Huebner, PhD; Kimberly Kelly, PhD; Gustavo Leone, PhD; Michael Ostrowski, PhD; Yuri Pekarski, PhD; Danilo Perrotti, MD, PhD; Christoph Plass, PhD; Fredika Robertson, PhD; Amanda Toland, PhD). - DNA repair and genomic stability (Samir Acharya, PhD; Richard Fishel, PhD; Joanna Groden, PhD; Kay Huebner, PhD; Helen Pace, PhD; Deborah Parris, PhD; Christoph Plass, PhD; Christoph Schmutte, PhD; Marshal Williams, PhD) - Immunology and immunogenetics (Carlo Croce, MD; Ronald Glaser, PhD; Jonathon Godbout, PhD; Kay Huebner, PhD; Tim Huang, PhD; James Lang, PhD; Danilo Perrotti, MD, PhD; Christoph Plass, PhD; Phillip Popovich, PhD; Virginia Sanders, PhD; Caroline Whitacre, PhD) - Bacterial and viral pathogenesis (Richard Fishel, PhD; Louise Fong, PhD; John Gunn, PhD; John Hughes, PhD; Deborah Parris, PhD; William Lafuse, PhD; James Shaw, PhD) DEPARTMENT OF NEUROLOGICAL SURGERY E. Antonio Chiocca, MD, PhD, Chair Completing its second full year in 2006, the Department of Neurological Surgery was again recognized in U.S.News & World Report’s listing of top-ranked clinical programs. Three neurosurgeons – Mario Ammirati, MD, MBA, Ehud Mendel, MD, FACS, and Atom Sarkar, MD, PhD – joined E. Antonio Chiocca, MD, PhD, Louis Caragine Jr., MD, PhD, John McGregor, MD, and Carole Miller, MD. Department faculty performed 1,439 neurosurgical procedures in 2006, including gamma knife radiosurgery, intensity-modulated fractionated stereotactic radiation therapy (IMRT) using a Peacock system, and angiographic procedures (compared to 836 in 2005). In addition, Mariano Viapiano, PhD, joined Yoshinaga Saeki, MD, PhD, Sean Lawler, PhD, and Balveen Kaur, PhD, as research faculty in the Dardinger Laboratory for Neuro-Oncology and Neurosciences, sharing an appointment in the Center for Molecular Neurobiology. Also in 2006, the Department participated in three clinical trials, received research funding exceeding $1.6 million and authored 38 publications, double the number from 2005. Ongoing Research Programs Research Accomplishments of 2006 • Identification of specific microRNAs as causative for solid and hematopoietic tumors • Discovery of novel DNA repair-based cellular mechanisms for defense against retroviral infection • New combined bioinformatics methods for systems biology analysis • Description of epigenetic regulation of tumor-suppressor genes in lung and head and neck tumors 84 Ohio State University Medical Center Dardinger Laboratory for Neuro-Oncology and Neurosciences – E. Antonio Chiocca, MD, PhD, codirects this laboratory along with Herbert Newton, MD, of the Department of Neurology, and oversees the research conducted within. Here are some highlights from 2006: • The laboratory of Yoshinaga Saeki, MD, PhD, develops therapeutic strategies for neurological, neoplastic and genetic disorders. One ongoing study involves the development and applications of herpes simplex virus (HSV)-based amplicon vectors, which are high-capacity plasmid-based vectors with full HSV infection machinery, for gene therapy and neuroscience research. A sec- ond research area involves the development and applications of engineered oncolytic HSV vectors for cancer therapy. A third project examines the roles of G protein-coupled receptors that upregulate cAMP signaling in the axonal outgrowth of neurons and neuronal differentiation of neural progenitor cells. • Balveen Kaur, PhD, and her lab team study changes that occur in the microenvironment of gliomas in response to treatment, hoping to learn how treatment strategies can be exploited to maximum potential. A major focus is investigating novel mechanisms to disrupt the changes in vascular biology that result from tumors to develop therapeutic strategies that may be used alone or in combination with oncolytic viruses (OV) to augment existing treatment modalities. Her team also studies the extracellular matrix of tumors to develop ways to enhance viral spread and infection to improve therapy. The Kaur lab is attempting to identify potential biomarkers in patient serum that will reflect the ongoing replication of OV in solid tumors. • Sean Lawler, PhD, and his lab team study cell signaling mechanisms in central nervous system disorders (cancer and neurodegeneration) to develop novel therapeutic approaches. Using a threedimensional cell culture system, they are investigating migration and invasion of glioma cells, mechanisms that present a major challenge in brain tumor therapy. They have identified a number of small-molecule drugs that block invasion and are testing these in animal glioma models. They also have identified genes that may be important in migration and invasion. In addition, Lawler’s team is examining the role of the microtubule-associated protein, tau, in neurodegeneration to explore a potential link between amyloid and tau, which may be critical in Alzheimer’s disease progression. • The lab team of Mariano Viapiano, PhD, studies the composition of the extracellular matrix (ECM) of gliomas. The ECM presents a major barrier to cell movement in the adult central nervous system, but invasive gliomas are impervious to inhibitory signals from the neural ECM and produce altered versions of matrix molecules that may help their invasive capacity. Viapiano’s team focuses on two families of matrix proteins: lecticans and link proteins. They are characterizing molecular events underlying the pro-invasive role of these proteins in gliomas. They also are developing reagents to disrupt the interactions of these proteins that promote glioma cell dispersion. By inactivating pro-invasive molecules in the glioma matrix, they can design strategies to limit disease spread and make these brain tumors therapeutically accessible. • Mario Ammirati, MD, MBA, and his microneurosurgical skull base laboratory develop surgical approaches to tumors at the base of the brain, educating residents in these techniques and partnering with private and non-private organizations to develop clinical technology. New surgical approaches that ask “What if?” in the operating room (e.g., “What if this tumor were approached from an angle rather than the conventional one?”) are explored in the laboratory on anatomical specimens under simulated operating room conditions and later translated to the clinical care of patients. Two ongoing projects are the quantification of exposure afforded by the endoscope and microscope in the endonasal-transphenoidal approach to the sella and suprasellar region, and investigating a navigational system and endoscope to remove the posterior wall of the internal auditory canal without disrupting the labyrinth via a retrosigmoid approach. • Ehud Mendel, MD, FACS, and his spine and spine cancer laboratory are evaluating physiologic forces that impact spinal health and methods to optimize the surgical therapy of spinal disorders. They are developing a patient-specific hybrid biomechanical model that uses a patient’s musclerecruitment pattern and spinal imaging to predict forces on spinal structures, and they are examining the relationship between spinal load and proinflammatory cytokine upregulation. In clinical studies, researchers are using kinematic measures of trunk motion and upright magnetic resonance imaging to evaluate biomechanical compromise under physiologic loading and thereby the extent of disorders of the lower back. Studies quantifying the relationship between physical and psychosocial occupational risk factors and low back disorders are also under way. 2007 Research Report 85 • The nanomedicine laboratory team of Atom Sarkar, MD, PhD, is investigating the relationship between single-molecule mechanics and disease states, particularly the micromechanical mechanisms that underlie the formation of pathologic fiber in Parkinson’s disease and that regulate the migration and spread of glioblastoma multiforme tumors. Both projects rely on atomic force microscopy (AFM), which can identify single molecules, place them under mechanical stress, and establish their mechanical stability. Such measurements are important to determine clinically relevant correlations between cell stiffness/elasticity and “aggressive” behavior. Parkinson’s is a disorder of the motor system involving genetic and environmental factors. The root of the illness is the formation and aggregation of α-synuclein fiber, a multi factorial process. Understanding the single-molecule mechanics for the α-synuclein protein will shed light on the protein’s stability and preventing fiber formation, guiding the design of molecular therapeutics. As for neoplastic cells in glioblastomas, cell motility is unpredictable. The cellular cytoskeleton is a filamentous system of “ropes, cables, and poles” that provides rigidity to the cell and determines its mechanical properties and motility. Glial fibrillary acidic protein is an important and abundant element of the intermediate filament network, one of three cytoskeletal components in the malignant astrocytes of these tumors. AFM data from experiments with single molecules can be tailored into a macroscopic model for investigating the role of force in the invasiveness of glioblastoma multiforme. Research Accomplishments of 2006 Department clinicians and researchers were active in professional organizations and received various institutional, regional and national awards for their expertise and accomplishments: - Louis Caragine, Jr., MD, PhD, received the residents’ annual Lawrence Mervis, MD, Teacher of the Year Award and was recognized in Who’s Who in Medicine & Health Care: Sixth Edition 2006-2007. - E. Antonio Chiocca, MD, PhD, served in the National Institutes of Health (NIH) Study Section for Developmental Therapeutics and on the National Cancer Institute (NCI) program project 86 Ohio State University Medical Center cluster review subcommittees C and D. He also was selected as one of the “Best Doctors in Ohio” by Columbus Monthly Magazine and elected to the American Society for Clinical Investigation, the Society of Neurological Surgeons, and as a fellow of the American Association for the Advancement of Science. - Clara Raquel Epstein, MD (second-year resident) was one of seven physicians nationally appointed to the Steering Committee of the American Medical Association’s (AMA) Minority Actions Consortium Hispanic Physician Outreach Initiative at the AMA’s annual meeting. - Jakob Godlewski, PhD (postdoctoral fellow), became the second recipient of the Jeffrey Thomas Hayden Foundation Endowed Fellowship, awarded as part of a $250,000 endowment to Ohio State over three years: The Jeffrey Thomas Hayden Foundation Endowed Fellowship Fund in Pediatric Brain Tumor at Ohio State’s James Cancer Hospital and Solove Research Institute. - Chris Karas, MD (third-year resident) was elected to the Alpha Omega Alpha Honor Medical Society (AOA). The only national honor medical society in the world, the AOA comprises members at any stage in their medical career, from students in medical schools through faculty. - Balveen Kaur, PhD, received the first grant award from the National Brain Tumor Foundation for a project titled “Anti-angiogenic treatment for enhancement of oncolytic virus efficacy.” Her article titled “Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis,” published with her colleagues at Emory in April 2005, was recognized as the most frequently read article in the journal Neuro-oncology in May 2006. - Kazuhiko Kurozumi, MD, PhD (postdoctoral researcher), won first prize in the Therapeutic Section of a poster contest staged by Ohio State’s Comprehensive Cancer Center at its eighth annual Scientific Meeting for an entry titled “Anti-angiogenic treatment enhances antitumor effects of an oncolytic virus in an experimental rat glioma model.” - John McGregor, MD, served as president of the Ohio State Neurological Society from 2004-06 and as Ohio representative, northwest quadrant, on the Council of State Neurological Societies since 1999. - Ehud Mendel, MD, FACS, received a $150,000 spine fellowship donation from George Skestos and a $75,000 spine fellowship donation from SYNTHES Spine. He also was named clinical codirector of Ohio State’s Spinal Dynamics and Ergonomics Laboratory. - Carole Ann Miller, MD, received a 2006 Excellence in Teaching Award from Ohio State’s College of Medicine. - Yoshinaga Saeki, MD, PhD, served in the NIH National Institute of Neurological Disorders and Stroke (NINDS), Neurological Sciences and Disorders B (NSD-B) Study Section. - Atom Sarkar, MD, PhD, received a co-appointment as assistant professor in the Department of Chemical & Biomolecular Engineering. - Joshua Shroll, American Brain Tumor Association (ABTA) summer fellow in the laboratory of Balveen Kaur, PhD, won the ABTA’s Lucien J. Rubinstein Award for identifying a secreted protein that can be used as a potential biomarker for oncolytic viral therapy of brain tumors. DEPARTMENT OF NEUROLOGY Michael Racke, MD, Chair The Department of Neurology focuses on education, clinical care and research of neurological diseases. The Department teaches the neurology clerkship in the College of Medicine and stimulates medical students in the neurosciences. Last year, four students from the College of Medicine matched in Neurology. In addition to the medical students, Neurology also instructs the Neurology house staff. Last year, six of eight returning Neurology residents scored higher than 90 percent, and seven of eight scored higher than 89 percent, on the in-service exam. Relating to clinical service, last year the Department of Neurology at University Hospital East was ranked 40th and the Department at University Hospital was ranked 44th in the nation by U.S.News & World Report. As for research, the Department participates in clinical trials in a number of areas, including epilepsy, stroke, movement disorders, neuromuscular diseases and multiple sclerosis. Ongoing Research Programs • The Department has a strong clinical and research program in spinal muscular atrophy, collaborating with Arthur Burghes, PhD, and Christine Beattie, PhD, in the Department of Neurosciences. • Department faculty are participating in four National Institutes of Health-funded clinical trials relating to stroke. • Investigators in the Department’s neuroimmunology research group are studying basic disease mechanisms in animal models and participating in parallel studies examining samples from patients with multiple sclerosis. The researchers are also involved with clinical trials. Research Accomplishments of 2006 • Yousef Mohammad, MD, led an effort in testing transcranial magnetic stimulation for treatment of migraine headache. The results of these studies were presented at the 2006 American Headache Society Meeting. This research attracted significant media attention and was featured in BBC news, The New York Times, Discovery and the London Times, plus other TV stations and magazines. A multicenter, placebo-controlled, randomized study is under way to confirm the efficacy of this novel therapy for aborting migraines. • The neuromuscular research group participated in a study examining the effects of etanercept on inclusion body myositis, continuing the Department’s long tradition of studying novel therapies in neuromuscular diseases. • The neuroimmunology research group spearheaded efforts to understand how the drug natalizumab increased the risk for multiple sclerosis patients to develop the devastating disease progressive multifocal leukoencephalopathy. Studies published in the Annals of Neurology and Archives of Neurology showed that natalizumab resulted in a dramatic reduction in immune surveillance of the brain. The researchers further confirmed that, after discontinuing natalizumab, the first patient 2007 Research Report 87 to experience an exacerbation of natalizumab was the patient who experienced the greatest return of immune cells in the cerebrospinal fluid, further confirming that one mechanism of natalizumab is to keep immune cells out of the central nervous system. DEPARTMENT OF NEUROSCIENCE James King, PhD, Interim Chair The Department of Neuroscience (DNS) was formed in 1999 in a reorganization of the basic science departments that created the School of Biomedical Science. The Department has grown from 10 to 20 full-time faculty, with 12 joint/courtesy appointments by faculty in other departments and colleges. Closely aligned with the Center for Molecular Neurobiology and the Neuroscience Graduate Studies Program, the DNS features outstanding research and teaching in the neurosciences. Research in the DNS is focused on understanding how the brain functions and using that knowledge to improve clinical treatment for those who suffer from neurological disease. Ongoing Research Programs • Molecular genetic studies of nervous system development • Preclinical testing of neuroprotective agents and neural transplantation • Studying a model of spinal muscular atrophy in the zebra fish • Understanding the basic mechanisms of cytoskeletal transport in axons • Studying basic cellular signaling mechanisms in the context of synaptic plasticity, aging and epilepsy • Probing the molecular basis for circadian rhythms in the brain and retina • Investigating the role of stem/progenitor cells in repair of the spinal cord and retina 88 Ohio State University Medical Center • Analyzing basic aspects of membrane channels that control neural activity Research Accomplishments of 2006 • Two new junior DNS faculty members, James Jontes, PhD, and Chen Gu, PhD, began work in the Center for Molecular Neurobiology with funding from the Burroughs Wellcome Fund and the National Multiple Sclerosis Society. • The spinal cord injury group continued its National Institutes of Health-sponsored research-training program and received excellent reviews. Dana McTigue, PhD, has assumed the role of principal investigator of this internationally recognized program. • Department faculty have been awarded $7.8 million in extramural funding for 2006 for basic research. Several funded research programs have application to diseases of the nervous system: epilepsy (Karl Obrietan, PhD); spinal muscular atrophy (Christine Beattie, PhD); aging of the brain (C. Glenn Lin, PhD); neuronal regeneration (Andy Fischer, PhD); and multiple sclerosis (Chen Gu, PhD). DEPARTMENT OF OBSTETRICS AND GYNECOLOGY Larry Copeland, MD, Chair Obstetrics and Gynecology has several divisions that conduct basic or clinical research. The Division of General Obstetrics and Gynecology focuses on clinical research, most of which is industry-supported and concentrates on hormonal therapies for pregnancy prevention and menopause management. The Division of Gynecologic Oncology is involved with clinical trials, mainly through the Gynecologic Oncology Group, as well as industrysupported drug-development trials. Most of these are aimed at preventing and treating ovarian, endometrial and cervical cancer. Basic researchers in this Division identify molecular targets associated with gynecologic malignancies. Scientists in the Division of Maternal-Fetal Medicine predominantly focus on clinical and basic research into preventing pre-term labor. In conjunction with the National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units, this Division also participates in clinical trials regarding fetal growth, diabetes in pregnancy, and preventing pre-eclampsia. Studies in the Division of Reproductive Biology and Vaccine Research focus primarily on developing and applying peptide vaccines. Research Accomplishments of 2006 • The Division of Gynecologic Oncology continued as a leading participant in the Gynecologic Oncology Group, the premier clinical trials group in this field. The Division maintains patient accrual within the top three study centers in the United States. Advances in this past year included studies to improve survival in ovarian cancer with the use of intraperitoneal chemotherapy, and preventing cervical cancer with vaccines. • The Division of Maternal-Fetal Medicine continued participation in clinical trials of the Multicenter Network of the Maternal-Fetal Medicine Units funded through the National Institute of Child Health and Human Development. Focus areas included evaluating vaginal births after cesarean section (VBAC). Additional research was directed at perinatal outcomes in women with preterm rupture of membranes. • The Division of Maternal-Fetal Medicine continued a basic research program directed by Douglas Kniss, PhD, and William Ackerman, MD, in mechanisms of preterm labor and regulating the cyclooxygenase cascade. • The Division of Reproductive Biology and Vaccine Research continued studies on the use of conformational peptides in developing vaccines to treat ovarian cancer. This research is directed by Pravin Kaumaya, PhD, in association with David Cohn, MD. • The Gynecologic Oncology Division continued to conduct basic and clinical research in the management and prognostic factors associated with endometrial adenocarcinoma. DEPARTMENT OF OPHTHALMOLOGY Thomas Mauger, MD, Chair The Department of Ophthalmology sees more than 60,000 patients a year, mostly in the William H. Havener Eye Institute at the Ohio State University Hospital Clinic in Cramblett Hall. The Department also has sites in Dublin and at Ohio State University Hospital East. Faculty are involved in multicenter clinical trials funded by the National Eye Institute, as well as by industry. Basic science studies include investigation of the biomechanical properties of the cornea and sclera, and fundamental studies of cerebral spinal fluid outflow facility and the impact on idiopathic intracranial hypertension. In addition, the Department’s Molecular Genetics Program studies uveal melanoma. Scientists are investigating the MET gene, which they believe is a crucial factor for the high selectivity of eye melanomas and other cancers that spread to the liver. They hope to identify molecular markers to monitor for metastatic disease. If they can identify at-risk patients, early intervention should lead to a better prognosis. Ongoing Research Programs • Cornea: - Optical profilometry and atomic force microscopy analysis of corneal surface topography: the effect of lamellar keratoplasty, excimer laser ablation and smoothing procedures - Standard surface anterior surface topography measurements quantify the discrepancy between Scheimpflug and Placido-based topographers - Evaluation of the utility of intra-operative topography to optimize corneal shape during penetrating keratoplasty: a clinical trial examining whether topography-guided suture placement will improve surgical outcomes in corneal transplant patients - Corneal wound healing and artificial anterior chamber cultures: femtosecond laser and longterm corneal cultures – examination of the effect of state-of-the-art Femtosecond anterior and posterior Lamellar surgery techniques 2007 Research Report 89 - Biomechanical and structural response of the cornea following Lamellar keratoplasty: an optical coherence tomography and Scheimpflug image analysis - High-resolution X-ray scattering analysis of the microfibril organization in cornea and optic nerve and its relation to biomechanical response - The study of central and peripheral corneal biomechanical response to swelling using topography, wave-front, viscoelastic properties and reflectivity coefficients in normal and LASIK populations • Glaucoma - The Ocular Hypertension Treatment Study (OHTS) evaluates the efficacy of placing ocular hypertensive patients on eye drops to delay or perhaps prevent them from developing glaucoma. - The OHTS Ancillary Genetic Study collects blood from OHTS patients for a national database that may help identify glaucoma genetic markers. - The Memantine Study evaluates the effective- ness and safety of oral Memantine in patients with glaucoma who are at risk for progression of optic nerve damage. - Researchers are comparing the current standard post-glaucoma filtration surgery antiproliferative treatment, Mitomycin-c, to novel antiproliferative drugs and determining their effect on fibroblast and epithelial cell proliferation in an in vitro culture model. - To understand the mechanism of laser trabecu- loplasty, which is wavelength independent and dependent on the rapid rate of temperature increase in the target tissue, with a total increase of less than 1°C, researchers are studying the irradiation-related functional relationships among the aqueous outflow pathway components, including the TM cells and the SCE cells using an in vitro model of the outflow pathway. • Neuro-Ophthalmology - Scientists are studying a cerebral spinal fluid outflow mechanism through human arachnoid granulations using both in vitro and ex vivo models, including postperfusion ultrastructural studies using fluorescent microparticle perfusion, TEM and immunohistochemistry. 90 Ohio State University Medical Center - Scientists are working to understand the role of vitamin A and its cerebral spinal fluid (CSF) metabolites to support a novel mechanism of idiopathic intracranial hypertension (IIH), a well-controlled CSF and blood study of newly presenting IIH subjects. • Pediatrics - In its sixth year of follow-up, the Early Treatment for Retinopathy of Prematurity trial continues to demonstrate the benefits of early treatment for high-risk prethreshold retinopathy of prematurity. - Corneal endothelium and ocular component change after strabismus surgery in children – This project investigates the influence of strabismus surgery on the corneal endothelial cells and ocular components in children. - Corneal endothelium change in type I diabetic children – This project investigates the influence of type I diabetes on corneal endothelial cells in children. It uses fMRI to explore normal and abnormal oculomotor function, as well as the neural basis of amblyopia and effects of pharmacological interventions. It also uses NIR (near infrared) to develop a test of visual function. • Retina - Age-Related Macular Degeneration (AMD) – This program evaluated treatment modalities for patients suffering from AMD, the leading cause of vision loss in the United States for people over age 60. Scientists are involved in many new and exciting National Institutes of Health- and industry-sponsored studies that investigate treatments including anti-VEGF (vascular endothelial growth factor) drugs. Another study will look at prevention of this disease using a combination of dietary supplements and vitamin therapies. It will evaluate the potential for lutein and omega-3 fatty acid nutritional supplements to impede the progression of AMD. - Molecular Genetics – This program examines uveal melanoma. Researchers are focusing on the MET gene (also known as hepatocyte growth factor receptor), which they believe to be a crucial factor for the high selectivity of eye melanomas and other cancers that spread to the liver. They hope to identify molecular markers to monitor for metastatic disease. If they can identify at-risk patients, early intervention should lead to a better prognosis. We are collaborating with Cheryl London, DVM, PhD, a veterinary oncologist who is involved in target therapy using the MET gene. - Diabetic Retinopathy – Three clinical trials are ongoing in this program. One compares laser therapy and intravitreal drug therapy to determine which is more effective in treating diabetic macular edema (DME), the leading cause of vision loss in the ever-increasing diabetic population. A second trial evaluates the effect of panretinal laser therapy for diabetic retinopathy on the development of DME. A third trial strives to determine the effectiveness of optical coherence tomography to detect DME at a stage at which clinical observation alone may not. This could allow new strategies for prevention of vision loss in this common malady. - Choroidal Melanoma MRI – In collaboration with the Department of Radiology, Ophthalmology is imaging its melanoma patients with a 1.5 tesla MRI with dynamic contrast and surface coil imaging. This is a novel approach to evaluate the radiological perfusion characteristic of choroidal circulation. The goal is to discover predictive parameters of choroidal circulatory blood flow that may correlate with tumor size, rate of growth, metastases and survival. Research Accomplishments of 2006 • Glaucoma – The Ocular Hypertension Treatment Study (OHTS) is entering its 14th year; a measurement of visual contrast sensitivity and additional patient surveys have been included into the study. Researchers also continued to enroll patients in the OHTS ancillary genetic study and planned to complete it by June 2007. The Memantine Study concluded patient follow-up in summer 2006 and is in the final phase of data collection. The Glaucoma Division also initiated three studies: “Autoimmune Mechanisms in Giant Cell Arteritis”; “Autoimmune Mechanisms in Glaucoma”; and “Autoimmune Mechanisms in Graves’ Disease.” Each focuses on the discovery of an antibody that could cause these diseases. If identified, it could lead to earlier diagnosis and better treatment. • Pediatrics – Investigators clarified the differences between look (voluntary) Optokinetic Nystagmus (OKN) and stare (involuntary) OKN (ARVO 2006). Based on fMRI, they mapped brain areas responsible for pursuit and saccadic eye movements. They also pursued IRB approval of the near infrared multidisciplinary and multi-institutional research project. • Retina – The Department secured a role in the National Institutes of Health- and National Eye Institute-sponsored Age-Related Eye Disease Study 2 (AREDS 2 Study). Ohio State site was selected as one of nearly 100 centers to participate in the nationwide study to see if a modified combination of vitamins, minerals and fish oil can further slow the progression of vision loss from age-related macular degeneration, the leading cause of vision loss in the United States for people over age 60. The Department also was selected as one of 21 sites nationwide to participate in the Comparison of Age-related Macular Degeneration Treatments Trial (the CATT Study), which will evaluate the relative efficacy and safety of treatment of subfoveal, neovascular AMD with lucentis and avastin. • Neuro-Ophthalmology – Researchers demonstrated that both the in vitro and ex vivo human models of the cerebral spinal fluid (CSF) outflow pathway through the arachnoid granulations mimic the physiological unidirectional flow, in addition to the bulk flow component, via vacuole transport. This work was initially reported in the journal Investigational Ophthalmology & Visual Science and will be detailed in future publications. Scientists also demonstrated that the en face probability-ofoccurrence maps of human arachnoid granulations (AG) are localized in a characteristic distribution with regions of high and low probability. These measurements provide age-related surface area quantification data in terms of absolute values as well as proportional area with respect to total brain area. Total brain surface area declines with age and must be considered when analyzing proportional AG area. Race has a statistically significant effect on AG surface area, with Caucasians having a smaller proportion of positive area. Females have a smaller proportion of surface area in most age groups. This data will be used as input for an in vitro CSF perfusion model. 2007 Research Report 91 • Jennifer Lewis, PhD, a U.S.-U.K. Fulbright Scholar in 2005-06, completed a research fellowship in the Structural Biophysics Laboratory at the School of Optometry and Vision Sciences, Cardiff University, Cardiff Wales and Guys, and the Department of Ophthalmology at St. Thomas’ Hospital in London. Her project was titled “Optical and X-ray studies of human and porcine cornea and the biomechanical response to Lamellar keratoplasty using femtosecond and mechanical microkeratome.” • Jennifer Wilding Bogucki, a third-year medical student, won first place at the fifth annual Ohio State University College of Medicine Graduate and PostGraduate Research Day for a poster entitled “Topographic data for normal donated human corneas.” • Andrew Schrader, an Ohio State University firstyear veterinary student, won the Deans’ Undergraduate Research Award to support his research. He also finished first in the Health Professions/Clinical Category at the Richard J. & Martha D. Denman Undergraduate Research Forum for a poster titled “Surface topography of the cornea following laser ablation with and without smoothing.” DEPARTMENT OF ORTHOPAEDICS Christopher Kaeding, MD, Interim Chair The Department of Orthopaedics has 15 full-time faculty, 65 auxiliary staff and nine adjunct faculty. Divisions in the Department include: Foot and Ankle; General Orthopaedics; Musculoskeletal Oncology; Spine; Sports Medicine; Total Joint Replacement; Trauma; Upper Extremity and Hand; and Research. Each Division is responsible for research, education and patient care related to its discipline. Each faculty member contributes by: providing high-quality patient care; instructing medical students, residents in Orthopaedics and/or Podiatry, and fellows in ortho- 92 Ohio State University Medical Center pedic subspecialties; researching orthopedic problems to identify causes, treatments and possible prevention; and providing public service in education, treatment and recovery options. Ongoing Research Programs • Basic science research in the Department of Orthopaedics focuses on engineering aspects of musculoskeletal conditions. The Orthopaedic BioMaterials Laboratory investigates the development and application of engineering materials to hard-tissue problems and the biomechanics of implantable fixation devices. The Ergonomics Laboratory explores the causes and prevention of spinal and industrial injuries. Both of these research thrusts are conducted in collaboration with colleagues in the colleges of Dentistry, Engineering, Medicine and Veterinary Medicine. • Clinical research has expanded to include studies in sports medicine, musculoskeletal trauma and spine. With the continuing expansion of clinical faculty, both in Orthopaedics and associated departments, new avenues of research are being developed. • Biological basic research is being conducted by Joel Mayerson, MD, of the Division of Oncology, who collaborated last year with Carl Morrison, MD, DVM, in the Department of Pathology, on genetic identification of tumor markers as a tool for predicting severity and prognosis. • Steve Lavender, PhD, seeks to quantify and model the biomechanics of the lumbar spine during push-pull industrial tasks to better understand the etiology of low back pain and thus give insight into preventing it. • Alan Litsky, MD, ScD, leads a biomaterials research program that is developing and testing new materials for orthopedic, dental and veterinary applications. They are developing and quantifying a technique to determine the source of polyethylene wear debris in total joint arthroplasties. • Joel Mayerson, MD, is a pioneer in the application of expandable total femoral implants designed to keep up with the growth of pediatric bone tumor patients. • Christopher Kaeding, MD, is a co-investigator in the first large, multi-institutional, prospective study of the functional outcomes following anterior cruciate ligament reconstruction. • Laura Pheiffer, MD, directs several projects quantifying the biomechanics of fracture fixation devices. • Josue Gabriel, MD, leads a study exploring the use of dynamic analysis modeling to predict injury to spinal structures. • Ajit Chaudhari, PhD, joined the faculty from Stanford University and is establishing a laboratory for using motion analysis techniques to analyze the causes and measure the effectiveness of treatment for sports medicine injuries. DEPARTMENT OF OTOLARYNGOLOGY – HEAD AND NECK SURGERY D. Bradley Welling, MD, PhD, Chair The Department of Otolaryngology – Head and Neck Surgery is nationally recognized in treating human communication disorders and head and neck malignancy. In 2006 it continued to expand, welcoming four new faculty members and opening two satellite offices to better serve the community. In addition, Otolaryngology residents continue the Department’s longstanding history of excellence, ranking in the 98th percentile as a residency group on the in-service training exam. For the 14th consecutive year, the department has been ranked by U.S.News & World Report among the top 20 Ear, Nose and Throat programs in the nation. Ongoing Research Programs • Molecular mechanisms of vestibular schwannoma formation • Virtual modeling of the temporal bone dissection • Intensification regimens for advanced-stage squamous cell carcinoma and chemoprevention for oral carcinoma • Mechanisms of pneumococcal otitis media Research Accomplishments of 2006 • Gregory Wiet, MD, continued work on a $1.87 million grant-funded project to develop and test an otologic surgical simulator. His work, with collaborator Don Stredney, PhD, was highlighted in a journal article titled “Multi-center testing of the virtual temporal bone dissector simulator.” The project is funded by the National Institutes of Health/National Institute on Deafness and Other Communication Disorders. The potential for education of all otolaryngology residents and for preoperative simulation with this technology is encouraging. • D. Bradley Welling, MD, PhD, continued work on his $1.62 million grant-funded project on phenotypic determinants of vestibular schwannomas. The project is funded by the National Institutes of Health and is directed at deciphering the underlying molecular causes of vestibular schwannomas. The work moved forward in 2006 in collaboration with Long-Sheng Chang, PhD, and a host of research colleagues. • James Lang, PhD, and David Schuller, MD, have secured funding through the Biomedical Research Commercialization Program (BRCP) to investigate head and neck squamous cell carcinomas. Jeffrey Chalmers, PhD, a professor of Chemical Engineering and member of Ohio State’s Comprehensive Cancer Center, is the lead principal investigator for the $3.5 million BRCP grant. DEPARTMENT OF PATHOLOGY Sanford Barsky, MD, Chair Faculty in the Department of Pathology are committed to furthering the understanding, knowledge, diagnosis and treatment of disease. They do this by educating students of all levels, conducting clinical, translational and basic research, and providing service to patients, the University and the community. Central tenets of this mission are mutual respect and citizenship by its faculty and staff, and the concept that excellence denotes exemplary achievements and continuous improvement of quality. 2007 Research Report 93 Ongoing Research Programs • The Department’s Experimental Pathology branch is undergoing a major expansion in terms of faculty recruitment and space. Experimental pathology is defined as disease-oriented hypothesis testing or generating research. The progress of this discipline parallels Ohio State University Medical Center’s Signature Programs in Cancer, Critical Care, Heart, Imaging, Neurosciences and Transplantation. In addition, within the Experimental Pathology branch are unique programs in image analysis and tissue banking. Research Accomplishments of 2006 • To complement Ohio State’s interest in imaging and informatics, the Department has a major interest in digital pathology. Research initiatives included developing an inexpensive scanner that will revolutionize digital pathology, target motion analysis (TMA) algorithms that will automate TMA interpretation, and the creation of TMAker that will automate TMA construction. • Another research achievement has been maintaining the Human Tissue Resource Network (HTRN), which collects, banks and distributes human tissue and fluid specimens for basic and translational research at Ohio State and associated clinical research programs throughout the United States. The HTRN consists of a prospective Tissue Procurement Service, Tissue Archive Service of diagnostic specimens, the Cancer and Leukemia Group B Pathology Coordinating Office, a Pathology Core Facility, the AIDS and Cancer Specimen Resource (ACSR), and an Adenoma Polyp Tissue Bank. HTRN services are funded by federal, corporate and Department research programs. Space support is provided by Ohio State’s Comprehensive Cancer Center. More information about each service can be obtained at http://www.pathology.osu.edu/htrn/default.htm. • Department investigators continued studying tumor invasion and metastasis using a novel human xenograft model of inflammatory breast cancer and human cancer cell myoepithelial interactions. 94 Ohio State University Medical Center • Ultraviolet (UV) carcinogenesis of squamous cell carcinoma (SCS) of the skin has been another area of research emphasis. Patients receiving solid organ transplants must undergo immunosuppressive therapy necessary to prevent their body from rejecting the new organ. Consequently, these patients are at a substantially increased risk to develop SCS. In fact, solid organ transplant recipients develop significantly more cases of SCC than the general population, and their tumors are more numerous and more aggressive. Pathology studies using the Skh-1 hairless mouse model of UVBinduced carcinogenesis demonstrated the importance of the CD4+ T cell in modulating the inflammatory response in the skin and in the development of UV-induced tumors. This cell type was chosen because immunosuppressive drugs modify its function. • The Department’s studies of bacterial pathogenesis strive to understand the biochemical, molecular and cellular basis of diseases caused by multidrug resistant emerging gram-positive pathogens commonly encountered in hospital-acquired infection. The goal is to develop an antimicrobial therapy to counteract bacterial multi-drug resistance problems, including those caused by their biofilm formation on abiotic and host tissue surfaces. DEPARTMENT OF PEDIATRICS Michael Brady, MD, Chair Based at Children’s Hospital in Columbus, the Department of Pediatrics has 221 full-time faculty and 59 part-time faculty representing 22 pediatric subspecialty disciplines. In affiliation with Children’s Hospital, the Department has an Accreditation Council for Graduate Medical Education (ACGME)-accredited residency-training program that accommodates 76 pediatric and 30 internal medicine/pediatric residents. In addition, 186 residents from other training programs (e.g., emergency medicine, family practice) in the Columbus area completed pediatric rotations at Columbus Children’s Hospital in 2006. Also last year, all of the 206 third-year medical students and 153 fourth-year medical students at Ohio State received their pediatric training at Columbus Children’s Hospital. Research activities in the Department are conducted under the auspices of Columbus Children’s Research Institute. Ongoing Research Programs • The laboratory group led by Lauren Bakaletz, PhD, is making progress toward understanding the molecular microbiology of non-typeable Hemophilus influenza. Her group has established a robust infrastructure to conduct preclinical studies for developing an otitis media vaccine. • In 2006, Jerry Mendell, MD, and colleagues in the Center for Gene Therapy initiated the first-ever phase I trial of AAV-based gene therapy for Duchenne’s muscular dystrophy. Other innovative molecular approaches for prevention and treatment of this fatal disorder are under way in the Center. • Keith Yeates, PhD, and colleagues continue their multi-center clinical investigations of recovery and outcome of pediatric traumatic brain injury. • The laboratory of Christopher Walker, PhD, leads a national effort to understand the immunobiology of hepatitis C using a variety of innovative models. • The Center for Injury Research and Policy under Gary Smith, MD, is dedicated to understanding the epidemiology, prevention, treatment, rehabilitation and biomechanics of pediatric acute injury. Much of the Center’s work impacts public policy. Research Accomplishments of 2006 • Advancing gene and cell-based research from the laboratory to potentially life-saving clinical use is a key priority for the Center for Gene Therapy. Using innovative molecular approaches, investigators including Center Director Jerry Mendell, MD, are analyzing and translating genetic manipulations to prevent and treat devastating childhood diseases such as the various forms of muscular dystrophy. In 2006, the Mendell group initiated the first-ever pediatric gene therapy trial for Duchenne’s muscular dystrophy using an adenoassociated virus-based vector. • A paper published in the journal Molecular Microbiology by Lauren Bakaletz, PhD, and her lab group identified for the first time a molecular mechanism by which non-typeable Haemophilus influenzae (NTHI) resists being destroyed by antimicrobial peptides. The paper is titled “The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition.” The researchers determined how NTHI requires a Sap (sensitive to antimicrobial peptides) system to keep the bacterium from being killed by antimicrobial peptides. Their findings also describe how a Sap transporter provides the necessary function in NTHI to survive against the body’s innate immune response. • In a publication titled “MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock,” Yusen Liu, PhD, and his team from the Center for Perinatal Research explored the role of MAP kinase phosphatase (MKP)-1 in animal models of inflammation. Liu compared gene-targeted Mkp-1 mice with wild-type mice in their response to LPS. The study, published in the Jan. 23, 2006, edition of The Journal of Experimental Medicine, showed that Mkp-1 knockout mice produced dramatically more proinflammatory cytokines and consequently developed severe low blood pressure, multiple organ failure and an increased chance of death. In addition to increasing how Mkp-1 serves as a crucial downregulator of endotoxic shock and inflammation, these findings may also provide a target for drug therapy. • Work in the laboratory of Rachel Altura, MD, titled “Dissecting the role of endothelial survivin(delta)Ex3 in angiogenesis,” identified the splice variant, Survivin xEx3, as a prominent regulator of angiogenesis (blood vessel formation), which is key to physiologic and pathophysiologic processes such as wound healing, vascular disease and neoplasia. This was published in the journal Blood. 2007 Research Report 95 • Joan Durbin, MD, PhD, and colleagues published “Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector” in the Journal of Virology. The team examined the weak stimulation of innate immune responses to RSV and explored the possibility of boosting the immune response by delivering the virus through an alternative viral vector system, notably the Newcastle disease virus (NDV). NDV is an attractive vector because of the lack of pre-existing immunity in the human population and, importantly, it has been proven safe in humans. Durbin’s findings indicate that the RSV F protein is more immunogenic when presented by NDV-F than by RSV alone. Ongoing Research Programs DEPARTMENT OF PHARMACOLOGY • Laura Bohn, PhD, has identified regulatory pathways of opioid and serotonergic receptors that are leading to novel opportunities for drug discovery – including analgesics with low addiction potential. • Howard Gu, PhD, has developed a mouse model expressing a functional dopamine transporter insensitive to cocaine – opening new approaches to the discovery of effective therapies for cocaine addiction. • Wolfgang Sadée, Dr.rer.nat., and his group, along with the Program of Pharmacogenomics, have discovered a series of functional polymorphisms in key candidate genes affecting central nervous system disorders and treatment outcomes, leading potentially to clinical biomarkers for guiding therapy of mental diseases. Wolfgang Sadée, Dr.rer.nat., Chair Research Accomplishments of 2006 The Department of Pharmacology focuses on the molecular pharmacology of central nervous system (CNS) agents, cancer chemotherapy and pharmacogenomics. One group of investigators – Laura Bohn, PhD, Howard Gu, PhD, Norton Neff, PhD, Wolfgang Sadée, Dr.rer.nat., and David Saffen, PhD – studies biochemical and genetic pathways contributing to drug addiction. Understanding how signaling pathways are triggered by drugs of abuse can lead to new approaches for treating drug addiction – research strongly supported by the National Institute on Drug Abuse. The Department also houses the Program in Pharmacogenomics, with a PGx Core laboratory featuring genotyping methodologies and tools for discovering functional genetic variants. This supports a series of basic and translational studies on CNS disorders, cardiovascular diseases, cancer, autoimmune disorders and attendant therapies. A Division on Clinical Trials has been performing industry-sponsored phase I and II drug trials. It is also a training ground for scientists and clinicians specializing in drug development – expertise of high value to the pharmaceutical industry. • Paul Blower, PhD, has developed a comprehensive approach for linking chemical structures with genomic data, including mRNA, microRNA and protein tissue profiles. Use of the NCI-60 panel of cell lines permits discovery of drug targets and anticancer drugs, as well as mechanisms of chemoresistance. • Using transgenic mouse models, Laura Bohn, PhD, addresses the regulation of opioid receptors with respect to pain control and side effects such as respiratory depression and gastrointestinal mobility. Her studies with the barrestin2 knockout mouse line have shed light on critical opioid and serotonin pathways. She has found that receptor regulation differs substantially in different tissues (central nervous system, gastrointestinal tract), and that this differential regulation translates to distinct action of opioid agonists. This has led to the discovery of opioid agents with properties suitable for developing analgesics with low adverse effects. • Roger Briesewitz, PhD, focuses on growth factor signaling pathways, their role in leukemia (specifically acute myeloid leukemia, or AML), and drug discovery targeting components of the growth factor signaling cascade. The main thrust of his 96 Ohio State University Medical Center work is to identify downstream factors mediating aberrant growth factor signaling in cancer and thus provide novel targets for anticancer drug discovery, including cyclin-dependent kinases (4 and 6). An inhibitor of CDK4,6 has shown exceptional promise in AML animal models and could proceed to clinical trials. • Howard Gu, PhD, has generated a mutant dopamine transporter (DAT) knockin mouse model in which DAT functions normally in dopamine transport but is highly insensitive to cocaine. Unexpectedly, the mutant DAT knockin mice are aversive to the actions of cocaine, indicating that the effects on norepinephrine transporter and serotonin transporter are opposite to those in DAT knockouts. This suggests that selective inhibition of cocaine binding to DAT (without affecting dopamine transport) is a viable strategy for treating cocaine addiction, leading toward discovery of novel therapies in drug addiction. • Working on Bardet-Biedl Syndrome (BBS), Kirk Mykytyn, PhD, has contributed to the understanding of monogenic disorders that have a broad spectrum of phenotypic manifestations. Therefore, Bardet-Biedl Syndrome is an excellent model to develop approaches for exploring the genetics of more complex disorders. His research indicates that the BBS proteins play a role in cilia development and function, potentially a main factor in the pathophysiology of several diseases. The underlying mechanism appears to involve protein trafficking into cilia. • Wolfgang Sadée, Dr.rer.nat., has developed a program in pharmacogenomics with outreach to diverse clinical areas, including central nervous system disorders, cardiovascular disease and cancer. He has developed an approach to finding functional polymorphisms that has been applied to candidate genes in these disease areas and in response to drug therapy. This approach has considerable promise for the development of clinically useful biomarkers. He has also initiated the development of a chemogenomics group, led by Paul Blower, PhD, for drug discovery and identification of drug targets and markers of cancer chemoresistance. • David Saffen, PhD, studies the genetics of central nervous system (CNS) disorders and calcium sig- naling of the M1 muscarinic receptor. His work has clarified signaling pathways of the transient receptor potential 6 (TRP6) channel, an important mediator of calcium signaling in the CNS. He is exploring TRPC6 as a potential drug target in the treatment of depression. Moreover, he has discovered a frequent variant of tryptophan hydroxylase 2 (TPH2), encoding a key enzyme in the synthesis of serotonin in the CNS. This functional variant could serve as a predictive biomarker. • Kirsten Raehal, a graduate student in the lab of Laura Bohn, PhD, was chosen to attend a highly competitive National Institute of General Medical Sciences-sponsored short course in Integrative Organ Systems Pharmacology at Michigan State and has been awarded an National Institutes of Health/National Institute on Drug Abuse F31grant. • Nick Berbari and Erin Newburn, graduate students serving respectively in the labs of Kirk Mykytyn, PhD, and Norton Neff, PhD, each received a competitive Ohio State University Presidential Scholarship award. • Wolfgang Sadée, Dr.rer.nat., was the invited keynote speaker at several scientific symposia in the United States and abroad. DEPARTMENT OF PHYSICAL MEDICINE AND REHABILITATION William Pease, MD, Chair Dodd Hall, the Rehabilitation Center for Ohio State University Medical Center, also houses the Department of Physical Medicine & Rehabilitation, which includes a Division of Rehabilitation Psychology and a Division of Pediatric Rehabilitation Medicine that is located at Columbus Children’s Hospital. Dodd Hall admitted 1,045 patients in 2006. The inpatient service is accredited by the Commission on the Accreditation of Rehabilitation Facilities (CARF), with specialty accreditation in rehabilitation of stroke, spinal cord injury and brain injury. The Traumatic Brain Injury 2007 Research Report 97 Network, an outpatient program specializing in the treatment of brain-injured patients with substance abuse problems, is also accredited by CARF. The Physical Medicine and Rehabilitation residency program, directed by Daniel Clinchot, MD, is accredited by the Accreditation Council for Graduate Medical Education for six positions each year. More than 230 graduates of the program are serving throughout the United States, Japan and Puerto Rico. Ongoing Research Programs • The Traumatic Brain Injury (TBI) Model System Program is part of a national network and database for brain injury treatment funded by the U.S. Department of Education, and W. Jerry Mysiw, MD, chairs the Health Committee for the national program. Research in brain injury also includes behavior and community reintegration and the impact of substance abuse on outcome. A special study of veterans of the Iraq and Afghanistan wars is under way in collaboration with Walter Reed Medical Center. • The Osteoporosis Prevention and Treatment Center is studying new treatments for bone weakness, as well as the effect of osteoporosis as a complication of disabling illnesses. • Areas of spinal cord injury (SCI) research emphasis include both the prevention and treatment of complications of SCI, including recovery of walking. • Predictors of disability caused by knee osteoarthritis are being studied through the National Institutes of Health-funded Osteoarthritis Initiative (OAI). • A database is being created from the Ohio-Indiana Veterans’ Amputation Project in conjunction with Ohio State’s School of Allied Medical Professions and Indiana University. Research Accomplishments of 2006 • In the Traumatic Brain Injury Model Systems, Ohio State is one of the most productive recruitment and follow-up sites among the 16 centers funded nationwide. John Corrigan, PhD, chairs the Model Systems Program Executive Committee. 98 Ohio State University Medical Center • The National Institutes of Health-funded multicenter clinical trial examining the effect of bodyweight supported treadmill training on recovery of ambulation following spinal cord injury completed subject enrollment under the direction of Lisa Fugate, MD, at the Ohio State site. This novel therapy is an important transfer of recent neuroscience research in activity-dependent plasticity to recovery of function in humans following trauma. More clinical trials are planned. • The Ohio State University site for the Osteoarthritis Initiative (OAI) completed its recruitment objectives in 2006 and represents the largest contributor of subjects for the five-year longitudinal study of knee osteoarthritis. • A recent study by Sharon McDowell, MD, has documented outcomes of rehabilitation of patients after brain tumor treatment. DEPARTMENT OF PHYSIOLOGY AND CELL BIOLOGY Muthu Periasamy, PhD, Chair Researchers in the Department of Physiology and Cell Biology work to understand cellular and molecular mechanisms that contribute to cancer, cardiovascular, gastrointestinal and neurological disease. Taking a systematic approach, researchers develop animal models to study human disease progression at the molecular level and its effect on organ physiology. The goal is to understand disease processes and identify molecular targets for clinical intervention. Faculty teach at Ohio State’s College of Medicine and colleges of Dentistry, Pharmacy, Optometry and Allied Medical Professions. They also participate in the Integrated Biomedical Graduate Program and mentor graduate students from five graduate programs at Ohio State. Ongoing Research Programs • Molecular mechanisms in heart failure and ventricular arrhythmia • The role of enteric nervous system in gut function • Breast and thyroid cancer therapy • Neuronal control of temperature and pain sensation • Molecular and cellular basis of spinal cord regeneration Research Accomplishments of 2006 • The lab team of George Billman, PhD, demonstrated that endurance exercise training (aerobic exercise, treadmill running) completely suppressed the induction of ventricular fibrillation in animals previously shown to be susceptible to this lethal cardiac arrhythmia. In contrast, the susceptibility to ventricular fibrillation was not altered in sedentary (time control) animals. They further demonstrated that exercise training improved autonomic regulation of the heart by increasing cardiac parasympathetic regulation and decreasing cardiac sympathetic activity. In particular, exercise training restored a more normal betaadrenergic regulation (by reducing beta2-adrenergic receptor sensitivity) in animals susceptible to ventricular fibrillation. It is likely that these changes in neural control of the heart contribute to the protection induced by exercise training. These studies demonstrate that exercise training could be used as a non pharmacological therapy for the management of life-threatening arrhythmias in patients recovering from a heart attack. Thus, exercise training could reduce the incidence of sudden cardiac death in these high-risk patients. • In a recent study, the lab team of Sandor Gyorke, PhD, identified a molecular defect in cardiac cells that may be a fundamental cause of heart failure, a progressive weakening of the heart that leaves the organ unable to pump blood through the body. Study results show that specialized proteins called ryanodine receptors (RyRs) malfunction in the failing heart. The RyRs are calcium channels that provide calcium required for shortening of the heart muscle. In heart failure, the RyRs become leaky, leading to calcium imbalances that prevent the heart from contracting effectively and relaxing adequately. Discovery of this mechanism suggests a potential target for treating heart failure. • Paul Jannsen, PhD, and his lab team developed a technique for assessing the relationship between intracellular calcium concentration and force generation in the heart. At the start of a heartbeat, calcium rapidly rises inside the myocytes, or muscle cells, of the heart and activates the molecular motors that are responsible for heart contraction. Using their new technique, in which they combine iontophoretic loading of a calcium indicator with potassium-induced contracture in isolated intact contracting myocardium, the researchers can investigate the sensitivity of the myofilaments for calcium. They recently observed that, when the heart beats faster, this relationship is altered so that the calcium sensitivity of the myofilaments decreases, allowing for faster relaxation of the heart and at faster heart rates. These results lend insight on how cardiac relaxation is governed and how this relationship may be altered in cardiac disease states. • Using site-directed mutagenesis to vary the affinity of troponin for calcium, the lab team of Jack Rall, PhD, determined that calcium controls the rate of cardiac muscle contraction solely by varying the number of force-generating cross-bridges. • Calsequestrin is a Ca2+ storage protein in the cardiac muscle and is involved in Ca2+ release. A naturally occurring mutation in cardiac calsequestrin (CASQ2) at amino acid 307 was discovered in a highly inbred family and hypothesized to cause catecholaminergic polymorphic ventricular tachycardia (CPVT). The lab team of Muthu Periasamy, PhD, developed a mouse model to establish a causal link between CASQ2D307H and the CPVT phenotype using an in vivo model. Their findings demonstrate that expression of mutant CASQ2D307H in the mouse heart results in abnormal myocyte Ca2+ handling and predisposes to complex ventricular arrhythmias similar to the CPVT phenotype seen in human patients. • The localization of neurons that express corticotropin-releasing factor and the receptor for corticotropin factor was determined for neurons in the “brain-in-the-gut.” Corticotropin-releasing factor (CRF) is a hormone of importance in the enteric nervous system, and it acts on the gastrointestinal tract to evoke the pathophysiological 2007 Research Report 99 changes in gut function in response to stress. Research by the lab team of Jackie Wood, PhD, has identified neurons that express CRF and the receptor (CRF1) using molecular and electrophysiological techniques. Their results show that CRF1 receptor mediates the excitatory actions of CRF and is involved in stress response. • Sissy Jhiang, PhD, and her lab team have developed a technique using the sodium iodide symporter gene (NIS) as an imaging reporter gene to monitor transgene expression in gene therapy clinical trials. Their goals are to improve targeted radioiodine therapy for patients with advanced thyroid cancer and to identify factors that selectively increase NIS expression and function to facilitate targeted radionuclide imaging and therapy for patients with breast cancer. • The lab team of Lyn Jakeman, PhD, has demonstrated that the inflammatory response to spinal cord injury differs in various inbred mouse strains and that different patterns of macrophage invasion are associated with either robust or failed regeneration of injured axons. This will lead to the use of genetic tools to identify genes associated with successful regeneration and repair after injury. • A major achievement in the laboratory of Arthur Strauch, PhD, was identifying a TGFbeta1 control element that regulates human myofibroblast differentiation during chronic fibrotic disease. The smooth muscle-like myofibroblast is the major collagen-secreting cell type involved in granulation tissue formation; it mediates wound-healing activity in the heart and lungs. TGFbeta1 controls expression of genes encoding type I collagen subunits as well as the smooth muscle contractile protein, alpha-actin. Chronic release of active TGFbeta1 after heart and lung transplant is responsible for dysfunctional remodeling of accepted organ grafts. A DNA-binding complex consisting of gene-activating proteins Sp1, SRF and Smads, plus repressors YB-1 and Pur alpha, is assembled at the TGFbeta control element in the smooth muscle alpha-actin and type I collagen promoters in myofibroblasts. In isolated human myofibroblasts, the DNA-binding complex undergoes dynamic reorganization in response to TGFbeta1. Significant changes in the association of activator and repressor proteins with the 100 Ohio State University Medical Center TGFbeta1 control element were observed after cardiac transplant in the mouse. • The lab teams of Dale Vandré, PhD, and John Robinson, PhD, developed methodology for the rapid isolation of highly enriched plasma membrane samples from human cells and tissue suitable for proteomics analysis of integral membrane proteins. Using these techniques, they identified dysferlin, a protein associated with limb girdle muscular dystrophy, as a major component of the apical plasma membrane of the placental syncytiotrophoblast. This plasma membrane forms the interface for nutrient transport between maternal blood and fetal blood. The researchers believe that dysferlin plays an important role in the formation and maintenance of this critical plasma membrane. They have submitted a manuscript to the Journal of Biological Chemistry describing this work. They were also preparing manuscripts describing the techniques in greater detail as well as the plasma membrane proteome of cultured human leukemic cells and the apical membrane of the syncytiotrophoblast. DEPARTMENT OF PSYCHIATRY AND OHIO STATE UNIVERSITY HARDING HOSPITAL Radu Saveanu, MD, Chair The first academic Department of Psychiatry at The Ohio State University was established in 1951. Today, the Department is one of the best in the nation, attracting patients, faculty, students and researchers from around the world. In 1996, the University Hospitals Board of Trustees formed a joint venture with Worthington’s Harding Hospital, the area’s only private hospital serving psychiatric patients. This integration allows clinicians to provide a comprehensive continuum of care in which they can develop and test new treatments and strategies to improve mental health. The Department and Ohio State’s Harding Hospital are in a $15 million psychiatric facility that houses clinical inpatient, outpatient, partial hospitalization and research programs. Their mission is: to provide outstanding psychiatric care; train residents, fellows and medical students in a spectrum of practice settings and patient populations; and conduct research in neuroscience, psychiatry and psychology. Ongoing Research Programs • Schizophrenia – Current projects include: identifying candidate genes associated with susceptibility for schizophrenia spectrum disorders, treatment response and outcome, as well as disease phenotypes, such as risk factors and cognitive deficits; and identifying genetic factors determining therapeutic response and side effects to second-generation antipsychotics in patients with acute psychotic episode. • Anxiety disorders – Researchers completed a project with an investigational agent for generalized anxiety disorder (GAD) and are engaged in negotiations for other projects with GAD. • Child and adolescent psychiatry – Researchers focus on child and adolescent disorders, including attention-deficit/hyperactivity disorder, bipolar disorder and other mood disorders. • Pharmacogenetics – This program in psychiatric genetics involves studying the role of opioids in nicotine addiction and signaling mechanisms involved in the neuroprotective/neurorestorative action of gangliosides. • Mood disorders – Researchers are investigating aspects of the assessment and treatment of mood disorders, particularly bipolar spectrum disorders, in school-aged children. Research Accomplishments of 2006 • The Department of Psychiatry in 2006 took on all aspects of clinical trials originally initiated with Pediatric Clinical Trials, Inc., which began in 2005. The Department’s new Clinical Trials Program has completed or is participating in seven clinical studies involving one phase II and six phase III trials, five of which will continue through 2007. These projects have led to a major study involving a new investigational antidepressant for major depression, and six other trials are being negotiated in bipolar disorder, schizophrenia, major depression and possibly generalized anxiety disorder. These include a greater number of phase II trials. • John Campo, MD, studies pediatric functional abdominal pain, its relationship with anxiety and depression, and its treatment using medication and brief cognitive behavioral psychotherapy in traditional medical settings. He also focuses on improving psychopharmacologic management of common pediatric mental disorders in specialty and primary care settings. • L. Eugene Arnold, MD, continued a clinical trial using zinc to treat attention-deficit/hyperactivity disorder (ADHD) and furthered his work on the National Institute of Mental Health’s multisite Multimodal Treatment Study of ADHD (the MTA). He also chaired the steering committee for Research Units in the Pediatric Psychopharmacology (RUPP) Autism Network, which is completing a multisite study of adding parent training in behavior management to medication for irritability in children with autism spectrum disorders. • Mary Fristad, PhD, ABPP, is site-principal investigator for a four-site National Institute of Mental Health (NIMH)-funded study to determine the longitudinal course of manic symptoms and the development of bipolar spectrum disorders in a large cohort of children being treated in outpatient clinics. She also has NIMH funding to write the treatment manual and training materials for a psychosocial treatment that proved efficacious in a recently completed NIMH clinical trial. • Janice Kiecolt-Glaser, PhD, has expanded her interdisciplinary collaborations on psychological influences of immune function to examine the role that serotonin and cytokine genes may play in interactions with stress and inflammation among older adults – that is, how genes interact with chronic stressors to enhance risk for adverse mental and physical health changes. Her work is supported by a new grant from the National Institute on Aging. Another of her National Institutes of Health-funded studies addresses whether a restorative hatha yoga session can produce positive changes in endocrine and immune function compared to a metabolically equivalent control condition. 2007 Research Report 101 DEPARTMENT OF RADIATION MEDICINE Research Accomplishments of 2006 Nina Mayr, MD, Chair • Functional and anatomical imaging-based tumor outcome prediction – Work has continued on this National Institutes of Health-funded research that focuses on developing an imaging-based predictive assay for cervical cancer (PI: Nina Mayr, MD). Scientists made five presentations on the subject in 2006, including three at the Annual Scientific Meeting of the American Society of Therapeutic Radiology and Oncology. Presenting were Mayr, Jian Wang, PhD, John Grecula, MD, and Hualin Zhang, PhD. Updated results were presented in a Special Focus Session titled “Cancer: Early Predictors of Tumor Responses” at the 2006 Annual Scientific Meeting of the Radiological Society of North America. This pioneering work, published in the American Journal of Roentgenology, demonstrated for the first time that tumor regression occurs in non-linear fashion and supported the use of three-dimensional imagingbased tumor volumetry instead of simple diameter-based measurements for response assessment and outcome prediction in cervical cancer. • Radiation Medicine established further collaborations with the departments of Radiology, Pathology, and Obstetrics and Gynecology, along with the College of Public Health, to develop a personalized multimodality algorithm for response monitoring and prediction of tumor response to cytotoxic therapy in patients with cervical cancer. The Department also is expanding this concept with other tumors. John Grecula, MD, has applied for a grant to help develop imaging-based predictive paradigms in lung cancer therapy. The research focuses on radiation therapy targeting and includes the continuation of tumor target delineation (Nilendu Gupta, PhD) with the development of an anatomical atlas for target delineation in radiation therapy. Manual and automated methods, as well as standardization of delineation techniques for tumor targets and normal tissue volumes, are being intensively investigated and developed in Radiation Medicine. Radiation Medicine serves cancer patients through applied medical research, education of medical specialists and personalized use of the latest radiation technology and treatments. The 73-member team includes five attending physicians, five medical physicists, four radiation oncology residents, two physics residents and a support staff of radiation dosimetrists, therapists, nurses and students. They work in a 33,000-square-foot facility that is housed in Ohio State’s James Cancer Hospital and Solove Research Institute and includes a treatment planning CT (computed tomography) simulator, access to molecular imaging-based therapy planning with positron emission tomography/computed tomography (PET/CT), and 3-dimensional treatment planning for conformal and intensity modulated radiation therapy and stereotactic radiosurgery. It also has two dual energy and two 6 MV linear accelerators, one with single-fraction stereotactic radiosurgery and fractionated central nervous system stereotactic radiotherapy capabilities, an intraoperative electron beam linear accelerator, and an upgraded gamma knife. The Brachytherapy Program offers permanent or temporary applications as well as intraoperative procedures and novel radionuclide therapies. Ongoing Research Programs • The Department’s research programs have focused on the use of anatomical and functional tumor imaging for radiation therapy planning and therapy-response monitoring, and on radiation therapy targeting delineation for radiation therapy delivery, outcome prediction and tumor-response modeling of radiation therapy and multimodality cancer therapy. 102 Ohio State University Medical Center • The team further concentrated on computational methods of radiation therapy optimization, including more effective dose prescription in highly focused radiation therapy for brain lesions treated with the Gamma Knife (Simon Lo, MD, and Joseph Montebello, MD) and other technologies (Nina Mayr, MD). Work on novel methodologies of Gamma Knife dosimetry planning, which can reduce the dose to normal brain tissue, was presented at the 2006 Annual Meeting of the Society of Neuro-Oncology. Results on the use of radiation grid therapy, a novel modality to improve dose delivery in treating melanoma and debulking large tumors, was presented at the Annual Meeting of the American Society of Therapeutic Radiology and Oncology by Hualin Zhang, PhD. • Research by Jian Wang, PhD, on response modeling expanded after his initial pioneering work on theoretical radiobiological modeling studies of prostate cancer response. The model with parameters established by Wang provides the most consistent interpretation of clinical data available for prostate cancer. It also provides a unifying hypothesis for resolving longstanding controversies regarding radio sensitivity and extremely low clonogen numbers in prostate cancer, with full consideration of various clinical effects. The Department has published two articles and presented several abstracts at national conferences regarding this work, which has expanded to include serial imaging-based tumor outcome databases in cervical cancer to model biophysical aspects of tumor response. High correlations have been found between temporal tumor response dynamics and ultimate tumor control and treatment outcome. A kinetic model has been developed to describe the tumor radiosensitivity and regression process of cervical cancer during radiation therapy. Abstracts have been presented on the subject at the American Society of Therapeutic Radiology and Oncology, the Annual Biomedical Imaging Research Opportunity Workshop, and the International Society for Magnetic Resonance Imaging in Medicine. DEPARTMENT OF RADIOLOGY Michael Knopp, MD, PhD, Chair The Department of Radiology’s mission is to: achieve national distinction in education, scholarship and public service; educate professionals in basic and clinical medical imaging sciences as well as allied medical professions; create and disseminate knowledge and technology; and provide solutions for improving health. Radiology has five divisions: Diagnostic Radiology (includes Interventional Radiology, Neuroradiology, Breast Imaging, Thoracic, Abdominal, Musculoskeletal, and NonVascular Interventional); Imaging Research; Nuclear Medicine and Molecular Imaging; Radiobiology; and Regional Radiology. In 2002, Radiology had eight funded projects for a total of $1.2 million. By 2004, it had 23 projects totaling $18.7 million. The Department had obtained another $13.1 million in funded awards/projects by 2006. As one of Ohio State University Medical Center’s Signature Programs, Imaging focuses on personalized health screening/appraisal, disease detection and characterization, imaging-based therapies and therapeutic response assessment. Ongoing Research Programs The Department’s technological advancements of 2006 impacted clinical care, the market and future opportunities. Being able to leverage research and training will strengthen global medical expertise. Here is a sampling of successes from the past year: • Biomedical Structural, Functional and Molecular Imaging Enterprise – In 2003, the state of Ohio awarded Michael Knopp, MD, PhD, a $9.1 million Third Frontier Grant and $8 million in Biomedical Research and Technology Transfer (BRTT) funding to create the Wright Center of Innovation (WCI) in Biomedical Imaging. The project (with funding through 2008) is also known as the Biomedical Structural, Functional and Molecular Imaging 2007 Research Report 103 Enterprise at Ohio State. It is designed to advance biomedical imaging technology. In the spring of 2006, the state announced continued funding of nearly $8 million for three years to lay the foundation for the next level of hybrid positron emission tomography/magnetic resonance (PET/MR) imaging and imaging-based therapy. • Cancer and Leukemia Group B (CALGB) Core Lab – Radiology is in its fourth year as a core lab for the CALGB. The lab facilitates standardized data acquisition, data transmission, quality control, storage, post-processing and analysis for CALGB. It also implements post-processing algorithms as desired by the imaging committee and provides technical infrastructure to enable rapid transfer and storage of the studies. An additional submission for $1.4 million is under consideration. Michael Knopp, MD, PhD, is principal investigator. • Imaging Response Assessment Teams (IRAT) in Cancer Centers – A National Cancer Institute award of $738,813 for three years to Ohio State’s Comprehensive Cancer Center (OSUCCC), in conjunction with the Department of Radiology, supports development of imaging-assessment methods to reveal early biologic, noninvasive response to cancer treatment. This award, announced in 2005, is helping develop, implement and validate response-assessment imaging methodologies in the OSUCCC in collaboration with Radiology’s Division of Imaging Research, which includes the Wright Center of Innovation in Biomedical Imaging. Ohio State is one of eight cancer centers nationwide to receive this grant. Michael Knopp, MD, PhD, is principal investigator. • Core Lab for Imaging Post-Processing and Analysis – The Department continues to work with Novartis Pharmaceuticals as the imaging core lab and to collaborate in advanced imaging methodologies for clinical trials. Including Novartis, 22 clinical trials with total funding of about $1.2 million are under way within the Department. • Advancing Imaging Technology as a Credential Biomarker for Clinical Drug Development – This award from Pfizer, Inc. was announced at the end of 2006 with Michael Knopp, MD, PhD, as principal investigator. The project includes all aspects of imaging and drug development with a focus on 104 Ohio State University Medical Center establishing imaging surrogates and biomarkers for therapeutic response prediction and assessment. • Molecular-Level Research – Scientists in the Division of Radiobiology, which is directed by Altaf Wani, PhD, study molecular mechanisms of DNA damage and repair. The Division has more than $1.5 million in research funding. Projects include: examining genomic instability in cancer pathogenesis, with a focus on the regulation of DNA damage processing in the native environment of normal and cancerous cells, and on delineating mechanisms of cross-talk between molecular pathways that control cellular homeostasis; characterizing and quantifying, in human tissue and cell types, the mechanisms of chemopreventive agents, cancer therapy in combination with chemical and radiological agents, modulation of genes and proteins regulating cellular proliferation and apoptosis (cell death), and genetic damage repair; studying anticancer topoisomerase poisons, including analysis of potential new drugs, and proteomic analysis of post-translational modifications of topoisomerases associated with drug exposure and disruption of cancer-cell metabolism by anticancer drugs. DEPARTMENT OF SURGERY E. Christopher Ellison, MD, Chair The Department of Surgery delivers high-quality patient care, contributes medical innovations through translational research and clinical outcomes studies, and educates medical students and postgraduate trainees. The Department interacts with all six Ohio State University Medical Center Signature Programs – Cancer, Critical Care, Heart, Imaging, Neurosciences and Transplantation – and comprises eight surgical specialty divisions: Cardiothoracic Surgery; Critical Care, Trauma and Burns; General and Gastrointestinal Surgery; General Vascular Surgery; Pediatric Surgery; Plastic Surgery; Surgical Oncology; and Transplantation. The Center for Minimally Invasive Surgery, a multidisciplinary entity within the Department, develops and implements laparoscopic surgical technologies and procedures. The Ohio State University Comprehensive Wound Care Center provides for interaction between basic scientists and clinicians, enhancing translational research and patient care. Research funding received from July 1, 2005, through June 30, 2006 by Department scientists through The Ohio State University Research Foundation was more than $7.3 million. Investigators received more than $4 million from the National Institutes of Health. Ongoing Research Programs • Cancer – Basic and clinical studies are investigating cancer immunology and gene-nutrient interactions in breast cancer. • Cardiovascular – Basic, preclinical and clinical studies are focusing on heart failure, myocardial infarction, stroke, atherosclerosis, antioxidant nutrition and mitochondrial dysfunction. • Critical Care – Cytomegalovirus infection and sepsis are key areas of investigation. • Transplantation – Mechanistic studies of the immune basis of organ allograft rejection, in combination with clinical trials, are evaluating the efficacy of novel immunosuppressive agents in organ transplant recipients. • Wound Healing – Molecular, preclinical and clinical studies are directed toward understanding the fundamental mechanisms (focusing on oxygen) underlying dermal wound healing and its impairment. Research Accomplishments of 2006 • Cancer – William Carson III, MD, has reported on a phase I trial of interferon and interleukin-12 in patients with metastatic cancer, proving that it is possible to enhance the responsiveness of peripheral blood mononuclear cells to interferons. His group has also demonstrated the utility of a novel flow cytometric assay to monitor the immune response to exogenously administered cytokines such as interleukin-2, which is used to treat patients who have metastatic melanoma. Carson’s group also demonstrated that natural killer cells in the body’s immune system produce T cell-recruiting chemokines in response to antibody-coated tumor cells, and that this response is enhanced by stimulatory factors such as interleukin-21 and CpG oligodeoxynucleotides. Pedram Ghafourifar, PhD, reported that tamoxifen induces oxidative stress and mitochondrial apoptosis by stimulating mitochondrial nitric oxide synthase. • Cardiovascular – Sampath Parthasarathy, PhD, celebrated the Klassen Research Day with Nobel laureate Louis Ignarro as guest scientist. Parthasarathy reported on how salicylic acid might be generated in plasma from aspirin. The ability of long-term treatment with aspirin to retard atherosclerosis might be dependent on the generation of free salicylic acid, a scavenger of free radicals. Pedram Ghafourifar, PhD, reported on how mitochondrial cytochrome c reacts with nitric oxide via S-nitrosation. Sashwati Roy, PhD, developed a laser-capture-based technique to study single myocytes from spatially resolved regions of the infarcted myocardium. She and Chandan Sen, PhD, also reported on a genomic study performing transcriptome analysis of the ischemia-reperfused remodeling myocardium. Benjamin Sun, MD, developed a way to label skeletal myoblasts with an oxygen-sensing spin probe for non-invasive monitoring of in situ oxygenation and cell therapy in the heart. Sen also reported on the beneficial effects of the lesser known vitamin E a-tocotrienol against strokerelated neurodegeneration. • Critical Care – Charles Cook, MD, and Ronald Ferguson, MD, PhD, reported on a novel mechanism by which critically ill surgical patients develop fibroproliferative acute respiratory distress syndrome. Cook’s work also identified carbamoyl phosphate synthase-1 as a marker of mitochondrial damage and depletion in the liver during sepsis. He reported on how lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta may trigger reactivation of latent cytomegalovirus. 2007 Research Report 105 • Transplantation – Ronald Pelletier, MD, and Ronald Ferguson, MD, PhD, reported on how steroid withdrawal in low-risk kidney transplant recipients is safe and ameliorates many of the unwanted side effects of steroid use. Their studies also demonstrated that myoglobinuria with myoglobin cast formation can occur following rapamycin administration and may aid development of unexpected severe acute renal dysfunction. Amer Rajab, MD, PhD, and Ferguson reported that excellent graft survival with a significantly lower incidence of acute rejection can be achieved using a steroid-free maintenance immunosuppressive protocol consisting of Neoral and sirolimus. Lisa Yee, MD, and Anne VanBuskirk, PhD, shed light on how memory cytotoxic T-lymphocytes are restimulated and function to prevent disease, especially post-transplant lymphoproliferative disorders. • Wound Healing – Sashwati Roy, PhD, and Chandan Sen, PhD, produced the first in vivo evidence that strategies to influence the redox environment of the wound site may have a bearing on healing outcomes. At low doses (endogenous as well as therapeutic), oxidants stimulated wound angiogenesis. Sen and Roy reported on the presence of a normoxic-setpoint in biological tissues and have characterized the regulation of such setpoint. Their group also reported on the wound site neutrophil transcriptome in response to psychological stress in young men. Stress tilted the genomic balance toward genes encoding proteins responsible for cell cycle arrest, death and inflammation. Additional reports by this group include characterization of changes in the transcriptome of the subcutaneous adipose of obese diabetic mice in response to dietary supplements. In collaboration with the Mathematical Biosciences Institute, this group provided insight on microarray analysis of gene expression and data mining. 106 Ohio State University Medical Center DEPARTMENT OF UROLOGY Robert Bahnson, MD, Director The Department of Urology is dedicated to achievement in patient care, urologic education and research. The goal of the faculty is to provide state-ofthe-art care to patients in an environment that fosters education of residents and medical students. The Department is committed to research that translates into more effective treatments for patients with urologic disorders. Recent accomplishments include establishing an internationally recognized robotic prostatectomy center, a voidingdisorders practice with a complete range of diagnostic capabilities, and a kidney stone management program with ambulatory treatment using lithotripsy and ureteral endoscopy. A recent Accreditation Council for Graduate Medical Education (ACGME) review of the Department’s residency program resulted in a commendation, full accreditation with a five-year review cycle (the longest period that can be granted), and a permanent increase in resident complement to three per year. Research Accomplishments of 2006 • Biodistribution of Dietary Tomato and Soy Phytochemicals in the Human Prostate – Consumption of tomato-based products and soy has been correlated with reduced risk for prostate cancer in human epidemiologic studies. But because human diets are complex, more evidence is needed to prove that these foods can help prevent cancer. Work by Ohio State researchers Steven Clinton, MD, PhD, Kamal Pohar, MD, and Robert Bahnson, MD, has ranged from molecular studies using cells grown in the laboratory to animal models for cancers, as well as human studies. These studies demonstrated that soy and tomato products contain many phytochemicals, or natural compounds that affect several components of the carcinogenic cascade. • Tomato-Soy Drink Tested Against Prostate Cancer – Using a $1.27 million federal grant, a team of researchers developed a promising tomato juice containing soy that has been favorably tested for palatability. A new $1.2 million grant from the National Cancer Institute is allowing the team to test this product in men with prostate cancer. The first study will focus on those planning to undergo prostatectomy. • Chemoprevention of Prostate Cancer – Robert Bahnson, MD, continues to work with Steven Clinton, MD, PhD, on chemoprevention of prostate cancer. • MEAL Study – Robert Bahnson, MD, and Electra Paskett, PhD, MSPH, a professor of Public Health, are serving as co-chairs for the Men’s Eating and Living (MEAL) study, a pilot trial of diet change among prostate cancer patients. SCHOOL OF ALLIED MEDICAL PROFESSIONS Deborah Larsen, PhD, Director The School of Allied Medical Professions (SAMP) has 33 regular faculty members (eight clinical, 11 probationary tenuretrack and 14 tenured) and 14 auxiliary/staff appointments with teaching/research responsibilities. Research ranges from basic science to translational, examining health promotion and disease prevention, mechanisms of injury and recovery, and long-term health-related quality of life. Funding for SAMP research also spans the spectrum from small foundational grants to National Institutes of Health (NIH) R01s. The School has seen an increase in research productivity in the past few years, with $2.075 million in research awards in new or ongoing grants during 2006. SAMP faculty were principal investigators (PIs) on two R01s and one R21, participated in two multi-centered, NIH-funded clinical trials as site PI, were co-investigators on three additional R01s, and were PIs on two nationally funded training grants. Publications for SAMP faculty are consistent with their diversity of scholarship and include basic science, clinical and educational arenas. Ongoing Research Programs • D. Michele Basso, PhD, an associate professor of Physical Therapy, focuses on recovery of motor function after central nervous system (CNS) injury, including the ability to not only assess normal and aberrant behavior but also to analyze structures within the CNS for response to injury and contribution to recovery. She was funded on four R01s during 2006, one as PI and three as Co-PI. • John Buford, PhD, associate professor of Physical Therapy, is involved in multiple neuroscience areas of inquiry, including: neural control of movement, specifically the neurophysiological basis of reaching; model of carpal tunnel syndrome; neuroanatomy of the reticulospinal system; and innovative approaches to teaching neuroscience. He received a renewal of his R01 in 2005 and was Co-PI on a second NIH grant. • Deborah Larsen, PhD, director of SAMP, focuses on recovery of function after stroke and related neural mechanisms. She was the site principal investigator on two multicenter clinical trials (described later) that were completed in 2005 with a no-cost extension into 2006, one with its primary outcome paper published in the Journal of the American Medical Association in 2006. • Deborah Heiss, PhD, associate professor and interim director of Physical Therapy, studies the treatment of low back pain, for which she received an R21 grant from the NIH in 2006. 2007 Research Report 107 Research Accomplishments of 2006 SCHOOL OF BIOMEDICAL SCIENCE • Steve Wilson, PhD, associate professor emeritus of Allied Medicine, in collaboration with Mark Sothman, PhD, at Indiana University, was awarded $1 million to establish the Ohio State UniversityIndiana University Center for Traumatic Amputee Rehabilitation Research program to study the needs of the Department of Defense and the Department of Veterans Administration related to the rehabilitation and health care of veterans and amputee war-related injuries. • EXCITE (Extremity Constraint Induced Therapy Evaluation), the largest multi-centered clinical trial of a rehabilitation technique for upper extremity recovery after stroke, was completed in 2006 and demonstrated significant improvement of upper extremity function among patients using this technique: Wolf et al, JAMA 2006;296(17):20952104. Ohio State was one of seven universities to participate. Deborah Larsen, PhD, director of SAMP, was site PI at Ohio State. • John Borstad, PhD, assistant professor in Physical Therapy, was awarded $168,739 from The Susan G. Komen Breast Cancer Foundation to study Three-Dimensional Analysis of Shoulder Motion Limitations Following Treatment for Breast Cancer. Caroline Whitacre, PhD, Director The School of Biomedical Science (SBS) promotes research to advance medical knowledge and to educate the next generation of biomedical scientists and health professionals. The School’s academic mission is to foster excellence in education and research education. The School encompasses six basic science departments in Ohio State’s College of Medicine: Biomedical Informatics; Molecular Virology, Immunology and Medical Genetics; Molecular and Cellular Biochemistry; Neuroscience; Pharmacology; and Physiology and Cell Biology. The School has been reorganized to better integrate biomedical research activities across the Medical Center in order to align with the Medical Center’s six Signature Programs and enhance the research and education missions. Representatives of the Signature Programs have been appointed to the School’s executive committee. SBS FACULTY NUMBER 160 140 120 100 80 60 40 20 0 2000 2001 2002 2003 2004 *2005 *2006 *2007 * Includes Research Track 108 Ohio State University Medical Center TOTAL GRANT FUNDING Integrated Biomedical Science Graduate Program (IBGP) In 2001 this program replaced the traditional departmental PhD graduate programs at Ohio State’s Medical Center. It is organized with a much more interdisciplinary approach to investigate the causes, biological mechanisms and cures of human disease. Students prepare for careers in biomedical research with an understanding of disease mechanisms that integrates information from several disciplines. This graduate program has a strong faculty membership that is Medical Center-wide and from 23 departments at Ohio State. They bring research and teaching expertise, along with research resources available to graduate students. The IBGP is recognized as one of the top PhD programs in the nation by virtue of a training grant awarded by the National Institutes of Health to support this program. Graduate/Postgraduate Research Day This is an annual event featuring biomedical research conducted by students/trainees at the Medical Center. Participation is open to students in the College of Medicine, including graduate, medical and MD/PhD students, as well as postdoctoral fellows and researchers, and clinical residents and fellows. Held each spring, Research Day features a poster display and awards presented for outstanding research, as well as presentations by worldrenowned researchers and experts in biomedical science. The Research Day is intended for and organized by Medical Center students/trainees. Postdoctoral Research Office Postdoctoral scholars contribute to the dynamic research enterprise of the College of Medicine. The office helps postdoctoral students maximize this pivotal stage in their professional development. As a resource for current and prospective postdoctoral scholars, faculty advisors and administrative staff, the office enhances the postdoctoral experience. Undergraduate Biomedical Science Program This exciting new undergraduate program has been created for high-ability students who are eligible for Ohio State’s honors program and who have an interest in conducting medical research and studying human disease. The first class of Biomedical Science students started in autumn quarter of 2005; approximately 20-25 freshmen enter the major each year. The goal is to provide a strong science background and involve students in biomedical research that will prepare them to enter a graduate program in research, medical school, dental school or other areas of health care. SBS FACULTY FUNDING 45.0 Faculty Growth More than 50 new faculty have been added to the School since its inception in 2000. The growth in faculty has more than doubled annual extramural funding from $18 million to $40 million. This extramural funding provides resources to support the School’s fundamental mission of improving health care by acquiring scientific knowledge and translating it to clinical applications. DOLLARS (MILLIONS) 40.0 35.0 30.0 25.0 20.0 15.0 10.0 5.0 0.0 FY 2000 FY 2001 FY 2002 FY 2003 FY 2004 FY 2005 FY 2006 TIU + Center Funding 2007 Research Report 109 esearc Technology Commercialization and Partnerships (TCP) AT OHIO STATE UNIVERSITY MEDICAL CENTER 110 Ohio State University Medical Center DIRECTOR: HENRY ZHENG, PHD, MBA The Technology Commercialization and Partnerships (TCP) Council works to improve collaboration between Ohio State University Medical Center faculty, researchers and inventors and the University’s Technology Licensing and Commercialization (TLC) team, as well as between the Medical Center and industry. The overall goal is to accelerate commercialization of technologies created at the Medical Center and to foster partnerships that bolster sponsored research, development and formation of startup companies or technology licensing. TCP objectives are to create an entrepreneurial organizational culture that promotes innovation and economic development, and to enhance the Medical Center’s status as a leader in medical innovation and discovery. Ohio State scientists who believe they have technologies or capabilities with strong commercial potential should work closely with the TLC team, which will lead the development of intellectual property from discovery through commercialization activities that could take many forms. Scientists are encouraged to connect early and often with both the TLC and the TCP teams. In 2006, the TCP Council included: Henry Zheng, PhD, MBA, director; Caroline Whitacre, PhD; Michael Bissell, MD, PhD, MPH; Jean Schelhorn, PhD; Andrew Hansen, PhD; Tim Cain, PhD; Jennifer Yucel, PhD; Kim Saunders; Darian Torrance; and Wendy Philips. Technology commercialization is important because it makes the latest scientific and technological discoveries available to the public and improves lives, objectives that are in line with Ohio State’s research and public service missions. The Federal Bayh-Dole Act of 1980 allows universities to elect title to inventions stemming from federally supported research and, through this, to jump-start commercialization. The TCP also emphasizes the importance of internal communications regarding TCP activities. The TCP Web site (http://medicine.osu.edu/tcp/index.cfm) includes helpful technology transfer-related infor- mation and links – a place where major events and tech transfer outcomes are frequently updated. In addition, a Web-based newsletter highlights TCP developments and major related events within and outside the Medical Center. The TCP Council also hosts seminars, workshops, colloquiums and roundtable discussions to provide personal interaction among invited speakers and Ohio State faculty/researchers. On Dec. 4, 2006, TCP hosted the first Industry Collaboration Symposium at Ohio State’s newly opened Biomedical Research Tower. The symposium attracted more than 300 attendees representing almost 90 companies and organizations from as far away as Germany, South Korea, England and India. By showcasing faculty research, Medical Center research capabilities and technologies available for commercialization to industry partners, the symposium demonstrated a desire to work with industry and other development organizations to promote technology transfer and economic development. A post-symposium survey of attendees suggests that this was a highly successful event that will create momentum for industry-academia collaboration in technology development and commercialization. Besides fostering closer collaboration with external organizations, TCP also collaborates with TLC on many activities, including workshops, panel discussions, the Industry Collaboration Symposium, marketing and technology cultivation. Members of the TLC life sciences team play an important role in helping faculty move their technologies from laboratory to commercialization. The TLC team is led by Jean Schelhorn, PhD, associate vice president for Commercialization, who joined Ohio State in early 2006. The mission of TLC is to: foster a University-wide entrepreneurial culture; catalyze faculty, staff and student inventions; maximize the value of Ohio State developments; and accelerate the transition of new developments into products, services, and new or expanded jobs. Schelhorn is Ohio State’s primary spokesperson on commercialization and builds collaborations with public and private entities to com- 2007 Research Report 111 mercialize intellectual property and foster economic growth. Before joining Ohio State, she was vice president for intellectual property strategy and development at Battelle, one of the premier technology development and commercialization companies in the world. Through team efforts among faculty, researchers, inventors, TLC and the Medical Center, more and more technologies are being disclosed and protected each year, and more commercialization successes are being generated. The success stories span new licensing activity, new technology-based startup company formation, and attraction of companies to the region to be in proximity to the Medical Center’s research capability and researchers. Two major commercialization success stories at Ohio State in 2006 are: achieving an institutional Technology Commercialization score high enough to merit a large monetary award from the state’s fiscal 2007 TCIA Fund; and earning national recognition for a Columbus-based biopharmaceutical company, OncoImmune Inc., an Ohio State University technology commercialization company that is developing drugs for diseases with no effective treatment. Using data supplied by universities, the Ohio Board of Regents staff determined that Ohio State University secured one of the three highest technology commercialization index scores in the state and thus recommended that the University be awarded $150,000 from the fiscal 2007 Technology Commercialization Incentive Awards (TCIA) Fund, a performance-based program under Economic Growth Challenge in Ohio’s higher education budget. The TCIA Fund rewards public and private universities for technology transfer to Ohio business and industry that results in the commercialization of new products, processes and services. Ohio’s Third Frontier Commission, with counsel from the Third Frontier Advisory Board, establishes eligibility criteria for colleges and universities applying for TCIA incentive funding. According to information reported by TLC, OncoImmune, Inc. was spun out of Ohio State in 2000 and holds rights to proprietary technologies from Ohio State and the University of Michigan. Diseases for which the company is developing potentially life-saving drugs include multiple sclerosis, tuberous sclerosis and cancer. The company expected to start clinical trials for tuberous sclerosis in 2007 and for multiple sclerosis in 2008. OncoImmune, Inc.’s multiple sclerosis drug is based on a novel patent-pending gene target and will treat early- and late-stage patients while complementing existing therapies. OncoImmune, Inc. discovered that rapamycin, an FDA-approved cancer drug, restores the function of a pair of faulty genes, offering a possible treatment for tuberous sclerosis and cancer. The company expects to move the novel formulation to market for these indications. For its innovative work in drug development, OncoImmune, Inc. was nominated by Ohio State and subsequently chosen to participate in the 2006 University Start-Ups National Showcase. The company has received funding from the National Institutes of Health and the state of Ohio, and it recently secured series-A venture capital. Technology Commercialization and Partnerships Team in 2006 - (seated from left): Henry Zheng, PhD, MBA; Caroline Whitacre, PhD; Jean Schelhorn, PhD; and (standing from left): Wendy Philips; Tim Cain, PhD; Darian Torrance; Andrew Hansen, PhD. 112 Ohio State University Medical Center Medical Center Information Warehouse: BRINGING ENTERPRISE-GRADE INFORMATICS TO THE RESEARCH COMMUNITY “The Information Warehouse at Ohio State is a critical component of the infrastructure that enables establishment of our wound human tissue bank for receiving tissues from clinics nationwide. It is also pivotal in enabling patient-based wound healing research involving genome-wide screening.” – Chandan Sen, PhD, professor and vice chairman (research) of Surgery, and executive director of the Ohio State University Wound Center. 2007 Research Report 113 DIRECTOR: JYOTI KAMAL, PHD Information is the foundation of innovation. As valuable as any tangible facility or monetary asset, the data that flows throughout an academic medical center is an indispensable key to advancement. At Ohio State University Medical Center, the Information Warehouse (IW) curates data from systems throughout the institution and provides a broad array of informatics services to help researchers turn that data into knowledge that will become the future of medicine. WHAT IS THE IW? The IW is a comprehensive data-warehousing facility providing data-integration, data-management and data-mining services to a diversity of customers across the clinical, education and research sectors of the Medical Center. While the IW is a shared service, in recent years leadership has charged the IW with expanding support for the research community by providing an informatics environment that facilitates both translational research and the development of personalized medicine. The IW maintains a scalable, multifaceted data system spanning 14 years of clinical and financial history. The IW also provides enterprise-grade data-management services so that researchers who use these services can free more time to focus on science. The IW also helps investigators with the informatics segments of their research proposals, thereby saving time and enhancing credibility. “The Information Warehouse is essentially an infrastructure service for the Medical Center,” says IW Director Jyoti Kamal, PhD. “We are always looking for ways to help our colleagues do their jobs better. Over the past several years we have been working hard to meet the special needs of our research colleagues.” NEW DEVELOPMENTS “Our Medical Center strategy is to use a centralized imaging data repository that adheres to all the stringent requirements, from reliability to data safety. With this new tool in the IW, imaging data can be accessed by authorized users via specific criteria in a Over the past year the IW developed many innovations and services to amplify research productivity at the Medical Center. These include IW infrastructure developments targeting common research community needs, deployment of several extensible pilot projects, and the establishment of key partnerships within the Medical Center. process that complies with HIPPA and other regulations. This is a major step forward in our quest to use imaging data in clinical research and for advancing personalized health care.” – Michael Knopp, MD, PhD, chair of Radiology and leader of the Imaging Signature Program. 114 Ohio State University Medical Center TRANSLATIONAL RESEARCH INFORMATICS ARCHITECT In partnership with the Department of Biomedical Informatics (BMI), Chief Information Officer Herb Smaltz, PhD, has created a joint position between the IW and the BMI to help coordinate informatics services offered to the research community. The translational research informatics architect (TRA) will help manage specific research efforts and will help guide the strategic development of new informatics capabilities and services to better meet the research community’s needs. In January 2007, Philip Payne, PhD, was selected for the role of TRA and is already making a substantial impact on research informatics at OSUMC. This partnership with BMI will strengthen the Medical Center’s ability to offer innovations and effective informatics services that will enhance research productivity. HONEST BROKER PROTOCOL In 2006 the IW’s Honest Broker Protocol was approved by Ohio State’s Institutional Review Board. Through this protocol, the IW is able to provide de- identified clinical data for research purposes. This mechanism can streamline the research process for many kinds of retrospective work while still ensuring the privacy of patients. In fiscal year 2007 the IW began processing many requests for data under this protocol. In the coming year it will expand the protocol to encompass new types of clinical information from its comprehensive repository and to implement new mechanisms for access that will enable investigators to explore the data more spontaneously, increasing opportunities for unanticipated discoveries. Figure 1: The Information Warehouse takes in data from a vast array of Ohio State University Medical Center information sources (more than can be shown here), cleanses it, arranges it into efficient datamarts and provides access to the data through a selection of presentation, mining and analysis tools. Through this integration, the IW helps to establish a translational research environment at the Medical Center by electronically linking the bench to the bedside. 2007 Research Report 115 Figure 2: The Information Warehouse produces a broad spectrum of custom data-management and data-mining applications. This capability is particularly valuable when commercial offerings do not provide the specialized functionality that research efforts often demand. The primary goal of these services is not only to help researchers manage their data, but also to help them turn that data into knowledge. 116 Ohio State University Medical Center COMPREHENSIVE WOUND CENTER PULMONARY PORTAL Working with the Comprehensive Wound Center (CWC) and CWC Executive Director Chandan Sen, PhD, the IW has constructed a richly featured informatics pipeline to support wound- and tissue-based research. Envisioned as a core service that would support many projects (including efforts outside of the Wound Center), the pipeline is a framework of custom applications, automated multisite data-collection processes, open source tools and commercial products, all integrated into the IW’s comprehensive clinical data repository. The pipeline provides tools for managing tissue data, annotating the samples with contextual information, analyzing linked microarray expression data and performing full-featured statistical analysis on all the encapsulated and linked information. The system also collects detailed wound-related data from more than 100 wound centers nationwide through a partnership with National Healing Corporation. In the future the IW expects to extend the application of such frameworks to support other research efforts within the Medical Center. In partnership with Clay Marsh, MD, and the Pulmonary Translational Core Group, the IW has developed a technology framework and a methodology that enables IW developers to rapidly produce Web-based applications in support of clinical studies. Providing data collection, management and reporting functionality, these applications give research staff an efficient means to enroll and track patients through even complex studies while reducing opportunities for error through many innovative features. The IW plans to extend this technology to support more groups within the Medical Center in the coming year. In the future this technology will be integrated with the commercial clinical trials management system when that product is selected and installed. PACS IMAGE INTEGRATION To make the Medical Center’s PACS image archives more accessible for research, the IW has implemented an image integration server that enables investigators to extract sets of images based on clinical parameters stored in the IW’s comprehensive clinical repository. By integrating a standardized ontology text search mechanism, images can also be selected according to generalized medical concepts mentioned in the free-text of physicians’ reports. This mechanism provides an efficient means to ensure that images extracted for a study all share a common medical linkage. PARTNERS IN RESEARCH Recognizing that innovation and agility in medical informatics are critical in today’s translational research environment, Medical Center leaders support development of powerful informatics capabilities within the institution, and the IW is a cornerstone of that effort. “Drawing information from the past, we strive in the present to enable our research colleagues to create the medicine of the future,” Kamal says. “Our job is to help them do their jobs better.” 2007 Research Report 117 AT OHIO STATE UNIVERSITY MEDICAL CENTER 118 Ohio State University Medical Center esearc Translational Research The National Institutes of Health (NIH) recognizes translational research – converting basic science discoveries into innovative clinical care – as a powerful medical resource that could be bolstered by a stronger national research infrastructure. An “NIH Roadmap for Medical Research” published by the NIH’s Office of Portfolio Analysis and Strategic Initiatives acknowledges that “growing barriers between clinical and basic research, along with the ever-increasing complexities involved in conducting clinical research, are making it more difficult to translate new knowledge to the clinic – and back to the lab bench” – thus limiting professional interest in the field and “hampering the clinical research enterprise.” To counter this dilemma, the NIH in October 2006 launched a re-engineering effort to develop a new discipline of clinical and translational research by establishing a Clinical and Translational Science Awards (CTSA) Consortium that began with 12 academic health centers around the country. More than 50 others, including The Ohio State University, are applying for membership in the consortium, which when fully implemented in 2012 will comprise some 60 institutions. The NIH says Consortium members will “serve as a magnet that concentrates basic, translational and clinical investigators, community clinicians, clinical practices, networks, professional societies and industry to facilitate development of professional interactions, programs and research projects.” Although not yet funded by the NIH, the CCTS is an inclusive and functioning entity that engages all faculty, staff and students from Ohio State’s seven health sciences colleges and the University community in general who share a common vision about clinical and translational science. In the College of Medicine, Rebecca Jackson, MD, and Chandan Sen, PhD, associate dean for translational and applied research, have played key roles alongside more than 50 faculty in establishing the CCTS as a means of supporting the national effort. Jackson and Sen note that Ohio State offers many strengths for this endeavor, including the close proximity of multiple academic disciplines geared toward research, a host of shared resources that collectively enhance research capabilities, and approximately 1 million patient visits per year to the Medical Center. The CCTS, they say, will be the new academic home for clinical and translational science at Ohio State and is open to all in the community who are interested or engaged in interdisciplinary research that directly benefits the community by translating science into better health care. Ohio State has already taken a major step to support this cause. Planning efforts by multiple schools and colleges at the University, along with Ohio State’s Medical Center and Children’s Hospital in Columbus, have led to the establishment of a Center for Clinical and Translational Science (CCTS) led by Rebecca Jackson, MD, associate dean for clinical research. The Center’s mission is to improve quality of health care for the community by creating a transformative clinical and translational science discipline that stands at the core of the University’s academic culture. 2007 Research Report 119 Clinical Trials Research Ohio State University Medical Center excels in clinical trials because of collaborative relationships among clinical and basic science faculty, and also through relationships with research centers and institutes on campus and around the world. 120 Ohio State University Medical Center DEPARTMENT OF ANESTHESIOLOGY All Ohio State University clinical trials are overseen by the University’s Office of Responsible Research Practices (ORRP), a unit of The Ohio State University Office of Research. ORRP supports the University’s goals of promoting the ethical conduct of research involving human and animal subjects. The ORRP provides administrative support for: Neural bowel physiology and dysfunction in inflammatory bowel disease PI: Fievos Christofi, PhD esearc RESPONSIBLE RESEARCH PRACTICES • Faculty, staff and student education on the responsible conduct of research • Pre-review of protocol submissions for compliance with regulatory requirements • The University’s research review boards • Implementation of the University’s Conflict of Interest Policy • Conduct of the University’s classified research and contract activities For more information about ORRP, visit www.orrp.ohio-state.edu. INSTITUTIONAL REVIEW All clinical trials at Ohio State are carefully evaluated, approved and monitored by an Institutional Review Board (IRB) under the ORRP. Ohio State has three IRBs – one each for biomedical sciences, cancer, and behavioral and social sciences. These boards are staffed by physicians, scientists, patient advocates, clergy, community members and other healthcare providers who are collectively responsible for overseeing the protection of human subjects in research. In addition, the Clinical Trials Office (CTO) of Ohio State’s Comprehensive Cancer Center (OSUCCC) facilitates development and implementation of all OSUCCC clinical trials. By providing regulatory processing, subject recruitment, financial development, data collection and protocol-management services, the CTO fosters a supportive environment conducive to conducting clinical trials in a methodologically sound, expedient and cost-effective manner. Correlation between cerebral spinal fluid and plasma biochemical markers with the clinical outcome due to spinal cord ischemia during thoracoabdominal aortic aneurism repair: a prospective analysis PI: Hamdy Elsayed-Awad, MD Destiny trial: effects of mitral valve annuloplasty with the geoform ring on left ventricular geometry and function in patients with cardiomyopathy PI: Nadia Nathan, MD An investigation of the reproducibility of functional MRI of the human brain during hand movement, tingling and pain stimulations PI: Robert Small, MD National Awake Intubation PI: Sergio Bergese, MD Clinical validation of the MEDRAD monitor PI: Sergio Bergese, MD A randomized, double-blind, placebo-controlled study of the dose-effects of neostigmine in spinal anesthesia PI: Yun Xia, MD, PhD DEPARTMENT OF EMERGENCY MEDICINE Infected elders in the ED: outcomes and processes of care PI: Jeffrey Caterino, MD A randomized, double-blind, double-dummy, placebo-controlled, 4 X 4 factorial design trial to evaluate Telmisartan 20, 40 and 80 mg tablets in combination with Amlodipine 2.5, 5 and 10 mg capsules after eight weeks of treatment in patients with stage I PI: Robert Guthrie, MD Although enrollment in clinical trials at Ohio State is 16 percent higher than the national average, the University is working to increase patient participation. Validation of a mortality prediction model for acutely decompensated heart failure patients PI: Brian Hiestand, MD ACTIVE CLINICAL TRIALS Induction of mild hypothermia in rescued cardiac arrest patients using traditional cooling techniques versus medivance arctic sun temperature management system PI: Michael Sayre, MD Hundreds of researchers are involved in hundreds of active clinical trials at Ohio State University Medical Center. Here are some of the most significant current studies as reported by clinical departments: 2007 Research Report 121 DEPARTMENT OF FAMILY MEDICINE Multicenter, randomized, double-blind study to evaluate the efficacy and safety of ezetimibe Simvastatin and niacin (extended release tablet) co-administered in patients with type IIa or type IIb hyperlipidemia PI: Patrick Fahey, MD Multicenter, randomized, double-blind, placebo-controlled, phase III trial to evaluate the efficacy and safety of Saxagliptin (BMS-477118) in combination with Metformin in subjects with type 2 diabetes who have inadequate glycemic control on metformin alone PI: William Miser, MD Multicenter, randomized, double-blind study to compare the effects of 24 weeks of treatment with LAF237 (50 mg qd, 50 mg bid or 100 mg qd) to placebo in drug naïve patients with type 2 diabetes PI: William Miser, MD Double-Blind, randomized, controlled, multicenter study to evaluate the safety, tolerability and immunogenicity of a refrigerator-stable formulation of Zoster Vaccine Live (Oka/Merck) PI: William Miser, MD Six-week, multicenter, randomized, parallel-group treatment regimen study to evaluate the efficacy of initial high dose valsartan monotherapy (160 mg) or combination therapy (valsartan + hydrochlorothiazide 160/12.5 mg) PI: Patrick Fahey, MD DEPARTMENT OF INTERNAL MEDICINE Division of Cardiovascular Medicine A CHF trial investigating outcomes of exercise (HF-ACTION) PI: William Abraham, MD Chronicle implantable cardioverter defibrillator (Reduce-HF) PI: Garrie Haas, MD Hemodynamically guided home self-therapy in severe heart failure patients (Homeostasis II) PI: Garrie Haas, MD The role of diagnosis and treatment of sleep apnea in the acute exacerbation of heart failure PI: Rami Khyat, MD Multicenter automatic defibrillator implantation trial II (MadditII) PI: Charles Love, MD Trial to assess chelation therapy (TACT) PI: Raymond Magorien, MD Reduction of infarct expansion and ventricular remodeling with Erythropoietin after acute MI (REVEAL) PI: Subha Raman, MD Division of Digestive Health Phase 1B pilot study evaluating oral administration of freezedried black raspberries in patients with Barrett esophagus PI: Gary Stoner, PhD; Co-PI: John Fromkes, MD Division of Endocrinology, Diabetes and Metabolism Group study comparing the efficacy and safety of intravenous zoledronic acid, 5 mg once yearly, and oral risedronate, 5 mg daily, in the prevention and treatment of corticosteroidinduced osteoporosis PI: Rebecca Jackson, MD Clinical center for clinical trials and observational study of the Women’s Health Initiative PI: Rebecca Jackson, MD Protein kinase a and its role as a mediator of both nf1- and nf2-associated schwann cell tumorigenesis PI: Lawrence Kirschner, MD, PhD Center for stress and wound healing - core D: Endocrinology core. PI: William Malarkey, MD Prevention of cardiovascular disease in diabetes mellitus-clinical center network PI: Kwame Osei, MD BARI II: Revascularization and glycemic control in NIDDM PI: Kwame Osei, MD Exenatide in islet cell transplantation in non-human primate model PI: Kwame Osei, MD 122 Ohio State University Medical Center Division of General Internal Medicine Division of Immunology A phase I/II open label, dose-escalation trial to explore the safety and efficacy of ICL670 in patients with iron overload resulting from hereditary hemochromatosis PI: Mark Wurster, MD A randomized, phase III, controlled, double-blind, parallel group, multicenter study to evaluate the safety and efficacy of rituximab in combination with methotrexate (mtx) compared to mtx alone in methotrexate-naive patients with active rheumatoid arthritis Co-PIs: Ronald Whisler, MD, & Kevin Hackshaw, MD Added effect of home blood pressure monitors in indigent adults PI: Robert Murden, MD Warfain therapy using computer-assisted guidance PI: Harrison Weed, MD Division of Hematology and Oncology Phase II study of efficacy and tolerability of GW572016 in patients with advanced hepatocellular and biliary carcinomas PI: Tony Bekaii-Saab, MD Phase II CRC study of flavopiridol administered as 30-minute loading dose followed by four-hour continuous infusion in patients with previously treated B-cell chronic lymphocytic leukemia (CLL) or prolymphocytic leukemia arising from CLL PI: John Byrd, MD Phase I study of decitabine in combination with valproic acid in patients with selected hematologic malignancies PI: Guido Marcucci, MD Abatacept in rheumatoid arthritis PI: Kevin Hackshaw, MD Humira efficacy response optimization study in subjects with active rheumatoid arthritis PI: Kevin Hackshaw, MD Efficacy and safety of GW406381, 5 mg, 10 mg, 25 mg and 50 mg administered orally once daily in adults with rheumatoid arthritis PI: Kevin Hackshaw, MD Division of Infectious Diseases Adult AIDS Clinical Trials Unit PI: Susan Koletar, MD Family-centered, community-based, coordinated HIV care program (FACES) PI: Susan Koletar, MD Phase I-II study of inhaled doxorubicin (doxorubicin HCl inhalation solution, resmycin) plus IV docetaxel and cisplatin in patients with locally advanced or metastatic unresectable non-small cell lung cancer PI: Gregory Otterson, MD Phase II, dose-escalating, placebo-controlled, double-blind parallel group study in HIV treatment-experiences in patients to evaluate the safety, tolerability and efficacy of PA103001-04 PI: Susan Koletar, MD Phase II study of histone deacetylase inhibitor SAHA (vorinostat) in patients with metastatic thyroid carcinoma PI: Manisha Shah, MD A phase III, randomized, double-blind comparative study of micafungin PI: Julie Mangino, MD Division of Human Genetics AIDS Education and Training Center PI: Michael Para, MD Frequency and clinical spectrum of germline PTEN mutations in a population-based series of incident breast cancer cases in central Ohio PI: Charles Shapiro, MD Dose optimization trial of three doses of tipranvir boosted with low-dose ritonavir in multiple antiretroviral drug-experienced subjects PI: Michael Para, MD Endocrine Neoplasia Repository PI: Richard Kloos, MD Vicriviroc (SCH417690) in combination treatment with optimized ART regimen in experienced subjects PI: Michael Para, MD The Columbus-area HNPCC (hereditary nonpolyposis colon cancer) study PI: Albert de la Chapelle, MD, PhD Characterization of mutations in the PMS2 gene in samples from the Human Cancer Genetics Sample Bank PI: Albert de la Chapelle, MD, PhD 2007 Research Report 123 Division of Nephrology African-American study of kidney disease and hypertension (AASK) cohort study (2002-2007) PI: Lee Hebert, MD Prospective, multinational, multicenter, double-blind, randomized, active-controlled trial to compare the effects of lotrel (amlodipine/benazepril) to benazepril and hydrochlorothiazide combined on the reduction of cardiovascular morbidity and mortality in patients with high-risk hypertension PI: Anil Agarwal, MD Trial to reduce cardiovascular events with Aranesp® (darbepoetin alfa) therapy PI: Anil Agarwal, MD Randomized, double-blind, placebo-controlled, four-arm parallel-group, multicenter, multinational safety and efficacy trial of 100 mg and 300 mg of LJP 394 in systemic lupus PI: Lee Hebert, MD Erythematosus (SLE) patients with a history of renal disease (2004-2007) PI: Lee Hebert, MD Prospective, randomized, double-blind, active-controlled, parallel group, multicenter trial to assess the efficacy and safety of mycophenolate mofetil (MMF) in inducing response and maintaining remission in subjects with lupus nephritis (2005-2009) PI: Brad Rovin, MD Phase III, randomized, double-blind, placebo-controlled, multicenter study to evaluate the efficacy and safety of rituximab in subjects with ISN/RPS class III or IV Lupus (2006-2008) PI: Brad Rovin, MD Randomized, double-blind, placebo-controlled study of XL784 administered orally to subjects with albuminuria due to diabetic nephropathy (2206-2007) PI: Christopher Valentine, MD Division of Pulmonary, Allergy, Critical Care and Sleep Medicine Study of acid reflux and asthma (multicenter trial) PI: John Mastronarde, MD Exercise-induced bronchoconstriction in college athletes Co-PIs: Jonathan Parsons, MD, & John Mastronarde, MD BMI and ICU outcomes PI: James O’Brien, MD ICU-acquired paresis and clinical outcomes (multicenter consortium) PI: Naeem Ali, MD DEPARTMENT OF NEUROLOGICAL SURGERY Phase III randomized evaluation of convection enhanced delivery of IL 13-PEQQR compared to gliadel wafer with survival endpoint in gliobastoma multiforme patients at first recurrence PI: E. Antonio Chiocca, MD, PhD Phase 1B study of ADV-tk + Valacyclovir gene therapy in combination with standard radiation therapy for malignant gliomas PI: E. Antonio Chiocca, MD, PhD Phase II trial involving patients with recurrent PCNSL (primary central nervous system lymphoma) treated with carboplatin/BBBD (blood brain barrier disruption) by adding rituxan (rituximab), an anti-CD-20 antibody, to the treatment regimen PI: John McGregor, MD DEPARTMENT OF NEUROLOGY Clinical trial of IGF-1 in amyotrophic lateral sclerosis PI: Steven Nash, MD Normal aged brains for the neurodegenerative disease brain tissue repository - Buckeye Brain Bank PI: Douglas Scharre, MD Proteonomics of HIV-associated emphysema PI: Philip Diaz, MD A multicenter, double-blind, randomized study comparing the combined use of interferon beta 1-a and glatiramer acetate to either agent alone in patients with relapsing remitting multiple sclerosis (CombiRx-Phase III) PI: Kottil Rammohan, MD Long-term oxygen therapy trial in COPD (multicenter trial) PI: Philip Diaz, MD SPS3 secondary prevention of small subcortical strokes PI: Andrew Slivka, MD Performance of CPAP and bi-Flex in treating SDB in a heart failure population PI: Rami Khayat, MD Phase II trial of dose intensive Temozolomide in combination with Erlotinib (Tarceva) for patients with recurrent malignant astrocytomas PI: Herbert Newton, MD 124 Ohio State University Medical Center Phase III, multicenter, randomized, double blind, placebo-controlled study of the effect of the daily treatment with MPC7869 on measures of cognitive and global function in subjects with mild to moderate dementia of the Alzheimer’s type PI: Douglas Scharre, MD DEPARTMENT OF OBSTETRICS AND GYNECOLOGY Multicenter prospective clinical study to evaluate the performance and clinical predictive value of the Invader HPV HR molecular assay and Invader HPV 16/18 molecular assay for the defection of human papillomavirus in cervical cytology samples PI: Deborah Bartholomew, MD Phase II study of gemcitabine/carboplatin/bevacizumab in platinum sensitive recurrent ovarian, fallopian tube or primary peritoneal cancer patients PI: Larry Copeland, MD Manipulation of vascular endothelial growth factor (VEGF) to regulate reproductive efficiency PI: Douglas Danforth, PhD STTARS trial – supplental seventeen alpha hydroxy progesterone to prevent preterm birth in twins and triplets PI: Jay Iams, MD Phase 1b active immunotherapy with HER-2 multi-epitope vaccine PI: Pravin Kaumaya, PhD Open label extension study to evaluate the safety and tolerability of ranibizumab in subjects with CNV secondary to AMD who have completed previous treatment with ranibizumab PI: Robert Chambers, DO Open label, multicenter trial of maintenance intravitreous injections of Macugen given every six weeks for 48 weeks in subjects with subfoveal neovascular AMD initially treated with a modality resulting in maculopathy improvement PI: Alan Letson, MD A 48-month, multicenter, randomized, double-masked, placebo-controlled clinical study to evaluate the effectiveness and safety of oral Memantine in daily doses of 20 mg and 10 mg in patients with chronic open-angle glaucoma PI: Paul Weber, MD Ocular Hypertension Treatment Study (OHTS) PI: Paul Weber, MD A study to evaluate the clinical and microbial efficacy of 0.6% ISV-403 compared to vehicle in the treatment of bacterial conjunctivitis PI: Thomas Mauger, MD DEPARTMENT OF ORTHOPAEDICS Double-blind, multicenter, phase III study comparing the efficacy and safety of OMS 103HP with vehicle in patients undergoing allograft ACL reconstruction Co-PI: Christopher Kaeding, MD A prospective, multicenter, open-label study to evaluate the safety and efficacy of the 28-day oral contraceptive DR-1021 PI: Lisa Keder, MD Double-blind, multicenter, phase III study comparing the efficacy and safety of OMS 103HP with vehicle in patients undergoing autograft ACL reconstruction Co-PI: Christopher Kaeding, MD A placebo-controlled, randomized, double-blind, parallel group, at-home exploratory study to evaluate the efficacy and safety of intranasally administered PT-141 in subjects with female sexual arousal disorder PI: Jonathan Schaffir, MD Fixation methods using alternative implants for the treatment of hip fractures (FAITH) PI: Laura Phieffer Hip fracture evaluation with alternatives of total hip arthroplasty versus hemi-arthroplasty (HEALTH) PI: Cornel Van Gorp, MD DEPARTMENT OF OPHTHALMOLOGY Multicenter, randomized trial of lutein, zeaxanthin and w-3 fatty acids in age-related macular degeneration PI: Robert Chambers, DO Embolic events detected with transesophageal echocardiography (TEE) and transcranial doppler (TCD) during total knee arthoplasty with use of RIA: a blinded, randomized controlled clinical trial PI: Cornel Van Gorp, MD Peribulbar triamcinolone for diabetic macular edema PI: Frederick Davidorf, MD 2007 Research Report 125 DEPARTMENT OF OTOLARYNGOLOGY – HEAD AND NECK SURGERY Optimal unilateral otosclerosis treatment: hearing aid versus stapedectomy PI: Edward Dodson, MD; Co-PI: D. Bradley Welling, MD, PhD Trial of lymphatic mapping and sentinel node lymphadecectomy for patients with T1 or T2 clinical N0 oral cavity squamous cell carcinoma PI: David Schuller, MD Incorporation of intensity modulated radiotherapy and submandibular gland transfers to minimize treatment morbidity PI: David Schuller, MD Phase Ib pilot study evaluating oral administration of freezedried black raspberries in presurgical patients with oral squamous cell carcinoma PI: Amit Agrawal, MD Pilot study evaluating long-term oral administration of freezedried black raspberries in postsurgical Appalachian oral cancer patients (pending) PI: Amit Agrawal, MD DEPARTMENT OF PATHOLOGY Validation of semi-automated digital imaging algorithms for ER/PR and Her-2 immunoreactivity in human breast cancer PI: Sanford Barsky, MD Correlative FISH studies for a multicenter, double blind, randomized, parallel-group study of the efficacy and safety of two lenalidomide dose regimens in subjects with relapsed or refractory B-cell chronic lymphocytic leukemia PI: Nyla Heerema, PhD Quantitative CD42 flow analysis for CLL and ALL clinical CALGB studies PI: Gerard Lozanski, MD Validation of the VERSANT HIV-1 RNA 3.0 assay (bDNA) using the VERSANT 440 molecular system PI: Preeti Pancholi, PhD Clinical evaluation of the XPERT SA/MRSA assay PI: Preeti Pancholi, PhD Multidrug resistant Acinetobacter baumannii synergy testing PI: Preeti Pancholi, PhD Evaluation and validation of SELDI-based diagnostic test PI: Haifeng Wu, MD 126 Ohio State University Medical Center DEPARTMENT OF PEDIATRICS Trial of automated risk appraisal for adolescents (TARAA) PI: Kelly Kelleher, MD Postconcussive symptoms in children with mild head injury PI: Keith Yeates, PhD Phase I clinical trial of rAAV2.5-CMV-mini-dystrophin gene vector in Duchenne muscular dystrophy PI: Jerry Mendell, MD The stress response system, health risk behaviors and decision-making in adolescent girls PI: Kathy Pajer, MD Growth hormone in cystic fibrosis PI: Dana Hardin, MD Changes in lung structure and function in children with cystic fibrosis PI: Robert Castille, MD DEPARTMENT OF PHARMACOLOGY Phase I study to evaluate the safety, tolerability and pharmacokinetics of a single intravenous dose of CBR-2092 in healthy volunteers PI: Glen Apseloff, MD Ascending single dose study of the safety, tolerability, pharmacokinetics and pharmacodynamics of NRI-022 administered orally to healthy postmenopausal women. PI: Glen Apseloff, MD Study in healthy male and female subjects to assess the pharmacokinetic characteristics and relative bioavailability of hydromorphone following administration of 3 new palladone melt extrusion multiparticulate formulations in the fed and fasted state. PI: Glen Apseloff, MD Double-blind, randomized, single and multiple ascending dose study to determine the safety, tolerability and pharmacokinetics of GSK232802 PI: Glen Apseloff, MD Phase I study of the safety, tolerability and pharmacokinetics of a single intravenous dose of ETI-204 and its potential interaction with ciprofloxacin PI: Glen Apseloff, MD Phase 1, randomized, placebo-controlled, double-blind study to evaluate the safety and tolerability of a single subfascial/intramuscular injection of ALGRX 4975 in healthy male subjects PI: Glen Apseloff, MD DEPARTMENT OF PHYSICAL MEDICINE AND REHABILITATION Efficacy of the individual placement and support (IPS) model for clients with disability and substance use disorders PI: John Corrigan, PhD Effectiveness of a brief educational intervention for reducing substance abuse after TBI PI: John Corrigan, PhD, & Jennifer Bogner, PhD Thrust and meniscal lesions in knee OA in the OAI PI: Rebecca Jackson, MD Multinational, multicenter, double-blind, randomized, placebocontrolled, parallel group study comparing the efficacy of intravenous zoledronic acid in preventing secondary osteoporotic fractures after a hip fracture PI: Velimir Matkovic, MD, PhD Self-regulation in co-occurring TBI and substance abuse PI: Jennifer Bogner, PhD Phase III, randomized, six-month, double-blind trial in subjects with bipolar 1 disorder to evaluate the continued safety and maintenance of effect of ziprasidone plus a mood stabilizer (vs. placebo plus a mood stabilizer) following a minimum of two months of response to open-label treatment with both agents PI: Stephen Pariser, MD Open-label, dose-titration, long-term safety study to evaluate concerta at doses of 36 mg., 54 mg., 73 mg., 90 mg. and 108 mg per day in adults with attention-deficit/hyperactivity disorder, protocol #12-304 (the “Study”) PI: Dan Martin, MD Randomized, double-blind, placebo-controlled, multicenter study to evaluate the efficacy, safety and tolerablity of licarbazepine in the treatment of manic episodes of bipolar 1 disorder over six weeks PI: Radu Saveanu, MD 52-week, open-label extension study to evaluate the efficacy, safety and tolerablity of licarbazepine in the treatment of manic episodes of bipolar 1 disorder PI: Radu Saveanu, MD Noradrenergic modulation of cognition PI: Sharon McDowell, MD DEPARTMENT OF RADIATION MEDICINE DEPARTMENT OF PSYCHIATRY AND OHIO STATE UNIVERSITY HARDING HOSPITAL Phase II study of submandibular salivary gland transfer to the submental space prior to start of radiation treatment for prevention of radiation-induced xerostomia in head and neck cancer patients PI: John Grecula, MD Randomized, double-blind, placebo-controlled, parallel-group study of the efficacy, safety and tolerability of XBD173 in patients with generalized anxiety disorder PI: Radu Saveanu, MD Phase III, randomized, double-blind, multicenter, placebo-controlled, parallel-group, forced-dose titration, safety and efficacy study of NRP104 in adulkts with ADHD (one year) PI: Radu Saveanu, MD Long-term, open-label, single-arm study of NRP104 30 mg., 50 mg. or 70 mg. per day in adults with ADHD (one year) PI: Radu Saveanu, MD Four-week, double-blind, placebo-controlled phase III trial evaluating the efficacy, safety and pharmacokinetics of flexible doses of oral ziprasidone in children and adolescents with bipolar 1 disorder (manic or mixed) PI: Lacramioara Spetie, MD 26-week, open-label extension study evaluating the safety and tolerability of flexible doses of oral ziprasidone in children and adolescents with bibolar 1 disorder (manic or mixed) PI: Lacramioara Spetie, MD Phase II randomized trial of surgery followed by chemoradiotherapy plus 225 (cetuximab) for advanced squamous cell carcinoma of the head and neck PI: John Grecula, MD Phase II/III randomized trial of two doses (phase III standard vs. high) and two high-dose schedules (phase II-once vs. twice daily) for delivering prophylactic cranial irradiation for patients with limited disease small cell lung cancer PI: John Grecula, MD Phase II trial of motexafin gadolinium with whole brain radiation therapy followed by stereotactic radiosurgery boost in the treatment of patients with brain metastases PI: John Grecula, MD Phase III randomized, open-labeled, comparative study of standard whole brain radiation therapy with supplemental oxygen, with or without concurrent RSR13 (efaproxiral), in women with brain metastases from breast cancer PI: John Grecula, MD 2007 Research Report 127 DEPARTMENT OF RADIOLOGY Aside from many clinical trials within the Department, Radiology supports a number of trials outside the Department, including National Cancer Institute phase I and II studies. Radiology’s Imaging Research Division has been instrumental in researching contrast agents for patient care. The Division is a core research lab for Novartis Pharmaceuticals Corporation, the Cancer and Leukemia Group B (CALGB) and others. Development of an integrated dynamic breast imaging system for the early detection of breast cancer PI: Stephen Povoski, MD Analysis of a novel strategy which suppresses aggressive (CD4-independent) CD8+ T cell-initiated hepatocyte rejection PI: Ginny Bumgardner, MD, PhD DEPARTMENT OF UROLOGY PI: Michael Knopp, MD, PhD In regard to the CALGB, its “impact” in obtaining further outside funding has exceeded initial expectations of $500,000 to $800,000. Clinical trials in conjunction with Novartis Pharmaceuticals Corporation total more than $1 million in budgeted research. They support clinical studies, education and training of post-docs and graduate research associates. The Department’s imaging research team, led by Michael Knopp, MD, PhD, will build on the work of the Wright Center and Imaging Core Lab to expand services to Ohio State’s Comprehensive Cancer Center as part of the Imaging Response Assessment Team (IRAT) award. IRAT provides resources for advanced imaging response assessments of in-house trials. It also supports investigator-initiated multicenter trials. Neuroendocrine studies in intestitial cystitis PI: Jason Gilleran, MD A phase II trial combining tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. PI: Robert Bahnson, MD SCHOOL OF ALLIED MEDICAL PROFESSIONS (Two clinical trials were completed in 2006 as no-cost extensions. Both were multi-institutional and NIH-funded, focusing on stroke recovery.) The Constraint Induced Therapy Evaluation Site PI: Deborah Nichols-Larsen, PhD DEPARTMENT OF SURGERY Prevention of internal hernias during laparoscopic Roux-en-Y gastric bypass with bioabsorbable Seamguard material PI: Dean Mikami, MD Randomized, double-blind, placebo-controlled, dose-ranging, multicenter study to evaluate the cardiovascular and cerebrovascular effects of MC-1 in patients undergoing high-risk coronary artery bypass graft (CABG) surgery PI: Benjamin Sun, MD Comparison of primary patency between Gore-Tex Propaten vascular grafts and thin walled Gore-Tex stretch vascular grafts PI: Patrick Vaccaro, MD Phase I trial of IL-21 in combination with weekly paclitaxel and trastuzumab in patients with HER2-positive malignancies PI: William Carson, III, MD 128 Ohio State University Medical Center Motor map plasticity in constraint therapy for stroke Site PI: Deborah Nichols-Larsen, PhD Here are some of the most significant current clinical trials as reported by centers and institutes at Ohio State: COMPREHENSIVE CANCER CENTER Phase II randomized trial of sequentially administered CPT-11 and mitomycin C in patients with advanced esophageal and stomach cancer PI: Miguel Villalona, MD Phase III study of daunorubicin and cytarabine +/- G3139 (Genasense, Oblimersen Sodium, NSC # 683428, IND # 58842), a BCL2 antisense oligodeoxynucleotide, in previously untreated patients with acute myeloid leukemia (AML) >/= 60 years PI: Guido Marcucci, MD Dose-escalation study of flavopiridol (NSC 649890) administered as a 30-minute loading dose followed by a four-hour infusion in patients with previously treated B-cell chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) PI: Thomas Lin, MD, PhD Phase I study of decitabine in combination with valproic acid in patients with selected hematologic malignancies PI: Guido Marcucci, MD Phase II study of efficacy and tolerability of GW572016 in patients with advanced hepatocellular and biliary carcinomas PI: Tony Bekaii-Saab, MD Phase II study of bevacizumab and interferon-alpha-2b in metastatic malignant melanoma PI: William Carson III, MD CENTER FOR MINIMALLY INVASIVE SURGERY Novel repair techniques of gastrointestinal perforations PI: Jeffrey Hazey, MD Transgastric bacterial contamination PI: Jeffrey Hazey, MD Diagnostic translumenal endoscopic peritoneoscopy PI: Jeffrey Hazey, MD The evaluation of the Sightline ColonoSight System PIs: W. Scott Melvin, MD; Dean Mikami, MD Transgastric bacterial contamination PI: W. Scott Melvin, MD DOROTHY M. DAVIS HEART AND LUNG RESEARCH INSTITUTE Prevention of internal hernias during laparoscopic roux-en-y gastric bypass with bioabsorable SEAMGUARD material (BSG) PI: Dean Mikami, MD CHF trial investigating outcomes of exercise PI: William Abraham, MD Lap-band adjustable gastric banding system: a post-market study proposal PI: Bradey Needleman, MD Chronicle implantable cardioverter defibrillator (Reduce-HF) PI: Garrie Haas, MD Hemodynamically guided home self-therapy in severe heart failure patients (Homeostasis II) PI: Garrie Haas, MD Reduction of infarct expansion and ventricular remodeling with Erythropoietin after acute MI (REVEAL) PI: Subha Raman, MD Study of acid reflux and asthma (multi-center trial) PI: John Mastronarde, MD Proteonomics of HIV-associated emphysema PI: Philip Diaz, MD Laparoscopic gastric bypass (EEA-LGB): a prospective randomized comparison of the 3.5 mm vs. 4.8 mm circular stapler for creation of the gastrojejunostomy in prevention of staple line hemorrhage during laparoscopic gastric bypass PI: Bradley Needleman, MD THE NISONGER CENTER FOR MENTAL RETARDATION AND DEVELOPMENTAL DISABILITIES Multicenter, double-blind, randomized, placebo-controlled, parallel-group study with three fixed doses of Aripiprazole in the treatment of children and adolescents with autistic disorder PI: Michael Aman, PhD Long-term oxygen therapy trial in COPD (multicenter trial) PI: Philip Diaz, MD Performance of CPAP and bi-Flex in treating SDB in a heart failure population PI: Rami Khayat, MD 2007 Research Report 129 DARDINGER NEURO-ONCOLOGY CENTER Phase III randomized evaluation of convection enhanced delivery of IL 13-PEQQR compared to gliadel wafer with survival endpoint in gliobastoma multiforme patients at first recurrence PI: E. Antonio Chiocca, MD, PhD Phase Ib study of ADV-tk + Valacyclovir gene therapy in combination with standard radiation therapy for malignant gliomas PI: E. Antonio Chiocca, MD, PhD Phase II trial involving patients with recurrent primary central nervous system lymphoma treated with carboplatin/blood brain barrier disruption, by adding Rituxan (Rituximab), an anti-CD-20 antibody, to the treatment regimen PI: John McGregor, MD Phase I/II study of intra-arterial carboplatin and oral temodar for the treatment of metastatic brain tumors PI: Herbert Newton, MD Phase II trial of continuous dose temozolomide in patients with newly diagnosed anaplastic oligodendroglioma and mixed oligoastrocytoma PI (local): Herbert Newton, MD 130 Ohio State University Medical Center Phase V gliasite radiation therapy system registry protocol for the treatment of resectable malignant brain tumors PI (local): Herbert Newton, MD Phase III randomized, double-blind study comparing human corticotropin-releasing factor (hCRF) to dexamethasone for control of symptoms associated with peritumoral brain edema in patients with primary malignant glioma PI (local): Herbert Newton, MD Phase III randomized, double-blind, dexamethasone-sparing study comparing human corticotropin-releasing factor (hCRF) to placebo for control of symptoms associated with peritumoral brain edema in patients with malignant brain tumor who require chronic administration of high-dose dexamethasone PI (local): Herbert Newton, MD Open-label, extended-use study of human corticotropinreleasing factor (hCRF) intended for patients who participate in the dexamethasone-sparing studies NTI 0302 or NTI 0303 PI (local): Herbert Newton, MD Faculty Serving on NATIONAL STUDY SECTIONS AND REVIEW PANELS Ohio State Medical Expertise Tapped for Service 2007 Research Report 131 Many Ohio State University faculty members have been invited to serve on National Institutes of Health (NIH) study sections or national-level grant-review panels in the medical sciences. Here is a listing: Yong Xia, MD - Grant Reviewer • American Heart Association • American Diabetes Association Jay Zweier, MD - Grant Reviewer • NIH Study Section – Ad Hoc Grant Reviewer DAVIS HEART AND LUNG RESEARCH INSTITUTE (DHLRI) Rita Alevriadou, PhD (see Internal Medicine – Cardiovascular Medicine) • NIH, NHLBI (National Heart, Lung and Blood Institute) BTSS (Bioengineering, Technology and Surgical Sciences) – Member Estelle Cormet-Boyaka, PhD • USDA (United States Department of Agriculture) National Research Initiative – Ad Hoc Reviewer Elliott Crouser, MD – Ad Hoc Grant Reviewer • Burroughs Wellcome Foundation • American Lung Association (Ohio Chapter) • Society of Critical Care Medicine, Vision Grants Sandor Gyorke, PhD (see Physiology & Cell Biology) – Ad Hoc Grant Reviewer • Electrical Signaling, Ion Transport Study Section • NIH, CVA (Cardiovascular) Study Section • NIH, NHLBI Study Section Periannan Kuppusamy, PhD - Grant Reviewer • NIH, Division of Research Resources Study Section • NIH/NCI (National Cancer Institute) Study Section • NIH, Reparative Medicine Study Section • NIBIB (National Institute of Biomedical Imaging and Bioengineering) Study Section Clay Marsh, MD – Ad Hoc Grant Reviewer • NIH, NHLBI, LCMI (Lung Cellular, Molecular and Immunology) Study Section – Ad Hoc Grant Reviewer • NIH, NHLBI, LIRR (Lung Injury, Repair and Remodeling) study section – Ad Hoc Grant Reviewer • NIH, NIAID (National Institute of Allergy and Infectious Diseases), CORT (Center of Research Translation) review panel – Ad Hoc Grant Reviewer • NIH, NHLBI, P01 Review Panel • Sarnoff Foundation for Cardiovascular Sciences John Mastronarde, MD - Program Grant Reviewer for Clinical Research Centers • Wellcome Trust (UK) Mark Wewers, MD - Grant Reviewer • NIH, LCMI (Lung Cellular and Molecular Immunobiology) study section – Ad Hoc Grant Reviewer • NIH, NIGMS, Glue Grant Review Committee • British Lung Foundation – Ad Hoc Grant Reviewer • Alpha-1 Foundation, Grants Advisory Committee 132 Ohio State University Medical Center INSTITUTE FOR BEHAVIORAL MEDICINE RESEARCH (IBMR) Charles Emery, PhD • NIH Center for Scientific Review Special Emphasis Panel: Risk, Prevention & Health Behavior Integrated Review Group – Chair • Scientific Advisory Council, American Association of Cardiovascular and Pulmonary Rehabilitation William Malarkey, MD • National Academy of Science - Institute of Medicine Panel on Stress Gulf Wars and Health Outcomes Virginia Sanders, PhD • NIH - ZRG1-F07 Review Panel for Pre- and Post-Doctoral Fellowships and AREA grants – Member • NIH - IMM-B (03) – Special Emphasis Panel – Ad Hoc Committee • NIH - NINDS Training grant (T32) Review Panel – Ad Hoc Committee Janice Kiecolt-Glaser, PhD • Ad Hoc NIA Panel for RFP on aging and inflammation Randy Nelson, PhD • Biotechnology and Biological Sciences Research Council – Ad Hoc Member • NIH Chronic Fatigue Syndrome – Ad Hoc Member • NINDS Interdisciplinary Center Core (P-30)/Neuroscience Blueprint – Regular Member • United States - Israel Binational Science Foundation – Ad Hoc Member Ning Quan, PhD • Center for Scientific Review – Neuroendocrinology, Neuroimmunology and Behavior Study Section – Permanent Member Ronald Glaser, PhD • Institute of Medicine’s Committee on Military Research • President – Academy of Behavioral Medicine Research, Institute of Medicine Committee • Member of National Space Biomedical Research Institute (NSBRI), Immunology, Infection and Hematology Team, Houston, Texas NISONGER CENTER L. Eugene Arnold, MD • NIH Review Committee - Center Grant, and NIMH (National Institute of Mental Health) Data and Safety Monitoring Board – Ad Hoc Committee INTERNAL MEDICINE Cardiovascular Medicine B. Rita Alevriadou, PhD • NIH, National Heart, Lung and Blood, BTSS (Bioengineering, Technology and Surgical Sciences) – Regular Reviewer Subha Raman, MD, MS • NIH, Grant Reviewer: Member of Special Emphasis Panel of the National Institute of Diabetes and Digestive and Kidney Diseases ZDK1 GRB-7 (01) – Ad Hoc Reviewer • NIH NIDDK Grant Reviewer: PAR-04-065 – Research Grants for Clinical Studies of Kidney Diseases – Ad Hoc Reviewer • NIH NIDDK Grant Reviewer: Review of Revised Applications for PAR-04-065 – Ad Hoc Reviewer Sanjay Rajagopalan, MD • NIH, National Heart, Lung and Blood Institute: (CCIS) Clinical Cardiovascular and Integrative Sciences – Permanent Member • Special Emphasis Panel National Institute of Environmental Sciences, Particle Center Grants Review Panel – Ad Hoc Member • Flight Attendant Medical Research Institute (FAMRI), Grants Review – Panel Member • American College of Cardiology Young Investigator Awards Selection Panel – Permanent Member Endocrinology, Diabetes & Metabolism Rebecca Jackson, MD • NIH, Osteoarthritis Initiative Steering Committee • NIH, Women’s Health Initiative (WHI), Case-Control Analyte Working Group - Chair • NIH, Women’s Health Initiative (WHI), Executive Committee – Vice Chair • NIH, WHI Executive Committee (MW PI Chair) • NIH, WHI, Calcium and Vitamin D Committee, Co-Chair Richard Kloos, MD • Panel Member to develop ATA Guidelines for the Management of Thyroid Nodules and Differentiated Thyroid Cancer • ATA Clinical Affairs Committee Member William Malarkey, MD • NIH, CCAM (National Center for Complementary and Alternative Medicine) – Review of Strategic Plan • National Academy of Sciences – Institute of Medicine Committee on Stress, Gulf War and Health Kwame Osei, MD • NIH, Scientific Study Section, Metabolism Subcommittee B • National Medical Association of Black Physicians, Endocrinology Section – Chair • American Federation of Clinical Research, Endocrinology Section – Chair • International Society of Hypertension in Blacks, Obesity and Metabolism Section – Chair Matthew Ringel, MD • ATA (American Thyroid Association) Executive Council Member • ATA Research Committee • ATA Member Executive Steering Committee for Clinical Research in Thyroid Cancer • National Advisory Board for ThyCa, Thyroid Cancer Survivors Organization • Endocrine Society Membership Committee Dara Schuster, MD • NIH Loan Repayment Application Program – Ad Hoc Reviewer General Internal Medicine Catherine Lucey, MD • American Board of Internal Medicine Board of Directors, Reviewer • National ACP Associates Scientific Competition, Reviewer Hematology & Oncology Clara D. Bloomfield, MD • CALBG (Cancer and Leukemia Group B), Correlative Sciences Committee Member • CALGB Board of Directors • CALBG Leukemia Core Committee Member • CALGB Nominating Committee for CALGB Group Statistician Chair John Byrd MD • NCI (National Cancer Institute), RAID Grant Program – Ad Hoc Reviewer • CALGB Clinical Research & Jr. Faculty Awards Program Review Committee • CALGB Lymphoma Committee Member • CALGB Faculty and Young Investigator Grants Review Committee • CALGB Young Investigator Grants Review Committee • CALGB Leukemia Correlative Science Committee – ViceChair • CALGB Leukemia Committee – Member 2007 Research Report 133 • NCI (National Cancer Institute) Subcommittee H-Clinical Groups, – Member • NCI Loan Repayment Special Interest Panel – Ad Hoc Reviewer Michael Caligiuri, MD • NCI Board of Scientific Counselors for Clinical Sciences – Member • NCI Adolescent & Young Adult Oncology Progress Review Group – Co-Chair • NCI Translational Research Working Group – Member • CALGB Board of Directors – Member • CALGB Leukemia Correlative Science – Committee Chair Steven Clinton, MD, PhD • CALGB Cancer Control and Health Outcomes Core – Committee Member • CALGB Prevention Subcommittee – Member • CALGB GU Committee – Liaison • National Institutes of Health – National Cancer Institute, Oncological Sciences Integrated Review Group, Center for Scientific Review, Chemoprevention and Dietary Prevention Study Section – Member Steven Devine, MD • CALGB Transplant Committee – Member Richard Love, MD • National Institute of Oncology, International Advisory Committee, Rabat, Morocco – Chair Guido Marcucci, MD • National Institute of Environmental Health Sciences – Special Emphasis Panel Member • NCI PO1 Clinical SEP Review Panel • NCI Tumor Microenvironment Consortium – Grant Application Reviewer Charles Shapiro, MD • CALGB Symptom Intervention Committee – Chair • CALGB Breast Core Committee – Cadre Member Miguel Villalona, MD • NCI Investigational Drug Steering Committee – Member • NCI Clinical Trial Design Task Force – Member • NCI Conflict of Interest Task Force – Member • NIH, NIAID Study Section, Host Interactions with Bacterial Pathogens – Ad Hoc Reviewer • Executive Advisory and Review Board, Great Lakes Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research Joanne Turner, PhD • NIH NIA (National Institute on Aging) Special Emphasis Panel ZAG1 ZIJ-5: Aging and Inflammation – Ad Hoc Reviewer • NIH NIA Cellular Mechanisms in Aging and Development • (CMAD) – Ad Hoc Reviewer John Gunn, PhD • NIH, NIAID Bacterial Pathogenesis Study Section – Permanent Member Nephrology Kevin Hackshaw, MD • NIH, Chronic Fatigue Syndrome Special Emphasis Panel – Ad-Hoc Reviewer Lee Hebert, MD • NIH DSMB (Data and Safety Monitoring Board) for Autoimmune Disease Trials – Permanent Reviewer • Kidney Disease Improving Global Outcomes (KDIGO) – Permanent Reviewer Brad Rovin, MD • NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases) Study Sections – Ad Hoc Reviewer Anil Agarwal, MD • American Society of Nephrology/NephSap Review Board • NIH Data and Safety Monitoring Board (DSMB) for Autoimmune Disease Trials – Permanent Reviewer ANESTHESIOLOGY Fedias Christofi, PhD • VA Research and Development Awards CDA02 – Grant Reviewer Infectious Diseases BIOMEDICAL INFORMATICS Larry Schlesinger, MD • Chair, NIH, NIAID Study Section, ZAI1 DDS-M (C2), Tuberculosis Research Unit – Member • NIH Study Section, ZRG1 F13 20 L, Infectious Diseases and Microbiology Joel Saltz, MD, PhD • National Library of Medicine (BLIRC) Biomedical Library and Informatics Review Committee 134 Ohio State University Medical Center EMERGENCY MEDICINE Mark Angelos, MD • NIH, Myocardial Ischemia and Metabolism Study Section – Temporary Member • Special Emphasis Panel ZRG1 SSS-W (10) Cardiovascular Devices – Permanent Member Michael Sayre, MD • Special Emphasis Panel ZRG1 SSS-W (10) Cardiovascular Devices – Temporary Member FAMILY MEDICINE Tom Best, MD, PhD • Canadian Institutes of Health Research, Review Panel • U.S. Army Research Institute of Environmental Medicine, Scientific Review Panel Larry Gabel, PhD • Department of Health and Human Services Standing Grant Peer Review Panel - American Academy of Family Physicians Foundation Research Grant – Peer Review Panel W. Fred Miser, MD • Department of Health and Human Services Standing Grant Peer Review Panel - American Academy of Family Physicians Foundation Research Grant – Peer Review Panel Randell Wexler, MD, MPH, FAAFP • Department of Health and Human Services Standing Grant Peer Review Panel - American Academy of Family Physicians Foundation Research Grant – Peer Review Panel MOLECULAR AND CELLULAR BIOCHEMISTRY Charles Bell, PhD • Congressionally Directed Medical Research Program in Breast Cancer - Concept/Cell Biology I Panel • U.S. Army Medical Research and Materiel Command National Review Panel Department of Defense National Review Panel Arthur Burghes, PhD • NIH Reviewer – Ad Hoc Member • ALS Grant – Research Reviewer Tsonwin Hai, PhD • NIH Tumor Cell Biology Study Section – Permanent Member • NIH Cellular Aspect of Diabetes and Obesity Study Section – Ad Hoc Member Jeff Kuret, PhD • Alzheimer’s Association, Initial Review Board of the Medical and Scientific Advisory Council, Research Grant Program • NIH Center for Scientific Review, Synapses, Cytoskeleton and Trafficking (SYN) Study Section – Ad Hoc Member Jiyan Ma, PhD • NIH Special Emphasis Panel, ZRG1 IDM-B (90) Studies of Transmissible Spongiform Encephalopathy – Ad Hoc Reviewer Kamal Mehta, PhD • NIH, Atherosclerosis and Inflammation of the Cardiovascular System (AICS) Study Section – Permanent Member Michael Ostrowski, PhD • NCI Program Project Grant Reviews, Molecular and Cellular Biology Programs – Ad Hoc Member • NIH Skeletal Biology, Development and Disease (SBDD) Study Section – Permanent Member Mark Parthun, PhD • NIH, Molecular Genetics A Study Section – Permanent Member • National Science Foundation – Ad Hoc Reviewer MOLECULAR VIROLOGY, IMMUNOLOGY AND MEDICAL GENETICS Denis Guttridge, PhD • NIH/NIAMS SMEP Study Section (Ad Hoc) • DOD, Breast Cancer Research Program/Molecular Biology and Genetics Panel • NIH/NINDS ZNS1 SRB-E • NIH/NIAMS SMEP Study Section (Ad Hoc) Tim Hui-Ming Huang, PhD • Reviewer, National Research Program for Genomic Medicine, Taiwan • Reviewer, NIH Study Section – Cancer Genetics • Site Visit, NCI Intramural Program, Laboratory of Population Genetics • Reviewer, NIH Study Section – Cancer Genetics • Reviewer, NIH Study Section – Cancer Genetics Ramana Davuluri, PhD • Ad Hoc Reviewer, NIH Study Section – ZCA1 • Ad Hoc Reviewer, National Science Foundation – DBI: Plant Genome Research • Program Committee Member, Interface 2007: the 39th Symposium on the Interface of Statistics, Computing Science and Applications, May 23-27, Philadelphia. Organized the invited session entitled “Integrative Systems Biology in Cancer Research” John Gunn, PhD • NIAID BACP Study Section, Member • NIAID BACP-B (09) F Special Emphasis Panel, Chair 2007 Research Report 135 Michael Freitas, PhD • NIH-NCI, Innovative Technologies for Molecular Analysis of Cancer • NIH-NCI, Application of Emerging Technologies for Cancer Research • NIH-NCI, Exfoliated Cells and Circulating DNA in Cancer Detection and Diagnosis • NIH, Biological Chemistry and Macromolecular Biophysics Small Business Activities [SBIR/STTR] Special Emphasis Panel • NIH-NCI, Innovations in Cancer Sample Preparation Christoph Plass, PhD • Leukemia & Lymphoma Society Career Development Grants Review Pearlly Yan, PhD • DOD Breast Cancer Research Program Synergistic Idea Award (Ad Hoc) Gustavo Leone, PhD • AACR Program Committee – Member of Cell Cycle Subcommittee of the Cellular and Molecular Biology Section • American Cancer Society, Cell Cycle & Growth Control Peer Review Committee Caroline Whitacre, PhD • NIH - Hypersensitivity, Autoimmunity and ImmuneMediated Diseases Study Section Virginia Sanders, PhD • Member, Fellowship Study Section, NIH (ZRG1 F07) • Special Emphasis Panel, ZRG1 IMM B (03) • Special Emphasis Panel/Scientific Review Group, ZNS1 SRBM (39) Amy Lovett-Racke, PhD • NINDS "Clinical Neuroimmunology and Brain Tumor" study section Joanna Groden, PhD • Cancer Genetics Study Section – Chair • NIH Cancer Genetics Study Section – Chair • DOD Ovarian Cancer Review Panel for Concept Awards • DOD Breast Cancer Review Panel for Career Development • DOE Committee of Visitors, Biological Sciences Review Panel Deborah Parris, PhD • Florida Biomedical Sciences Grant Review • NIH - Postdoctoral Fellowship Study Section, Genomes and Genomics, Ad Hoc NEUROLOGY John Kissel, MD • American Academy of Neurology, Committee of Sections, Executive Committee – Member • CDC Care Considerations – Member 136 Ohio State University Medical Center • NIH/FDA Symposium on SMA – Chair • MDA Medical Advisory/Grant Review Panel NEUROSCIENCE Christine Beattie, PhD • NIH, NINDS (National Institute of Neurological Disorders and Stroke) ZNS1 SRB-P – Ad Hoc Member • NSF – Ad Hoc Grant Reviewer Michael Beattie, PhD • NIH ZRG1 BDCN-L (50)(R) Study Section – Ad Hoc Member R. Thomas Boyd, PhD • NIH, ZRG 1, FO3B – Study Section Member Anthony Brown, PhD • NIH, Study Section – Ad Hoc Member • NSF (National Science Foundation) – Ad Hoc Grant Reviewer Richard Burry, PhD • NIH, CSR ZRG1 CDF-4 S10, SIG Microscopes – Study Section Member Jacqueline Bresnahan, PhD • NIH Study Section – Ad Hoc Member • Scientific Advisory Council, Christopher Reeve (Paralysis) Foundation Andrew Fischer, PhD • NSF – Ad Hoc Grant Reviewer Paul Henion, PhD • NSF – Ad Hoc Grant Reviewer • NIH, CSR DEV-2 Study Section – Ad Hoc Member C. Glenn Lin, PhD • Alzheimer’s Association Grant Review Committee James Jontes, PhD • NSF – Ad Hoc Grant Reviewer Dana McTigue, PhD • Uniformed Services University of the Health Sciences Brain and Spinal Grant – Grant Reviewer • NIH NSD-C (Neurological Sciences and Disorders – C) Grant Reviewer – Ad Hoc Member • Health Research Council of New Zealand – Grant Reviewer John Oberdick, PhD • NIH, NDPR (Neurodifferentiation, Plasticity & Regeneration) Study Section Member • MDCN-K 90 S, Special Emphasis Panel – Ad Hoc Member • NSF – Ad Hoc Grant Reviewer Karl Obrietan, PhD • NIH Study Section – Ad Hoc Member Michael Xi Zhu, PhD • NIH Study Section ZRG1 MDCN-C – Ad Hoc Member • NIH Study Section ZRG1 MDCN-B 02 – Ad Hoc Member OBSTETRICS AND GYNECOLOGY Douglas Kniss, PhD • NIH Pregnancy and Perinatology Study Section – Ad Hoc Reviewer OTOLARYNGOLOGY D. Bradley Welling, MD, PhD • Congressionally Directed Medical Research Program, Department of Defense, Neurofibromatosis Research Program – Ad Hoc Reviewer James Lang, PhD • NIH/NCI Special Emphasis Panel ZCA1 RTRB-A M2 – Ad Hoc Reviewer • NIH/NCI Special Emphasis Panel ZCA1 RTRB-A M1 – Ad Hoc Reviewer • NIH/NCI Special Emphasis Panel ZCA1 RTRB-A 01 – Ad Hoc Reviewer • NCI K99/R00 Review Committee – Ad Hoc Reviewer • NIH National Center for Research Resources COBRE Awards – Ad Hoc Reviewer • NIH/NCI IRG Study Section NCI-F Institutional Training Grant (T32) and “Pathway to Independence” K99/R00 Awards – Permanent Reviewer Thomas Demaria, PhD • NIH Auditory Systems (AUD) Study Section – Permanent Member PATHOLOGY Sanford Barsky, MD • California Breast Cancer Research Program – Regular Study Section Member • NIH, NCI – Ad Hoc Reviewer • Department of Defense Breast Cancer Research Program – Ad Hoc Reviewer Tatiana Oberyszyn, PhD • NIH/NCI Cancer Etiology Study Section – Permanent Member PEDIATRICS Rachel Altura, MD • American Institute of Biological Sciences – Reviewer, Blood and Cancer Panel for DoD (Department of Defense) • American Institute of Biological Sciences – Reviewer, Childhood Cancer Panel for DoD John Barnard, MD • Gastrointestinal Cell and Molecular Biology Study Section – Permanent Member Jeff Bartlett, MD • NIH GTIE (Gene Therapy and Inborn Errors) Study Section – Permanent Member • ACGT (Alliance for Cancer Gene Therapy) Review Panel – Permanent Member Carl Bates, MD • NIH, Urologic and Kidney Development and Genitourinary Diseases Study Section – Ad Hoc Reviewer John Bauer, PhD • NIH/NIHLBI, Cardiac Contractility and Heart Failure Study Section – Regular Member • NIH/NIHLBI, Metabolism and Myocardial Ischemia and Metabolism Study Section – Ad Hoc Member • American Heart Association, Committee 5B Study Section – Regular Member • National Heart Association Study Section – Regular Member Michael Brady, MD • American Academy of Pediatrics Committee on Infectious Diseases (Red Book Committee) John Campo, MD • AACAP (American Academy of Child and Adolescent Psychiatry) Grant Review Committee Joan Durbin, MD, PhD • Review Panel for the Virology B Study Section – Permanent Member Heithem El-Hodiri, PhD • NIH, Special Emphasis Panel ZRG1 GGG-T52: Genomic and Genetic Analysis in Xenopus Ihuoma Eneli, MD, MS • HRSA (Health Resources and Services Administration), Innovative Approaches to Promoting Healthy Weight in Women, Division of Independent Review – Ad Hoc Member Vijay Pancholi, PhD • Grant Agency of the Academy of Sciences of the Czech Republic – Grant Reviewer • European Research Area (ERA)-net PathoGenomics – Grant Reviewer Gerard Nuovo, MD • NIH Study Section on HIV-1 Pathogenesis – Ad Hoc Reviewer 2007 Research Report 137 Timothy Feltes, MD • NHLBI Pediatric Heart Disease Clinical Research Network – Protocol Review Committee Kim McBride, MD • NIH, Clinical and Integrative Cardiovascular Sciences – Ad Hoc Reviewer William Gardner, PhD • NIH Review Panel ZNS1 SRB-H 25 (Research on Research Integrity Review) – Review Panel Chair • NIMH Review Panel SNRS (Mental Health Services in NonStandard Settings) – Study Section Member James Mulick, PhD • Organization for Autism Research, Scientific Council – Permanent Member Judy Groner, MD • NICHD (National Institute of Child Health and Human Development) DC Initiative to Reduce Infant Mortality in Minority Populations – Ad Hoc Grant Review Thomas Gross, MD, PhD • NIH, NAIAD - Immune Tolerance Network, EBV Review Committee Brett Hall, PhD • Congressionally Directed Medical Research Programs Breast Cancer Con-CET-2 Panel (DoD Breast Cancer) Gail Herman, MD, PhD • NIH, Genetics of Health & Disease Study Section – Chair Timothy Hoffman, MD • NIH, Loan Repayment Program Pediatric and Clinical Research Programs • NIH, Pregnancy and Obesity Special Study Section Sudarshan Jadcherla, MD, F.R.C.P.(I), DCH • NIH, NIDDK, Study Section ZDK1-GRB8-M1, GI and Nutrition Grants – Reviewer Brian Kaspar, PhD • Clinical Neuroplasticity and Neurotransplantation (CNNT) – Ad Hoc Member • International Motor Neuron Disease Association – Ad Hoc Scientific Review Panel • Kentucky Science and Technology State Foundation – Ad Hoc Scientific Reviewer Jiayuh Lin, PhD • Congressionally Directed Medical Research Programs (CDMRP) Breast Cancer Research Program – Review Committee (Ad Hoc) Carlo diLorenzo, MD • Thrasher Research Fund – Ad Hoc Reviewer Joel Mayerson, MD • National Comprehensive Cancer Network, Bone Cancer National Committee, James Cancer Hospital Representative • Clinical Orthopaedics and Related Research, Bone 138 Ohio State University Medical Center Robert Munson, PhD • Canadian Institutes of Health Research – Ad Hoc Reviewer • NIH, Vaccines Against Microbial Diseases (VMD) Study Section – Ad Hoc Reviewer • VA Merit Committee for Infectious Diseases-B – Ad Hoc Reviewer Leif Nelin, MD • Cooperative Grants Program of the U.S. Civilian Research and Development Foundation – Ad Hoc Scientific Reviewer Stephen Qualman, MD • Food and Drug Administration Center for Biologics Evaluation and Research Office of Vaccines Research • NIH, Review Site Visit, Laboratory of Immunobiochemistry, Laboratory of Respiratory Viral Diseases • NIH, NIDDK Special Emphasis Panel Brady Reynolds, PhD • NIH, National Institute on Drug Abuse, Imaging – Science Track Award for Research Transition (I/START) Steve Roach, MD • Department of Defense Tuberous Sclerosis Complex Research Program (TSCRP) FY 2007 Integration Panel – Permanent Member Phil Scribano, DO, MSCE • Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics Scientific Review Committee Amanda Termuhlen, MD • Coordinating Committee, ASBMT (American Society for Blood and Marrow Transplantation), Pediatric Special Interest Group, planning for ASBMT International Meeting Veronica Vieland, PhD • NIH, Center for Inherited Disease Research Access Committee – Member Christopher Walker, PhD • NIH, Center for Scientific Review, Study Section Member, Ancillary Studies for Clinical Trials Keith Yeates, PhD, ABPP/CN • Biobehavioral and Behavioral Sciences Subcommittee, NICHD Initial Review Group – Permanent Member • March of Dimes Birth Defects Foundation – Special Reviewer Chack-Yung Yu, D.Phil. • NIH NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases) P30 Rheumatic Disease Center Core Grants, ZAR1 EHB-D 01 • NIH NIAID (National Institute of Allergy and Infectious Diseases), Special Emphasis Panel ZAI KS-I (M1) – Program Grant (P01) Reviewer • Hong Kong University of Science and Technology, Candidate for the Headship of the Department of Biology – External Reviewer Program Project Grant in Chronic DTH and IFN-Gamma in Human Graft Arteriosclerosis • NIH, NHLBI, Special Emphasis Panel, Boston Biomedical Institute, Program Project Grant in Dynamics of the Vascular Smooth Muscle Cytoskeleton – Member • NIH/NHLBI, Atherosclerosis and Inflammation in the Cardiovascular Sciences (AICS), NIH Study Section – Permanent Member Jackie Wood, PhD • NIH Digestive Diseases Commission PHARMACOLOGY PHYSICAL MEDICINE AND REHABILITATION Laura Bohn, PhD • NIH National Institute on Drug Abuse-NIDA-K Training and Career Development Subcommittee – Permanent Member • National Science Foundation, Signal Transduction and Cellular Regulation Panel I: Apoptosis, G-Proteins & Kinases – Permanent Member • Burroughs-Wellcome Fund Grant Review (International; UK) – Ad Hoc Reviewer John Corrigan, PhD • National Academy of Sciences, Institute of Medicine’s Committee on Traumatic Brain Injury – Member Ernest Johnson, MD • Warm Springs-Roosevelt Foundation – Permanent Member Norton Neff, PhD • NIH Study Section Special Emphasis Panel/Scientific Review Group 01 ZAI1 BDP-A • NIH Study Section Special Emphasis Panel/Scientific Review Group 05 ZRG1 NCF-D • Reviewer for Oak Ridge-Associated Universities PSYCHIATRY PHYSIOLOGY AND CELL BIOLOGY L. Eugene Arnold, MD • Loan Repayment Program Review Panel – Permanent Member • Special Emphasis Center Grant Application Review Committee – Ad Hoc Member Sandor Gyorke, PhD • NIH NHLBI Member Special Review Committee • Temporary Member, ESTA Study Section Lyn Jakeman, PhD • NINDS Specialized Neuroscience Research Program Review Mary Fristad, PhD, ABPP • NIH, NIMH, Special Emphasis Panel, IRG – Ad Hoc Member Janice-Kiecolt-Glaser, PhD • NIH, NIA Panel – Ad Hoc Reviewer John Campo, MD • Grants Oversight Committee for the Academy of Child and Adolescent Psychiatry Sissy Jhiang, PhD • NIH ICER Study Section Beth Lee, PhD • NIH Special Emphasis Panel on Bone Cell Biology, Skeletal Biology Structure and Regeneration Study Section – Permanent Member Muthu Periasamy, PhD • NIH, Cardiac Contracility, Hypertrophy and Heart Failure Robert Stephens Jr., PhD • NIH Somatosensory and Chemosensory Systems – Permanent Member Arthur Strauch, PhD • National Heart, Lung and Blood Institute, National Institutes of Health, Member Special Emphasis Panel, Yale University, RADIOLOGY Michael Knopp, MD, PhD • ICMICS (In Vivo Cellular and Molecular Imaging Center) Study Section of the National Cancer Institute Altaf Wani, PhD • NIH NAEHS (National Advisory Environmental Health Sciences) Council Steven D’Ambrosio, PhD • NIH, NIEHS Centers & Programs Review Committee – Ad Hoc Member 2007 Research Report 139 Robert Snapka, PhD • BMCT (Basic Mechanisms of Cancer Therapeutics) Study Section – Ad Hoc Member • NIH NINDS Roadmap HTS Assay Development – Permanent Member SCHOOL OF ALLIED MEDICAL PROFESSIONS (SAMP) Deborah Givens Heiss, DPT, PhD, OCS • NIH MRS (Musculoskeletal Rehabilitation Sciences) Study Section – Invited Reviewer • Ontario Workplace Safety and Insurance Board Research Advisory Council, Toronto, Ontario, Canada – External Grant Reviewer D. Michele Basso, PT, EdD • NIH Study Section, Brain Disorders and Clinical Neuroscience Integrated Review Group – Ad Hoc Member • Craig Neilsen Foundation – Grant Reviewer Jane Case-Smith, EDD, OTR/L, FAOTA • NIH Study Section Member, Motor Function, Speech and Rehabilitation SURGERY Gail Besner, MD, Division of Pediatric Surgery • NIH, Surgery, Anesthesiology and Trauma Study Section – Permanent Member Gayle Gordillo, MD, Division of Plastic Surgery • Plastic Surgery Educational Foundation – Ad Hoc Member • American Society of Plastic Surgery – Ad Hoc Member Sampath Parthasarathy, PhD, Division of Cardiothoracic Surgery • National VA Cardiovascular Review Section – Permanent Member Chandan Sen, PhD, Division of General and Gastrointestinal Surgery • SBTS-E Cardiovascular Devices Study Section – Ad Hoc Member • Surgery, Anesthesia and Trauma – Ad Hoc Member • Vascular Cell Molecular Biology – Ad Hoc Member Anne VanBuskirk, PhD, Division of Surgical Oncology • American Heart Association Committee 4A Microbiology/Immunology – Permanent Member • American Society for Transplantation Grants/Awards Committee – Permanent Member • NIH/NIAID, Allergy Immunology, Transplantation Research Committee – Ad Hoc Member • Special Emphasis Panel on Innovative Grants in Immune Tolerance – Ad Hoc Member Lisa Yee, MD, Division of Surgical Oncology • DOD BCRP Concept-Endocrinology – Ad Hoc Member • Susan G. Komen Foundation Postdoctoral Fellowship – Permanent Member Center for Molecular Neurobiology (CMN) NIH Permanent Study Section Members Ginny Bumgardner, MD, PhD, Division of Transplantation • NIH, Special Emphasis Panel, Human Pancreatic Islet Cell Resource Centers – Ad Hoc Member • NIH, Transplantation, Tolerance and Tumor Immunology – Ad Hoc Member Tsonwin Hai, PhD (see Molecular & Cellular Biochemistry) Jeff Kuret, PhD (see Molecular & Cellular Biochemistry) Mark Seeger, PhD Anthony Brown, PhD (see Neuroscience) Charles Cook, MD, Division of Critical Care, Trauma and Burns • NIH NIDCR (National Institute of Dental and Craniofacial Research) Special Emphasis Panel for PPG Review – Ad Hoc Member NIH Ad Hoc Reviewers Christine Beattie, PhD (see Neuroscience) Michael Zhu, PhD (see Neuroscience) John Oberdick, PhD (see Neuroscience) Paul Henion, PhD (see Neuroscience) Anthony Young, PhD Pedram Ghafourifar, PhD, Division of General Vascular Surgery • American Heart Association Peer-Review Committee – Permanent Member • Breast Cancer Foundation Review Panel – Ad Hoc Member • Mitochondria and Neurodegeneration, Neurodegeneration and Biology of Glia – Ad Hoc Member • Neurodegeneration, Neuroinflammation, Oxidant Stress and Mitochondria – Ad Hoc Member 140 Ohio State University Medical Center NSF-Ad Hoc Reviewers Paul Henion, PhD (see Neuroscience) Harald Vaessin, PhD Christine Beattie, PhD (see Neuroscience) James Jontes, PhD (see Neurosciences) Research Core Facilities Ohio State University Medical Center offers more than 20 core research laboratories for shared use by health-sciences investigators. Clinical research faculty, basic scientists and students all benefit from the shared cost of these resources, and the research environment at Ohio State benefits from the economies of scale that enable timely acquisition of new instrumentation and technologies. 2007 Research Report 141 Analytical Cytometry – This laboratory, directed by Jeffrey Chalmers, PhD, is a joint venture between Ohio State’s Comprehensive Cancer Center and the Medical Center’s Davis Heart and Lung Research Institute. It provides basic and clinical investigators with flow cytometric hardware and software for cell characterization and sorting. The laser-based technique is useful to quantitate intracellular and extracellular properties of cells, bacteria, chromosomes and other biological particles. http://heartlung.osu.edu/flowcytometry/index.cfm Behavioral Measurement – This shared resource, led by Barbara Andersen, PhD, assists in integrating behavioral research into the broad research goals of Ohio State’s Comprehensive Cancer Center. It also provides researchers in cancer prevention and control with population-based data retrieval, consultation for patient accrual procedures and locations, identification or adaptation of existing measures of key behavioral constructs, and guidance with behavioral data collection methodology and personnel. http://www.behavioralm.osu.edu Behavioral Phenotyping – This new facility in the Biomedical Research Tower vivarium is led by Laura Bohn, PhD, and Matthew During, MD, PhD. It offers specialized equipment and collaborative expertise for monitoring an array of behavioral responses. A partial list of behaviors and monitoring equipment includes a multifunction video system for digital monitoring of operant and preference conditioning. Monitoring of diurnal patterns, locomotion, feeding, social interactions, learning and memory, anxiety and depression is also available. Many of these tasks can be accomplished through a newly purchased Clever Systems analysis suite. On-site training is available by appointment. Biomedical Informatics – Computers play an everincreasing role in the analysis of biologically derived data. Led by Joel Saltz, MD, PhD, the Biomedical Informatics Core Laboratory applies distributed and parallel computing techniques to data retrieval and integration, imaging, simulation, medical informatics and computational biology. Its personnel also develop middleware and optimizations to enable 142 Ohio State University Medical Center Grid computing in the biological, medical and physical sciences. http://medicine.osu.edu/informatics/ Biostatistics Core – Led by Dave Jarjoura, PhD, this resource helps researchers identify collaborators to prepare grants, create and maintain databases, analyze data, develop methodologies and publish results. It assists them in all aspects of grant proposal development, experimental design, sample size determination, data management, statistical analysis, development and application of statistical methodologies, and manuscript preparation. http://www-biostat.med.ohio-state.edu/ Campus Microscopy and Imaging Facility (CMIF) – The Campus Microscopy and Imaging Facility (CMIF), under director Richard Burry, PhD, serves University faculty, staff and students as well as researchers outside Ohio State. It offers a full range of microscopes, and support instrumentation allows cell and tissue preparation with immunocytochemistry, in situ hybridization, freeze-fracture, cryoultramicrotomy, scanning and transmission electron microscopy. http://cmif.osu.edu Center for Knowledge Management – Ohio State’s Center for Knowledge Management (CKM), housed in the John A. Prior Health Sciences Library, is one of the nation’s most comprehensive repositories of global biomedical knowledge and intellectual capital. The CKM provides cost-effective access to biomedical knowledge, identifies and makes available knowledge and key research findings, expedites packaging of information content as reusable and sharable resources, facilitates understanding and helps incorporate information resources into work processes. The interim director for this resource is Susan Kroll, MLS. http://www.med.ohio-state.edu/knowledge/ Clinical Trials Office (CTO) – The CTO, under the leadership of James Thomas, MD, PhD, facilitates development and implementation of all Ohio State University Comprehensive Cancer Center clinical trials, including regulatory processing, subject recruitment, data collection and protocol-management services. www.osuccc.osu/9178.cfm The General Clinical Research Center (GCRC) program is funded by the National Center for Research Resources in the National Institutes of Health. The GCRC program provides indirect financial support to principal investigators of components essential to clinical research: hospitalization and ancillary laboratory costs, and salaries of key personnel, including nurses, research bionutritionists, administrators, core laboratory staff, biostatisticians and computer personnel. The program enables flexibility in the design, accessibility and scope of research. This facilitates rapid initiation of novel protocols and pilot studies. William Malarkey, MD, directs this core. www.gcrc.osu.edu/ Microarray – The Microarray resource, led by Chang-Gong Liu, PhD, offers genome-wide analysis of multiple genes using Affymetrix GeneChips. Services include mRNA transcriptional profiling, microRNA/non-coding small RNA transcriptional profiling, single nucleotide polymorphism genotyping, genomic DNA gain/loss detection on BAC CGH Array, microRNA genomic gain/loss on oligo CGH array, consultation, RNA characterization, and microarray processing and data analysis. A satellite Ohio State University Comprehensive Cancer Center Affymetrix microarray facility is housed at Columbus Children’s Research Institute. www.dnaarrays.org Laser Microdissection Pressure Catapulting Molecular Analysis Facility – Led by Sashwati Roy, PhD, this facility contains a robotized PALM MicroLaser system containing PALM MicroBeam and PALM RoboStage/RoboMover for high throughput sample collection. Procurement of another device, specifically directed at community service, is in process. The facility enables molecular analyses of laser captured tissue material. Services include standardization of novel techniques related to tissue processing, staining, fixation and capture, with the goal being to preserve nucleic acid and protein integrity of the laser-captured tissue. Capture and analysis of tissue down to the resolution of a single cell population (cutting precision 0.6 micron) from in vivo tissue sections are routinely performed. In addition, the facility has developed a novel way to rapidly identify and capture human blood vessels from clinical samples in a manner that makes highdensity screening of the transcriptome possible. MicroMD – A premier microfabrication facility for developing bioMEMS devices (microelectromechanical systems), the Ohio MicroMD Laboratory facilitates a broad range of research and development activities and is the nation’s first technologically integrated facility dedicated to developing therapeutic applications for BioMEMS. This core is led by Robert Davis, PhD. www.micromd.ohio-state.edu/information/overview.html Leukemia Tissue Bank – Directed by Clara D. Bloomfield, MD, this resource provides central collection, processing and repository for samples collected from leukemia patients treated on Ohio State University protocols. These samples are available to investigators within Ohio State’s Comprehensive Cancer Center and to outside collaborators who examine cellular and molecular properties of leukemia. http:www.osuccc.osu.edu/11167.cfm Microscopy – The Microscopy Core Lab in Ohio State’s Davis Heart and Lung Research Institute provides technology to visualize minute details of the subcellular organization of living cells and tissues. In addition to fluorescent microscopes fully equipped for optimized magnification, time-lapse video microscopy and multi-channel visualization, the lab offers other instrumentation, including multiphoton confocal microscopy that allows investigators to probe delicate living cells or tissues longer and deeper without damaging samples. This lab is directed by Thomas Clanton, PhD. http://heartlung.osu.edu/hlri/corelabs/microcore.jsp Molecular Cytogenetics – Directed by Nyla Heerema, PhD, the Molecular Cytogenetics Shared Resource provides molecular cytogenetic technology and classical banded metaphase cytogenetics. Services include metaphase karyotyping of human and mouse tissue, fluorescence in situ hybridization (FISH) using many different types of probes and tissues, and multicolor spectral karyotyping (SKY). http://www.cytogenetics.osu.edu/ 2007 Research Report 143 Mouse Phenotyping – Researchers using animal models of human cancer receive pathology support from this resource, which specializes in morphologic characterization of newly produced lines of genetically engineered mice. Supervised by interim leader Michael Lairmore, DVM, PhD, it also offers such services as necroscopy, slide preparation, semiquantitative histopathology for experimental studies, morphometry, hematology, clinical chemistry, consultation and referral. http://www.osumc.osu.edu/mouse_phenotyping Nucleic Acid – This resource, led by Hansjurg Alder, PhD, provides instrumentation and expertise for DNA sequencing, genotyping, real time polymerase chain reaction (PCR), RNA/DNA extraction, imaging and DNA synthesis support. It also consults and assists in experimental design, supports development of novel methodologies and applications relevant to cancer research, and functions as a training and education center. www.osuccc.osu.edu/9168.cfm Pharmacoanalytical – This resource offers two LC/MS systems for quantitation of analytes and identification of metabolites in biological matrices. This equipment is primarily used for quantitation of parent drug and metabolites in clinical specimens. This resource is led by James Dalton, PhD. www.osuccc.osu.edu/psr/ Pharmacogenomics – Led by Wolfgang Sadée, Dr.rer.nat., the Core Laboratory of Ohio State’s Program in Pharmacogenomics supports intermediate scale genotyping for use in clinical association studies. Genotyping panels covering nearly 1,000 polymorphisms are available, targeting genes implicated in cancer, cardiovascular and central nervous system disorders, as well as drug metabolism and transport. The Core Laboratory also has developed a rapid approach for discovery of functional polymorphisms in candidate genes as potential markers for assessing disease and therapy outcomes. http://pharmacogenomics.osu.edu/section1.html 144 Ohio State University Medical Center Proteomics – A shared resource of Ohio State’s Comprehensive Cancer Center and the Medical Center’s Davis Heart and Lung Research Institute, the Proteomics lab provides researchers with instrumentation, expertise and services needed to identify proteins, protein modifications and protein biomarkers in biological samples. It can identify proteins from 1D and 2D gels using electrophoresis and imaging equipment, robotic sample handlers and mass spectrometers. The lab is directed by Kari Green-Church, PhD, and Douglas Kniss, PhD. http://heartlung.osu.edu/hlri/corelabs/proteomics.jsp Tissue Procurement – Part of the Human Tissue Resource Network at Ohio State, this resource procures and provides researchers with malignant and normal tissues from solid tumors. Under the direction of Scott Jewell, PhD, Tissue Procurement staff provide quality control of the research specimen and interact with pathologists and investigators to better assist in procurement of tissues and to foster hypothesis-driven cancer research. http://www.osuccc.osu.edu/97970.cfm Transgenic Animal Facility – Led by Anthony Young, PhD, and Akihira Otoshi, MD, PhD, this facility provides transgenic and gene-targeted mice and other related services to the Ohio State biomedical research community. Jointly operated by the Center for Molecular Neurobiology, University Laboratory Animal Resources and Columbus Children’s Research Institute, the service includes three animal vivariums, a DNA preparation laboratory, consultation and primary care of animals. http://cmn.osu.edu/1521.cfm; http://transgeniccore.ccri.ws/index.asp X-ray Crystallography – This shared resource, led by Charles Bell, PhD, houses equipment and computational resources for collecting single crystal macromolecular X-ray diffraction data for determining X-ray crystal structures of proteins and other macromolecules at atomic resolution. Cryogenic devices are available for low-temperature data collection. Affiliated Cores Cell Manipulations Laboratory (CML) – The CML is a cleanroom for FDA GTP-compliant processing of “more than minimally manipulated” human tissues. The facility can accommodate two concurrent and fully segregated projects. CML services may be tailored to researcher needs and range from “cleanroom use only” to “full service production.” Led by Tom Leemhuis, PhD, the CML is located at The Cincinnati Children’s Hospital Medical Center and is a shared resource with Ohio State’s Comprehensive Cancer Center and Columbus Children’s Hospital. www.cincinnatichildren’s.org/research/div/exphematology/translational/cml/ Translational Trials Development and Support Lab – The Cincinnati Children’s Hospital Medical Center Translational Trials Development and Support Lab, which collaborates with Ohio State’s Comprehensive Cancer Center and Columbus Children’s Hospital, provides assays in support of translational research. Molecular-based assays include LAM and LM vector insertion, mycoplasma and multiple PCR-based assays. Cellular-based assays include clonogenicity, Fanconi Anemia complementation, endotoxin and tailored assays for protein function, as well as a normal donor cell repository for IRB-approved research. It is led by Lilith Reeves, MS. www.cincinnatichildren’s.org/research/div/exphematology/translational/ttdsl.htm ed cleanroom for producing viral vectors for phase I/II clinical studies. Led by Han van der Loo, PhD, this core is a shared resource with Ohio State’s Comprehensive Cancer Center and Columbus Children’s Hospital. www.cincinnatichildren’s.org/ research/div/exphematology/translational/vpf/vvc/ default.htm. The Viral Vector Core at Columbus Children’s Research Institute supplies recombinant adeno-associated virus (rAAV) and recombinant adenovirus (rAd) vectors of uniform quality to individual research laboratories. The core is a full-service facility, capable of generating vectors beginning with a cDNA sequence and ending with the synthesis and purification of gene transfers that have passed rigorous quality assurance assays. The core supports production of six novel rAAV vector serotypes (rAAV1, rAAV2, rAAV4, rAAV5, rAAV8 and rAAV9). It is led by Reed Clark, PhD. http://columbuschildrens.com/GD/Templates/Pages/ Childrens/Research/ResearchCenter.aspx?page=5060 Viral Vector Core – The Cincinnati Children’s Hospital Medical Center Viral Vector Core produces research-grade retroviral and lentiviral vectors, generates stable producer lines, and offers non-GMP quality control testing, including vector titer by functional assay or PCR, mycoplasma, sterility, RCR and RCL testing. Retrovirus for clinical application is produced in the Vector Production Facility, a validat- 2007 Research Report 145 University Resources The Ohio State University is at the forefront of biomedical technology and multidisciplinary research, in large part due to the depth and breadth of resources available on campus for basic and clinical scientists. By sharing these esoteric resources, the University can exploit economies of scale, decrease dependency on outside resources and increase opportunities for collaboration. 146 Ohio State University Medical Center FOR BIOMEDICAL RESEARCH esearc The Ohio State University Research Foundation (OSURF), led by Executive Director Robert Killoren, promotes development, implementation and coordination of sponsored research at Ohio State by overseeing sponsored projects and personnel associated with them, including financial resources, equipment inventory and compliance issues. http://rf.osu.edu The Technology Licensing and Commercialization (TLC) team, led by Jean Schelhorn, PhD, oversees protection of intellectual property via patent, copyright and/or trademark, marketing University technologies, negotiating license agreements, consulting about intellectual property on sponsored research projects, and ensuring compliance with federal regulations related to intellectual property. http://otl.osu.edu University Laboratory Animal Resources, led by Director William Yonushonis, is a centralized program that supports the humane, legal, proper and scientifically valid use of animals in scientific research at Ohio State. http://ular.osu.edu Biosafety Level 3 Facilities are located on Ohio State’s main and west campuses. A Biosafety Level 3 Facility is located in the Biomedical Research Tower to safely handle potentially infectious agents with the intent to guarantee biocontainment of pathogens under study. Campus Chemical Instrument Center (CCIC), was founded in 1981 as a unit of The Ohio State University Office of Research. Under the direction of Ming-Daw Tsai, PhD, the center focuses on providing state-of-the-art research facilities for the entire campus in three areas: nuclear magnetic resonance, mass spectrometry and proteomics. Chemistry-Mass Spectrometry Facility, a CCICrelated unit, is able to analyze small organic molecules using either positive ion electrospray ionization or electron impact ionization analysis. Chemistry NMR (Nuclear Magnetic Resonance) Laboratory, managed by Karl Vermillion, provides access to four Bruker NMR spectrometers. Plant-Microbe Genomics Facility serves the entire campus, as well as researchers in the state of Ohio and beyond, by providing resources to study genomes – from DNA sequence to protein expression. Microscopic and Chemical Analysis Research Center (MARC) is a University wide instrument service laboratory that supports research through elemental chemical analysis capabilities ranging from nanometer scale microscopic imaging to bulk chemical composition. Bio Medical Mass Spectrometry Facility, directed by Kenneth Chan, PhD, performs pharmacoanalytical services and assists investigators in solving pharmacoanalytical problems. Based at the College of Pharmacy. http://mass-spectrometrylab.osu.edu/facility.html Center for Materials Research, led by David Rigney, PhD, CMR associate director and professor of Materials Science and Engineering, promotes interdisciplinary scholarship among faculty, facilitates industrial partnerships, encourages interdisciplinary undergraduate and graduate education, provides a mechanism for major cooperative research funding, and creates central multi user research facilities for the study of familiar and new materials. http://www.physics.ohio-state.edu/cmr Ohio Supercomputer Center (OSC). Under executive director Stanley Ahalt, PhD, the OSC provides a high-performance computing and communications infrastructure for a diverse statewide community, including members of educational, academic research, industry and state government institutions. In collaboration with this community, OSC evaluates, implements and supports new and emerging information. Within OSC, the Biomedical Applications Research Group involves an interdisciplinary group of research and computer scientists and clinicians whose goals include applying high-performance computing to biomedicine and applying advanced interface technology for virtual exploration of complex computational data. Don Stredney, research scientist and director, interface lab, is also a member of the Experimental Therapeutics Program at the OSUCCC. 2007 Research Report 147 Collaboration and cooperation are the watchwords for research partnerships, not only within Ohio State, but also extending into the national and international scientific community. As partnerships are forged among OSU divisions and departments and with other academic institutions and corporations, the opportunities for science to advance increase exponentially. Over the past five years, external biomedical research support at Ohio State increased by 31.5 percent to reach $160.2 million in 2006. 148 Ohio State University Medical Center esearc Research Partnerships INTERDISCIPLINARY UNIVERSITY PROGRAMS The OSU Mathematical Biosciences Institute (MBI) – Based in the OSU Department of Mathematics, and in collaboration with the Department of Statistics, the MBI is the first center of its kind created by the National Science Foundation (NSF). A $10 million grant from the NSF initiated the Institute. The mission of the MBI, led by Avner Friedman, PhD, is to: develop mathematical theories, statistical methods and computational algorithms for solving fundamental problems in the biosciences; to involve mathematical scientists and bioscientists in the solutions of these problems; and to nurture a community of scholars through the education and support of students and researchers in mathematical biosciences. Institute partners include the following universities: Arizona State, Case Western Reserve, COSNet, Drexel, Florida State, Howard, Indiana-Purdue, Iowa State, Michigan State, New Jersey Institute of Technology, Ohio, California at Irvine, Cincinnati, Georgia, Iowa, Maryland Baltimore County, Michigan, Minnesota and Vanderbilt. design of virtual bone dissection simulations, biomechanics of bone adaptation, magnetic resonance imaging and spectroscopy, corneal topography, and biocompatibility of novel implant materials are all studied in the Department of Biomedical Engineering. Fifteen departmental faculty members in biomedical engineering, plus more than 60 participating faculty researchers, collaborate through the Department, providing extensive resources for research. Among these are researchers in Ohio State’s Davis Heart and Lung Research Institute and the Ohio Nanotech West Laboratory, with multiple resources dedicated to bioMEMS. In addition to the technical and clinical research facilities across campus and at the Medical Center, research is also conducted at Columbus Children’s Hospital. OSU Bionutrition and Food Safety Research Institute (BFSRI) – A one-of-a-kind research institute, the BFSRI integrates traditional nutrition study with contemporary biology. Linking agriculture, food systems and public health research programs, BFSRI encourages the study of dietary nutrition and genetic interactions to define risks for disease and to develop strategies for disease prevention. EXTERNAL PARTNERSHIPS BIOMEDICAL ENGINEERING DEPARTMENT “The discipline of biomedical engineering lies at the forefront of the medical revolution. Advances in biomedical engineering are accomplished through interdisciplinary activities that integrate the physical, chemical, mathematical and computational sciences with engineering principles to study biology, medicine and behavior.” (National Institutes of Health working definition of Biomedical Engineering, July 1997). Directed by Richard Hart, PhD, the Department of Biomedical Engineering at Ohio State was established in 2005 following more than 30 years of biomedical engineering research housed in a multidisciplinary Center. Current studies include biomicroelectromechanical systems (bioMEMS), imaging, biomechanics, biomaterials and tissue engineering research directed primarily toward cardiovascular, orthopedic and vision applications. Breakthroughs in nanotechnology for cell transplants, microfabrication of biodegradable polymers for drug delivery, Battelle Memorial Institute – Battelle Memorial Institute is a global science and technology enterprise that develops and commercializes new products and processes for governmental and commercial applications. It has a vast science and technology reach and conducts $3 billion in annual research and development. Battelle partners with OSU in a number of ways. From collaboration on new therapy-delivery technologies to building the world’s fastest supercomputer, OSU and Battelle have joined forces for biomedical research and to create commercial applications for new technology, including nanotechnology and biomedical informatics. Battelle and OSU are founding partners of the Columbus Business Technology Center. Intuitive Surgical, Inc. – The Medical Center is one of the two original sites in the world to study the advanced surgical robotics systems developed by Intuitive Surgical, including its daVinci® system. In 2007 Research Report 149 addition to the studies that led to FDA-approval of robotics for heart surgery, Ohio State researchers are perfecting tiny cameras with multiple lenses, which when inserted into a patient’s chest, provide a three-dimensional image of the heart. General Electric Medical Systems – The Ohio State University Medical Center and GE, in a unique corporate-academic partnership called the OSU Advanced Biomedical Imaging Institute are developing new technologies that provide unprecedented views into the inner workings of the human body and make many invasive diagnostic techniques obsolete. iMEDD – The principal user of the Ohio MicroMD Laboratory at OSU’s Science Village is iMEDD, an emerging drug delivery company that employs micro-engineering technology to develop improved drug delivery through nanotechnology. Philips Medical Systems – Ohio State and Case Western Reserve University have teamed with Philips to develop and commercialize one of the first high-power (7-tesla) magnetic resonance imaging systems in the world and to develop breakthrough technologies in molecular imaging used for research on cancer, cardiovascular disease, Alzheimer’s disease and other metabolic diseases of genetic origin. Siemens - Siemens Medical Solutions, Germany, along with Battelle Memorial Institute and the state of Ohio, is part of a $15 million program to develop innovative strategies and technologies to better prevent, detect and treat lung cancer. The effort is designed to position Ohio as an international leader in fighting the world’s deadliest malignancy. Led by Michael Caligiuri, MD, director of Ohio State’s Comprehensive Cancer Center, the project includes a number of researchers at the University. State of Ohio – Ohio’s Third Frontier Project aims to establish a high-tech, knowledge economy in the state by supporting leading-edge research and development. As part of this project, the Biomedical Research Commercialization Program (BRCP) (formerly called the Biomedical Research and Partnership) funds innovative projects with commercial potential in biomedicine and biotechnology. 150 Ohio State University Medical Center The BRCP has funded several Medical Center programs, including: • a $6.5 million cardiovascular bioengineering enterprise • a $1.49 million study on the genetics of gastrointestinal cancer led by Albert de la Chapelle, MD, PhD, and subcontracted from Case Western Reserve University • a $6 million biomedical informatics synthesis platform led by Joel Saltz, MD, PhD • a $17.1 million biomedical structural, functional and molecular imaging enterprise led by Michael Knopp, MD, PhD • a $7.9 million biomedical structural, functional and molecular imaging enterprise (part 2) led by Michael Knopp, MD, PhD • an $8 million comprehensive program for the prevention, detection and treatment of lung cancer led by Michael Caligiuri, MD • a $4.25 million commercialization platform for immunotherapeutic treatment of multiple sclerosis led by Caroline Whitacre, PhD • a $556,834 stem cell and tissue engineering institute led by Larry Lasky, MD United States Surgical Corporation – U.S. Surgical established the Center for Minimally Invasive Surgery at Ohio State in 1995 with a multimillion dollar donation, which enabled OSU surgeons to develop and teach the use of advanced laparoscopic and robotic techniques to other health professionals. In addition to the companies mentioned above, Ohio State’s Medical Center gratefully acknowledges the following companies that made significant research awards to the Medical Center through The Ohio State University Research Foundation during 2006: Abbott Laboratories Acorda Therapeutics Alcon Labs, Inc. Alexion Pharmaceuticals, Inc. Algorx Pharmaceuticals, Inc. American Lung Association Amgen, Inc. Amylin Corp. Astrazeneca, L.P. Aventis Pharmaceuticals, Inc. Battellepharma, Inc. Bausch & Lomb / Paragon Vision Sciences Berlex Labs, Inc. Biogen Idec, Inc. Biomec, Inc. Boehringer Ingelheim Pharmaceuticals, Inc. Booz Allen Hamilton, Inc. Bristol-Myers Squibb Co. Bristol-Myers Squibb Pharmaceutical Research Institute Britstol-Myers/Sanofi Pharmaceutical Partnership Cancervax Carotech Berhad Celgene Corp. Chiron Corp. Ciba Vision Ciphergen Biosystems, Inc. Cogentech, Inc. Covance, Inc. Cumbre Pharmaceuticals, Inc. Eli Lilly & Co. Elusys Therapeutics, Inc. Farnam Companies, Inc. Favrille, Inc. Forest Labs, Inc. Fondazione Adrioano Buzzati-Traverso Fort Dodge Labs Fujisawa Healthcare, Inc. Genzyme Corp. Glaxosmithkline Human Genome Sciences, Inc. Idec Pharmaceuticals Corp. Immunex Corp. Igenix Pharmaceutical Services, Inc. Inspire Pharmaceuticals, Inc. InterHealth Neutraceuticals, Inc. Intermune, Inc. Ivax Corp. Janssen Pharmaceutical Products, L.P. Johnson & Johnson Vision Care, Inc. Kosan Biosciences, Inc. Lipsome/Rhone Poulenc Rorer Pharmaceutical Lupus Clinical Trials Consortium, Inc. MDS Pharma Services Medtronic, Inc. Merck & Co., Inc. Merial, Ltd. MGI Pharma, Inc. Millennium Pharmaceuticals, Inc. Myogen, Inc. Neopharm, Inc. Novartis Pharmaceuticals Corp. Neurologix, Inc. Novo Nordisk Pharmaceuticals Omeros Vorp. Ortho Biotech Products, L.P. Peninsula Pharmaceuticals, Inc. Pfizer, Inc. Pharmacia & Upjohn, Inc. Pharmacia, Inc. Pharmacyclics, Inc. Pharmanet, Inc. Phoenix Scientific, Inc. PPD Development PRA International Purdue Pharma, L.P. Quintiles Transnational Corp. Respironics, Inc. Roche Labs, Inc. Ross Products Division Sanofi Winthrop Pharmaceuticals Sanofi-Aventis Savacor, Inc. Schwarz Biosciences, Inc. Science Applications International Corp. Serono, Inc. Serono/Pfizer Shire Pharmaceutical Development, Inc. Siemens Sigma-Tau Healthscience, SPA Supergen, Inc. Targacept, Inc. Teva Neuroscience, Inc. Thoratec Corp. Ucb Pharma, Inc. Vaxgen, Inc. Warner-Lambert Co. Worldwide Clinical Trials, Inc. Wyeth Pharmaceuticals, Inc. Wyeth-Ayerst, Res. Zivena, Inc. 2007 Research Report 151 PHILANTHROPIC SUPPORT Corporate Partners Partnerships at the Medical Center can also encompass corporate and foundation philanthropy. Such support from a company or its charitable foundation provides vital funds for supplies, equipment or other new technology, research personnel and other activities required for quality medical research. Corporate contributors in 2006 include: Abercrombie & Fitch Management Co.; Armor Fire Protection, Inc.; Battelle Memorial Institute; Cardinal Health; C.G. Therapeutics, Inc.; Cheryl & Company; Cordis Corporation; EBI LP (division of Biomet); Kroger Company; Lorillard Tobacco Company; PGA Tour, Inc.; and Schnipke Engraving Company, Inc., among many others. Private Donations During this reporting period, $36.9 million ($13.4 million in philanthropy and $23.5 million in private grant support) was received through private gifts and grants supporting medical research. These funds include support for chair and professorship positions, enhancing faculty recruitment and retention efforts of distinguished medical faculty who spearhead medical research efforts. These private donations come from grateful patients and their families, from alumni and from other individual or organization friends in the community, in addition to this corporate support. Of these research dollars, more than $2 million was raised by patients, their families and friends, and by volunteers who are mobilized to increase awareness about a disease and raise funds toward medical research to eradicate it or improve treatments. An individual or family can make a significant impact by funding medical research on a particular disease as recognized by Jay and Kathy Tolkan Worly of Bexley, Ohio, who funded two endowments at $50,000 each for cancer research – one for breast cancer and the other for lung cancer. Another local individual, George A. Skestos, established The Justine Skestos Chair in Minimally 152 Ohio State University Medical Center Invasive Neurological Spinal Surgery at $1.5 million in honor of his wife and to advance a particular medical specialty. Ohio residents from outside central Ohio often support Ohio State’s academic medical research, as did the many members of the Fraternal Order of Eagles – Grand Aerie (through their Ohio State Eagles Charity Fund). They supported $100,000 in medical research in diabetes, cervical cancer, cardiovascular disease and spinal cord injury. Some individuals accomplish their philanthropic goals through their personal estate planning, as did the late Leo H. Faust and Judge Grace Heck Faust of Urbana, Ohio, who provided almost $90,000 each to cardiology, psychiatry, cancer and ophthalmology. The Medical Center’s academic medical research reputation has resulted in personal philanthropy in support of research in cancer, ophthalmology, spinal cord injuries, cardiology, neurological diseases, diabetes and orthopedics, to name a few. ENDOWMENTS Establishing a named endowment that can support significant research year after year is often a motivating factor in philanthropy. The medical center has more than 400 endowed funds for medical research or chair and professorship positions established through philanthropy. These unique endowments not only enhance our quality of medical research and recognize the innovative research of the faculty recipient of the chair or professorship, but they also honor the person for which the endowment is named through the generosity of the donor. CHAIRS AND PROFESSORSHIPS Endowed or deignated chairs and professorships, representing 51 percent of the Medical Center’s gift endowment and valued at $118 million in gift principal, support research efforts at the Medical Center and include the following: Chair or Professorship Fund Carl M. and Grace C. Baldwin Chair in Ophthalmology (Ophthalmology ) Battelle Professorship in Inhalation Therapeutics (Inhalation Therapeutics) Barbara J. Bonner Chair in Lung Cancer Research (Cancer) John G. Boutselis M.D. Chair in Gynecology (Gynecology) Warren Brown Family Designated Professorship in Leukemia Research (Cancer) Dr. John D. and E. Olive Brumbaugh Chair in Brain Research and Teaching (Neurosurgery) H. William Clatworthy Jr. Professorship in Pediatric Surgery (Pediatric Surgery) Jeg Coughlin Chair in Childhood Cancer Development Therapeutics (Pediatric Cancer) Charles Austin Doan Chair of Medicine (MedicineUnrestricted) George T. Harding III MD Endowed Chair in Psychiatry (Psychiatry) William H. Havener MD Chair in Ophthalmology Research (Ophthalmology) Irene D. Hirsch Chair in Ophthalmology (Ophthalmology) Fred A. Hitchcock Professorship in Environmental Physiology (Physiology) Leonard J. Immke Jr. and Charlotte L. Immke Chair in Cancer Research (Cancer) Jay J. Jacoby MD, PhD, Chair in Anesthesiology (Anesthesiology) Dr. Arthur G. and Mildred C. James – Richard J. Solove Chair in Surgical Oncology (Surgical Oncology) Henry G. Cramblett Chair in Medicine (Pediatrics) Dr. Ernest W. Johnson Professorship (Physical Medicine & Rehabilitation) Dardinger Family Endowed Chair in Oncological Neurosurgery (Cancer-Oncological Neurosurgery) Donald G. Jones MD and Patsy P. Jones Designated Professorship in OB/Gynecology (Gynecology) Esther Dardinger Endowed Chair in NeuroOncology (Cancer-NeuroOncology) Luther M. Keith Professorship in Surgery (Surgery) Dorothy M. Davis Chair in Cancer Research (Cancer) William C. and Joan E. Davis Cancer Research Professorship (Cancer) Karl P. Klassen Chair of Thoracic Surgery (Thoracic Surgery) Frank J. Kloenne Chair in Orthopedic Surgery (Orthopedic Surgery) Klotz Chair in Cancer Research (Cancer) S. Robert Davis Chair of Medicine (MedicineUnrestricted) Richard J. and Martha D. Denman Professorship (Epilepsy) Department of Pathology Professorship (Pathology) Ralph W. and Helen Kurtz Chair in Hormonology (Hormonology) Helen C. Kurtz Chair in Neurology (Neurology) Ralph W. Kurtz Chair in Pathology (Pathology) 2007 Research Report 153 Lois Levy Professorship for Cancer (Cancer) Dave Longaberger Endowed Chair in Urology (Urology with Cancer) John H. and Mildred C. Lumley Medical Research Chair (Heart, Cancer, Arthritis) Torrence A. Makley Research Professorship (Ophthalmology) John L. Marakas Nationwide Insurance Enterprise Foundation Chair in Cancer Research (Cancer) Frank E. and Mary W. Pomerene Professorship in the Prevention and Treatment of Infectious Diseases (Infectious Disease) Warner M. and Lora Kays Pomerene Chair in Medicine (Primary Care) Ray W. Poppleton Research Chair (Orthopedics & Spinal Cord) Harry C. and Mary Elizabeth Powelson Professorship of Medicine (Medicine-Unrestricted) John A. Prior Professorship (Pulmonary) John G. and Jeanne Bonnet McCoy Chair in The Ohio State University Heart Center (Heart) Richard L. Meiling Chair of Obstetrics and Gynecology (Obstetrics/Gynecology) Dr. Samuel T. and Lois Felts Mercer Professorship of Medicine and Pharmacology (Pharmacology) Gilbert and Kathryn Mitchell Chair (Cancer, Heart, Kidneys and Eyes) Max Morehouse Chair in Cancer Research (Cancer) Martha Morehouse Chair in Immunology and Rheumatology (Immunology/Rheumatology) Novartis Pharmaceuticals Corporation Chair for Clinical Research (Clinical Research) Marion Rowley Designated Chair (Cancer) Joseph M. Ryan M.D. Chair in Cardiovascular Medicine (Cardiology) Samuel Saslaw Professorship in Infectious Diseases (Infectious Disease) Robert A. and Martha O. Schoenlaub Cancer Research Chair (Cancer) Donald A. Senhauser MD Professorship in Pathology (Pathology) Justine Skestos Chair in Minimally Invasive Neurological Spinal Surgery (Neurological Surgery) Sarah Ross Soter Endowed Chair in Women’s Cardiovascular Health at OSU Heart Center (Heart) Stefanie Spielman Chair in Breast Imaging (Cancer) James W. Overstreet Chair in Cardiology Fund (Cardiology) Martha G. and Milton Staub Chair for Research in Ophthalmology (Ophthalmology) William Greenville Pace III and Joanne Norris Collins-Pace Chair for Cancer Research (Cancer) Julius F. Stone Chair in Cancer Research (Cancer) William Greenville Pace III Endowed Chair in Cancer Research (Cancer) University Pathology Services Anatomic Pathology Professorship (Pathology – Anatomic) 154 Ohio State University Medical Center University Pathology Services Clinical Pathology Professorship (Pathology – Clinical) Clayton C. Wagner Parkinson’s Disease Professorship in Neurotherapeutics (Parkinson’s Disease) William D. and Jacqueline L. Wells Chair in Imaging Research (H&L Imaging Research) Bert C. Wiley M.D. Endowed Chair in Physical Medicine and Rehabilitation (Physical Medicine & Rehabilitation) Judson D. Wilson Professorship in Orthopedic Surgery (Orthopedics) James Hay and Ruth Jansson Wilson Professorship in Cardiology Fund (Cardiology) Dr. Benjamin and Helen Wiltberger Memorial Chair in Orthopaedic Surgery (Orthopedics) Lucius A. Wing Chair of Cancer Research and Therapy (Cancer) John W. Wolfe Chair in Human Cancer Genetics (Cancer) John W. Wolfe Chair in Cancer Research (Cancer) John W. Wolfe Professorship in Cardiovascular Research (Cardiovascular Medicine) Robert M. Zollinger Chair of Surgery (Surgery) Frederick P. Zuspan Chair in Obstetrics and Gynecology (Obstetrics & Gynecology) 2007 Research Report 155 Educational Initiatives PROMOTING BIOMEDICAL RESEARCH An essential component of Ohio State University Medical Center’s mission is education – instructing the students of today and training the biomedical researchers of tomorrow. Academic medical centers that promote research attract top medical students who want to train in an environment where exemplary research is conducted and applied to advancements in patient care. 156 Ohio State University Medical Center GRADUATE AND POSTGRADUATE RESEARCH PROGRAMS The Health Sciences Major is nationally recognized as a leader in practice-based allied healthcare education. The School of Allied Medical Professions offers undergraduate programs in athletic training, circulation technology, health information and management system, medical and radiological technology, medical dietetics and respiratory therapy. Students in all areas of study are involved in research. The undergraduate health sciences major, the School’s newest program, was established to prepare students for entry-level career opportunities in health care or for entry into graduate and professional programs. Masters of Science Program This program prepares registered, certified and/or licensed health professionals for expanded roles in research, teaching, administration and professional practice. A flexible study program designed around individual student needs offers five areas of emphasis: Advanced Professional Practice, Education, Management, Health Informatics and Gerontology. esearc UNDERGRADUATE RESEARCH PROGRAMS The Biomedical Sciences Major prepares OSU honors students for a career and/or further study in medicine or biomedical science. This program gives these undergraduates early training in conducting sound biomedical research, as well as an opportunity to participate in research projects at OSU Medical Center. MEDICAL STUDENT RESEARCH PROGRAMS Medical Scientist (MD/PhD)Program – The classic combined degree for physician-scientists, the MD/PhD program combines clinical training leading to the MD degree with training in research culminating in the PhD degree. The OSU College of Medicine’s Medical Scientist Program (MSP) allows for a synchronized MD/PhD program over a sevenyear period. The curriculum minimizes redundancy and optimizes students’ use of time without compromising educational quality in either area. The Independent Study Program (ISP) – This is a flexible option for obtaining the medical degree that allows students to arrange a study schedule best suited for them. The broad faculty expertise provides a large range of research disciplines and projects for students to explore in laboratory rotations early in their training. Carnegie Initiative on the Doctorate (CID) The Carnegie Initiative on the Doctorate is a program of the Carnegie Foundation for the Advancement of Teaching, a policy center devoted to strengthening teaching and learning at America’s colleges and schools. The Foundation began a three-year examination of teaching and learning in medical education and nursing education in July 2004. The CID is a multiyear research and action project to support academic departments’ efforts to more purposefully structure their doctoral programs, foster discipline-based conceptual work and design experiments in a small number of selected departments. Carnegie will collect, examine and disseminate findings from this discussion and related experiments. This initiative recognizes outstanding graduate programs across the nation. It provides funding in six areas: Chemistry, Education (educational psychology and curriculum and instruction), English, History, Mathematics and Neuroscience. OSU Medical Center received the top recognition level of Partner for its neuroscience graduate program, and The Ohio State University received the top recognition in the other five areas as well. OSU was the only university in the country to receive the top recognition in all six areas. PHD PROGRAM IN HEALTH AND REHABILITATION SCIENCES This interdisciplinary program was approved to begin classes in autumn 2005. Its purpose is to prepare healthcare professionals to enter academic positions and contribute to the advancement of knowledge in their fields within the allied health disciplines. 2007 Research Report 157 GRADUATE/POSTGRADUATE RESEARCH DAY INTERDISCIPLINARY GRADUATE PROGRAMS The Graduate and Postgraduate Research Day is an annual forum in which graduate and postgraduate trainees can offer poster presentations of their research and interact with top national researchers. OSU hosted its sixth annual Graduate and Postgraduate Research Day on March 29, 2007. All College of Medicine/Health Sciences faculty, staff and students are invited to this event, which features posters representing the research contributions of Medical Center graduate, medical and MD/PhD students, postdoctoral trainees, residents and postdoctoral fellows. The 2007 event also featured presentations from world-renowned researchers, including Judah Folkman, MD, professor of Cell Biology at Harvard Medical School and director of the Vascular Biology Program at Children’s Hospital in Boston, and Sandra Porter, PhD, director of Education and co-director of Research at Geospiza, Inc. The research day is organized by the Postdoctoral Research Scholars Association, Bennett Society, Landacre Society, and Medical Scientist Program. With the comprehensive range of OSU colleges on the same campus and a distinguished faculty that interacts on numerous levels and within multiple collaborative research initiatives, several OSU graduate-level programs are competitively sought by students from around the world: INTEGRATED BIOMEDICAL SCIENCE GRADUATE PROGRAM Led by Allan Yates, MD, PhD, Ohio State’s Integrated Biomedical Science Graduate Program (IBGP) guides students from understanding in the basic sciences and the complex mechanisms of human disease to medical applications of that knowledge while preparing them as biomedical scientists. The program’s graduate faculty has more than 200 members from 19 departments. The course sequence focuses on grant writing and mock-NIH review, a process that later serves as the basis for the student’s doctoral candidacy examination. The IBGP has attained national prominence, having been one of only five programs of its kind profiled as “exemplary” in the Jan. 26, 2001, issue of the journal Science. 158 Ohio State University Medical Center The Neuroscience Graduate Studies Program emphasizes a multidisciplinary approach, with faculty from traditional areas such as neuroanatomy, neurochemistry, neuropharmacology, neurophysiology, neuropsychology and others. The Molecular, Cellular and Developmental Biology Interdisciplinary Graduate Program involves collaborative efforts of more than 125 faculty members from 25 departments and colleges to offer a course of study encompassing the molecular, cellular and organismic levels of organisms. The Ohio State Biochemistry Program is an interdisciplinary biochemistry program offering the PhD, with faculty members from Biochemistry, Molecular & Cellular Biochemistry and Chemistry. The faculty includes more than 80 members from more than 20 departments across campus. The Biophysics Graduate Program is an interdisciplinary program that prepares students for careers in structural biology and molecular biophysics, biological spectroscopy and imaging, cellular and integrative biophysics and computational bioinformatics. 2006 Sponsored Research Grants BY DEPARTMENT Nearly $160.2 million in outside funding was awarded in 2006 to scientists pursuing research projects within Ohio State University Medical Center’s academic departments. Included in this section of the research report are grants received during 2006 for $100,000 or more. They are listed by academic department. A complete list of grants, including those under $100,000, is published at www.medicalcenter.osu.edu. This funding reflects a growing confidence in the expertise and ability of Ohio State University medical researchers to advance the frontiers of health-science knowledge. 2007 Research Report 159 PI NAME TITLE SPONSOR AWARD Anesthesiology Fedias Christofi Purinergic regulation of enteric neural reflexes National Institute of Diabetes and Digestive and Kidney Diseases $511,425 Yun Xia GDNF in the enteric nervous system National Institute of Diabetes and Digestive and Kidney Diseases $130,113 Umit Catalyurek Massive-scale semantic graphs (MSSG) University of California $158,417 Umit Catalyurek SciDAC Institute: Combinatorial scientific computing and petascale simulations (CSCAPES) US Department of Energy $130,000 Joel Saltz ARCH DEV - caGrid 1.0 design and implementation Booz Allen Hamilton, Inc. $735,075 Joel Saltz caBIG architecture workspace Booz Allen Hamilton, Inc. $776,355 Douglas Knutson Pre-doctoral training in primary care (Teaching to the CORE) Health Resources & Services Administration $214,477 Douglas Post Patient-centered communication during chemotherapy National Cancer Institute $105,110 Gunjan Agarwal Atomic force microscope National Center for Research Resources $253,510 Philip Binkley Eliminating barriers to effective training in clinical investigation National Center for Research Resources $669,785 Yeong-Renn Chen Myocardial injury associated with mitochondria-derived oxygen free radical(s) National Heart, Lung, and Blood Institute $375,000 Glen Cooke Pharmacogenetic antiplatelet strategies in CHD patients National Heart, Lung, and Blood Institute $136,571 Curt Daniels Registry to evaluate early and longterm pulmonary arterial hypertension (PAH) disease management (REVEAL Registry) Anonymous $108,750 Nicholas Flavahan Alpha2C adrenergic receptors and cutaneous circulation National Heart, Lung, and Blood Institute $328,471 Nicholas Flavahan Mechanisms of vascular dysfunction in vibration injury National Institute for Occupational Safety and Health $253,776 Guanglong He In vivo EPR imaging of myocardial oxygen consumption National Heart, Lung, and Blood Institute $328,471 Biomedical Informatics Family Medicine Internal Medicine Cardiovascular Medicine 160 Ohio State University Medical Center PI NAME TITLE SPONSOR AWARD Govindasamy Ilangovan Heat shock proteins, nitric oxide and oxygen consumption in the heart National Heart, Lung, and Blood Institute $361,375 Periannan Kuppusamy Development of spin probes for cell-tagging and oximetry National Institute of Biomedical Imaging and Bioengineering $262,777 Periannan Kuppusamy In vivo EPR imaging and redox status and thiols in tumor National Cancer Institute $299,274 Periannan Kuppusamy Novel methods for in vivo imaging of tissue oxygenation National Institute of Biomedical Imaging and Bioengineering $262,776 Yunbo Li Induction of cellular antioxidants and cardioprotection National Heart, Lung, and Blood Institute $291,974 Zhenguo Liu Effects of nitric oxide on stem cell differentiation National Heart, Lung, and Blood Institute $133,434 Subha Raman Iron and atherosclerosis National Heart, Lung, and Blood Institute $164,236 Orlando Simonetti Cardiovascular MRI and CT application research Siemens $197,420 Yong Xia Superoxide generation from endothelial NO synthase National Heart, Lung, and Blood Institute $291,974 Jay Zweier Inflammation and repair in cardiac ischemia-reperfusion Johns Hopkins University Jay Zweier Measurement of free radical generation in the heart National Heart, Lung, and Blood Institute $641,734 Jay Zweier Oxygen radicals and nitric oxide in postischemic injury National Heart, Lung, and Blood Institute $481,354 Jay Zweier Protection of ischemic myocardium University of Louisville $190,235 Jay Zweier Proton electron double resonance imaging (PEDRI) of free radicals National Institute of Biomedical Imaging and Bioengineering $1,009,832 $448,500 Endocrinology, Diabetes and Metabolism Lawrence Kirschner Carney complex: a model for PKA-mediated tumorigenesis National Cancer Institute $269,346 Kwame Osei Exenatide in islet cell transplantation in non-human primate model Eli Lilly and Company $267,498 Kwame Osei Prevention of cardiovascular disease in diabetes mellitus - clinical center network Case Western Reserve University $248,457 Joseph Pinzone BP1 and nuclear hormone signaling in breast cancer National Cancer Institute $133,650 Matthew Ringel Akt intracellular localization in thyroid cancer National Cancer Institute $259,491 2007 Research Report 161 PI NAME TITLE SPONSOR AWARD Matthew Ringel Anti-metastatic effect of MCIP1 in thyroid cancer National Cancer Institute $125,549 Belinda Avalos-Copelan The G-CSF receptor and ubiquitination National Institute of Diabetes and Digestive and Kidney Diseases $182,483 Robert Baiocchi Mechanism of resistance to histone deaceylase inhibitor-induced apoptosis in Epstein-Barr virus associated malignancies V Foundation $100,000 Jeffrey Chalmers Advanced biomedical devices for disease diagnosis and therapy Ohio Department of Development $525,000 Jeffrey Chalmers High performance magnetic cell sorting National Cancer Institute $338,825 Clara Bloomfield CALGB Foundation protocol capitation projects 1997 Cancer & Leukemia Group B Foundation $128,780 Clara Bloomfield CALGB University of Chicago services agreement University of Chicago $305,280 Clara Bloomfield Cancer and Leukemia Group B The Ohio State University National Cancer Institute $374,894 Kristie Blum Targeting transcriptional repression in CLL National Cancer Institute $135,076 William Blum Experimental therapeutics in acute leukemias National Cancer Institute $129,720 John Byrd B-CLL biology: impact of combination chemotherapy Mayo Foundation for Medical Education and Research John Byrd Hu1D10 in CLL: clinical and laboratory studies National Cancer Institute John Byrd Targeting PDK1/AKT in CLL Leukemia & Lymphoma Society $260,000 Michael Caligiuri CALGB leukemia tissue bank supplement University of Chicago $224,740 Michael Caligiuri Cancer and leukemia group B leukemia correlative science National Cancer Institute $1,012,609 Michael Caligiuri IL-15 characterization through experimental immunology National Cancer Institute $279,200 Michael Caligiuri Innate immunity: Elucidation/ modulation - cancer therapy National Cancer Institute $1,921,456 Michael Caligiuri Oncology training grant National Cancer Institute $437,284 Michael Caligiuri The Ohio State University comprehensive cancer support grant National Cancer Institute $3,825,067 Hematology and Oncology 162 Ohio State University Medical Center $440,428 $305,171 PI NAME TITLE SPONSOR AWARD Tong Chen Evaluation of the chemopreventive effects of strawberries on esophageal cancer development California Strawberry Commission Steven Clinton Tomato-soy juice for prostate cancer National Cancer Institute Steven Clinton Vitamin D status and prostate cancer Purdue University $225,233 Sherif Farag QMS technology to deplete T-cell alloreactivity National Institute of Allergy and Infectious Diseases $347,343 Michael Grever Chronic lymphocytic leukemia research consortium $159,500 $187,411 University of California at San Diego $662,237 Michael Grever Phase I trials of anticancer agents National Cancer Institute $602,401 Thomas Lin A novel dosing schedule of flavopiridol in CLL National Cancer Institute $288,324 Thomas Lin A phase 1 study of the AKT inhibitor 17-AAG in CLL National Cancer Institute $288,324 Thomas Lin A phase I study of single agent flavopiridol in B-NHL National Cancer Institute $257,065 Thomas Lin Monoclonal antibody therapy in B-cell CLL National Cancer Institute $131,814 Richard Love Adjuvant hormonal therapy in Vietnamese breast cancer National Cancer Institute $263,370 Richard Love Luteal adjuvant oophorectomy in Vietnamese breast cancer National Cancer Institute $838,100 Guido Marcucci Pharmacological modulation of epigenetic changes in AML National Cancer Institute $299,274 Michael Pereira Preclinical efficacy and intermediate biomarker assays National Cancer Institute $2,117,529 Pierluigi Porcu Combined cytokine-monoclonal antibody therapy in lymphoma National Cancer Institute $135,513 Forrest Ravlin Chemoprevention of GI tract cancers with berries Cooperative State Research, Education and Extension Service Laura Rush Aberrant DNA methylation in acute myeloid leukemia National Cancer Institute $120,148 Manisha Shah Targeting RAF and VEGF signaling in thyroid cancer National Cancer Institute $198,261 Gary Stoner Prevention of esophageal cancer with berries National Cancer Institute $497,266 $444,787 2007 Research Report 163 PI NAME TITLE SPONSOR AWARD Miguel Villalona Early therapeutics development with a phase II emphasis National Cancer Institute $653,131 Miguel Villalona Phase I dose escalation trial to evaluate the safety, pharmacokinetics and pharmacodynamics of KOS-1584 in patients with advanced solid tumors Kosan Biosciences, Inc. $139,530 Susan Whitman FLT3 genotypes in acute myeloid leukemia National Cancer Institute $143,575 FcRn binds and transports albumin National Institute of Allergy and Infectious Diseases $656,941 Adult AIDS clinical trials unit National Institute of Allergy and Infectious Diseases Immunology Clark Anderson Infectious Diseases Susan Koletar $1,587,829 Michael Para AIDS education and training center University of Pittsburgh $212,000 Larry Schlesinger Altered M. tuberculosis mannosylation and the macrophage National Institute of Allergy and Infectious Diseases $385,521 Larry Schlesinger Lung innate immune responses to F tularensis: a central role for the macrophage University of Chicago $546,877 Kurt Stevenson Ohio State health network infection control collaborative: epi-centers for prevention of healthcare-related infections Centers for Disease Control and Prevention $394,073 Joanne Turner CD8 T cells and immunity to tuberculosis in old mice National Institute on Aging General Clinical Research Center (GCRC) National Center for Research Resources AASK cohort study National Institute of Diabetes and Digestive and Kidney Diseases $318,291 General Internal Medicine Fred Sanfilippo William Malarkey $2,955,771 Nephrology Lee Hebert $159,993 Lee Hebert Genetic and clinical risk for human SLE nephritis National Institute of Diabetes and Digestive and Kidney Diseases $112,500 Todd Pesavento A randomized, controlled trial of homocysteine (FAVORIT) Rhode Island Hospital $146,961 Brad Rovin Lupus clinical trials consortium 164 Ohio State University Medical Center Lupus Clinical Trials Consortium, Inc. $100,000 PI NAME TITLE SPONSOR AWARD Pulmonary, Allergy, Critical Care and Sleep Medicine Naeem Ali TSP-1: A mediator of sepsis-induced lung injury National Center for Research Resources $130,316 Thomas Clanton Redox mechanisms of respiratory muscle stress adaptation National Heart, Lung, and Blood Institute Philip Diaz Alveolar macrophage proteomics in HIV-induced emphysema National Heart, Lung, and Blood Institute $381,390 Philip Diaz Long-term oxygen treatment trial (LOTT) regional clinical center National Heart, Lung, and Blood Institute $121,965 Andrea Doseff Molecular mechanisms of apoptosis in monocytes National Heart, Lung, and Blood Institute $239,200 Andrea Doseff Regulation of apoptosis by the interaction of Casp-3 with PKC and small heat shock proteins NSF Molecular & Cellular Biosciences $139,600 Rami Khayat The role of diagnosis and treatment of sleep apnea in the acute exacerbation of heart failure Respironics, Inc. $167,250 Valery Khramtsov In vivo imaging and spectroscopy of pH and thiols status National Institute of Biomedical Imaging and Bioengineering $156,448 John Mastronarde Asthma clinical research center American Lung Association $164,914 John Mastronarde Exercise as an anti-inflammatory therapy for asthma National Center for Research Resources $118,997 Patrick Nana-Sinkam Regulation of prostacyclin in pulmonary hypertension National Heart, Lung, and Blood Institute $131,490 Patrick Nana-Sinkam The obese critical III: process and outcome disparities National Heart, Lung, and Blood Institute $130,248 Mark Wewers Macrophage inflammasome regulation National Heart, Lung, and Blood Institute $357,941 Karen Wood Lung alloimmunity after bone marrow transplantation National Heart, Lung, and Blood Institute $130,518 $343,555 Molecular and Cellular Biochemistry Charles Bell Structural studies of RecA-DNA complexes National Institute of General Medical Sciences $252,060 Arthur Burghes Survival motor neuron genes in spinal muscular atrophy National Institute of Neurological Disorders and Stroke $334,780 Kalpana Ghoshal The role of microRNA in hepatocarcinogenesis National Cancer Institute $171,000 2007 Research Report 165 PI NAME TITLE SPONSOR Tsonwin Hai ATF3 and iNOS in islet destruction and graft rejection National Institute of Diabetes and Digestive and Kidney Diseases $254,918 Tsonwin Hai ATF3 in beta cell signaling, gene expression and destruction National Institute of Diabetes and Digestive and Kidney Diseases $279,067 Tsonwin Hai Beta cell transcriptional network in stress response American Diabetes Association, Inc. $100,000 Russell Hille Structure/activity studies of two molybdenum enzymes National Institute of General Medical Sciences $240,879 Russell Hille Studies of environmentally relevant molybdenum enzymes National Institute of Environmental Health Sciences $312,047 Samson Jacob A research proposal to test novel nucleotide analogs in the DNA methylation machinery Supergen, Inc. $150,000 Samson Jacob Alcohol-induced epigenetic changes in the liver genome National Institute on Alcohol Abuse and Alcoholism $225,224 Samson Jacob DNA methylation and chromatin modifications: Mechanisms and applications in cancer therapy National Cancer Institute $2,248,455 Samson Jacob Molecular mechanisms of diet-induced carcinogenesis National Cancer Institute $288,427 Jeffrey Kuret Structure and genesis of tau filaments National Institute on Aging $269,345 Kamal Mehta Role of protein kinase Cbeta in diet-induced hypercholesterol mice National Heart, Lung, and Blood Institute $286,353 Type B histone acetyltransferases and the assembly of chromatin structure National Institutes of Health $303,750 Jill Rafael-Fortney DLG and CASK at the mammalian neuromuscular junction National Institute of Arthritis and Musculoskeletal and Skin Diseases $180,409 Daniel Schoenberg Hormonal regulation of mRNA stability National Institute of General Medical Sciences $365,954 Saïd Sif Effects of human SWI/SNF-associated PRMT5 on lymphomagenesis National Cancer Institute $236,250 Sung Ok Yoon Mechanisms of crosstalk between NGF receptors, TrkA and p75 National Institute of Neurological Disorders and Stroke $337,595 Mark Parthun AWARD Molecular Virology, Immunology and Medical Genetics George Calin Roles of microRN as in familial chronic lymphocytic leukemia CLL Global Research Foundation $100,000 Jean-Leon Chong Determine how action of PTEN in stromal fibroblasts suppresses development of mammary epithelial tumors US Department of Defense $605,342 166 Ohio State University Medical Center PI NAME TITLE SPONSOR AWARD Carlo Croce ALL fusion proteins: associated with multiprotein complexes and role in transcription National Cancer Institute $298,467 Carlo Croce Role of Fhit in apoptosis and susceptibility to therapy Thomas Jefferson University $317,040 Carlo Croce The microRNA genes in hematopoietic/ mesenchimal/neural development and neoplasias Fondazione Adrioano Buzzati-Traverso $100,000 Ramana Davuluri Genomewide discovery and analysis of alternative promoters National Human Genome Research Institute $325,000 Albert de la Chapelle BAALC in neurogenesis and hematopoiesis National Cancer Institute $299,274 Matthew During An immunological approach to alter the function of neuronal genes National Institute of Neurological Disorders and Stroke $308,848 Matthew During Gene therapy of neurological disorders and viral vector development Neurologix, Inc. $250,000 Richard Fishel Functional studies of the meiotic MutS homologs hMSH4-hMSH5 National Institute of General Medical Sciences $267,488 Richard Fishel Human mismatch repair proteins and carcinogenesis National Cancer Institute $355,444 Richard Fishel Recombination/repair complex in human cells National Institute of General Medical Sciences $373,259 Louise Fong Cell proliferation and esophageal carcinogenesis in zinc-deficient rats American Institute for Cancer Research $143,565 Louise Fong Chemoprevention of upper aerodigestive tract cancer by dietary zinc National Cancer Institute $272,249 Ronald Glaser Stress, the immune system and basal cell carcinoma National Cancer Institute $809,342 Joanna Groden Characterization of tumor suppression by the APC gene National Cancer Institute $264,600 Joanna Groden Genetics of gastrointestinal cancer Case Western Reserve University Joanna Groden Mouse models of gastrointestinal cancer National Cancer Institute $1,405,108 Denis Guttridge Cytokines and NF-kappaB regulation of cancer cachexia National Cancer Institute $153,753 Denis Guttridge NF-kappaB regulation of muscle wasting in cancer cachexia National Cancer Institute $239,456 Tim Hui-Ming Huang Interrogating epigenetic changes in cancer genomes National Cancer Institute $1,451,281 Kay Huebner Wwox as a critical signal mediator in breast cancer National Cancer Institute $147,626 $190,217 2007 Research Report 167 PI NAME TITLE SPONSOR Gustavo Leone E2F3 and embryonic development National Institute of Child Health and Human Development $324,077 Amy Elizabeth Lovett-Racke Characterizing therapeutic targets for multiple sclerosis National Multiple Sclerosis Society $121,849 Amy Elizabeth Lovett-Racke Role of T-bet in immune-mediated demyelinating disease National Multiple Sclerosis Society $110,790 Michael Ostrowski Genetic analysis of the breast tumor microenvironment National Cancer Institute Michael Ostrowski Mi: Modulating osteoclast gene expression and function National Institute of Arthritis and Musculoskeletal and Skin Diseases $154,368 Michael Ostrowski Molecular analysis of ras oncogene activated gene expression National Cancer Institute $153,828 Deborah Parris Coordination of HSV lagging strand synthesis National Institute of General Medical Sciences $299,408 Deborah Parris Suppression of RNA interference by herpes simplex virus National Institute of Allergy and Infectious Diseases $164,235 Yuri Pekarsky MicroRNAs targeting TCL1 as therapeutic agents for B-CLL CLL Global Research Foundation $100,000 Danilo Perrotti Role of RNA binding protein in BCR/ABL leukemogenesis National Cancer Institute $239,456 Christoph Plass DNA methylation as a diagnostic marker in AML National Cancer Institute $288,427 Christoph Plass DNA methylation as a therapeutic target in chronic lymphocytic leukemia Leukemia & Lymphoma Society $117,000 Christoph Plass DNA methylation in AML Leukemia & Lymphoma Society $200,000 Phillip Popovich Macrophage heterogeneity in spinal cord injury National Institute of Neurological Disorders and Stroke $390,920 Phillip Popovich T-cell functions in the injured spinal cord National Institute of Neurological Disorders and Stroke $403,961 Virginia Sanders Neuromodulation of the antibody response National Institute of Allergy and Infectious Diseases $360,084 Virginia Sanders Training program in integrative immunobiology National Institute of Allergy and Infectious Diseases $231,072 Amanda Toland Identification of Aurora-A interacting cancer susceptibility genes American Cancer Society, Inc. Caroline Whitacre Effect of pregnancy on EAE and MS National Institutes of Health 168 Ohio State University Medical Center AWARD $1,336,146 $180,000 $337,595 PI NAME TITLE SPONSOR AWARD Caroline Whitacre Migration inhibitory factor in the progression of EAE National Institute of Allergy and Infectious Diseases $224,250 Nicola Zanesi Mouse models of the most common human cancers Sidney Kimmel Foundation for Cancer Research $100,000 Antonio Chiocca Biology of tauopathies studied with HSV amplicons National Institute of Neurological Disorders and Stroke $277,728 Antonio Chiocca Gene therapy of brain tumors Massachusetts General Hospital $274,160 Antonio Chiocca Imaging transcriptional activation of gliomas National Cancer Institute $145,987 Antonio Chiocca Interdisciplinary tumor complexity modeling National Cancer Institute $1,125,756 John Kissel Six month (26 weeks) clinical trial of gentamicin in Duchenne muscular dystrophy subjects with stop code mutations Columbus Children’s Research Institute $108,400 Kottil Rammohan Combination therapy using mycophenylate mofetil (CellCept(R)) and human interfer on beta 1a (Rebif (R)) in early treatment of multiple sclerosis Serono/Pfizer $150,000 Kottil Rammohan Study to evaluate the safety and efficacy of rituximab in adults with primaryprogressive multiple sclerosis Genentech, Inc. $128,054 Douglas Scharre A randomized, multicenter, double-blind, placebo-controlled, 18-month study of the efficacy of zaliproden in patients with mild-to-moderate dementia of the Alzheimer’s type Sanofi Winthrop Pharmaceuticals $117,626 Douglas Scharre Study of the effect of daily treatment with MPC-7869 on measures of cognitive and global function in subjects with mild to moderate dementia of the Alzheimer’s type PPD Development $142,016 Candice Askwith Molecular and functional analysis of the acid-sensing ion channels NSF Integrative Organismal Biology Christine Beattie Genetic and molecular regulation of motor axon pathway formation NSF Biological Sciences Christine Beattie Spinal muscular atrophy: is it a motor axon disease? National Institute of Neurological Disorders and Stroke Neurological Surgery Neurology Neuroscience $137,181 $360,000 $337,595 2007 Research Report 169 PI NAME TITLE SPONSOR AWARD Christine Beattie The generation of transgenic zebra fish carrying mutations in the zebra fish sod1 gene Amyotrophic Lateral Sclerosis Association $130,000 Michael Beattie New York State SCI research program animal modeling The Burke Medical Research Institute $695,516 Michael Beattie Recovery of sacral spinal reflexes after transplantation National Institute of Neurological Disorders and Stroke $333,079 Jacqueline Bresnahan Mechanisms of secondary damage after spinal cord injury National Institute of Neurological Disorders and Stroke $326,782 Anthony Brown Axonal transport of neurofilaments National Institute of Neurological Disorders and Stroke $307,872 John Enyeart Properties of ion channels that control secretion National Institute of Diabetes and Digestive and Kidney Diseases $274,455 Andrew Fischer Muller glia and neuronal regeneration in the retina National Eye Institute Chen Gu Targeting and function of voltage-gated potassium channel in myelinated axons National Multiple Sclerosis Society $137,500 Paul Henion Development of distinct neural crest and hematopoietic subpopulations National Institute of General Medical Sciences $283,070 James Jontes Role of protocadherins in neural development studied in living zebra fish embryos Burroughs Wellcome Fund $100,000 Chien-Liang Glenn Lin Consequence of RNA oxidation National Institute on Aging $190,613 Stuart Mangel Chloride transporter function in the retina National Eye Institute $919,821 Stuart Mangel Neuronal plasticity in the retina National Eye Institute $727,467 Dana McTigue Facilities of research in spinal cord injury National Institute of Neurological Disorders and Stroke $510,004 Dana McTigue Oligodendrocyte progenitors in spinal cord injury repair National Institute of Neurological Disorders and Stroke $273,664 John Oberdick Ohio State Neuroscience Center Core National Institute of Neurological Disorders and Stroke $492,412 Karl Obrietan CREB and synaptic reorganization National Institute of Neurological Disorders and Stroke $273,878 Karl Obrietan MAPK signaling and circadian timing National Institute of Mental Health $252,060 Michael Xi Zhu High throughput screening of ligands of TRP channels National Institute of Neurological Disorders and Stroke 170 Ohio State University Medical Center $299,000 $185,625 PI NAME TITLE SPONSOR AWARD Nisonger Center Michael Aman The OSU RUPP-PI project National Institute of Mental Health $473,136 Margaretha Izzo Access Tomorrow: using E-mentoring, web-based and assistive technologies for increasing achievement and transition outcomes US Department of Education $199,463 Margaretha Izzo Enhanced academic achievement and transition outcomes through technology: phase 2, stepping stones of technology innovation US Department of Education $261,900 Obstetrics and Gynecology William Ackerman Lipid bodies and prostaglandin production in labor National Institute of Child Health and Human Development $122,472 Jeffrey Fowler Clinical trials in gynecologic oncology American College of Obstetricians and Gynecologists $313,991 Jay Iams Multicenter network of maternal-fetal medicine units National Institute of Child Health and Human Development $758,949 Jay Iams Vaginal ultrasound cerclage trial University of Alabama at Birmingham $144,632 Phenotypic determinants of vestibular schwannomas National Institute on Deafness and Other Communication Disorders $314,697 Leona Ayers Cooperative tissue bank of HIV positive malignancies National Cancer Institute $538,481 Sanford Barsky The bone marrow stem cell origin of human breast cancer using transgenic mouse models Army Medical Research Acquisition Activity $112,500 Sanford Barsky The bone marrow stem cell origin of lung BAC/PAC Joan’s Legacy: The Joan Scarangello Foundation $100,000 Rolf Barth Molecular targeting of EGFR for the treatment of gliomas National Cancer Institute $288,428 Wendy Frankel Cooperative human tissue network National Cancer Institute $866,397 Scott Jewell A randomized, double-blinded, placebocontrolled, phase III trial comparing docetaxel and prednisone with and without bevacizumab (IND#7921, NSC#704865) in men with hormone-refractory prostate cancer Cancer & Leukemia Group B Foundation $463,293 Scott Jewell CALGB Pathology coordinating office (PCO) base funding University of Chicago $684,229 Otolaryngology D Bradley Welling Pathology 2007 Research Report 171 PI NAME TITLE SPONSOR AWARD Scott Jewell Cancer and Leukemia Group B Pathology coordinating office University of Chicago $403,937 Larry Lasky Commercialization of a 3D modular perfusion bioreactor system for laboratory use Case Western Reserve University $300,000 Larry Lasky Engineered hematopoietic cell self-renewal and death National Heart, Lung, and Blood Institute Yang Liu CD24 polymorphism and multiple sclerosis National Institute of Neurological Disorders and Stroke $258,260 Yang Liu Hunting for novel x-linked breast cancersuppressor genes in mouse and human Army Medical Research and Materiel Command $448,500 Yang Liu Selective modulation of cancer immunity and autoimmunity National Cancer Institute $265,363 Yang Liu Tumor burden and T-cell immunity National Cancer Institute $270,783 Tatiana Oberyszyn Role of the EP1 prostanoid receptor in UV carcinogenesis National Cancer Institute $303,230 Tatiana Oberyszyn Therapeutic immunosuppression, inflammation and skin cancer National Cancer Institute $259,492 Vijay Pancholi Role of eukaryotic-type STK and PPPL in GAS pathogenesis National Institute of Allergy and Infectious Diseases $259,073 Thomas Prior Sen. Paul D. Wellstone Muscular Dystrophy Cooperative Research Center: Seattle University of Washington $300,000 Haifeng Wu Evaluation and validation of SELDI-based diagnostic tests for thrombotic thrombocytopenic purpura Ciphergen Biosystems, Inc. $149,500 Allan Yates Integrative training in biomedical systems National Institute of General Medical Sciences $157,167 Rachel Altura Inhibition of beta-cell loss by surviving gene targeting Juvenile Diabetes Research Foundation International $100,210 Lauren Bakaletz Antimicrobial peptides & innate immunity in otitis media National Institutes of Health $352,096 Lauren Bakaletz Assessment of the relative ability of pediatric immune serum pools directed against either the P.O.E.T. or STREPTORIX formulations of GSK’S pneumococcal polysaccharide-protein D conjugate vaccine to protect against ascending otitis media in a chinchilla Anonymous $214,476 Pediatrics 172 Ohio State University Medical Center $152,010 PI NAME TITLE SPONSOR AWARD Lauren Bakaletz Determinants of H. influenzae in otitis media National Institutes of Health $455,326 Lauren Bakaletz Studies on the biology and immunogenicity of NTHi The University of Iowa $236,529 John Barnard TGF-beta regulation of intestinal epithelial cells National Institutes of Health $305,059 Jeffrey Bartlett AAV vector targeting strategies for gene therapy National Institutes of Health $297,833 Carl Bates Role of FGF receptors in the developing kidney National Institutes of Health $313,652 Gail Besner HB-EGF and protection of the intestines from injury - 505005 Anonymous Gail Besner Role of NO and endothelin in human NEC National Institutes of Health $317,378 Katherine Blackmore Women, Infant and Children’s Nutrition Program (WIC) Columbus Health Department $427,335 Michael Brady Family AIDS Clinic and Educational Services (FACES) Health Resources & Services Administration $662,634 Michael Brady NICHD institutional training for pediatricians National Institutes of Health $229,922 Jeffrey Bridge Quality of care for adolescent suicide attempters National Institutes of Health $128,148 John Campo Anxiety and recurrent abdominal pain in children National Institutes of Health $495,372 John Campo Brief CBT for pediatric abdominal pain and anxiety National Institutes of Health $200,880 Paul Casamassimo Northeast Center for Research to Reduce Oral Health Disparities Boston University Marcel Casavant Poison control bioterrorism preparedness Ohio Department of Health Long-Sheng Chang Phenotypic determinants in vestibular schwannomas Ohio State University Research Foundation $101,556 Long-Sheng Chang The role of drosophila merlin in the control of mitosis exit and development US Army Medical Research $207,776 Reed Clark Characterization of CNS-compartmentalized env genes Children’s Hospital of Philadelphia $132,328 Reed Clark HIV vaccine design and development teams National Institutes of Health Reed Clark International AIDS Vaccine Initiative Anonymous $200,000 $155,164 $215,000 $6,792,629 $756,408 2007 Research Report 173 PI NAME TITLE SPONSOR Reed Clark Novel prophylactic HIV vaccines based on rAAV vectors Children’s Hospital of Philadelphia $295,813 Daniel Coury HMG hospital-based regional child find grant Ohio Department of Health $128,000 Daniel Coury Leadership education in neuro-developmental and related disabilities program Maternal & Child Health Bureau Heithem El-Hodiri The role of Rx in retinal progenitor cell development National Institutes of Health $389,927 Emilio Flano Gamma-herpesvirus infection of dendritic cells National Institutes of Health $277,326 Roger Friedman Angioedema study 2006 Anonymous $103,932 Haiyan Fu AAV-mediated gene therapy for neurological disorders of MPS IIIB using a knock-out mouse model The Sanfilippo Research Foundation $100,000 William Gardner Authorship and conflict of interest in clinical trials National Institutes of Health $263,656 Julie Gastier-Foster St. Baldrick’s Acute Lymphoblastic Leukemia Reference Laboratory National Childhood Cancer Foundation $130,000 Cynthia Gerhardt Sibling and parent bereavement from childhood cancer National Institutes of Health $627,960 Judith Groner Can changing how mom eats prevent obesity in toddlers? National Institutes of Health $144,690 Judith Groner Secondhand smoke and cardiovascular dysfunction in children Flight Attendant Medical Research Institute $108,500 Brett Hall High throughput screening for a novel class of anticancer agents that deregulate tumor-stromal cell interactions Elsa U. Pardee Foundation $100,406 Mark Hall Monocyte pyrin expression in human sepsis - 500205 National Institutes of Health $132,300 Dana Hardin Increased gluconeogenesis is one cause of CFRD National Institutes of Health $572,118 Gail Herman Molecular studies of x-linked chondrodysplasia punctata National Institutes of Health $278,010 Gail Herman Regional Genetics Center at Children’s Hospital Ohio Department of Health $325,000 Robert Hoffman Major affiliates to support research activities (TrialNet) TrialNet - George Washington University 174 Ohio State University Medical Center AWARD $400,000 $118,223 PI NAME TITLE SPONSOR AWARD Sudarshan Jadcherla Pathophysiology of aerodigestive reflexes in infants – 387105 National Institutes of Health $275,163 Jane Jarboe Early Childhood Education Program Franklin County Board MR/DD $367,470 Brian Kaspar Engineering AAV for enhanced retrograde transport National Institutes of Health $324,579 Brian Kaspar Motor neurons from neural progenitors Project A.L.S. $100,000 Brian Kaspar Therapeutic strategies for ALS Anonymous $200,000 Kelly Kelleher Cross design synthesis: combining evidence about antidepressants and suicidality National Institutes of Health $301,058 Kelly Kelleher Trial of automated risk assessment for adolescents National Institutes of Health $416,450 Bryce Kerlin Patient and family support group for hemophilia clinic Cascade Hemophilia Consortium $123,540 Lawrence Leguire Amblyopia Registry Ohio Optometric Association $143,959 Jiayuh Lin Inhibition of constitutive Stat3 pathway in breast cancer cells by a novel lowmolecular-weight compound Susan G. Komen Breast Cancer Foundation $125,000 Jiayuh Lin PDK-1/AKT pathway as a therapeutic target using a novel small molecular compound, OSU-03012, in childhood rhabdomyosarcoma Elsa U. Pardee Foundation $100,000 Yusen Liu MKP-1 in regulation of inflammatory cytokine production National Institutes of Health $285,138 Paul Martin Galgt2, dystroglycan and muscle extracellular matrix National Institutes of Health $274,553 Paul Martin Glycosyltransferase therapy for myopathies National Institutes of Health $312,400 Paul Martin Identification of novel bioactive glycans on dystroglycan National Institutes of Health $191,700 Kim McBride Genetics of congenital left-sided heart defects National Institutes of Health $132,343 Douglas McCarty Recombinant AAV gene therapy vector recombination, integration and genotoxicity National Institutes of Health $319,500 Richard McClead Telemedicine in transport decisions Ohio Board of Regents $100,000 Karen McCoy Asthma Clinical Research Center Ohio State University Research Foundation $122,778 2007 Research Report 175 PI NAME TITLE SPONSOR AWARD Karen McCoy CF Therapeutic Development Network Cystic Fibrosis Foundation $108,000 Karen McCoy Cystic Fibrosis Center Cystic Fibrosis Foundation $173,467 Kirk McHugh A genetic model of urogenital development and obstruction National Institutes of Health $305,059 Kirk McHugh Co-regulatory model of smooth muscle myogenesis National Institutes of Health $269,910 Jerry Mendell Clinical thresholds and gene transfer in DMD and LGMD University of Pittsburgh $610,224 Jerry Mendell Early screening and diagnosis of Duchenne muscular dystrophy Centers for Disease Control and Prevention $250,000 Jerry Mendell Gentamicin trial in Duchenne and limb girdle dystrophies (grant transfer) National Institutes of Health $428,558 Jerry Mendell Immunogenicity of golden retriever and normal dogs to rAAV vectors carrying mini-dystrophin Muscular Dystrophy Association, Inc. $194,962 Jerry Mendell Phase I study of mini-dystrophin gene in AAV Anonymous $657,182 Jerry Mendell Vascular approach to gene therapy for muscular dystrophy Children’s National Medical Center James Mulick The OSU RUPP-PI Project Ohio State University Research Foundation $133,159 Robert Munson NTHi Type IV pili: expression and vaccine potential National Institutes of Health $451,937 Robert Munson Protein glycosylation in haemophilus ducreyi National Institutes of Health $180,000 Leif Nelin Cationic amino acid transporters and lung NO production National Institutes of Health $332,000 Akihira Otoshi CCC Transgenic Facility Ohio State University Research Foundation $127,837 Akihira Otoshi Genetic analysis of the microenvironment in breast tumor progression Ohio State University Research Foundation $218,333 Kathi Pajer HPA axis function in adolescent antisocial females National Institutes of Health $604,961 Mark Peeples Gene therapy for cystic fibrosis University of North Carolina $198,172 Mark Peeples Generation of a single-cycle virus to study the pathogenesis of nipah virus National Institutes of Health $177,500 Mark Peeples Translation regulation in sendai virus National Institutes of Health $278,735 176 Ohio State University Medical Center $224,596 PI NAME TITLE SPONSOR AWARD Stephen Qualman Acute Lymphocytic Leukemic Reference Laboratory National Childhood Cancer Foundation $416,780 Stephen Qualman ALL – specimen banking National Childhood Cancer Foundation $380,396 Stephen Qualman BPC – specimen banking National Childhood Cancer Foundation $415,553 Stephen Qualman COG Neuroblastoma Reference Laboratory National Childhood Cancer Foundation $150,000 Stephen Qualman COG studies of gene amplification in rhabdomyosarcoma University of Pennsylvania $157,079 Stephen Qualman COG – specimen banking Anonymous $573,350 Stephen Qualman Pediatric CHTN: new scope/technology aids cancer research National Institutes of Health $828,867 Mark Ranalli Comprehensive Sickle Cell Treatment Center Ohio Department of Health $143,704 Brady Reynolds Impulsivity at different stages of adolescent smoking National Institutes of Health $211,320 Gary Smith Ohio CODES (Crash Outcome Data Evaluation System) Ohio Department of Public Safety $194,848 Gary Smith The medical and economic impact of motorized recreational vehicle-related traumatic brain injury in Ohio Ohio Department of Public Safety $110,000 Olivia Thomas Wellness Block Grant I - pregnancy prevention Franklin County Children Services $124,844 Veronica Vieland Bayesian reanalysis of a multisite gene-mapping study of cleft-lip/cleft palate National Institutes of Health $144,000 Christopher Walker AAV vectors for prevention and therapy of HCV infection National Institutes of Health $442,522 Christopher Walker Dendritic cell mediated induction of antiHCV immunity New York University School of Medicine $258,513 Christopher Walker HCV replication and immunity in chimpanzees National Institutes of Health $915,804 Christopher Walker HCV-specific T-cell responses in chimpanzees National Institutes of Health $954,969 Christopher Walker Immunological strategies for curing chronic hepatitus virus infections Emory University $677,967 Stephen Welty Clara cell function and CCSP in hyperoxic lung injury National Institutes of Health $297,833 2007 Research Report 177 PI NAME TITLE SPONSOR AWARD Gregory Wiet Validation/dissemination virtual temporal bone dissection National Institutes of Health $315,480 Huiyun Xiang Work-related injuries among immigrant workers Centers for Disease Control and Prevention $162,754 Keith Yeates A neurobehavioral late-effects in pediatric brain tumors Children’s Hospital Medical Center Cincinnati $106,687 Keith Yeates Child and family sequelae of preschool brain injury Children’s Hospital Medical Center Cincinnati $167,466 Keith Yeates Outcomes of traumatic brain injury in children National Institutes of Health $105,451 Keith Yeates Social outcomes in pediatric traumatic brain injury National Institutes of Health $842,827 Chack-Yung Yu Variations of complement in immunity and disease National Institutes of Health $290,988 Glen Apseloff A double-blind, randomized, single and multiple ascending dose study to determine the safety, tolerability and pharmacokinetics of GSK232802 GlaxoSmithKline Glen Apseloff A randomized pilot study to assess pharmacokinetic characteristics and relative bioavailability of hydromorphone following administration of 3 different hydromorphone formulations Covance, Inc. Glen Apseloff Ascending single dose study of the safety, tolerability, pharmacokinetics and pharmacodynamics of NRI-022 administered orally to healthy postmenopausal women Wyeth Pharmaceuticals, Inc. $744,891 Glen Apseloff Phase I study of the safety, tolerability and pharmacokinetics of a single intravenous dose of ETI-204 and its potential interaction with ciprofloxacin EluSys Therapeutics, Inc. $330,978 Glen Apseloff Phase I study to evaluate the safety, tolerability and pharmacokinetics of a single intravenous dose of CBR-2092 in healthy volunteers Cumbre Pharmaceuticals, Inc. $410,766 Glen Apseloff Study in healthy male and female subjects to assess the pharmacokinetic characteristics and relative bioavailability of hydromorphone Covance, Inc. $238,126 Pharmacology 178 Ohio State University Medical Center $110,303 $306,290 PI NAME TITLE SPONSOR AWARD Laura Bohn Morphine tolerance in Barrestin-2KO mice: beyond pain National Institute on Drug Abuse $163,906 Laura Bohn Physiological implications of opioid receptor regulation National Institute on Drug Abuse $255,476 Roger Briesewitz Cooperation of Flt3 ITD and p15INK4b inactivation in leukemogenesis Leukemia & Lymphoma Society $235,445 Arturo Cardounel Methylarginines and vascular injury National Heart, Lung, and Blood Institute $338,702 Howard Haogang Gu Cocaine and monoamine transporters National Institute on Drug Abuse $328,950 Howard Haogang Gu Mechanism of drug addiction National Institute on Drug Abuse $288,068 Wolfgang Sadee Genetic and epigenetic regulation of addiction genes National Institute on Drug Abuse $366,130 Wolfgang Sadee Quantitative assessment of cis-acting polymorphisms affecting CETP mRNA expression Pfizer, Inc. $220,000 Physical Medicine and Rehabilitation John Corrigan Efficacy of the individual placement and support (IPS) model for clients with disability and substance use disorders Wright State University $113,474 John Corrigan Ohio regional TBI model system National Institute on Disability and Rehabilitation Research $364,957 John Corrigan Reliability and predictive validity of the OSU method of detecting prior TBI Centers for Disease Control and Prevention $164,916 Physiology and Cell Biology George Billman Effects of a novel compound on susceptibility to ventricular fibrillation induced by myocardial ischemia in a conscious canine model of sudden death Sanofi-Aventis $250,000 Jack Boulant Neural control of temperature regulation National Institute of Neurological Disorders and Stroke $342,081 Sandor Gyorke Controlled and uncontrollable calcium release in heart National Heart, Lung, and Blood Institute $358,683 Sandor Gyorke Ryanodine receptor channels in heart failure National Heart, Lung, and Blood Institute $360,133 Lyn Jakeman Axons and the extracellular matrix in spinal cord injury National Institute of Neurological Disorders and Stroke $270,076 Paul Janssen Cardiac contraction-relaxation coupling National Heart, Lung, and Blood Institute $364,967 2007 Research Report 179 PI NAME TITLE SPONSOR AWARD Paul Janssen Cardiac relaxation: frequency-dependent relaxation and load-induced remodeling American Heart Association $100,000 Paul Janssen Myofilament calcium sensitivity in health and disease National Heart, Lung, and Blood Institute $102,708 Sissy Jhiang Sodium iodide symporter as an imaging reporter gene National Institute of Biomedical Imaging and Bioengineering $324,077 Sissy Jhiang X-SPECT(TM) for functional & anatomic imaging in vivo National Center for Research Resources $349,90 Beth Lee Dynamics of the actin cytoskeleton in osteoclasts National Institute of Arthritis and Musculoskeletal and Skin Diseases $263,120 Beth Lee Regulation of mRNA stability in kidney epithelia National Institute of Diabetes and Digestive and Kidney Diseases $314,304 Jack Rall Myofibrillar determinants of striated muscle relaxation National Institute of Arthritis and Musculoskeletal and Skin Diseases $263,039 Jackie Wood Function of the enteric nervous system National Institute of Diabetes and Digestive and Kidney Diseases $306,973 Jackie Wood Purinergic neurogenic mucosal secretion National Institutes of Health $291,974 Mark Ziolo NOS1/NOS3 functional effects on cardiac myocyte function National Heart, Lung, and Blood Institute $375,000 L. Eugene Arnold Pilot explorations of zinc effects in ADHD National Institute of Mental Health $218,434 Mary Fristad Longitudinal assessment of manic symptoms (LAMS) National Institute of Mental Health $396,908 Janice Kiecolt-Glaser Depression, stress, aging and proinflammatory cytokines National Institute on Aging $184,500 Janice Kiecolt-Glaser Psychoneuroimmunology and mind-body interventions National Center for Complementary and Alternative Medicine $248,178 Electra Paskett Appalachian Cancer Center Network University of Kentucky $237,077 Electra Paskett Breast cancer prevention through nutrition program The Breast Cancer Research Foundation Electra Paskett Cancer information service Wayne State University Electra Paskett Ohio patient navigation program American Cancer Society, Inc. $480,000 Electra Paskett Reducing cervical cancer in Appalachia National Cancer Institute $1,626,982 Christopher Weghorst Chemoprevention of oral cancer in Appalachia American Cancer Society, Inc. Psychiatry Public Health 180 Ohio State University Medical Center $250,000 $142,391 $249,600 PI NAME TITLE SPONSOR AWARD Radiology Michael Knopp Advancing imaging technology as a credentialed biomarker for clinical drug development Pfizer, Inc. $700,000 Michael Knopp The biomedical structural, functional and molecular imaging enterprise – part two Ohio Department of Development Robert Snapka Proteomics of topoisomerase-DNA cleavage complexes National Cancer Institute $229,930 Altaf Wani Cross-talking pre-incision events of eukaryotic NER National Institutes of Health $346,719 Altaf Wani DNA damage responses following genotoxin exposure National Institute of Environmental Health Sciences $343,306 $7,878,957 School of Allied Medical Professions Michele Basso Sparing and exercise training promote recovery in SCI National Institute of Neurological Disorders and Stroke $283,920 John Borstad Three-dimensional analysis of shoulder motion limitation following treatment for breast cancer Susan G Komen Breast Cancer Foundation $168,739 John Buford Reticulospinal control of reaching National Institute of Neurological Disorders and Stroke $283,715 Deborah Heiss Efficacy of therapeutic exercise for recurrent back pain National Institute of Child Health and Human Development $204,142 Stephen Wilson Creating an Indiana-Ohio Center for Traumatic Amputation Rehabilitation Research Indiana University-Purdue University Indianapolis Benjamin Sun Surgical treatment for ischemic heart failure (STICH) Duke University $100,650 Ginny Bumgardner Hypoxia pre-conditioning of pancreatic islets for intrahepatic transplantation National Institute of Diabetes and Digestive and Kidney Diseases $218,980 William Carson III A program of immune-based treatments for cancer National Cancer Institute $163,858 William Carson III Modulation of tumor CEA levels for an antiCEA vaccine National Cancer Institute $288,324 William Carson III Therapy of melanoma with bortexomib and interferon-alpha National Cancer Institute $265,363 William Carson III Tumor immunology National Cancer Institute $198,449 $200,000 Surgery 2007 Research Report 181 PI NAME TITLE SPONSOR AWARD Charles Cook Bacterial sepsis and reactivity of latent cytomegalovirus National Institute of General Medical Sciences $285,187 Charles Cook The immunobiology of murine allograft acceptance National Institute of Allergy and Infectious Diseases $282,522 Pedram Ghafourifar Heart mitochondrial NOS and hypoxia/reoxygenation American Heart Association – Ohio Valley Affiliate Gayle Gordillo Role of macrophages in hemangioendothelioma development National Institute of General Medical Sciences $126,588 Sampath Parthasarathy Dietary oxidized lipids and atherosclerosis National Institute of Diabetes and Digestive and Kidney Diseases $619,245 Sampath Parthasarathy NGS: A services-oriented framework for next generation data analysis centers NSF Computer & Information Sciences & Engineering $154,000 Sampath Parthasarathy Oxidation hypothesis – paradoxes and pitfalls National Heart, Lung, and Blood Institute $701,656 Amer Rajab Rapid four-day steroid withdrawal with thymoglobulin induction and maintenance immunosuppression with sirolimus and mycophenolate in primary cadaveric renal transplantation Wyeth Pharmaceuticals, Inc. $164,297 Sashwati Roy Regulator of diabetic wound healing InterHealth Nutraceuticals, Inc. $165,000 Chandan Sen Oxygen-sensitive signaling in primary cardiac fibroblast National Heart, Lung, and Blood Institute $328,471 Chandan Sen Redox control of wound healing National Institute of General Medical Sciences $270,076 Anne VanBuskirk IFN-gamma and TGF-beta interactions in post-transplant lymphoproliferative disorder (PTLD) Roche Organ Transplantation Research Foundation $193,887 Lisa Yee A dose-response study of omega 3 fatty acids supplements in women at high risk for breast cancer University of Chicago $146,319 Lisa Yee HER-2/neu and dietary fat: genenutrient interactions in breast cancer National Cancer Institute $156,936 TOTAL: 182 Ohio State University Medical Center $114,121 $148,871,106 PROJECTS FUNDED THROUGH OHIO’S THIRD FRONTIER PROJECT Launched in 2002, Ohio’s Third Frontier Project is a 10-year, $1.1 billion initiative to expand the state’s research capabilities, promote scientific innovation and create companies that will foster more jobs. The initiative funds various grants, including Biomedical Research and Commercialization Partnership (BRCP) awards and Wright Center of Innovation (WCI) awards. Both types support early research and proof of principle in projects that are usually public/private collaborations. Here is a list of BRCPor WCI-funded collaborations involving Ohio State University Medical Center and partners: Wright Center of Innovation (WCI) and Biomedical Research and Technology Transfer (BRTT) Partnership Awards Researcher Partners Project Title Award Michael Knopp, MD, PhD Philips Medical Rexon The Biomedical, Structural, Functional and Molecular Imaging Enterprise $17.1 million BRTT Partnership Awards Caroline Whitacre, PhD OncoImmune, Ltd. Cleveland Clinic Commercialization platform of immunotherapeutics for multiple sclerosis $4.25 million Michael Caligiuri, MD Battelle Zivena The prevention, detection and treatment of lung cancer $8 million Joel Saltz, MD, PhD LabBook Biomedical informatics synthesis platform $6 million Mauro Ferrari, PhD Battelle iMeDD, Inc. The cardiovascular bioengineering enterprise Albert de la Chapelle,MD, PhD, and Charis Eng, MD, PhD (subcontract with CWRU) Athersys Case Western Reserve University (sponsor) Genetics of gastrointestinal cancer Larry Lasky, MD Case Western Reserve University (subcontract) Stem Cell and Tissue Engineering Institute (SCTEI) $6.5 million $1.49 million $.56 million Biomedical Research and Commercialization Program (BRCP) Award Michael Knopp, MD, PhD Phillips Medical Systems of Cleveland Cardinal Health, Inc. The Biomedical, Structural, Functional and Molecular Imaging Enterprise $7.9 million Total: $51.8 million 2007 Research Report 183 GRANTS FROM INTERNAL FUNDING SOURCES The Davis/Bremer Medical Research Endowment Davis/Bremer Grant Awards – 2006 Juan Crestanello, MD Surgery Effect of ischemic preconditioning on mitochondrial function and on mitochondrial free oxygen radical production Ulysses Magalang, MD Internal Medicine Adiponectin as an endogenous anti-inflammatory agent David Feldman, MD, PhD Internal Medicine Adrenergic signaling and genomic perturbations in a nonischemic cardiomyopathy Zhenguo Liu, MD, PhD Cardiovascular Medicine Hyperglycemia may impair the differentiation of bone marrow stem cells into endothelial cells: a novel mechanism for the development of cardiovascular dysfunction in diabetes mellitus Peter Muscarella, MD Surgery Preclinical assessment of a novel PI3K/Akt inhibitor as therapy for pancreatic cancer Rulong Shen, MD Pathology The role of precancer stem cells in tumor angiogenesis Karen Wood, MD Internal Medicine Heat shock protein 27: alloimmunity and apoptosis 2006 STRATEGIC INITIATIVE GRANTS Ohio State University Medical Center Strategic Initiative Grants (SIGs) are competitive awards internally funded from a pool of money contributed by four departments (Ophthalmology, Emergency Medicine, Pathology and Radiology) to support outcomes research by their faculty. Four years ago the Ohio State University medical director’s office assumed the expenses for some house staff in these departments. The practice dollars that had been underwriting some of these salaries were then redirected into the SIG pool to support research. In 2006, the Medical Center awarded nine SIGs collectively totaling $697,129. Strategic Initiative Grant Recipients in Calendar Year 2006 Principal Investigator Department Project Title Carlos Alexandre Andrade Torres, MD Emergency Medicine Unraveling the inotropic effect of Pyruvate Michael Sayre, MD Emergency Medicine Pilot study using indirect calorimetry to describe resting metabolic rate of resuscitated cardiac arrest patients during hypothermia therapy Frederick Davidorf, MD Ophthalmology MET oncogene as therapeutic target for uveal melanoma Jeffrey Caterino, MD Emergency Medicine Infected elders in the ED: outcome and processes of care 184 Ohio State University Medical Center Mark Angelos, MD Emergency Medicine Cellular mechanisms of early reactive oxygen species formation in myocardial reperfusion Vijay Pancholi, PhD Pathology Targeting a eukaryotic-type signaling system in staphylococcus aureus to harness the bacterial drug resistance and biofilm formation Sanford Barsky, MD Pathology Clues to the stem cell origin of human cancers by studying a registry of organ transplant recipients who later developed secondary solid cancers Jian-Xin Gao, MD, PhD Pathology Identification of early diagnosis marker for cancer Brian Hiestand, MD Emergency Medicine Comparison of multidetector coronary computed tomography and conventional stress imaging in ED chest pain patients RESEARCH INVESTMENT FUND (RIF) AWARDS Ohio State University Medical Center’s Research Investment Fund (RIF) Advisory Committee is dedicated to the institution’s longterm research strategies and to supporting investment in research and research infrastructure. The Committee considers applications in the areas of new faculty start-up, faculty salary support, faculty bridge funding, faculty retention packages, equipment purchase, core facility support and matching funds for grant proposals. Applicants are asked to provide a comprehensive description of the research and the proposed use of funds. The Committee, supported by Ohio State’s College of Medicine and led by the vice dean for research, carefully considers each request. Research Investment Fund Approved Funding Cumulative through 12/31/2006 FY’04-FY’11 Amount Percentage of Total $838,886 16.22% 175,000 3.38% 117,180 2.27% 2,317,662 44.82% 906,233 17.52% 176,651 3.42% Faculty Retention 415,000 8.02% Shared Equipment 224,987 4.35% $5,171,599 100.00% Type of Expense Bridge Funds Core Facility Support Facilities Support Personnel Faculty Recruitment & Start-Up Matching Funds Program Expansion Totals (see research investment funds pie chart, next page) 2007 Research Report 185 RESEARCH INVESTMENT FUNDS BY TYPE OF EXPENSE Core Facility Support 3% Facilities Support Personnel 2% Bridge Funds 16% Shared Equipment 4% Faculty Recruitment & Start-up 45% Faculty Retention 8% Program Expansion 4% Matching Funds 18% CRISAFI-MONTE PRIMARY CARE CARDIOPULMONARY GRANT PROGRAM The Crisafi-Monte Research Endowment supports physician investigators in Family Medicine and/or Primary Care, supporting teaching, research and scholarship in diseases of the heart, lungs and related disorders. Grants are awarded for one or two years, with a maximum total of $40,000. During 2006, Patricia Schwirian, PhD, RN, Judith Groner, MD, and William Mizer, MD, were awarded $18,868 from the CrisafiMonte Fund to supplement a National Institutes of Health award of $247,500 (plus $41,915 from the Children’s Research Institute) for a project titled Can changing how mom eats prevent obesity in toddlers? 186 Ohio State University Medical Center Selected High-Impact Publications BY DEPARTMENT, CENTER OR INSTITUTE Publications listed in this section are considered the most significant of the year by their departments, centers or institutes at Ohio State, but they represent only a portion of the thousands of publications by Ohio State medical researchers that appeared in scientific journals during 2006. For a complete list, visit our Web site at www.medicalcenter.osu.edu. 2007 Research Report 187 (Note: In this listing, IF = impact factor) Centers and Institutes COMPREHENSIVE CANCER CENTER (of 643 publications) Hajitou A, Trepel M, Lilley CE, Soghomonyan S, Alauddin MM, Marini FC, Restel BH, Ozawa MG, Moya CA, Rangel R, Sun Y, Zaoui K, Schmidt M, von Kalle C, Weitzman MD, Gelovani JG, Pasqualini R, Arap W. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell 2006;125(2):385398. (IF: 29.431) Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libe R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 2006;38(7):794-800. (IF: 25.797) Prentice RL, Caan B, Chlebowski RT, Patterson R, Kullelr LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, Paskett ED, Lawrence P, Robbins J, Rossouw JE, Sarto GE, Shikany JM, Stafanick ML, Thomson CA, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Wassertheil-Smoller S, Whitlock E, Yano K, Adams-Campbell L, Anderson GL, Assaf AR, Beresford SAA, Black HR, Brunner RL, Brzyski RG, Ford L, Gass M, Hays J, Heber D, Heiss G, Hendrix SL, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Kotchen JM, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Henderson MM. Low-fat eating pattern and risk of invasive breast cancer: The Women’s Health Initiative randomized controlled dietary modification trial. JAMA 2006;295(6):629-642. (IF: 23.332) Fedele M, Visone R, De Martino I, Troncone G, Palmieri D, Arra C, Melillo RM, Helin K, Croce CM, Fusco A. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell 2006;9(6):459-471. (IF: 18.725) Porter PL, Barlow WE, Yeh IT, Lin MG, Yuan XP, Donato E, Sledge GW, Shapiro CL, Ingle JN, Haskell CM, Albain KS, Roberts JM, Livingston RB, Hayes DF. p27(Kip1) and cyclin E expression and breast cancer survival after treatment with adjuvant chemo therapy. J Natl Cancer Inst 2006;98(23):17231731. (IF: 15.171) Yu J, Wei M, Becknell B, Trotta R, Liu S, Boyd Z, Jaung MS, Blaser BW, Sun J, Benson DM, Jr., Mao H, Yokohama A, Bhatt D, Shen L, Davuluri R, Weinstein M, Marcucci G, Caligiuri MA. Pro- and anti-inflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity 2006;24(5):575-590. (IF: 15.156) Dorrance AM, Liu S, Yuan W, Becknell B, Arnoczky KJ, 188 Ohio State University Medical Center Guimond M, Strout MP, Nakamura T, Yu L, Rush LJ, Weinstein M, Leone G, Wu L, Ferketich A, Whitman SP, Marcucci G, Caligiuri MA. MII partial tandem duplication induces aberrant Hox expression in vivo via specific epigenetic alterations. J Clin Invest 2006;116(10):2707-26. (IF: 15.053) Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med 2006;203(4):1033-1043. (IF: 13.965) Ollila S, Sarantaus L, Kariola R, Chan P, Hampel H, HolinskiFeder E, Macrae F, Kohonen-Corish M, Gerdes AM, Peltomaki P, Mangold E, de la Chapelle A, Greenblatt M, Nystrom M. Pathogenicity of MSH2 missense mutations is typically associated with impaired repair capability of the mutated protein. Gastroenterology 2006;131 (5):1408-17. (IF: 12.386) Hartman TR, Qian S, Bolinger C, Fernandez S, Schoenberg DR, Boris-Lawrie K. RNA helicase A is necessary for translation of selected messenger RNAs. Nat Struct Mol Biol 2006;13(6):509-516. (IF: 12.19) Paschka P, Marcucci G, Ruppert AS, Mrózek K, Chen H, Kittles RA, Vukosavljevic T, Perrotti D, Vardiman JW, Carroll AJ, Kolitz JE, Larson RA, Bloomfield CD. Adverse prognostic significance of KIT mutations in adult myeloid leukemia with inv (16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol 2006;24(24):3904-3911. (IF: 11.81) Monk JP, Phillips G, Waite R, Kuhn J, Schaaf LJ, Otterson GA, Guttridge D, Rhoades C, Shah M, Criswell T, Caligiuri MA, Villalona-Calero MA. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol 2006;24(12):1852-1859. (IF: 11.81) CENTER FOR KNOWLEDGE MANAGEMENT JOHN A. PRIOR HEALTH SCIENCES LIBRARY Knutson D, Cain T, Hurtubise L and Kreger C. Lessons learned: developing e-learning to teach physical examination. The Clinical Teacher 2006;3(3)163-169. DOROTHY M. DAVIS HEART AND LUNG RESEARCH INSTITUTE (of 678 publications) Sutton MG, Plappert T, Hilpisch KE, Abraham WT, Hayes DL, Chinchoy E. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Circulation 2006;113(2):266-72. (IF: 11.632) Wani MA, Haynes LD, Kim J, Bronson CL, Chaudhury C, Mohanty S, WaldmannTA, Robinson JM, Anderson CL. Familial hypercatabolic hypoproteinemia caused by FcRn deficiency due to mutant b2-microglobulin gene. Proc Natl Acad Sci 2006;103:5084-5089. (IF: 10.231) Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, Younce C, Binkley PF, Kolattukudy PE. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res 2006;98(9):1177-85. (IF: 9.408) Wei Q, XiaY. Proteasome inhibition downregulates endothelial nitric oxide synthase phosphorylation and function. J Biol Chem 2006;281:21652-21659. (IF: 5.854) Roy S, Khanna S, Kuhn D, Rink C, Williams W, Zweier JL, Sen C. Transcriptome analysis of the ischemia-reperfused remodeling myocardium: temporal changes in inflammation and extracellular matrix. Physiological Genomics 2006;25(3):364374. (IF: 4.636) Chen YR, Chen CL, Yeh A, Liu X, Zweier JL. Direct and indirect roles of cytochrome b in the mediation of superoxide generation and NO catabolism by mitochondrial succinatecytochrome c reductase. J Biol Chem 2006;281(19):13159-68. (IF: 5.854) Cooke GE, Liu-Stratton Y, Ferketich AK, Moeschberger ML, Frid DJ, Magorien RD, Bray PF, Binkley PF, GoldschmidtClermont PJ. Effect of platelet antigen polymorphism on platelet inhibition by aspirin, clopidogrel or their combination. J Am Coll Cardiol. 2006;47(3):541-6. (IF: 9.200) Montague CR, Hunter MG, Gavrilin MA, Phillips GS, Goldschmidt-Clermont PJ, Marsh CB. Activation of estrogen receptor-α reduces aortic smooth muscle cell differentiation. Circ Res 2006;99(5):477-84. (IF: 9.408) Martin MM, Lee EJ, Buckenberger JA, Schmittgen TD, Elton TS. MicroRNA-155 regulates human angiotensin II type 1 receptor expression in fibroblasts. J Biol Chem 2006;281:18277-18284. (IF: 5.854) CENTER FOR MICROBIAL INTERFACE BIOLOGY (CMIB) Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Gyorke S. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res 2006;98(9):1151-8. (IF: 9.408) (of 19 publications) Bratasz A, Weir NM, Parinandi NL, Zweier JL, Sridhar R, Ignarro LJ, Kuppusamy P. Reversal to cisplatin sensitivity in recurrent human ovarian cancer cells by NCX-4016, a nitroderivative of aspirin. Proc Natl Acad Sci USA 2006;103,39143919. (IF: 10.231) Kulkarni MM, McMaster WR, Kamysz E, Kamysz W, Engman DM, McGwire BS. The surface-metalloprotease of the parasitic protozoan, Leishmania, protects against antimicrobial peptide-induced apoptotic killing. Molecular Microbiology 2006;62(5): 1484-1497. (IF: 6.203) Li H, Liu X, Cui H, Chen YR, Cardounel AJ, Zweier JL. Characterization of the mechanism of cytochrome P450 reductase-cytochrome P450-mediated nitric oxide and nitrosothiol generation from organic nitrates. J Biol Chem 2006;281(18):12546-54. (IF: 5.854) Seveau S, Tham TN, Payrastre B, Hoppe AD, Swanson JA, Cossart P. A FRET analysis to unravel the role of cholesterol in Rac1 and PI 3-kinase activation in the InlB/Met signaling pathway. Cellular Microbiology 2006, Nov 28. (IF: 6.333) Babu GJ, Bhupathy P, Petrashevskaya NN, Wang H, Raman S, Wheeler D, Jagatheesan G, Wieczorek D, Schwartz A, Janssen PM, Ziolo MT, Periasamy M. Targeted overexpression of sarcolipin in the mouse heart decreases sarcoplasmic reticulum calcium transport and cardiac contractility. J Biol Chem 2006;281(7):3972-9. (IF: 5.854) Gadd ME, Broekemeier KM, Crouser ED, Kumar J, Graff G, Pfeiffer DR. Mitochondrial iPLA2 activity modulates the release of cytochrome c from mitochondria and influences the permeability transition. J Biol Chem 2006;281(11):6931-9. (IF: 5.854) Flaherty DK, Vesosky B, Beamer GL, Stromberg P, Turner J. Exposure to Mycobacterium avium can modulate established immunity against Mycobacterium tuberculosis infection generated by Mycobacterium bovis BCG vaccination. Journal of Leukocyte Biology 2006;80:1262-1271. (IF: 4.627) Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myoinositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. Journal of Immunology 2006;177:1805-1816 (IF: 6.387) Parsa KVL, Ganesan LP, Rajaram MVS, Gavrilin MA, Balagopal A, Mohapatra NP, Wewers MD, Schlesinger LS, Gunn JS, Tridandapani S. Macrophage proinflammatory response to Francisella novicida infection is regulated by the SH2 domaincontaining inositol 5’ phosphatase SHIP. PLOS Pathogens 2006;2:0681-0690 (IF: 8.389) 2007 Research Report 189 Gavrilin MA, Bouakl I, Knatz M, Duncan MW, Hall JS, Gunn J, Wewers MD. Internalization and phagosome escape required for live Francisella to induce human monocyte IL-1β processing and release. PNAS 2006;103:141-146. (IF: 10.231) Gu C*, Zhou W, Puthenveedu MA, Xu M, Jan YN, Jan LY*. The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron 2006;52:803-816 (* = co-corresponding author). (IF: 14.304) Rappleye CA, Goldman WE. Defining virulence genes in the dimorphic fungi. Annual Review of Microbiology 2006;60:281303. (IF: 13.412) Massa SM, Xie Y, Yang T, Harringtion AW, Kim ML, Yoon SO, Kraemer R, Moore LA, Hempstead BL, Longo FM. Small, Nonpeptide p75NTR ligands induce survival signaling and inhibit proNGF-induced death. Journal of Neuroscience 2006; 26:5288-5300. (IF: 7.506) CENTER FOR MINIMALLY INVASIVE SURGERY (of seven publications) Lucas ME, Muller F, Rudiger R, Henion PD, Rohrer H. The bHLH transcription factor Hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development 2006;133: 4015-4024. (IF: 7.603) Dunkin BJ, Martinez J, Bejerano P, Smith CD, Chang K, Livingstone AS, Melvin WS. Thin-layer ablation of human esophageal epithelium using a bipolar radiofrequency balloon device. Surgical Endoscopy 2006;20:125-130. (IF: 1.746) Yin H, Laguna KA, Li G, Kuret J. Dysbindin homolog CKBP1 is an isoform-selective binding partner of human casein kinase-1 isoforms. Biochemistry 2006;45:5297-5308. (IF: 3.848) Li J, Zhu J, Melvin WS, Bekaii-Saab TS, Chen CS, and Muscarella P. A structurally optimized celecoxib derivative inhibits human pancreatic cancer cell growth. Journal of Gastrointestinal Surgery 2006;10(2):207-214. (IF: 2.29) INSTITUTE OF BEHAVIORAL MEDICINE RESEARCH Davis SS Jr., Goldblatt MI, Hazey JW, Melvin WS. Unexpected gastrointestinal tract conditions. Current Problems in Surgery 2006; 43(2):77-118. (IF: 1.560) Ellison EC, Sparks J, Verducci JS, Johnson JA, Muscarella P, Melvin WS. Fifty-year appraisal of gastrinoma: recommendations for staging and treatment. Journal of American College of Surgeons 2006;202:897-905. (IF: 2.621) Smith CD, Bejarno P, Melvin WS, Patti M, Muthusamy R, Dunkin BJ. Endoscopic ablation of intestinal metaplasia containing high-grade dysplasia in esophagectomy patients using a balloon-based ablation system. Surgical Endoscopy 2006; [Epub ahead of print] (IF: 1.746) CENTER FOR MOLECULAR NEUROBIOLOGY (of 23 publications) Carrel TL, McWhorter ML, Workman E, Zhang H, Wolstencroft EC, Lorson C, Bassell G, Burghes AHM, Beattie CE. SMN function in motor axons is independent of functions required for snRNP biogenesis. Journal of Neuroscience 2006;26:11014-11022. Highlighted in This Week in the Journal. (IF: 7.506) Jontes JD, Phillips GR. Selective stabilization and synaptic specificity: a new cell-biological model. Trends in Neurosciences 2006;29:186-191. (IF: 14.325). 190 Ohio State University Medical Center (of 61 publications) Glaser R, Litsky M, Padgett D, Baiocchi R, Yang E, Chen M, Yeh P-E, Green-Church KB, Caligiuri M, Williams MV. EBV-encoded dUTPase induces immune dysregulation: implications for the pathophysiology of EBV-associated disease. Virology 2006;346(1):205-218. (IF: 3.08) Yang EV, Sood AK, Chen M, Yang L, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh P-E, Lemeshow S and Glaser R. Norepinephrine upregulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2 and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Research 2006;66(21):10357-10364. (IF: 7.6) Kin N and Sanders VM. It takes nerve to tell T and B cells what to do. J Leuk Biol, 2006;79:1093-1104. (IF: 4.6) Kin N and Sanders VM. CD86 stimulation on a B cell activates the PI3K/Akt and PLCgamma2/PKCalpha/beta signaling pathways. J Immunol 2006;176:6727-6735. (IF: 6.387) Pongratz G, McAlees JW, Conrad DH, Erbe RS, Haas KM and Sanders VM. The level of IgE produced by a B cell is regulated by norepinephrine in a p38 MAPK- and CD23-dependent manner. J Immunol 2006;177:2926-2938. (IF: 6.387) Nelson RJ, Trainor BC, Chiavegatto S & Demas GE. Pleiotropic contributions of nitric oxide to aggressive behavior. Neuroscience and Biobehavioral Reviews 2006;30: 346-355. (IF: 7.44) Bailey MT, Engler H, Sheridan JF. Stress induces the translocation of cutaneous and gastrointestinal microflora to secondary lymphoid organs of C57BL/6 mice. Journal of Neuroimmunology 2006;171(1-2):29-37. (IF: 4.6) Huang AS, Beigneux A, Weil ZM, Kim PM, Molliver ME, Blackshaw S, Nelson RJ, Young SG, & Snyder SH. D-aspartate regulates melanocortin formation and function: behavioral alterations in D-aspartate oxidase-deleted mice. Journal of Neuroscience 2006;26:2814-2819. (IF: 7.5) Weil ZM, Pyter LM, Martin LB & Nelson RJ. Perinatal photoperiod organizes adult immune responses in Siberian hamsters (Phodopus sungorus). American Journal of Physiology 2006;290:R1714-R1719. (IF: 3.94) Pyter LM, Trainor BC & Nelson RJ. Testosterone and photoperiod interact to affect spatial learning and memory in adult male white-footed mice (Peromyscus leucopus). European Journal of Neuroscience 2006;23:3056-3062. (IF: 3.94) Rong Chen, Tilley MR, Hua W, Fuwen Z, Fu-Ming Z, San C, Ning Q, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci USA 2006;103: 9333-9338, 2006. (IF: 10.23) Fulci G, Breymann L, Gianni D, Kurozumi K, Rhee SS, Yu J, Kaur B, Louis DN, Weissleder R, Caligiuri MA, Chiocca EA. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proceedings of the National Academy of Sciences USA 2006;103(34):12873-12878. (IF:10.231) Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, Moeniralm S, Saeki Y, Carette JE, Weissleder R, Vandertop WP, van Beusechem VW, Dirven CMF, Chiocca EA. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Molecular Therapy 2006;14(6):779-788. (IF: 5.443) Newton HB, Figg GM, Slone W, Bourekas E. Incidence of infusion plan alterations after angiography in patients undergoing intra-arterial chemotherapy for brain tumors. Journal of Neurooncology 2006;78(2):157-160. (IF: 1.581) Sarkar A, Caamano S, Fernandez JM. The mechanical fingerprint of a parallel polyprotein dimer. Biophysical Journal E-publication December 2006. (IF: 4.507) Suzuki M, Kasai K, Saeki Y. Plasmid DNA sequences present in conventional HSV amplicon vectors cause rapid transgene silencing by forming inactive chromatin. Journal of Virology 2006;80(7):3293-3300. (IF: 5.178) DARDINGER NEURO-ONCOLOGY CENTER (of 61 publications) Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenol types. Cancer Research 2006;66(18):9054-9064. (IF: 7.616) Bourekas EC, Figg GM, Slone W, Newton HB. Incidence and complication rate of incidental aneurysms discovered during intra-arterial chemotherapy of brain tumors. AJNR American Journal of Neuroradiology 2006;27(2):297-299. (IF: 2.463) Chakrabarti I , Burton AW, Rhines L, Mendel E. Percutaneous vertebroplasty of myelomatous kyphotic wedge fracture in the presence of previous posterior instrumentation: case report. Journal of Neurosurgery 2006;5(2):168-171. (IF: 2.446) THE NISONGER CENTER FOR MENTAL RETARDATION AND DEVELOPMENTAL DISABILITIES (of 20 publications) Arnold LE, Aman MG, Cook AM, Witwer AN, Hall KL, Thompson S, Ramadan Y. Atomoxetine for hyperactivity in autism spectrum disorders: placebo-controlled crossover pilot trial. J Amer Academy of Child & Adolesc Psychiatry 2006;45(10):1196-1205. Lecavalier, L. Behavior and emotional problems in young people with pervasive developmental disorders: relative prevalence, effects of subject characteristics and empirical classification. Journal of Autism and Developmental Disorders 2006;36, 101-1114. (IF: 2.375) Friedman A, Tian JP, Fulci G, Chiocca EA, Wang J. Glioma virotherapy: effects of innate immune suppression and increased viral replication capacity. Cancer Research 2006;66(4):2314-2319. (IF: 7.616) 2007 Research Report 191 Lecavalier, L., et al. The validity of the Autism Diagnostic Interview – Revised. American Journal on Mental Retardation 2006;111,199-215. (IF: 1.640) Monreal G, Gerhardt MA. Left ventricular assist device support induces acute changes in myocardial electrolytes in heart failure. ASAIO J 2006 (e-pub ahead of print). (IF: 1.15) Martens, MA, Jones, L, Reiss, S. Organ transplantation, organ donation and mental retardation. Pediatric Transplantation 2006;10,658-664. (IF: 1.424) Verhey JF, Nathan NS. Utilizing FEM-software to quantify preand post-interventional cardiac reconstruction data based on modeling data sets from surgical ventricular repair therapy (SVRT) and cardiac resynchronization therapy (CRT). BioMedical Engineering OnLine 2006;5:58. (IF: 1.17) Departments and Schools DEPARTMENT OF ANESTHESIOLOGY Wang GD, Wang XY, Hu H-Z, Fang XC, Liu S, Gao N, Gao X, and Xia Y. Platelet-activating factor in the enteric nervous system of guinea pig small intestine. Am J Physiol. 2006;291(5):G928-G937. (IF: 3.47) Brewer AJ, Lane ES, Ross P, Hachwa B. Misdiagnosis of perioperative myocardial ischemia: the effects of electrocardiogram filtering. Anesth Analg. 2006;103(6):1632-1634. (IF: 2.40) DEPARTMENT OF BIOMEDICAL INFORMATICS Chen Z, Suntres Z, Palmer J, Guzman J, Javed A, Xue J, Yu JG, Cooke HJ, Awad H, Hassanain HH, Cardounel AJ, Christofi FL. Cyclic AMP signaling contributes to neural plasticity and hyperexcitability in AH sensory neurons following intestinal trichinella spiralis-induced inflammation. International Journal of Parasitology 2006;(doi:10.1016/j.ijpara). (IF: 3.35) Dzwonczyk R, del Rio CL, Sai-Sudhakar C, Sirak J, Michler R, Sun B, Kelbick N, Howie M. Vacuum-assisted apical suction devices induce passive electrical changes consistent with myocardial ischemia during off-pump coronary artery bypass graft surgery. European J Cardiothoracic Surg 2006; 30:873-876. Goldman E, Fisher JL. Discrepancies in cancer mortality estimates. Archives of Medical Research 2006;37:548-551. (IF: 1.38) Guzman J, Yu JG, Suntres Z, Bozarov A, Cooke H, Javed N, Auer H, Palatini J, Hassanain HH, Cardounel AJ, Javed A, Grants I, Wunderlich JE, Christofi FL. ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm Bowel Dis 2006;12(8):766-789. (IF: 3.01) Hassanain H, Gregg D, Marcelo Zweier, JL, Souza HP, Selvakumar B, Ma Q, Moustafa-Bayumi M, Binkley PF, Zweier J, Flavahan NA, Morris M, Dong C, and Goldschmidt-Clermont P. Hypertension caused by transgenic overexpression of Rac 1. Antioxidant and Redox Signaling 2006;9(1):91-100. (IF: 4.23) Liu S, Gao N, Hu H-Z, Wang X, Wang G-D, Fang X, Xia Y, Wood JD. Distribution and chemical coding of corticotropin releasing factor-immunoreactive neurons in the guinea pig enteric nervous system. J Comp Neurol 2006;494:63-74. (IF: 3.86) (of 40 publications) Chen J, Yuan B. Detecting functional modules in the yeast protein-protein interaction network. Bioinformatics 2006;22:22832290. (IF: 6.019) Devine KD, Boman EG, Heaphy R, Bisseling R, Catalyurek UV. Parallel hypergraph partitioning for scientific computing. Proceedings of 20th International Parallel and Distributed Processing Symposium (IPDPS) 2006. Digital Object Identifier 10.1109/IPDPS.2006.1639359 Ioshikhes IP, Albert I, Zanton SJ, Pugh BF. Nucleosome positions predicted through comparative genomics. Nature Genetics 2006;38(10):1210-1215. (IF: 25.707) Kumar VS, Rutt B, Kurc TM, Catalyurek UV, Chow S, Lamont S, Martone M, Saltz J. Large image correction and warping in a cluster environment. Proceedings of the 2006 ACM/IEEE Conference on Supercomputing (SC2006) Nov. 2006; page(s):38 Digital Object Identifier 10.1109/SC.2006.39. (Citeseer rating: 1.25, top 13.4%) Kurc TM, Janies DA, Johnson A, Langella S, Oster S, Hastings SL, Habib F, Camerlengo T, Ervin DW, Catalyurek UV, Saltz J. An XML-based system for synthesis of data from disparate databases. Journal of the American Medical Informatics Association 2006;13:289-301. (IF: 4.339) Saltz JH, Oster S, Hastings SL, Langella S, Sanchez W, Kher M, Covitz P. CaGrid: design and implementation of the core architecture of the cancer biomedical informatics grid. Bioinformatics 2006;22:1910-1916. (IF: 6.019) Division of Anatomy (of six publications) Weichel J, Bolte IV JH. Response of reclined post-mortem human subjects to frontal impact. Society of Automotive Engineers 2006;01-0647. 192 Ohio State University Medical Center Bartsch AJ, Bolte IV JH, Litsky AS, Herriot RG, McFadden JD. Application of anthropomorphic test device crash test kinetics to post-mortem human subject lower extremity testing. Society of Automotive Engineers 2006;01-0251. Tung K, Raman SV, King MA, DePhilip RD. Correlation of magnetic resonance imaging with histopathology in arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C). Clinical Anatomy 2006;19:44-50. DEPARTMENT OF FAMILY MEDICINE (of 44 publications) Coyle JD, Miser WF. Race determines appropriate weight to use in Cockroft-Gault equation in diabetic patients (abstract). American Journal of Kidney Disorders 2006; 47:A25. (IF: 4.412) McDougle L, Gabel LL, Stone L. Future of family medicine workforce in the United States. Family Practice 2006;23:8-9. (IF: 1.167) Pressler JM, Heiss DG, Buford JA, Chidley JV. Between-day repeatability and symmetry of multifious cross-sectional area measured using ultrasound imaging. Journal of Orthopaedic and Sports Therapy 2006;36:10-18. Fahey P, Cruz-Huffmaster D, Blincoe T, Welter C, Welker MJ. Analysis of downstream revenue to an academic medical center from a primary care network. Academic Medicine 2006; 81(8):702-707. (IF: 1.94) Shaw JM, Herriott RG, McFadden JD, Donnelly BR, Bolte IV JH. Oblique and Lateral Impact Response of the PMHS Thorax. Fiftieth Stapp Car Crash Conference Journal, November 2006. Wexler RK, Feldman D. Antihypertensive drugs for CVD. Journal of Family Practice October 2006;55:889-891 (IF: 1.327) DEPARTMENT OF EMERGENCY MEDICINE Toumi H, Best TM. The myotendinous junction: acute changes and adaptation to stretch injury. Journal of Anatomy 2006;208: 459-470. (IF: 2.010) (of 13 publications) Hallstrom A, Rae TD, Sayre MR, Christenson J, Anton AR, Mosesso Jr VN, Ottingham LV, Olsufka M, Pennington S, White L, et al. Manual chest compressions vs. use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest. Journal of the American Medical Association 2006;295(22):2620-2628. (IF: 23.332) Aufderheide T, Hazinski MF, Nichol G, Smith Steffens S, Buroker A, McCune R, Stapleton E, Nadkarni V, Potts J, Ramirez RR, Eigel B, Epstein A, Sayre M, Halperin H, Cummins RO. Community lay rescuer automated external defibrillation programs: key state legislative components and implementation strategies. Circulation 2006;113(9):1260-70. (IF: 12.563) Angelos MG, Kutala VK, Torres CA, He G, Stoner J, Mohammad M, Kuppusamy P. Hypoxic reperfusion of the ischemic heart and oxygen radical generation. American Journal Of Physiology - Heart and Circulatory Physiology 2006;290:H341-H347. (IF: 3.56) Wexler RK, Aukerman G. Non-pharmacologic treatment of hypertension. American Family Physician 2006;73:1953-1958. (IF: 1.251) Toumi H, Hegge J, Subbotin V, Noble M, Herweijer H, Best TM, Hagstrom JE. Rapid intravascular injection into skeletal muscle: a damage-assessment study. Molecular Therapy 2006;13(1):229-236. (IF: 5.443) DEPARTMENT OF INTERNAL MEDICINE Division of Cardiovascular Medicine (of 76 publications) Abraham WT. Cardiac resynchronization therapy is important for all patients with congestive heart failure and ventricular dyssynchrony. Circulation 2006;114(24):2692-2698. (IF: 11.632) Stoner JD, Clanton TL, Aune SE, Angelos MG. O2 delivery and redox state are determinants of compartment-specific reactive oxygen species in myocardial reperfusion. American Journal of Physiology - Heart and Circulatory Physiology 2007 (Jan.) 292(1):H109-16. (IF: 3.56) Baliga RR, Ranganna P, Pitt B, Koelling TM. Spironolactone treatment and clinical outcomes in patients with systolic dysfunction and mild heart failure symptoms: a retrospective analysis. Journal of Cardiac Failure 2006;12:250-256. (IF: 2.935) Diercks DB, Peacock FW, Hiestand BC, Chen AY, Pollack CV, et al. Frequency and consequences of recording an electrocardiogram > 10 minutes after arrival in an emergency room in nonST-segment elevation acute coronary syndromes (from the CRUSADE Quality Initiative). American Journal of Cardiology 2006;97(4):437-42. (IF: 3.14) Cooke GE, Liu-Stratton Y, Ferketich AK, Moeschberger ML, Frid DJ, Magorien RD, Bray PF, Binkley PF, GoldschmidtClermont PJ. Effect of platelet antigen polymorphism on platelet inhibition by aspirin, clopidogrel or their combination. Journal of American College of Cardiology 2006; 47(3): 541-546. (IF: 9.2) 2007 Research Report 193 Daoud EG, Nademanee K, Fuenzalida C, Tomassoni GF, Schuger C, Chisner M, Simones M, Schwartz M, Reeve H. Clinical experience with tiered atrial therapies and atrial arrhythmia prevention algorithms in a dual chamber cardioverter defibrillator. Journal of Cardiovascular Electrophysiology 2006;17(8):852-856. (IF: 3.285) Gumina RJ, Murphy JG, Rihal CS, Lennon RJ, Wright RS. Longterm survival after right ventricular infarction. American Journal of Cardiology 2006;98:1571-1573. (IF: 3.059) Iyengar S, Feldman DS, Cooke GE, Leier CV, Raman SV. Detection of coronary artery disease in orthotopic heart transplant recipients with 64-detector row computed tomography angiography. The Journal of Heart and Lung Transplantation 2006;25(11):1363-1366. (IF: 2.992) Raman SV, Shah M, McCarthy B, Garcia A, Ferketich AK. Multi-detector row cardiac CT accurately quantifies right and left ventricular size and function compared to cardiac magnetic resonance. American Heart Journal 2006;151(3):736-744. (IF: 3.552) Division of Digestive Health (of six publications) Fang X, Hu HZ, Gao N, Liu S, Wang GD, Wang XY, Xia Y, Wood JD. Neurogenic secretion mediated by the purinergic P2Y1 receptor in guinea-pig small intestine. Eur J Pharmacol 2006;536(1-2):113-122. (IF: 2.477) Gao N, Hu HZ, Zhu MX, Fang X, Liu S, Gao C, Wood JD. The P2Y purinergic receptor expressed by enteric neurons in guinea-pig intestine. Neurogastroenterol Motil 2006;18(4):316323. (IF: 2.566) Gao N, Hu HZ, Liu S, Gao C, Xia Y, Wood JD. Stimulation of adenosine A1 and A2A receptors by adenosine 5’-monophosphate (AMP) in the submucosal plexus of guinea-pig small intestine. Am J Physiol Gastrointest Liver Physiol 2006; [Epub ahead of print]. (IF: 3.472) Kresty L, Frankel W, Hammond C, Baird M, Mele J, Stoner G and Fromkes J. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries. Nutrition and Cancer 2006;54(1):148-156. (IF: 2.426) Liu S, Gao N, Hu HZ, Wang X, Wang GD, Fang X, Gao X, Xia Y, Wood JD. Distribution and chemical coding of corticotropin releasing factor-immunoreactive neurons in the guinea-pig enteric nervous system. J Comp Neurol 2006;494(1):63-74. (IF: 3.855) Wang GD, Wang XY, Hu HZ, Fang XC, Liu S, Gao N, Xia Y. Platelet-activating factor in the enteric nervous system of the guinea-pig small intestine. Am J Physiol Gastrointest Liver Physiol 2006;291(5):G928-937. (IF: 3.472) 194 Ohio State University Medical Center Division of Endocrinology, Diabetes and Metabolism (of 25 publications) Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SAA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O’Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D. Calcium plus vitamin D supplementation on risk for fractures. New Engl J Med 2006;354:669-683. (IF: 44.016) Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller, LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, Paskett E, Phillips L, Robbins J, Rossouw JE, Sarto GE, Shikany JM, Stefanick ML, Thomson CA, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Wassertheil-Smoller S, Whitlock E, Yano K, Adams-Campbell L, Anderson GL, Assaf AR, Beresford SAA, Black HR, Brunner RL, Brzyski RG, Ford L, Gass M, Hays J, Heber D, Heiss G, Hendrix SL, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Kotchen JM, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Henderson MM. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:629-642. (IF: 23.494) Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libe R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 2006;38:794-800. (IF: 25.797) Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, Sherman S, Haugen B, Corone C, Molinaro E, Elisei R, Ceccarelli C, Pinchera A, Wahl RL, Leboulleux S, Ricard M, Yoo J, Busaidy NL, Delpassand E, Hanscheid H, Felbinger R, Lassmann M, Reiners C. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. JCEM 2006;91:926-932. (IF: 6.020) Kirschner, LS. Emerging treatment strategies for adrenocortical carcinoma: a new hope. J Clin Endocrinol Metab. 2006;91:14-21. (IF: 6.020) Hänscheid H, Lassmann M, Luster M, Thomas SR, Pacini F, Ceccarelli C, Ladenson PW, Wahl RL, Schlumberger M, Ricard M, Driedger A, Kloos RT, Sherman SI, Haugen BR, Carriere V, Corone C, Reiners C. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med 2006;47:648-654. (IF: 4.684) Shanafelt TD, Byrd JC, Call TG, Zent CS, Kay NE. Narrative review: initial management of newly diagnosed, early-stage chronic lymphocytic leukemia. Ann Intern Med 2006;145(6):435-447. (IF: 13.254) Mrózek E, Kloos RT, Ringel MD, Kresty L, Snider P, Arbogast D, Kies M, Munden R, Busaidi N, Klein MJ, Sherman SI, Shah M. Phase II study of celecoxib in metastatic differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2006;Mar 7, [E-pub ahead of print.] (IF: 6.020) Monk JP, Phillips G, Waite R, Kuhn J, Schaaf LJ, Otterson GA, Guttridge D, Rhoades C, Shah M, Criswell T, Caligiuri MA, Villalona-Calero MA. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol 2006;24(12):1852-1859. (IF: 11.81) Division of General Internal Medicine Division of Human Genetics (of seven publications) (of 16 publications) Ledford CH, Headley A, Hollein A, Houghton B, Picchioni M, Pokala S, Speer A, Whelton, S. Journal Watch from the Alliance of Clinical Education, Annual Review of Medical Education Articles: 2004-2005. Teaching and Learning Medicine 2006;18(3):273-277. Division of Hematology and Oncology (of 98 publications) Porter PL, Barlow WE, Yeh IT, Lin MG, Yuan XP, Donato E, Sledge GW, Shapiro CL, Ingle JN, Haskell CM, Albain KS, Roberts JM, Livingston RB, Hayes DF. p27(Kip1) and cyclin E expression and breast cancer survival after treatment with adjuvant chemotherapy. J Natl Cancer Inst 2006;98(23):17231731. (IF: 15.171) Tada Y, Brena RM, Hackanson B, Morrison C, Otterson GA, Plass C. Epigenetic modulation of tumor suppressor CCAAT/enhancer binding protein alpha activity in lung cancer. J Natl Cancer Inst 2006;98(6):396-406. (IF: 15.171) Yu J, Wei M, Becknell B, Trotta R, Liu S, Boyd Z, Jaung MS, Blaser BW, Sun J, Benson DM Jr., Mao H, Yokohama A, Bhatt D, Shen L, Davuluri R, Weinstein M, Marcucci G, Caligiuri MA. Pro- and anti-inflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity 2006;24(5):575-590. (IF: 15.156) Dorrance AM, Liu S, Yuan W, Becknell B, Arnoczky KJ, Guimond M, Strout MP, Nakamura T, Yu L, Rush LJ, Weinstein M, Leone G, Wu L, Ferketich A, Whitman SP, Marcucci G, Caligiuri MA. MII partial tandem duplication induces aberrant Hox expression in vivo via specific epigenetic alterations. J Clin Invest 2006;116(10):2707-2726. (IF: 15.053) Narod S, Lubinski J, Ghadirian P, Lynch HT, Moller P, Foulkes W, Rosen B, Kim-Sing C, Isaacs C, Domchak S, Sun P, Hereditary Breast Cancer Clinical Study Group (Wagner T, Ainsworth P, Chudley A, Eisen A, Golcrist D, Lemire E, Provencher D, Pasini B, Bellati C, Couch F, Daly M, Eng C, Fishman D, Karlan B, McLennan J, McKinnon W, Merajver S, Neuhasen S, Pasche B, Olopade O, Osborne M, Sweet K, Saal H, Tung N, Weitzel J, Wood M). Screening mammography and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Lancet Oncol 2006;7(5):402-406. (IF: 23.878) Pezzolesi M, Li Y, Zhou XP, Pilarski R, Shen L, Eng C. Mutationpositive and mutation-negative patients with Cowden and Bannayan-Riley-Ruvalcaba syndrome associated with distinct 10q haplotypes. Am J Hum Genet 2006;79. (IF: 12.649) Tekin M, Ozturk Hismi B, Fitoz S, Yalcinkaya F, Ekim M, Kansu A, Ertem M, Deda G, Tutar E, Arsan S, Zhou XP, Pilarski R, Eng C, Akar N. A germline PTEN mutation with manifestations of prenatal onset and verrucous epidermal nevus. Am J Med Genet 2006;PartA140A:1472-1475. (IF: 12.649) Sarquis M, Agrawal S, Shen L, Pilarski R, Zhou XP, Eng C. Distinct expression profiles for PTEN transcript and its splice variants in Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome. Am J Hum Genet 2006;79:23-30. (IF: 12.649) Ollila S, Sarantaus L, Kariola R, Chan P, Hampel H, HolinskiFeder E, Macrae F, Kohonen-Corish M, Gerdes AM, Peltomaki P, Mangold E, de la Chapelle A, Greenblatt M, Nystrom M. Pathogenicity of MSH2 missense mutations is typically associated with impaired repair capability of the mutated protein. Gastroenterology 2006;131(5):1408-1417. (IF: 12.386) Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med 2006;203(4):1033-1043. (IF: 13.965) 2007 Research Report 195 (of three publications) the parasitic protozoan, Leishmania, protects against antimicrobial peptide-induced apoptotic killing. Mol Micro 2006; 62(5):1484-97. (IF: 6.203) Wu LC, Goettl VM, Madiai F, Hackshaw KV, Hussain SR. Reciprocal regulation of nuclear factor kappa B and its inhibitor ZAS3 after peripheral nerve injury. BMC Neurosci 2006;12,7(1):4. (IF: 2.733) Vesosky B, Flaherty DK, Turner J. Th1 cytokines facilitate CD8 T cell-mediated early resistance to infection with Mycobacterium tuberculosis in old mice. Infect Immun 2006; 74(6):3314-24. (IF: 3.933) Emery CF, Keefe FJ, France CR, Affleck G, Waters S, Fondow MD, McKee DC, France JL, Hackshaw KV, Caldwell DS, Stainbrook D. Effects of a brief coping skills training intervention on nociceptive flexion reflex threshold in patients having osteoarthritic knee pain: a preliminary laboratory study of sex differences. J Pain Symptom Manage. 2006;31(3)262-269. (IF: 2.309) Division of Nephrology Division of Immunology Birmingham DF, Nagaraja HN, Rovin BH, Spetie L, Zhao Y, Li X, Hackshaw KV, Yu CY, Malarkey WB, Hebert LA. Fluctuation in self-perceived stress and increased risk of flare in patients with lupus nephritis carrying the serotonin receptor 1A – 1019 G allele. Arth Rheum 2006;54:3291-3299. (IF: 7.421) Division of Infectious Diseases (of 13 publications) Gulick RM, Ribaudo HJ, Shikuma CM, Lalama C, Schackman BR, Meyer WA, Acosta EP, Schouten J, Squires KE, Pilcher CD, Murphy RL, Koletar SL, Carlson M, Reichman RC, Bastow B, Klingman KL, Kuritzkes DR, for the AIDS Clinical Trials Group A5095 Study Team. Three- vs. four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA 2006; 296:769-81. (IF: 23.49) Marion CL, Rappleye CA, Engle JT, Goldman WE. An alpha(1,4)-amylase is essential for alpha-(1,3)-glucan production and virulence in the Histoplasma capsulatum. Mol Micro 2006;62:970-83. (IF: 6.203) Rappleye CA, Goldman WE. Defining virulence genes in the dimorphic fungi. Ann Rev Micro 2006;60:281-303. (IF: 13.412) Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myoinositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol 2006;177:180516. (IF: 8.389) (of 20 publications) Agarwal A, Silver M, Reed J. et al. An open-label study of darbepoetin alfa administered once monthly for the maintenance of haemoglobin concentrations in patients with chronic kidney disease not receiving dialysis. J Int Med 2006;260:577-585. (IF: 4.040) Birmingham D, Nagaraja H, Rovin B, Spetie L, Zhao Y, Li X, Hackshaw K, Yu C, Marlarkey W, Hebert L. Fluctuation in selfperceived stress and increased risk of flare in patients with lupus nephritis carrying the serotonin receptor 1A-1019 G allele. Arthritis Rheum 2006;54:3291. (IF: 7.421) Birmingham D, Gavit K, McCarty S, Yu C, Rovin B, Hagaraja H, Hebert L. Consumption of erythrocyte CR1 (CD35) is associated with protection against systemic lupus erythematosus renal flare. Clin Exp Immunol 2006;143:274. (IF: 2.805) Nori U, Manoharan A, Yee J, Besarab A. Comparison of lowdose gentamicin with minocycline as catheter lock solutions in the prevention of catheter-related bacteremia. Am J of Kidney Diseases 2006;48:596-605. (IF: 4.412) Rovin B, Song H. Chemokine induction by the adipocytederived cytokine adiponectin. Clin Immu 2006;120(1):99-105. (IF: 2.805) Division of Pulmonary, Allergy, Critical Care and Sleep Medicine (of 81 publications) Parsa KVL, Ganesan LP, Rajaram MVS, Gavrilin MA, Wewers MD, Schlesinger LS, Gunn JS, Tridandapani S. Macrophage proinflammatory response to Francisella novicida infection is regulated by the SH2 domain-containing inositol 5’ phosphatase SHIP. PLOS Pathogens 2006;2:0681-90. (IF: 8.389) Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc Natl Acad Sci 2006;103(1):141146. (IF: 10.231)Bratasz A, Weir NM, Parinandi NL, Zweier JL, Sridhar R, Ignarro LJ, Kuppusamy P. Reversal to cisplatin sensitivity in recurrent human ovarian cancer cells by NCX-4016, a nitro derivative of aspirin. Proc Natl Acad Sci 2006;103(10):3914-3919. (IF: 10.231) Kulkarni MM, McMaster WR, Kamysz E, Kamysz W, Engman DM and McGwire BS. The major surface-metalloprotease of Ganesan LP, Joshi T, Fang H, Kutala VK, Roda J, Trotta R, Lehman A, Kuppusamy P, Byrd JC, Carson WE, Caligiuri MA, 196 Ohio State University Medical Center Tridandapani S. FcgammaR-induced production of superoxide and inflammatory cytokines is differentially regulated by SHIP through its influence on PI3K and/or Ras/Erk pathways. Blood 2006;Jul 15;108(2):718-725. (IF: 10.131) Wang X, Thomas B, Sachdeva R, Arterburn L, Frye L, Hatcher P G, Cornwell DG, Ma J. Mechanism of quinone toxicity involving arylation and induction of ER stress. Proc Natl Acad Sci USA 2006; 103:3604-3609. (IF: 10.231) Montague CR, Hunter MG, Gavrilin MA, Phillips GS, Goldschmidt-Clermont PJ, Marsh CB. Activation of estrogen receptor-alpha reduces aortic smooth muscle differentiation. Circ Res. 2006;99(5):477-484. (IF: 9.408) Hanft LM, Rybakova IN, Patel JR, Rafael-Fortney JA, Ervasti JM. 2006. Cytoplasmic gamma-actin contributes to a compensatory remodeling response in dystrophin-deficient muscle. Proc Natl Acad Sci, USA 2006;103:5385-5390. (IF: 10.231) Joshi MS, Julian MW, Huff JE, Bauer JA, Xia Y, Crouser ED. Calcineurin regulates myocardial function during acute endotoxemia. Am J Respir Crit Care Med. 2006;173(9):999-1007. (IF: 8.689) Qin S, Parthun MR. Recruitment of the type B histone acetyltransferase Hat1p to chromatin linked to DNA double strand breaks. Molecular Cellular Biology 2006;26:3649-3658. (IF: 7.093) Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, Wewers MD. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1beta and interleukin-18. Am J Respir Crit Care Med. 2006;174(9):10031010. (IF: 8.689) Huang W, Batra S, Korrapati S, Mishra V, Mehta KD. Selective repression of low-density lipoprotein receptor expression by SP600125: coupling of histone H3-Ser10 phosphorylation and Sp1 occupancy. Molecular Cellular Biology 2006;26(4):13071317. (IF: 7.093) Magro CM, Pope-Harman AL, Abbas AE, Ross P Jr. Lung transplantation: opportunities for research and clinical advancement. Am J Respir Crit Care Med. 2006;173(4):466467. (IF: 8.689) DEPARTMENT OF MOLECULAR VIROLOGY, IMMUNOLOGY AND MEDICAL GENETICS Parsa KV, Ganesan LP, Rajaram MV, Gaurilin MA, Balagopal A, Mohapatra NP, Wewers MD, Schlesinger LS, Gunn JS, Tridandapani S. Macrophage proinflammatory response to Francisella novicida infection is regulated by SHIP. PLoS Pathog;2006;2(7):e71. (IF: 8.389) Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, Lemeshow S, Glaser R. Norepinephrine upregulates the expression of vascular endothelial growth factor, Matrix Metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66(21):10357-10364. (IF: 7.616) DEPARTMENT OF MOLECULAR AND CELLULAR BIOCHEMISTRY (of 154 publications) He H, Jazdzewski K, Wei L, Liyanarachi S, Nagy R, Volinia S, Calin G, Liu C-G, Franssila K, Suster S, Kloos RT, Croce CM, de la Chapelle A. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA, 2005;102:19075-19080. (IF: 10.231) Costinean S, Zanesi N, Pekarsky Y, Esmerina T, Volinia S, Heerema N and Croce CM. Pre B-cell proliferation and lymphoblastic leukemia/high grade lymphoma in Eμ miR155 transgenic mice. Proc Natl Acad Sci, USA 2006;103:70247029. (IF: 10.231) Yoder K, Sarasin A, Kraemer K, McIlhatton M, Bushman F, Fishel R. The DNA repair genes XPB and XPD defend cells from HIV integration. Proc Nat Acad Sci USA 2006; 103:46224627. (IF: 10.231) (of 52 publications) Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Kennedy K, Hai T, Bolouri H, Aderem A. Systems biology approaches identify ATF3 as a negative regulator of innate immunity. Nature 2006;441:173-178. (IF: 29.273) Hartman TR, Qian S, Bolinger C, Fernandez S, Schoenberg DR, Boris-Lawrie K. RNA helicase A is necessary for translation of selected messenger RNAs. Nature Structural and Molecular Biology 2006;13:509-516. (IF: 12.19) Cheng ASL, Jin VX, Yan PS, Fan M, Leu Y-W, Chan MWY, Plass C, Nephew KP, Davuluri RV, Huang T H-M. Combinatorial analysis of transcription factor partners reveals recruitment of c-MYC to estrogen receptor-a responsive promoters. Mol Cell 2006;21:1-12. (IF: 14.971) Smith LT, Lin M, Brena RM, Lang JC, Schuller DE, Otterson GA, Morrison CD, Plass C. TCF21: Epigenetic regulation of the tumor-suppressor gene TCF21 on 6q23-q24 in lung and head and neck cancer. Proc Natl Acad Sci USA, 2006;103:982-987. (IF: 10.231) Wilson RC, Bohlen CJ, Foster MP, Bell CE. Structure of Pfu Pop5, an archaeal RNase P protein. Proc Natl Acad Sci USA 2006;103:873-878. (IF: 10.231) 2007 Research Report 197 DEPARTMENT OF NEUROLOGICAL SURGERY (of 38 publications) Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenol types. Cancer Research 2006;66(18): 9054-9064. (IF: 7.616) Chakrabarti I, Burton AW, Rhines L, Mendel E. Percutaneous vertebroplasty of myelomatous kyphotic wedge fracture in the presence of previous posterior instrumentation: case report. Journal of Neurosurgery 2006;5(2):168-171. (IF: 2.446) Friedman A, Tian JP, Fulci G, Chiocca EA, Wang J. Glioma virotherapy: effects of innate immune suppression and increased viral replication capacity. Cancer Research 2006;66(4):2314-2319. (IF: 7.616) Fulci G, Breymann L, Gianni D, Kurozumi K, Rhee SS, Yu J, Kaur B, Louis DN, Weissleder R, Caligiuri MA, Chiocca EA. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad of Sci USA 2006;103(34):12873-12878. (IF: 10.231) Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, Moeniralm S, Saeki Y, Carette JE, Weissleder R, Vandertop WP, van Beusechem VW, Dirven CMF, Chiocca EA. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Molecular Therapy 2006;14(6):779-788 (e-publication). (IF: 5.443) Sarkar A, Caamano S, Fernandez JM. The mechanical fingerprint of a parallel polyprotein dimer. Biophysical Journal (e-publication 2006); Biophysical Journal 2007;92(4). (IF: 4.507) Suzuki M, Kasai K, Saeki Y. Plasmid DNA sequences present in conventional HSV amplicon vectors cause rapid transgene silencing by forming inactive chromatin. Journal of Virology 2006;80(7):3293-3300. (IF: 5.178) DEPARTMENT OF NEUROLOGY (of 38 publications) Barohn RJ, Herbelin L, Kissel JT, King W, McVey AL, Saperstein DS, Mendell JR. Pilot trial of etanercept in the treatment of inclusion body myositis. Neurology 2006;66:S123-124. (IF: 5.065) 198 Ohio State University Medical Center Moore SA, Shilling CJ, Westra S, Wall C, Wicklund MP, Stolle C, Brown CA, Michele DE, Piccolo F, Winder TL, Stence A, Barresi R, King N, King W, Florence J, Campbell KP, Fenichel GM, Stedman HH, Kissel JT, Griggs RC, Pandya S, Mathews KD, Pestronk A, Serrano C, Darvish D, Mendell JR. Limb-Girdle muscular dystrophy in the USA. Journal of Neuropathology and Experimental Neurology 2006;65:995-1003. (IF: 4.471) Blum D, Meador K, Biton V, Fakhoury T, Shneker B, Chung S, et al. Cognitive effects of lamotrigine compared with topiramate in patients with epilepsy. Neurology 2006;67:400-406. (IF: 5.065) Stüve O, Marra CM, Jerome KR, Cook L, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Hemmer B, Monson NL, Racke MK. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 2006;59:743-747. (IF: 7.571) Tennakoon DK, Mehta RS, Ortega SB, Bhoj V, Racke MK, Karandikar NJ. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol 2006;176: 71197129. (IF: 6.387) Frohman EM, Racke MK, Raine CS. Multiple sclerosis: The plaque and its pathogenesis. New Engl J Med 2006;354:942955. (IF: 44.016) DEPARTMENT OF NEUROSCIENCE (of 53 publications) Jontes JD, Phillips GR. Selective stabilization and synaptic specificity: a new cell-biological model. Trends in Neurosciences 2006;29.4:186-191. (IF: 14.32 ) Gavrikov KE, Nilson JE, Dmitriev AV, Zucker CL, Mangel SC. Dendritic compartmentalization of chloride cotransporters underlies directional responses of starburst amacrine cells in retina. Proc Natl Acad Sci USA 2006;103.49:18793-18798. (IF: 10.231) Carrel TL, McWhorter ML, Workman E, Zhang H, Wolstencroft EC, Lorson C, Bassell GJ, Burghes AH, Beattie CE. Survival motor neuron function in motor axons is independent of functions required for small nuclear ribonucleoprotein biogenesis. Journal of Neuroscience 2006;26(43):1101411022. (IF: 7.5) Cheng HY, Obrietan K. Dexras1: Shaping the responsiveness of the circadian clock. Seminars in Cell & Developmental Biology. 2006;917.3:345-351. (IF: 6.49) Tian G, Lai L, Guo H, Butchbach MR, Chang Y, Lin CL. Translational regulation of glial glutamate transporter EAAT2 expression. Journal of Biological Chemistry 2006; Epub (IF: 5.85) Shan X, Lin C-L G. Quantification of oxidized Rnas in Alzheimer’s disease. Neurobiology of Aging 2006;27.5:657-662. (IF: 5.31) McTigue DM, Tripathi R, Wei P. NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. Journal of Neuropathology and Experimental Neurology 2006;65.4:406-420. (IF: 4.47) Ackerman WE IV, Hughes LH, Robinson JM, Kniss DA. In situ immunolabeling allows for detailed localization of prostaglandin synthesizing enzymes within amnion epithelium. Placenta 2006;27:919-923. (IF: 2.883) Schaffir J. Sexual intercourse at term and onset of labor. Obstet Gynecol 2006;107(6):1310-1314. (IF: 4.170) DEPARTMENT OF OPHTHALMOLOGY DEPARTMENT OF OBSTETRICS AND GYNECOLOGY (of 20 publications) (of 42 publications) Cohn DE, Valmadre S, Resnick KE, Eaton LE, Copeland LJ, Fowler JM. Bevacizumab and weekly taxane chemotherapy demonstrates activity in refractory ovarian cancer. Gynecol Oncol 2006;102:134-139. (IF: 2.251) Morrison C, Zanagnolo V, Ramirez N, Cohn DE, Kelbick N, Copeland LJ, Maxwell LG, Fowler JM. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged cases. J Clin Oncol 2006;24:2376-2385. (IF: 11.810) Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, Comeras I, LaJeunesse J, Nakagawa H, Westman JA, Prior TW, Clendenning M, Penzone P, Lombardi J, Dunn P, Cohn DE, Copeland L, Eaton L, Fowler J, Lewandowski G, Vaccarello L, Bell J, Reid G, de la Chapelle A. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 2006;66(15):7810-7817. (IF: 7.616) Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer: a GOG study. N Engl J Med 2006;354:34-43. (IF: 44.016) Durnwald CP, Rouse DJ, Leveno KJ, Spong CY, MacPherson C, Varner MW, Moawad AH, Caritis SN, Harper M, Wapner RJ, Sorokin Y, Miodovnik M, Carpenter M, Peaceman AM, O’Sullivan MJ, Sibai B, Langer O, Thorp JM, Ramin S, Mercer BM, Gabbe SG for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The Maternal-Fetal Medicine Units Cesarean Registry: safety and efficacy of a trial of labor in preterm pregnancy after a prior cesarean delivery. Am J Obstet Gynecol 2006;195(4):11191126. (IF: 3.083) Hundley AF, Yuan L, Visco AG. Skeletal muscle heavy-chain polypeptide 3 and myosin binding protein H in the pubococcygeus muscle in patients with and without pelvic organ prolapse. Am J Obstet Gynecol 2006;194:1404-1410. (IF: 3.083) Boyer KL, Herzog A, Roberts C. Automatic recovery of the optic nervehead geometry in optical coherence tomography.” IEEE Transactions on Medical Imaging 2006;25(5):553-570. (IF: 3.94) Diabetic Retinopathy Clinical Research Network: Diurnal variation in retinal thickening measurement by optical coherence tomography in center-involved diabetic macular edema. Archives of Ophthalmology 2006;124:521-527. (IF: 3.27) Grzybowski DM, Holman DW, Katz SE, Lubow M. In vitro model of cerebrospinal fluid outflow through human arachnoid granulations. Investigative Ophthalmology & Visual Science 2006;47(8):3664-3672. (IF: 3.64) Keltner JL, Johnson CA, Anderson DR, Levine RA, Fan J, Cello KE, Quigley HA, Budenz DL, Parrish RK, Kass MA, Gordon MO, and the Ocular Hypertension Treatment Study Group. The association between glaucomatous visual fields and optic nerve head features in the OHTS. Ophthalmology 2006;113(9):1603-12. (IF: 3.66) Kotecha A, Elsheikh A, Roberts C, Haogang Z, Garway-Heath DF. Corneal thickness- and age-related biomechanical properties of the cornea measured with the Ocular Response Analyzer. Investigative Ophthalmology & Visual Science 2006;47(12):5337-5347. (IF: 3.64) Levine RA, Demirel S, Fan J, Keltner JL, Johnson CA, Kass MA and the Ocular Hypertension Treatment Study (OHTS) Group. Asymmetries and visual field summaries as predictors of glaucoma in the Ocular Hypertension Treatment Study (OHTS). Investigative Ophthalmology & Visual Science 2006;47:38963903. (IF: 3.64) Warden SM, Pachydaki SI, Christoforidis JB, D’Amico DJ, Loewenstein JI. Choroidal neovascularization after epiretinal membrane removal. Archives of Ophthalmology 2006;124:521527. (IF: 3.27) 2007 Research Report 199 DEPARTMENT OF ORTHOPAEDICS (of 24 publications) phy and nasolaryngoscopy in children: a novel approach to evaluate glottal status. Dysphagia 2006;(1):75-81. (IF: 0.877) Bertone A, Lipson D, Kamei J, Litsky A, Weisbrode S. Effective bone hemostasis and healing using radiofrequency and conductive fluid. Clin Orthop Rel Res 2006;446:278-285. (IF: 1.528) Ozer E, Grecula JC, Agrawal A, Rhoades CA, Young DC, Schuller DE. Long-term results of a multimodal intensification regimen for previously untreated advanced resectable squamous cell cancer of the oral cavity, oropharynx or hypopharynx. Laryngoscope 2006;116(4):607-612. (IF: 1.617) Chaudhari AM, Lindenfeld TN, Andriacchi TP, Hewett TE, Noyes FR. Knee and hip loading patterns at different phases in the menstrual cycle: implications for the gender difference in ACL injury rates. American Journal of Sports Medicine, September 2006. (IF: 2.396) Smith LT, Lin M, Brena RM, Lang JC, Schuller DE, Otterson GA, Morrison CD, Smiraglia DJ, Plass C. Epigenetic regulation of the tumor-suppressor gene TCF21 on 6q23-q24 in lung and head and neck cancer. Proc Natl Acad Sci USA 2006;103:982987. (IF: 10.231) Fleshman, RL, Mayerson JL, Wakely Jr. PA. Fine needle aspiration biopsy of high-grade sarcoma with emphasis on neoadjuvant chemotherapy. Mod Pathol 2006;19(Suppl 1):58A. (IF: 3.426) DEPARTMENT OF PATHOLOGY Oleske DM, Lavender SA, Andersson GBJ, Morrissey M, Hahn JJ, Zold-Kilbourn P, Allen C, Taylor E. Risk factors for recurrent episodes of work-related low back disorders in an industrial population. Spine 2006;31(7):789-798. (IF: 2.187) Barsky SH, Karlin NJ. Mechanisms of disease: breast tumor pathogenesis and the role of the myoepithelial cell. Nature Clinical Practice Oncology 2006;3(3):138-151. (IF: 10.0) Phieffer LS, Goulet JA. Delayed unions of the tibia. J Bone & Jt Surg 2006;88A:205-216. (IF: 2.339) Shin CS, Chaudhari AM, Andriacchi TP. The influence of deceleration forces on ACL strain during single leg landing: a simulation study. Journal of Biomechanics, available online, June 2006. (IF: 2.364) Smith LT, Mayerson J, et. al. 20q11.1 amplification in giant cell tumor of bone: array CGH, FISH and association with outcome. Genes Chromosomes Cancer 2006;45(10):957-966. (IF: 3.937) DEPARTMENT OF OTOLARYNGOLOGY – HEAD AND NECK SURGERY (of 28 publications) Akhmametyeva E, Mihaylova M, Luo H, Kharzai S, Welling DB, Chang L-S. Regulation of the neurofibromatosis 2 gene promoter expression during embryonic development. Developmental Dynamics 2006;235(10):2771-2785. (IF: 3.333) Chang L-S, Jacob A, Lorenz M, Rock J, Akhmametyeva EM, Mihai G, Schmalbrock P, Chaudhury AR, Lopez R, Yamate J, John MR, Wickert H, Neff BA, Dodson EE, Welling DB. Growth of benign and malignant schwannoma xenografts in SCID mice. Laryngoscope 2006;116(11):2018-2026. (IF: 1.617) Jadcheria SR, Gupta A, Stoner E, Coley BD, Wiet GJ, Shaker R. Correlation of glottal closure using concurrent ultrasonogra- 200 Ohio State University Medical Center (of 129 publications) Byrd JC, Briggen JG, Peterson BL, Grever MR, Lozanski G, Lucas DM, Lampson B, Larson RA, Caligiuri MA, Heerema NA. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for riskadapted therapy. Journal of Clinical Oncology 2006;24(3):437443. (IF: 11.810) Jin H, Pancholi V. Identification and biochemical characterization of a eukaryotic-type serine/threonine kinase and its cognate phosphatase in streptococcus pyogenes: their biological functions and substrate identification. Journal of Molecular Biology 2006;357(5):1351-1372. (IF: 5.229) Li O, Chang X, Zhang H, Kocak E, Ding C, Zheng P, Liu Y. Massive and destructive T cell response to homeostatic cue in CD24-deficient lymphopenic hosts. Journal of Experimental Medicine 2006;203(7):1713-1720. (IF: 13.965) Richards JO, Chang X, Blaser BW, Caligiuri MA, Zheng P, Liu Y. Tumor growth impedes natural-killer-cell maturation in the bone marrow. Blood 2006;108(1):246-252. (IF: 10.131) Tober KL, Wilgus TA, Kusewitt DF, Thomas-Ahner JM, Maruyama T, Oberyszyn TM. Importance of the EP(1) receptor in cutaneous UVB-induced inflammation and tumor development. Journal of Investigative Dermatology 2006;126(1):205211. (IF: 4.406) Wang Y, Liu Y, Wu C, Zhang H, Zheng X, Zheng Z, Geiger TL, Nuovo GJ, Liu Y, Zheng P. Epm2a suppresses tumor growth in an immunocompromised host by inhibiting Wnt signaling. Cancer Cell 2006;10(3):179-190. (IF: 18.725) DEPARTMENT OF PEDIATRICS (of 348 publications) Mason KM, Bruggeman ME, Munson Jr. RS, Bakaletz LO. The nontypeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapDdependent potassium acquisition. Mol Micro 2006;62(5):13571372. (IF: 6.203) Martinez-Sobrido L, Gitiban N, Fernandez-Sesma A, Cros J, Mertz SE, Jewell NA, Hammond S, Flano E, Durbin RK, GarciaSastre A, Durbin JE. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. Journal of Virology 2006; 80(3):1130-1139. (IF: 5.178) Miller TM, Kim SH, Yamanaka K, Hester M, Umapathi P, Arnson H, Rizo L, Mendell JR, Gage FH, Cleveland DW, Kaspar BK. Gene transfer demonstrates that muscle is not a primary target for non-cell-autonomous toxicity in familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 2006;103(51):1954619551. (IF: 10.2) Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, Chang CH, Liu Y. MAP kinase phosphatase-1 controls innate immune responses and suppresses endotoxic shock. The Journal of Experimental Medicine 2006;203:131-140. (IF: 13.9) Nwomeh BC, Chisolm DJ, Caniano DA, Kelleher KJ. Racial and economic disparity in perforated appendicitis among children: where is the problem? Pediatrics 2006;117: 870-875. (IF: 4.272) Ballard RA, Truog WE, Cnann A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, Null DR, Hudak ML, Puri AR, Golombek SG, Courtney SE, Steward DL, Welty SE, Phibbs RH, Hibbs AM, Luan X, Wadlinger SR, Asselin JM, Coburn CE, NO CLD Study Group. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. New England Journal of Medicine 2006;355:343-353. (IF: 44.016) DEPARTMENT OF PHARMACOLOGY (of 34 publications) Blower PE, Cross KP. Decision tree methods in pharmaceutical research. Curr Top Med Chem 2006;6(1):31-39. Review. (IF: 4.400) Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. An opioid agonist that does not induce mu opioid receptor - arrestin interactions or receptor internalization. Mol Pharmacol 2006; e-pub ahead of print. (IF: 4.612) Li H, Liu X, Cui H, Chen YR, Cardounel AJ, Zweier JL. Characterization of the mechanism of cytochrome P450 reductase-cytochrome P450-mediated nitric oxide and nitrosothiol generation from organic nitrates. J Biol Chem 2006;281(18): 12546-12554 (also e-pub 2006). (IF: 5.854) Besco JA, Hooft van Huijsduijnen R, Frostholm A, Rotter A. Intracellular substrates of brain-enriched receptor protein tyrosine phosphatase rho (RPTPrho/PTPRT). Brain Res 2006;1116(1):50-57 (also e-pub 2006). (IF: 2.296) Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci USA 2006;103(24):9333-9338. (also e-pub 2006) (IF: 10.231) Neff NH, Wemlinger TA, Duchemin AM, Hadjiconstantinou M. Clozapine modulates aromatic L-amino acid decarboxylase activity in mouse striatum. J Pharmacol Exp Ther 2006;317(2):480-487 (also e-pub 2006). (IF: 4.098) Pinsonneault JK, Papp AC, Sadée W. Allelic mRNA expression of X-linked monoamine oxidase a (MAOA) in human brain: dissection of epigenetic and genetic factors. Hum Mol Genet 2006;15(17): 2636-2649 (also e-pub 2006). (IF: 7.764) Krejsa C, Rogge M, Sadée W. Protein therapeutics: new applications for pharmacogenetics. Nat Rev Drug Discov 2006;5(6):507-521. Review. (IF:18.775) Lim J-E, Papp A, Pinsonneault J, Sadée W, Saffen D. Allelic expression of serotonin transporter (SERT) mRNA in human pons: lack of correlation with the polymorphism SERTPR. Molecular Psychiatry 2006;11:649-662. (IF: 9.335) DEPARTMENT OF PHYSICAL MEDICINE AND REHABILITATION (of 25 publications) Dobkin B, Apple D, Barbeau H, Basso M, Behrman A. Deforge D, Ditunno J, Dudley G, Elashoff R, Fugate L, Harkema S, Saulino M. Scott M. Weight-supported treadmill vs. overground training for walking after acute incomplete SCI. Neurology 2006;66(4):484-493. (IF: 5.065) Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, Lewis CE, Limacher MC, Margolis KL, Mysiw WJ, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Schatz IJ, Snetselaar LG, Stevens VJ, Tinker LF, Trevisan M, Vitolins MZ, Anderson GL, Assaf AR, Bassford T, Beresford SAA, Black HR, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Granek I, Greenland P, Hays J, Heber D, Heiss G, Hendrix SL, Hubbell FA, Johnson KC, Kotchen JM. Low-fat dietary pattern and risk of cardiovascular disease: The Women’s Health Initiative randomized controlled dietary modification trial. JAMA 2006;295(6):655-666. (IF: 23.494) 2007 Research Report 201 Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, Mysiw WJ, et al. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the Women’s Health Initiative randomized trial. J Bone Min Res 2006;21(6):817-828. (IF: 6.527) Marras WS, Parakkat J, Chany AM, Yang G, Burr D, Lavender SA. Spine loading as a function of lift frequency, exposure duration and work experience. Clin Biomech 2006;21(4):345352. (IF: 1.501) Mysiw WJ, Bogner JA, Corrigan JD, Fugate L, Clinchot DM, Kadyan V. The impact of acute care medications on rehabilitation outcome after traumatic brain injury. Brain Injury 2006;20(9):905-911. (IF: 1.471) Sherwin E, Whiteneck G, Corrigan J, Bedell G, Brown M, Abreu B, DePompei R, Gordon W, Kreutzer J. Domains of a TBI minimal data set: community reintegration phase. Brain Injury 2006;20(4):383-389. (IF: 1.471) DEPARTMENT OF PHYSIOLOGY AND CELL BIOLOGY (of 58 publications) Di Barletta MR, Viatchenko-Karpinski S, Nori A, Memmi M, Terentyev D, Turcato F, Valle G, Rizzi N, Napolitano C, Gyorke S, Volpe P, Priori SG. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation 2006;14(10):1012-1019. (IF: 11.632) Terentyev D, Noria A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Gyorke S. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circulation Research 2006;98(9):1151-1158. (IF: 9.408) Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacol & Therap 2006;111:808-835. (IF: 8.357) DEPARTMENT OF PSYCHIATRY (of 71 publications) Arnold LE. Research Units on Pediatric Psychopharmacology (RUPP) Autism Network: a randomized, controlled crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Archives of General Psychiatry 2006. (IF: 12.642) McDougle CJ, Scahill L, Aman MG, McCracken JT, Tierney E, Davies M, Arnold LE, Posey DJ, Martin A, Ghuman JK, Shah B, Chuang SZ, Swiezy NB, Gonzalez NM, Hollway J, Koenig K, McGough J, Ritz L, Vitiello B. Risperidone for the core symptom domains of autism: results from the RUPP autism network study. American Journal of Psychiatry 2005;162:1142-1148. (IF: 8.286) Kowatch RA, Fristad MA, Birmaher B, Wagner KD, Findling RL, Hellander M, and the Child Psychiatric Workgroup on Bipolar Disorder. Treatment guidelines for children and adolescents with bipolar disorder. Journal of the American Academy of Child & Adolescent Psychiatry 2005;44(3):213-235. (IF: 4.113) Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. Biomed Central Pharmacology 2006;6:(1)6. McGuire L, Heffner K, Glaser R, Needleman B, Malarkey WB, Dickinson S, Lemeshow S, Cook C, Muscarella P, Melvin WS, Ellison EC, Kiecolt-Glaser J. Pain and wound healing in surgical patients. Annals of Behavioral Medicine 2006;31,165-172. Kim J-Y, Saffen D. Activation of M1 muscarinic acetylcholine receptors stimulates the formation of a multiprotein complex centered on TRPC6 channels. Journal of Biological Chemistry 2006;280:32035-32047. (IF: 5.854) Chan YC, Miller KM, Shaheen N, Votolati NA, Hankins MB. Worsening of psychotic symptoms in schizophrenia with addition of Lamotrigine: a case report. Schizophrenia Research 2006;78(2-3):343-345. (IF: 4.231) DEPARTMENT OF RADIATION MEDICINE (of 21 publications) Johnson BE, Crawford J, Downey RJ, Ettinger DS, Fossella F, Grecula JC, Jahan T, Kalemkerian GP, Kessinger A, Koczywas M, Langer CJ, Martins R, Marymont MH, Niell HB, Ramnath N, Robert F, Williams CC Jr., National Comprehensive Cancer Network (NCCN). Small cell lung cancer clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network 2006;4(6):602-622. (IF: N/A) 202 Ohio State University Medical Center Nag S, DeHaan M, Scruggs G, Mayr N, Martin EW. Long-term follow-up of patients of intrahepatic malignancies treated with iodine-125 brachytherapy. International Journal of Radiation Oncology Biology and Physics 2006; 64:736-744. (IF: 4.556) Ozer E, Grecula JC, Agrawal A, Rhoades CA, Young DC, Schuller DE. Long-term results of a multimodal intensification regimen for previously untreated advanced resectable squamous cell cancer of the oral cavity, oropharynx or hypopharynx. Laryngoscope 2006;116(4):607-612. (IF: 1.617) Wang JZ, Li XA, Mayr NA. Dose escalation to combat hypoxia in prostate cancer: a radiobiological study on clinical data. British Journal of Radiology 2006;79(947):905-911. (IF: 1.394) Zhang H, Johnson EL, Zwicker RD. Dosimetric validation of the MCNPX Monte Carlo simulation for radiobiologic studies of megavoltage grid radiotherapy. Int J Radiat Oncol Biol Phy 2006;66(5):1576-1583. (IF: 4.556) DEPARTMENT OF RADIOLOGY (of 52 publications) Eckstein F, Hudelmaier M, Wirth W et al. Double echo steadystate magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Annals of the Rheumatic Diseases 2006;65(4):433-441. (IF: 6.956) Giesel F, Bischoff H, von Tengg-Kobligk H, Weber M, Zechmann C, Kauczor H, Knopp MV. Dynamic contrast enhanced MRI of malignant pleural mesothelioma: a feasibility study of non-invasive assessment, therapeutic follow-up and possible predictor of improved outcome. Chest 2006;129(6):1570-1576. (IF: 4.008) Von Tengg-Kobligk H, Giesel F, Locklin J et al. Image fusion for abdominal radiofrequency ablation (RFA). American Journal of Roentgenology 2006. (IF: 2.209) DEPARTMENT OF SURGERY (of 57 publications) Moss RL, Dimmitt RA, Barnhart DC, Sylvester KG, Brown RL, Powell DM, Islam S, Langer JC, Sato TT, Brandt ML, Lee H, Blakely ML, Lazar EL, Hirschl RB, Kenney BD, Hackman DJ, Zelterman D, Silverman BL. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med 2006;354(21):2225-2234. (IF: 44.016) Magro CM, Pope-Harman AL, Abbas AE, Ross P Jr. Lung transplantation: opportunities for research and clinical advancement. Am J Respir Crit Care Med 2006;173(4):466467. (IF: 8.689) Gao R, Brigstock DR. A novel integrin alpha5beta1 binding domain in module 4 of connective tissue growth factor (CCN2/CTGF) promotes adhesion and migration of activated pancreatic stellate cells. Gut 2006;55(6):856-862. (IF: 7.692) Bloomston M, Zhou JX, Rosemurgy AS, Frankel W, MuroCacho CA, Yeatman TJ. Fibrinogen gamma overexpression in pancreatic cancer identified by large-scale proteomic analysis of serum samples. Cancer Res 2006;66(5):2592-2599. (IF: 7.616) Roda JM, Parihar R, Magro C, Nuovo GJ, Tridandapani S, Carson WE 3rd. Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res 2006;66(1):517-526. (IF: 7.616) Jia G, Heverhagen J, Polzer H et al. Dynamic contrast-enhanced magnetic resonance imaging as a biological marker to noninvasively assess the effect of finasteride on prostatic suburethral microcirculation. Journal of Urology 2006;176:2299-2304. (IF: 3.592) VanBuskirk AM, Lesinski GB, Nye KJ, Carson WE, Yee LD. TGF-beta inhibition of CTL re-stimulation requires accessory cells and induces peroxisome-proliferator-activated receptorgamma (PPAR-gamma). Am J Transplant 2006;6(8):1809–1819. (IF: 6.002) Knopp M, Balzer T, Esser M et al. Assessment of utilization and pharmacovigilance based on spontaneous adverse event reporting of gadopentetate dimeglumine as a magnetic resonance contrast agent after 45 million administrations and 15 years of clinical use. Investigative Radiology 2006;41(6). (IF: 3.173) Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal wound healing is subject to redox control. Mol Ther 2006;13(1):211220. (IF: 5.443) Li J, Wang Q, Zhu Q, El-Mahdy MA, Wani G, Prætorius-Ibba M and Wani AA. DNA damage binding component DDB1 participates in nucleotide excision repair through DDB2 DNA binding and cullin 4A ubiquitin ligase activity. Cancer Res 2006;66:8590-8597. (IF: 7.616) Cook CH, Trgovcich J, Zimmerman PD, Zhang Y, Sedmak DD. Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta triggers reactivation of latent cytomegalovirus in immunocompetent mice. J Virol 2006;80(18):9151-9158. (IF: 5.178) 2007 Research Report 203 DEPARTMENT OF UROLOGY SCHOOL OF ALLIED MEDICAL PROFESSIONS (of nine publications) (of 42 publications) Gilleran JP, Thaly RK, Chernoff AM. Rapid communication: bipolar PlasmaKinetic transurethral resection of the prostate: reliable training vehicle for today's urology residents. J Endourol 2006;20(9):683-687. Knudsen BE, Matsumoto ED, Chew BH, Johnson B, Margulis V, Cadeddu JA, Pearle MS, Pautler SE, Denstedt JD. A randomized, controlled, prospective study validating the acquisition of percutaneous renal collecting system access skills using a computer-based hybrid virtual reality surgical simulator: phase I. J Urol 2006;176(5):2173-2178. Patel V, Gosalbez R, Castelland M. A comparison between ureteral replacements using a transverse tabularized colonic tube or ileal ureter: experimental study in dogs. J Pediatric Surgery 2006;41(4):799-803. Montie JE, Abrahams NA, Bahnson RR, Eisenberger MA, ElGalley R, Herr HW, Hudes GR, Kuzel TM, Lange PH, Patterson A, Pollack A, Richie JP, Sexton WJ, Shipley WU, Small EJ, Trump DL, Walther PJ, Wilson TG. Bladder cancer: clinical guidelines in oncology. J Natl Compr Canc Netw 2006;4(10):984-1014. Motzer RJ, Bolger GB, Boston B, Carducci MA, Fishman M, Hancock SL, Hauke RJ, Hudes GR, Jonasch E, Kantoff P, Kuzel TM, Lange PH, Levine EG, Logothetis C, Margolin KA, Pohar K, Redman BG, Robertson CN, Samlowski WE, Sheinfeld J. Kidney cancer: clinical practice guidelines in oncology. J Natl Compr Canc Netw 2006;4(10):1072-81. Sahabbudin RM, Arni T, Ashani N, Arumuga K, Rajenthran S, Murali S, Patel V, Hemal A, Menon M. Development of robotic program: an Asian experience. World Journal of Urology 2006;24(2):161-164. 204 Ohio State University Medical Center Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Guiliani C, Light KE, Nichols-Larsen D. Effect of constraintinduced movement therapy on upper extremity function 3 to 9 months after stroke. The EXCITE randomized clinical trial. JAMA 2006;296(17);2095-2104. (IF: 23.494) Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. The Basso Mouse Scale for Locomotion (BMS) detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 2006;23:635-659. (IF: 2.574) Cordova ML, Dorrough J, Kious K, Ingersoll CD, Merrick MA. Prophylactic ankle bracing reduces rearfoot motion during sudden inversion. Scand J Med Sci Sports 2006; e-print ahead of publication, June 26. (IF: 2.151) Davidson AG and Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Experimental Brain Research 2006;173:25-39. (IF: 2.118) Kowalczyk N and Mazal J. Administrators’ perceptions of advanced skills required for radiologic technologists. Radiologic Technology 2006;77:4. Received Radiologic Technology Distinguished Author award. Statistical Year I N R E V I E W OHIO STATE UNIVERSITY MEDICAL CENTER RESEARCH AWARD DOLLARS 175 $163.3 D o l l a r s ( i n M i l $ ) $159.3 155 $160.2 $143.3 135 $121.8 115 $97.3 95 $79.6 $67.3 75 55 $48.1 $56.3 $56.7 1997 1998 35 1996 1999 2000 2001 2002 C a l e n d a r Source(s): Ohio State University Research Foundation e-Activity Reports 2003 2004 2005 2006 y e a r Notes: CY is Calendar Year (January 1 to December 31). Total research award dollars include direct, indirect, new, renewal and continuation funding processed/money received during the period indicated. The College of Medicine, the Office of Health Sciences, the School of Public Health, and Columbus Children’s Research Institute are reported. Separate budgeted research through the University Ledger is not included. OHIO STATE UNIVERSITY MEDICAL CENTER NIH RESEARCH AWARD DOLLARS D o l l a r s ( i n M i l $ ) 120 $112.1 110 $104.8 100 $94.2 $89.4 90 80 $76.3 70 $65.0 60 50 $49.8 40 2000 2001 2002 2003 C a l e n d a r 2004 2005 2006 y e a r Source(s): Ohio State University Research Foundation e-Activity Reports Notes: CY is Calendar Year (January 1 to December 31). NIH awards are the sum of the direct and indirect dollars awarded for the calendar year, not the life of the project. The College of Medicine, the Office of Health Sciences, the School of Public Health, and Columbus Children’s Research Institute are reported. CY 2004 – CY 2006 NIH research award dollars also include NIH awards from principal investigators who have joint appointments with other colleges and the College of Medicine. Separate budgeted research through the University Ledger is not included. 2007 Research Report 205 Statistical Year I N R E V I E W OHIO STATE UNIVERSITY MEDICAL CENTER AWARD DOLLARS INVOLVING HUMAN SUBJECTS D o l l a r s ( i n M i l $ ) 90 80 $85.8 $78.9 70 $74.6 60 $51.4 $68.1 $62.0 50 40 30 $33.8 $31.7 1997 1998 $39.8 $40.7 1999 2000 $26.2 20 1996 2001 C a l e n d a r 2002 2003 2004 2005 2006 y e a r Source(s): Ohio State University Research Foundation Notes: CY is Calendar Year (January 1 to December 31). Total award dollars involving human subjects include direct, indirect, new, renewal and continuation awards funded/processed during the period indicated. The College of Medicine, the Office of Health Sciences, and the School of Public Health are reported. Awards administered at Columbus Children’s Research Institute are not included. Separate budgeted research through the University Ledger is not included. OHIO STATE UNIVERSITY MEDICAL CENTER RESEARCH AWARD DOLLARS/SQUARE FOOT 425 D o l l a r s ( i n M i l $ ) $382 $420 $394 375 325 $371 $368 $308 275 $255 $221 225 $253 $220 $186 175 125 1996 1997 1998 1999 2000 2001 C a l e n d a r 2002 2003 2004 2005 2006 y e a r Source(s): Ohio State University Research Foundation; Medical Space Inventory Notes: CY is Calendar Year (January 1 to December 31). Total research award dollars include direct, indirect, new, renewal and continuation funding processed/money received during the period indicated. The College of Medicine, the Office of Health Sciences, and the School of Public Health are reported. Awards administered at Columbus Children’s Research Institute are not included. Separate budgeted research through the University Ledger is not included. Calculation: Total Research Award Dollars/Total Assigned Research Square Feet = Total Research Dollars Awarded per Square Foot. 206 Ohio State University Medical Center Contact Information For more information about projects or data in this report, direct correspondence to: Office of the Associate Vice President for Health Sciences Research Ohio State University Medical Center 260 Meiling Hall 370 W. 9th Ave. Columbus, Ohio 43210 Phone: (614) 292-2595 Fax: (614) 688-3427 www.medicalcenter.osu.edu College of Medicine Medicine Administration Wiley “Chip” Souba, MD, ScD, MBA, Dean 254 Meiling Hall 370 West 9th Avenue Columbus, Ohio 43210 Phone: (614) 292-2600 Fax: (614) 292-4254 Departments Anesthesiology David Zvara, MD, Chair N437 Doan Hall 410 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 293-5188 Fax: (614) 293-9643 Biomedical Informatics Joel Saltz, MD, PhD, Chair 3184 Graves Hall 333 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 292-4778 Fax: (614) 688-6600 Emergency Medicine Douglas Rund, MD, Chair 146 Means Hall 1654 Upham Drive Columbus, Ohio 43210 Phone: (614) 293-8176 Fax: (614) 293-6570 Neurology Michael Racke, MD, Chair 445 Means Hall 1654 Upham Drive Columbus, Ohio 43210 Phone: (614) 293-4036 Fax: (614) 293-9029 Family Medicine Mary Jo Welker, MD, Chair Rardin Center 2231 North High Street Columbus, Ohio 43210 Phone: (614) 293-2653 Fax: (614) 293-2715 Neuroscience James King, PhD, Interim Chair 4198 Graves Hall 333 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 688-8327 Fax: (614) 688-8742 Internal Medicine Michael Grever, MD, Chair 215 Means Hall 1654 Upham Drive Columbus, Ohio 43210 Phone: (614) 293-5661 Fax: (614) 293-6656 Obstetrics and Gynecology Larry Copeland, MD, Chair 505 Means Hall 1654 Upham Drive Columbus, Ohio 43210 Phone: (614) 293-8697 Fax: (614) 293-5877 Molecular and Cellular Biochemistry Michael Ostrowski, PhD, Chair 333 Hamilton Hall 1645 Neil Avenue Columbus, Ohio 43210 Phone: (614) 292-5451 Fax: (614) 292-4118 Ophthalmology Thomas Mauger, MD, Chair 5835 Cramblett Hall 456 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 293-8116 Fax: (614) 293-5602 Molecular Virology, Immunology and Medical Genetics Carlo Croce, MD, Chair 455D Comprehensive Cancer Center 410 W. 12th Avenue Columbus, Ohio 43210 Phone: (614) 292-3063 Fax: (614) 292-4080 Neurological Surgery E. Antonio Chiocca, MD, PhD, Chair N1021 Doan Hall 410 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 293-5444 Fax: (614) 293-4281 Orthopaedics Christopher Kaeding, MD, Interim Chair N1043 Doan Hall 410 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 293-2165 Fax: (614) 293-4755 Otolaryngology D. Bradley Welling, MD, PhD, Chair 4A Cramblett Hall 456 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 293-8706 Fax: (614) 293-3193 2007 Research Report 207 Pathology Sanford Barsky, MD, Chair 129 Hamilton Hall 1645 Neil Avenue Columbus, Ohio 43210 Phone: (614) 292-4692 Fax: (614) 688-5632 Psychiatry Radu Saveanu, MD, Chair 130F Neuroscience Facility 1670 Upham Drive Columbus, Ohio 43210 Phone: (614) 293-8283 Fax: (614) 293-2926 Pediatrics Michael Brady, MD, Chair Children’s Hospital 7th Floor Outpatient Care Center 700 Children’s Drive Columbus, Ohio 43205 Phone: (614) 722-4561 Fax: (614) 722-4565 Radiation Medicine Nina Mayr, MD, Chair 080 CHRI 300 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 293-0672 Fax: (614) 293-4044 Pharmacology Wolfgang Sadée, Dr.rer.nat, Chair 5078 Graves Hall 333 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 292-8608 Fax; (614) 292-7232 Physical Medicine and Rehabilitation William Pease, MD, Chair 1018 Dodd Hall 480 Medical Center Drive Columbus, Ohio 43210 Phone: (614) 293-3801 Fax: (614) 293-3809 Physiology & Cell Biology Muthu Periasamy, PhD, Chair 304 Hamilton Hall 1645 Neil Avenue Columbus, Ohio 43210 Phone: (614) 292-5448 Fax: (614) 292-4888 208 Ohio State University Medical Center Radiology Michael Knopp, MD, PhD, Chair 657 Means Hall 1654 Upham Drive Columbus, Ohio 43210 Phone: (614) 293-9998 Fax: (614) 293-9275 Surgery E. Christopher Ellison, MD, Chair 327 Means Hall 1654 Upham Drive Columbus, Ohio 43210 Phone: (614) 293-8701 Fax: (614) 293-4063 Urology Robert Bahnson, MD, Chair 4980 Cramblett Hall 456 W. 10th Avenue Columbus, Ohio 43210 Phone: (614) 293-3648 Fax: (614) 293-5363 Schools Allied Medical Professions Deborah Larsen, PhD, Director 106 Atwell Hall 453 W. 10th Avenue Columbus, Ohio 43210 Phone: (614) 292-5645 Fax: (614) 292-0210 Biomedical Science Caroline Whitacre, PhD, Director 1190 Graves Hall 333 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 292-8725 Fax: (614) 292-6226 Health Sciences Health Sciences Administration Fred Sanfilippo, MD, PhD, Senior Vice President and Executive Dean for Health Sciences 200 Meiling Hall 370 West 9th Avenue Columbus, Ohio 43210 Phone (614) 292-7755 Fax: (614) 688-8644 Center for Biostatistics Stanley Lemeshow, PhD, Director M200 Starling-Loving Hall 320 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 293-6901 Fax: (614) 293-6902 Center for Microbial Interface Biology Larry Schlesinger, MD, Director 1011 Biomedical Research Tower 460 West 12th Avenue Columbus, Ohio 43210 Phone: (614) 247-1564 Fax: (614) 292-9616 Center for Minimally Invasive Surgery W. Scott Melvin, MD, Director N729 Doan Hall 410 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 293-4499 Fax: (614) 293-7852 Center for Molecular Neurobiology Anthony Young, PhD, Director 206 Rightmire Hall 1060 Carmack Road Columbus, Ohio 43210 Phone: (614) 292-5670 Fax: (614) 292-5379 Comprehensive Cancer Center Michael Caligiuri, MD, Director A458 Starling-Loving Hall Columbus, Ohio 43210 Phone: (614) 293-7518 Fax: (614) 293-7520 Davis Heart and Lung Research Institute Jay Zweier, MD, Director 110 Heart and Lung Research Institute 473 West 12th Avenue Columbus, Ohio 43210 Phone: (614) 247-7766 Fax: (614) 247-7799 General Clinical Research Center William Malarkey, MD, Director Davis Medical Research Building 480 West 9th Avenue Columbus, Ohio 43210 Phone: (614) 293-8750 Fax: (614) 247-7799 Prior Health Sciences Library Susan Kroll, MLS, Director 280B Health Sciences Library 376 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 292-4851 Fax: (614) 292-1920 Institute for Behavioral Medicine Research Ronald Glaser, PhD, Director 2175 Graves Hall 333 West 10th Avenue Columbus, Ohio 43210 Phone: (614) 292-2252 Fax: (614) 688-3110 Nisonger Center for Mental Retardation and Developmental Disabilities Steven Reiss, PhD, Director 357 McCampbell Hall 1581 Dodd Drive Columbus, Ohio 43210 Phone: (614) 292-6705 Fax: (614) 292-3727 Office of Geriatrics and Gerontology Linda Mauger, Interim Director S2042 Davis Center 480 Medical Center Drive Columbus, Ohio 43210 Phone: (614) 293-4815 Fax: (614) 293-5612 Primary Care Research Institute Mary Jo Welker, MD, Director 2231 North High Street Columbus, Ohio 43210 Phone: (614) 293-2653 Fax: (614) 293-2715 2007 Research Report 209 ACKNOWLEDGMENTS Thank you to the following individuals for help in producing this report: LEADERSHIP: Fred Sanfilippo, MD, PhD – Senior Vice President and Executive Dean for Health Sciences; CEO, Ohio State University Medical Center Wiley “Chip” Souba, Jr., MD, ScD – Dean, College of Medicine Caroline Whitacre, PhD – Associate Vice President for Health Sciences Research; Vice Dean for Research; Director, School of Biomedical Science, Ohio State University College of Medicine Darian Torrance, CRA – Grants Facilitator, The Ohio State University Medical Center PRODUCTION TEAM: Editorial Management – Bob Hecker, Ron Shaull Graphic Design – Mary Jones Smith, Kathy Lillash Photography – Jim Brown, Roman Sapecki, Will Shively ADVISERS: College of Medicine Research Council Caroline Whitacre, PhD – Chair Michael Bissell, MD, PhD, MPH Jacqueline Bresnahan, PhD Melissa Briggs-Phillips, PhD Brad Harris, MHA Ron Henthorn Rebecca Jackson, MD James King, PhD Nancy Miller, JD Michael Para, MD Wendy Philips Kim Saunders, MHSA, LNHA Melissa Slivanya, BSBA Jill Springer, BSBA Angela Street-Underwood, MSA Darian Torrance, BA, CRA Henry Zheng, PhD, MBA 210 Ohio State University Medical Center This Time It IS Personal When Time magazine selected “You” as “Person of the Year” for 2006, it was recognizing that individuals armed with computers, cell phone cameras, iPods, personal digital assistants, personal blogs and a cornucopia of other communications mechanisms had taken control of information away from mass media and self-appointed experts. But that isn’t all the information explosion has wrought. Today, genetic information about each individual “You” can be used to enhance our health care in important new ways. Combined with a person’s distinctive family, social and environmental profile, new “personalized medicine” tools hold the promise of predicting disease as well as the risk of a particular course of treatment on a particular disease in a particular person. Until now, deciding which treatments have the best chance of success has been based on years of physician experience, slowly but steadily informed by research. But today, because of the convergence of genomics, nanotechnology and computational biology, what was once “breast cancer,” for example, is now treated as “Miranda’s breast cancer,” “Lakeisha’s breast cancer” or “Susan’s breast cancer” – each requiring different prevention strategies and therapies based on age, stage, lifestyle and, most significantly, genetic makeup. New research tools have made it possible for disciplines such as molecular biology, genetics and computer science, to name just a few, to work together more closely so diseases can be better understood by physicians as well as by basic and clinical researchers. Personalized medicine holds promise for treating an array of illnesses. Dr. Francis Collins, director of the National Human Genome Research Institute, predicts that, by 2010, the science of pharmacogenetics will be put to work to treat and even conquer diabetes, heart disease, Alzheimer’s, schizophrenia and many other diseases. It’s good that the public seems to embrace the personalized medicine concept, albeit without much sense of specificity. We at Research!America commissioned a national poll that found a good base of intuitive support for personalized medicine. But it’s not enough. Now the research community must let more people know what they can do as individuals to help assure that this research moves forward as rapidly as scientific opportunity warrants. And moving forward will require robust investment. The relationship between money well spent and the development of therapies that improve and extend life is a direct one. As cancer survivor and television news personality Sam Donaldson puts it: “I think the biggest challenge we face now is how to energize the American public to really get behind the effort to fund (medical) research.” Why is it such a challenge to turn favorable public opinion into favorable public investment? As with so many other public policy issues, it is a matter of more effectively spreading the word. In this case, the word is easy: Medical research saves lives and saves the government money. In the past two years, The Ohio State University has earned double-digit percentage increases in support from the National Institutes of Health, the federal agency responsible for funding most of the medical research paid for by taxpayers. Ohio State also has more than doubled its annual funding from the NIH over the past six years. In addition, the University has opened a biomedical research tower and recruited a number of acclaimed researchers. All it needs now is strong funding support to put a state-of-the-art facility and 21st-century brainpower to work. But the NIH budget has now been cut for the first time in 30 years. It would be a terrible irony if the successes realized in that time somehow lead policymakers to believe they can retract support and expect progress to continue. The promise of personalized medicine is within reach of patients and their families because of research conducted at academic institutions such as Ohio State’s Medical Center. But it won’t happen if NIH budget stagnation eliminates all but the conventional research and starves young investigators of opportunity. Flat or declining NIH budgets are not only bad for science but harmful to the economy, because medical breakthroughs have the promise of reducing healthcare costs. In addition, medical research drives strong regional economies that stimulate new biotech and information-technology businesses and keep locally educated young people from seeking better opportunities elsewhere. To assure continuing, dynamic public support for medical research, many more people – patients, family members, physicians and scientists – must participate in public policy. That means talking with community leaders and elected officials to make sure that researchers and their institutions operate in a fiscal, policy and intellectual environment that makes faster progress against fearsome diseases a much higher national priority. If more of us get involved, it won’t be long until that Time person of the year – “You” – will be healthier than ever before. By Mary Woolley, president of Research!America (Adapted from Frontiers magazine, Fall 2007) 2007 Research Report 211 Office of Health Sciences Associate Vice President for Research Ohio State University Medical Center 260 Meiling Hall 370 W. 9th Ave. Columbus, Ohio 43210 Phone: (614) 292-2595 Fax: (614) 688-3427 www.medicalcenter.osu.edu ©2007 Ohio State University Medical Center
© Copyright 2026