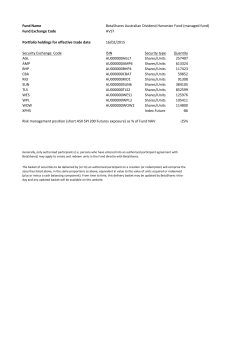
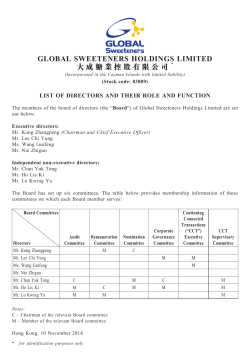
Labco DOCUMENT DE BASE
Free translation for information purposes only Labco A société anonyme with a board of directors and capital of €70,679,705.00 Registered office: – 60-62 rue d’Hauteville, 75010 Paris 448 650 085 R.C.S. Paris DOCUMENT DE BASE DISCLAIMER By accepting this document, you acknowledge, and agree to be bound by the following statements. This document is a translation of Labco’s document de base dated April 7, 2015 (the “document de base”). The document de base, in its original French version, is publicly available at www.amf-france.org and at www.labco.eu. This translation (the “Translation”) is provided for your convenience only and may not be reproduced, redistributed or passed on, directly or indirectly, to any other person or published in whole or in part for any purpose. It does not include the translations of certain sections of the document de base. This Translation has not been prepared for use in connection with any offering of securities. It does not contain all of the information that an offering document would contain. IN THE EVENT OF ANY AMBIGUITY OR CONFLICT BETWEEN THE CORRESPONDING STATEMENTS OR OTHER ITEMS CONTAINED HEREIN, THE FRENCH LANGUAGE DOCUMENT DE BASE SHALL PREVAIL. None of Labco or any of its respective officers, directors, employees or affiliates, or any person controlling any of them, assumes any liability which may be based on this Translation or any errors or omissions therefrom or misstatements therein, and any such liability is hereby expressly disclaimed. This Translation does not constitute or form part of any offer to sell or the solicitation of an offer to purchase securities, nor shall it or any part of it form the basis of, or be relied on in connection with, any contract or commitment whatsoever. Persons into whose possession of this Translation may come are required by Labco to inform themselves about and to observe any restrictions as to the distribution of this Translation. GENERAL INFORMATION In this document de base, unless stated otherwise, the term “Company” or “Labco” refers to Labco, a French société anonyme, headquartered at 60-62 rue d’Hauteville, 75010 Paris and registered with the registre du commerce et des sociétés de Paris under number 448 650 085, and the term “Group” refers collectively to the Company and its consolidated subsidiaries. This document de base contains information on the Group’s objectives and forecasts, and in particular Chapter 12 “Outlook for 2016-2017” and Chapter 13 “Profits forecasts or estimates”. Such information may be identified by the use of the future tense, the conditional mood or by forward-looking terms such as “think”, “aim”, “expect”, “intend”, “should”, “has the ambition of”, “consider”, “believe”, “wish”, “may” etc. This information is based on data, assumptions and estimates that the Company considers reasonable. It may be subject to change or alteration as a result of the uncertainties inherent to any business activity and to the economic, financial, competitive, regulatory and climatic environment. The Company does not undertake to publish updates of the objectives, forecasts and forward-looking information contained in this document de base, except when it is required by applicable law or regulations. In addition, the occurrence of certain risk factors presented in Chapter 4 “Risk factors” of this document de base may have an impact on the Group’s activities and on its ability to attain its objectives. Furthermore, attainment of objectives is also contingent on the success of the strategy presented in section 6.1. “Overview of the Group” of this document de base. The Company makes no representation and gives no warranty about the attainment of the objectives presented in this document de base. Investors are invited to carefully consider the risk factors presented in Chapter 4 “Risk factors” of this document de base before making their investment decision. The occurrence of some or all of these risks may have a negative impact on the Group’s business activities, financial condition, results of operation or objectives. In addition, other risk factors, which have not yet been identified at the date of this document de base or are considered as non-material by the Company, may have the same adverse effect, and investors may lose all or part of their investment. This document de base contains information relating to the markets in which the Group operates, the business segments in which the Group is active and the Group’s competitive position, particularly in Chapter 6 “Overview of Group businesses”. In addition to estimations prepared by the Group, the information underpinning the Group’s statements is taken primarily from a report commissioned by the Group from L.E.K. and from various supplementary sources (see Chapter 23 “Information derived from third parties, experts’ statements and declarations of interest” of this document de base). Some information contained in this document de base is information available to the public that the Group considers to be reliable but which has not been verified by an independent expert. The Company cannot guarantee that a third party using different methods to gather, analyze or calculate business segment data would obtain the same results. The Company and its shareholders make no representation and make no warranties as regards the accuracy of this information. Given the very rapid changes in the Group’s business’ sector in France and worldwide, it is possible that this information is inaccurate or out-of-date. Accordingly, trends in the Group’s business activities may depart from those presented in this document de base. The Company does not undertake to publish any updates of this information, except when it is required by applicable law or regulations. 1 DEFINITIONS In this document de base, the terms listed below have the following meaning: “acquisition” refers to the acquisition of a legal entity or business that operates one or more Laboratories, it being stipulated that in the event that several legal entities or businesses operating one or more Laboratories are acquired through a single acquisition, the number of acquisitions referred to in this document de base corresponds to the number of legal entities or businesses operating one or more Laboratories thus acquired. “AFEP-MEDEF Code” refers to the Corporate Governance Code for listed companies drafted by the Association Française des Entreprises Privées and Mouvement des Entreprises de France (France), as amended in June 2013. “Amended RCF” has the meaning set forth in section 10.4.4. “RCF” of this document de base. “API” refers to in-house associated professional biologists working within a SEL. “Aplitec” refers to the company Aplitec, the Company’s principal statutory auditor, headquartered at 4-14 rue Ferrus, 75014 Paris and registered with the registre du commerce et des sociétés de Parisunder number 702 034 802. “ARS” stands for Agence régionale de santé (regional health authority) in France. “ASL” stands for Azienda Sanitaria Local (i.e. the Italian regional health authority). “BSA” refers to a share purchase warrant issued by the Company. “CET” stands for the Contribution Économique Territoriale levy on businesses in France. “CNAM” refers to the Caisse Nationale d’Assurance Maladie (National Health Insurance Fund) in France. “COFRAC” refers to the Comité français d’accréditation (French accreditation body). “Collection Center” refers to a center for collecting or taking clinical samples, most of which will be sent to a Laboratory for analysis, but some are analyzed on site. Most Laboratories have a Collection Center on site. “CPA” refers to Clinical Pathology Accreditation (United Kingdom). “CPAM” refers to one or many Caisse(s) Primaire(s) d’Assurance Maladie (Local Health Insurance Fund) in France. “CPD” refers to continuing professional development, the new regulatory training system for health professionals and in particular doctors and clinical pharmacists. “CT” stands for computed tomography. “CVAE” stands for the Cotisation sur la Valeur Ajoutée des Entreprises levy on businesses in France. “Deep Dive” refers to the restructuring program implemented by the Group in France during 2012 and 2013. “Deloitte” refers to the company Deloitte et Associés, the Company’s principal statutory auditor, headquartered at 185, avenue Charles de Gaulle, 92200 Neuilly-sur-Seine and registered with the Nanterre Trade and Companies Register under number 572 028 041. “DNA” stands for deoxyribonucleic acid. 2 “EBITDA” stands for earnings before interest, tax, depreciation and amortization and represents operating income before non-recurring items restated for depreciation, amortization, impairment losses, and provisions net of reversals. “ERS” refers to the Entidade Reguladora de Saude (Portugal). “EURIBOR” stands for Euro Interbank Offered Rate. “FDHA” refers to the Federal Department of Home Affairs in Switzerland. “Financing EBITDA” refers to consolidated EBITDA published by the Company at the end of an accounting period, plus the following items: the full-year effect of acquisitions performed during the relevant period (net of disposals), the portion of EBITDA from equity-accounted companies in that period, and specific items such as share-based payments, transaction costs, redundancy and restructuring costs, and the amount of losses other than that related to the restructuring, recorded during the implementation of the new contracts in the United Kingdom, excluding the impact of the loss arising from the inability to recoup VAT paid on head-office costs passed on to Laboratory-owning companies. “Financing Net Debt” refers to net debt plus borrowing arrangement costs and undertakings to make earn-out payments in relation to acquisitions made on the date Financing Net Debt is calculated. “FTCE” stands for fixed-term employment contract. “FTE” stands for full-time equivalent. “GIE” refers to “Labco Gestion”, the groupement d’intérêt économique that is intended to house all the Group’s existing laboratory companies in France, and some of the Group’s foreign companies. “Group” refers collectively to the Company and its consolidated subsidiaries. “Group Company” refers to the Company or any other company or entity controlled directly or indirectly by the Company within the meaning of Article L.233-3 of the French Commercial Code. “High-Yield Bonds” has the meaning set forth in section 10.4.3. “High-Yield Bonds” of this document de base. “HIV” refers to human immunodeficiency virus. “IFRS” stands for International Financial Reporting Standards. “iLab” refers to the “iLab” mobile application which enables health professionals and partners of the Group’s laboratories to consult the testing catalogues and sampling handbooks, and to stay informed of any changes. “INAMI” refers to the Institut National d’Assurance Maladie-Invalidité (national health and disability insurance agency) in Belgium. “Indenture” refers to the high yield bond issuance agreement. “Integrated Diagnostics Center” refers to a location that combines three of the main disciplines required for establishing a clinical diagnosis: clinical testing, anatomical or cytopathology testing and medical imaging. The center may also provide complementary services for therapeutic purposes, such as day surgery or physiotherapy. “Integrated Medical Diagnostics” refers to an offer of services that combines three of the main disciplines required for establishing a clinical diagnosis: clinical testing, anatomical or cytopathology testing and medical imaging. “IRAP” refers to the Imposta Regionale sulle attività produttive (i.e. tax on production activities in Italy). “IRCCS” refers to the Istituto di Ricovero e Cura a Carattere Scientifico (i.e. a category of medical research body in Italy). 3 “ISO” stands for the International Organization for Standardization, and by extension for the international standards it has published. “IUI” stands for Intra-Uterine Insemination with the partner’s sperm. “IVF” stands for In Vitro Fertilization. “Labco” or the “Company” refers to Labco, a French société anonyme headquartered at 60-62 rue d’Hauteville in Paris (75010), registered with the registre du commerce et des sociétés de Parisunder number 448 650 085. “Laboratory” stands for a location where biological samples of human or animal origin are analyzed, these samples having been collected in a dedicated area at this location or originating from an external location such as another Laboratory or an external Collection Center, to assist mainly in the establishment of a clinical diagnosis or, where appropriate, the treatment of certain conditions. One company may operate several Laboratories. When required, the “site” of a clinical testing laboratory, as defined in the French public health Code, may be referred to as a Laboratory in this document de base, if it satisfies the conditions laid out above. “Laboratory doctor” refers to a professional who is qualified to own, manage or operate a clinical laboratory and who, depending on the country in which he operates, may or may not be a medical doctor. “Law of May 30, 2013” refers to French law no. 2013-442 adopted on May 30, 2013 implementing the clinical testing reforms. “LIS” stands for laboratory information system. “MAR” stands for medically assisted reproduction. “Mix effect” refers to the phenomenon by which the Group’s performance, and in particular organic growth in the Group’s business, is influenced by the combined effects of volumes and the evolution of the average price per test, which is in turn affected by the evolution of the prices and the nature of the clinical tests performed. “MRI” stands for magnetic resonance imaging. “NHS” refers to the National Health Service in the United Kingdom. “NSW” stands for non-salaried workers. “OECE” stands for open-ended employment contract. “PET” stands for Positron Emission Tomography technology. “RCF” has the meaning set forth in section 10.4.4. “RCF” of this document de base. “Revenue” refers to “total revenue” as shown in the Group’s consolidated financial statements. “SEL” refers, in France, to a company incorporated as a société d’exercice libéral of laboratory doctors if it mainly operates a clinical laboratory, or of doctors, if it exclusively operates an anatomical and cyto-pathology testing laboratory. “SNS” stands for the Serviço Nacional de Saúde (National Health Service in Portugal). “SPORT” stands for Strategic Procurement Optimization and Rationalization (the Group’s pan-European project which has allowed a reduction in procurement costs by cutting the number of its suppliers and by securing better commercial terms through the use of framework agreements, especially for purchases of the reagents used to perform the clinical tests). “TUPE” stands for the Transfer of Undertakings Protection of Employment (i.e. transfer of employees between the public sector and the private sector in the United Kingdom). 4 “UKAS” refers to the United Kingdom Accreditation Services. “UNCAM” refers to the Union Nationale des Caisses d’Assurance Maladie in France (French association of health insurance funds). “VAT” stands for Value Added Tax. “VAT Directive” refers to the Council Directive 2006/112/EC of November 28, 2006 on the common system of value added tax. 5 TABLE OF CONTENTS GENERAL INFORMATION ................................................................................................................................. 1 DEFINITIONS .................................................................................................................................................... 2 CHAPTER 1 PERSONS RESPONSIBLE FOR THE DOCUMENT DE BASE ............................................................... 14 1.1 1.2 1.3 PERSON RESPONSIBLE FOR THE DOCUMENT DE BASE ......................................................................................... 14 STATEMENT BY THE PERSON RESPONSIBLE FOR THE DOCUMENT DE BASE ............................................................... 14 PERSON RESPONSIBLE FOR THE FINANCIAL DISCLOSURE ...................................................................................... 14 CHAPTER 2 STATUTORY AUDITORS ................................................................................................................ 15 2.1 2.2 PRINCIPAL STATUTORY AUDITORS ................................................................................................................. 15 ALTERNATE STATUTORY AUDITORS................................................................................................................ 16 CHAPTER 3 SELECTED FINANCIAL INFORMATION ........................................................................................... 17 CHAPTER 4 RISK FACTORS .............................................................................................................................. 20 4.1 RISKS RELATED TO THE BUSINESS SECTORS AND MARKETS IN WHICH THE GROUP OPERATES ....................................... 20 4.2 RISKS RELATED TO THE GROUP’S TECHNOLOGY AND INTELLECTUAL PROPERTY RIGHTS .............................................. 27 4.3 RISKS RELATED TO THE GROUP AND ITS COMMERCIAL ACTIVITIES ......................................................................... 29 4.4 LEGAL, TAX AND INSURANCE RISKS................................................................................................................. 36 4.4.1 Risks related to disputes and litigation ......................................................................................... 36 4.4.2 Risks related to the protection of personal data and information security .................................. 37 4.4.3 Risks related to taxation ............................................................................................................... 37 4.4.4 Risks related to tax rules regarding the tax-deductibility of interests .......................................... 38 4.4.5 Risks related to VAT and French payroll tax ................................................................................. 38 4.5 FINANCIAL RISKS ........................................................................................................................................ 38 4.5.1 Credit or counterparty risk ............................................................................................................ 38 4.5.2 Exchange-rate risk ........................................................................................................................ 39 4.5.3 Interest-rate risk ........................................................................................................................... 39 4.5.4 Liquidity risk .................................................................................................................................. 40 4.5.5 Equity risk ...................................................................................................................................... 41 4.6 RISK MANAGEMENT POLICY AND POLICY ON INSURANCE .................................................................................... 41 4.6.1 Risk management ......................................................................................................................... 41 4.6.2 Policy on insurance ....................................................................................................................... 42 CHAPTER 5 INFORMATION ABOUT THE GROUP ............................................................................................. 44 5.1 HISTORY AND EVOLUTION OF THE GROUP........................................................................................................ 44 5.1.1 Business name .............................................................................................................................. 44 5.1.2 Company registration place and number ..................................................................................... 44 5.1.3 Date of incorporation and term of existence ................................................................................ 44 5.1.4 Headquarters, legal form and applicable legislation .................................................................... 44 5.1.5 Significant events in the development of the Group’s activities ................................................... 44 5.2 INVESTMENTS ........................................................................................................................................... 46 5.2.1 Historical investments ................................................................................................................... 46 5.2.1.1 5.2.1.2 5.2.2 5.2.2.1 5.2.2.2 5.2.3 Acquisitions and disposals of groups of companies, companies and/or businesses ................................. 47 Acquisitions of property, plant and equipment and intangible assets ...................................................... 49 Current investments ...................................................................................................................... 50 Acquisitions of groups of companies, companies and/or businesses ....................................................... 50 Acquisitions of property, plant and equipment and intangible assets ...................................................... 50 Future investments ....................................................................................................................... 50 CHAPTER 6 OVERVIEW OF GROUP BUSINESSES ............................................................................................. 51 6.1 OVERVIEW OF THE GROUP........................................................................................................................... 51 6.1.1 Group businesses .......................................................................................................................... 52 6.1.2 The Group’s economic advantages ............................................................................................... 53 6 6.1.3 The Group’s strategy ..................................................................................................................... 54 6.2 PRESENTATION OF SECTORS IN WHICH THE GROUP IS ACTIVE .............................................................................. 54 6.2.1 Background ................................................................................................................................... 54 6.2.2 Sector trends ................................................................................................................................. 55 6.2.2.1 6.2.2.2 6.2.2.3 6.2.2.4 6.2.2.5 6.2.2.6 6.2.2.7 6.2.3 6.2.4 6.2.4.1 6.2.4.2 6.2.4.3 6.2.4.4 Demographics ........................................................................................................................................... 55 Pricing conditions ...................................................................................................................................... 55 Sub-contracting and outsourcing .............................................................................................................. 56 Medicine and new technologies ............................................................................................................... 56 Quality Standards ...................................................................................................................................... 57 A personalized direct offering ................................................................................................................... 58 Consolidation ............................................................................................................................................ 58 Competitive features ..................................................................................................................... 58 Clinical Laboratory Services Market in Europe .............................................................................. 60 Overview ................................................................................................................................................... 60 Northern Europe segment ........................................................................................................................ 60 Southern Europe segment ........................................................................................................................ 65 Emerging markets ..................................................................................................................................... 67 6.3 GROUP STRATEGY ...................................................................................................................................... 67 6.4 DETAILED PRESENTATION OF THE GROUP ........................................................................................................ 70 6.4.1 Services offered by the Group ....................................................................................................... 70 6.4.1.1 6.4.1.2 6.4.1.3 6.4.1.4 6.4.2 6.4.2.1 6.4.2.2 6.4.2.3 6.4.3 6.4.3.1 6.4.3.2 6.4.3.3 6.4.3.4 Clinical laboratory tests ............................................................................................................................. 72 Anatomical pathology ............................................................................................................................... 74 Medical imaging ........................................................................................................................................ 74 MAR .......................................................................................................................................................... 76 The Group’s organizational models .............................................................................................. 76 Collection centers and clinical Laboratories .............................................................................................. 76 Sub-contracting and outsourcing laboratory services ............................................................................... 77 Export services .......................................................................................................................................... 78 Group’s operations by country ...................................................................................................... 79 Overview of Northern European market .................................................................................................. 79 Overview of Southern European market .................................................................................................. 84 Emerging countries ................................................................................................................................... 88 Germany.................................................................................................................................................... 89 6.4.4 External growth strategy .............................................................................................................. 89 6.4.5 Quality standards .......................................................................................................................... 92 6.4.6 Group’s customers ........................................................................................................................ 93 6.4.7 Invoicing and payment procedures ............................................................................................... 94 6.4.8 Group’s suppliers .......................................................................................................................... 95 6.4.9 Information Technology Systems .................................................................................................. 95 6.4.10 Sales forces, assistance in performing tests and establishing diagnostics ................................... 96 6.5 REGULATION............................................................................................................................................. 97 6.5.1 France ........................................................................................................................................... 97 6.5.2 United Kingdom .......................................................................................................................... 105 6.5.3 Belgium ....................................................................................................................................... 106 6.5.4 Switzerland ................................................................................................................................. 107 6.5.5 Spain ........................................................................................................................................... 107 6.5.6 Portugal ...................................................................................................................................... 108 6.5.7 Italy ............................................................................................................................................. 109 6.6 DATA ON WHICH ANY STATEMENT BY THE COMPANY WITH RESPECT TO ITS COMPETITIVE POSITION IS BASED .............. 110 CHAPTER 7 ORGANIZATION STRUCTURE ...................................................................................................... 111 CHAPTER 8 REAL ESTATE PROPERTIES, PLANTS AND EQUIPMENT ............................................................... 115 8.1 SIGNIFICANT EXISTING OR PLANNED PROPERTY, PLANT AND EQUIPMENT ............................................................. 115 8.1.1 Real estate properties ................................................................................................................. 115 8.1.2 Other property, plant and equipment ......................................................................................... 116 8.2 ENVIRONMENT AND SUSTAINABLE DEVELOPMENT .......................................................................................... 116 CHAPTER 9 OPERATING AND FINANCIAL REVIEW OF THE GROUP ................................................................ 117 7 9.1 OVERVIEW ............................................................................................................................................. 117 9.1.1 Introduction ................................................................................................................................ 117 9.1.2 Principal factors influencing the Group’s results from operating activities ................................ 119 9.1.2.1 9.1.2.2 9.1.2.3 9.1.2.4 9.1.2.5 9.1.3 9.1.3.1 9.1.3.2 9.1.3.3 9.1.3.4 9.1.3.5 9.1.3.6 9.1.3.7 9.1.3.8 9.1.3.9 9.1.3.10 9.1.3.11 9.1.3.12 9.1.3.13 General economic conditions and legal framework................................................................................ 119 Expansion in the Group’s network of clinical laboratories through acquisitions .................................... 121 Organic growth ....................................................................................................................................... 123 Cost structure and operating performance............................................................................................. 123 Seasonality .............................................................................................................................................. 124 Description of the key income statement items .......................................................................... 125 Revenue .................................................................................................................................................. 125 Cost of sales ............................................................................................................................................ 126 Payroll-related expenses ......................................................................................................................... 126 Share-based payments ............................................................................................................................ 126 Other operating expenses ....................................................................................................................... 127 Transaction costs for usual small size acquisitions ................................................................................. 127 EBITDA..................................................................................................................................................... 127 Depreciation, impairment losses and amortization, provisions and reversals ........................................ 127 Non-recurring income and expenses ...................................................................................................... 128 Net finance costs ................................................................................................................................ 128 Income tax expenses .......................................................................................................................... 128 Share of profit of associates ............................................................................................................... 128 Net profit from discontinued operations ............................................................................................ 128 9.1.4 Main accounting principles ......................................................................................................... 128 9.2 ANALYSIS OF THE RESULTS OF OPERATIONS FOR THE FINANCIAL YEARS ENDED DECEMBER 31, 2014 AND DECEMBER 31, 2013 ............................................................................................................................................................ 129 9.2.1 Overview ..................................................................................................................................... 129 9.2.2 Revenue ...................................................................................................................................... 130 9.2.2.1 9.2.2.2 9.2.3 9.2.4 9.2.5 9.2.6 9.2.7 9.2.8 9.2.8.1 9.2.8.2 Northern Europe ..................................................................................................................................... 130 Southern Europe ..................................................................................................................................... 131 Cost of sales ................................................................................................................................ 131 Payroll related expenses ............................................................................................................. 132 Share-based payments................................................................................................................ 132 Other operating expenses ........................................................................................................... 132 Transactions costs for usual small size acquisitions.................................................................... 133 EBITDA ........................................................................................................................................ 133 Northern Europe ..................................................................................................................................... 133 Southern Europe ..................................................................................................................................... 134 9.2.9 Depreciation, impairment losses and amortization, provisions and reversals ............................ 134 9.2.10 Non-recurring income and expenses........................................................................................... 134 9.2.11 Net finance costs ......................................................................................................................... 135 9.2.12 Income tax expenses ................................................................................................................... 135 9.2.13 Share of profit of associates........................................................................................................ 135 9.2.14 Net profit from discontinued operations .................................................................................... 135 9.2.15 Net profit..................................................................................................................................... 136 9.3 ANALYSIS OF THE RESULTS OF OPERATIONS FOR THE FINANCIAL YEARS ENDED DECEMBER 31, 2013 AND DECEMBER 31, 2012 ............................................................................................................................................................ 136 9.3.1 Overview ..................................................................................................................................... 136 9.3.2 Revenue ...................................................................................................................................... 137 9.3.2.1 9.3.2.2 9.3.3 9.3.4 9.3.5 9.3.6 9.3.7 9.3.8 9.3.8.1 9.3.8.2 9.3.9 Northern Europe ..................................................................................................................................... 137 Southern Europe ..................................................................................................................................... 138 Cost of sales ................................................................................................................................ 138 Payroll related expenses ............................................................................................................. 139 Share based payments ................................................................................................................ 139 Other operating expenses ........................................................................................................... 139 Transaction costs for usual small size acquisitions ..................................................................... 139 EBITDA ........................................................................................................................................ 139 Northern Europe ..................................................................................................................................... 140 Southern Europe ..................................................................................................................................... 140 Depreciation, amortization, impairment losses, provisions and reversals .................................. 140 8 9.3.10 9.3.11 9.3.12 9.3.13 9.3.14 9.3.15 Non-recurring income and expenses........................................................................................... 140 Net finance costs ......................................................................................................................... 141 Income tax expenses ................................................................................................................... 141 Share of profit of associates........................................................................................................ 141 Net profit from continuing operations ........................................................................................ 142 Net profit from discontinued operations .................................................................................... 142 CHAPTER 10 CAPITAL RESOURCES ................................................................................................................ 143 10.1 GENERAL PRESENTATION ........................................................................................................................... 143 10.2 PRESENTATION AND ANALYSIS OF THE MAIN WAYS IN WHICH THE GROUP USES CASH ............................................. 144 10.2.1 Acquisitions ................................................................................................................................. 144 10.2.2 Investments................................................................................................................................. 144 10.2.3 Payment of interests and repayment of debts ............................................................................ 144 10.2.4 Financing the working capital requirement ................................................................................ 144 10.3 THE COMPANY’S CONSOLIDATED CASH FLOWS ............................................................................................... 145 10.3.1 A business model that generates large amounts of cash ........................................................... 145 10.3.2 Group cash flows in the financial years ended December 31, 2013 and 2014 ............................ 145 10.3.3 Group cash flows from operating activities in the financial years ended December 31, 2013 and 2014 .................................................................................................................................................... 146 10.3.4 Group cash flows from investing activities for the financial years ended December 31, 2013 and 2014 .................................................................................................................................................... 146 10.3.5 Group cash flows from financing activities in the financial years ended December 31, 2013 and 2014 .................................................................................................................................................... 147 10.3.6 Group cash flows in the financial years ended December 31, 2012 and 2013 ............................ 147 10.3.7 Cash flows from operating activities ........................................................................................... 148 10.3.8 Cash flows from investing activities ............................................................................................ 148 10.3.9 Cash flows from financing activities ........................................................................................... 149 10.4 EQUITY AND FINANCIAL DEBT ..................................................................................................................... 149 10.4.1 Group equity ............................................................................................................................... 149 10.4.2 Financial liabilities....................................................................................................................... 150 10.4.3 High Yield Bonds ......................................................................................................................... 150 10.4.4 RCF .............................................................................................................................................. 152 10.4.4.1 10.4.4.2 10.4.4.3 10.4.4.4 10.4.4.5 10.4.4.6 10.4.4.7 10.4.4.8 10.4.5 10.4.5.1 10.4.5.2 10.4.5.3 10.4.5.4 10.4.5.5 10.4.5.6 10.4.5.7 10.4.5.8 Amount, usage, term .......................................................................................................................... 152 Interests, commitment fee ................................................................................................................. 153 Repayment of the principal ................................................................................................................ 154 Early repayment ................................................................................................................................. 154 Security interests and guarantees ...................................................................................................... 154 Undertakings - Restrictive clauses - Financial covenants .................................................................... 156 Accelerated maturity situations ......................................................................................................... 157 Applicable law – Competent courts .................................................................................................... 158 Intercreditor Agreement ............................................................................................................. 158 Ranking and Priority ........................................................................................................................... 158 Parallel debt ........................................................................................................................................ 159 Permitted Payments ........................................................................................................................... 159 Enforcement of security interests ...................................................................................................... 160 Release of guarantees and security interests ..................................................................................... 160 Additional debt ................................................................................................................................... 160 Amendments ...................................................................................................................................... 160 Applicable law ..................................................................................................................................... 161 10.4.6 Other financial debts................................................................................................................... 161 10.5 OFF-BALANCE SHEET COMMITMENTS ........................................................................................................... 161 10.6 RESTRICTION ON THE USE OF CAPITAL THAT HAS MATERIALLY INFLUENCED OR COULD MATERIALLY INFLUENCE, DIRECTLY OR INDIRECTLY, THE COMPANY’S BUSINESS ACTIVITY ....................................................................................................... 162 10.7 EXPECTED SOURCES OF FINANCING FOR FUTURE INVESTMENTS .......................................................................... 162 CHAPTER 11 RESEARCH & DEVELOPMENT, PATENTS AND LICENSES ............................................................ 163 CHAPTER 12 OUTLOOK FOR 2016-2017 ........................................................................................................ 164 CHAPTER 13 PROFIT FORECASTS OR ESTIMATES .......................................................................................... 165 9 13.1 FORECASTS ............................................................................................................................................. 165 13.1.1 Assumptions ................................................................................................................................ 165 13.1.2 Group forecasts for the financial year ending December 31, 2015 ............................................ 165 13.2 STATUTORY AUDITORS’ REPORT ON THE PROFITS FORECASTS............................................................................. 167 CHAPTER 14 ADMINISTRATIVE, MANAGEMENT AND SUPERVISORY BODIES AND GENERAL MANAGEMENT .................................................................................................................................................................... 169 14.1 MEMBERS OF THE ADMINISTRATIVE, MANAGEMENT AND SUPERVISORY BODIES AND GENERAL MANAGEMENT ............ 169 14.1.1 The board of directors ................................................................................................................. 169 14.1.1.1 14.1.1.2 14.1.1.3 Composition of the board of directors ............................................................................................... 169 Biographies of the members of the board of directors ...................................................................... 174 Declarations relating to the members of the board of directors ........................................................ 177 14.1.2 General Management ................................................................................................................. 177 14.1.3 Executive Committee .................................................................................................................. 177 14.2 CONFLICTS OF INTEREST IN THE ADMINISTRATIVE BODIES AND IN GENERAL MANAGEMENT ...................................... 177 CHAPTER 15 COMPENSATION AND BENEFITS .............................................................................................. 178 15.1 COMPENSATION AND BENEFITS OF ANY NATURE ALLOCATED TO DIRECTORS AND CORPORATE OFFICERS AND MEMBERS OF ADMINISTRATIVE, MANAGEMENT AND SUPERVISORY BODIES DURING THE FINANCIAL YEARS ENDING DECEMBER 31, 2013 AND DECEMBER 31, 2014........................................................................................................................................... 178 15.1.1 Summary of the compensation of the Chairman of the board and of the Chief Executive Officer in respect of the financial years 2013 and 2014 ............................................................................................. 178 15.1.2 Compensation and benefits of any nature allocated to the Chairman of the board and to the Chief Executive Officer ................................................................................................................................ 178 15.1.3 Compensation and benefits of any nature allocated to the directors......................................... 179 15.1.4 Subscription and share purchase options allocated by the Company or by any Group Company to the Chairman of the board and to the Chief Executive Officer ................................................................... 180 15.1.5 Subscription and share purchase options exercised by the Chairman of the board and by the Chief Executive Officer ................................................................................................................................ 180 15.1.6 Performance shares allocated to the Chairman of the board and to the Chief Executive Officer .... .................................................................................................................................................... 181 15.1.7 Performance shares that became available to the Chairman of the Board and to the Chief Executive Officer ......................................................................................................................................... 181 15.1.8 History of allocations of subscriptions and acquisitions of share purchase options ................... 181 15.1.9 History of allocations of acquisitions of share purchase warrants ............................................. 181 15.1.10 Share purchase options allocated to the top ten non-corporate officer employees ............... 182 15.1.11 History of allocations of free shares – Information on shares allocated for free .................... 182 15.1.12 Change in compensation of executive corporate officers and directors’ fees paid to members of the Board of directors after the initial public offering ............................................................................ 183 15.1.13 Employment contracts, retirement benefits and payments in the event of termination of the functions of the Chairman of the board and of the Chief Executive Officer ............................................... 184 15.2 AMOUNTS PLACED IN RESERVES BY THE GROUP TO PAY PENSIONS, RETIREMENTS OR OTHER BENEFITS TO SENIOR EXECUTIVES ........................................................................................................................................................ 185 CHAPTER 16 FUNCTIONING OF THE COMPANY’S ADMINISTRATIVE AND MANAGEMENT BODIES ............... 186 16.1 THE FUNCTIONING OF THE COMPANY’S ADMINISTRATIVE AND MANAGEMENT BODIES............................................ 186 16.1.1 Board of directors ....................................................................................................................... 186 16.1.1.1 Composition of the board of directors ............................................................................................... 186 16.1.1.2 Directors’ duties ................................................................................................................................. 189 16.1.1.3 Powers of the board of directors (Article 19 of the Articles of Association, Section II of the Internal Regulations) ............................................................................................................................................................ 191 16.1.1.4 Resolutions of the board of directors (Article 18 of the Articles of Association, Section IV of the Internal Regulations) ................................................................................................................................................ 192 16.1.1.5 Directors’ remuneration (Article 17 of the Articles of Association, Article 23 of the Internal Regulations) ............................................................................................................................................................ 192 16.1.2 General Management ................................................................................................................. 193 16.1.2.1 Chairman of the board of directors (Article 15 of the Articles of Association, Article 14 of the Internal Regulations) ............................................................................................................................................................ 193 10 16.1.2.2 Chief Executive Officer (Articles 21, 22, 24, 25 and 26 of the Articles of Association, Article 5 of the Internal Regulations) ................................................................................................................................................ 193 16.1.2.3 Deputy Chief Executive Officers (Articles 23 to 26 of the Articles of Association, Article 5 of the Internal Regulations) ................................................................................................................................................ 194 16.2 SERVICE CONTRACTS BETWEEN MEMBERS OF ADMINISTRATIVE, MANAGEMENT AND SUPERVISORY BODIES AND THE COMPANY OR ITS SUBSIDIARIES............................................................................................................................... 194 16.3 OBSERVERS (ARTICLE 20 OF THE ARTICLES OF ASSOCIATION AND ARTICLES 21.5 TO 21.8 OF THE INTERNAL REGULATIONS) . ............................................................................................................................................................ 194 16.4 COMMITTEES .......................................................................................................................................... 195 16.4.1 The Audit and Risks Committee .................................................................................................. 195 16.4.1.1 16.4.1.2 16.4.1.3 16.4.2 Composition of the Audit and Risks Committee (Articles 25 and 28 of the Internal Regulations) ..... 195 The remit of the Audit and Risks Committee (Articles 25 and 26 of the Internal Regulations) .......... 196 The functioning of the Audit and Risks Committee (Articles 25, 27 and 29 of the Internal Regulations) . ............................................................................................................................................................ 198 The Nominations and Compensation Committee ....................................................................... 198 16.4.2.1 The composition of the Nominations and Compensation Committee (Articles 25 and 32 of the Internal Regulations) ................................................................................................................................................ 198 16.4.2.2 The remit of the Nominations and Compensation Committee (Articles 25 and 30 of the Internal Regulations) ............................................................................................................................................................ 198 16.4.2.3 The functioning of the Nominations and Compensation Committee (Article 25, 31 and 33 of the Internal Regulations) ................................................................................................................................................ 200 16.4.3 The Strategy and Major Projects Committee .............................................................................. 200 16.4.3.1 Regulations) 16.4.3.2 Regulations) 16.4.3.3 Regulations) 16.5 16.6 The composition of the Strategy and Major Projects Committee (Articles 25 and 36 of the Internal ............................................................................................................................................................ 200 The remit of the Strategy and Major Projects Committee (Articles 25 and 34 of the Internal ............................................................................................................................................................ 200 The functioning of the Strategy and Major Projects Committee (Article 25, 35 and 37 of the Internal ............................................................................................................................................................ 201 DECLARATION RELATING TO CORPORATE GOVERNANCE.................................................................................... 201 INTERNAL CONTROL AND CORPORATE GOVERNANCE ....................................................................................... 201 CHAPTER 17 EMPLOYEES ............................................................................................................................. 203 17.1 PRESENTATION ........................................................................................................................................ 203 17.1.1 Number and breakdown of employees ....................................................................................... 203 17.1.2 Employment and working conditions.......................................................................................... 205 17.1.3 Training ....................................................................................................................................... 205 17.1.4 Compensation policy ................................................................................................................... 206 17.1.5 Employee representation ............................................................................................................ 206 17.2 EMPLOYEE INCENTIVE AND PROFIT-SHARING PLANS......................................................................................... 206 17.2.1 Employee incentive plan ............................................................................................................. 207 17.2.2 Employee profit-sharing plan ...................................................................................................... 207 17.3 MULTI-COMPANY EMPLOYEE SHARE OWNERSHIP PLANS .................................................................................. 207 17.4 INCENTIVES PAID TO EXECUTIVE CORPORATE OFFICERS AND DIRECTORS’ SHARE DEALINGS ....................................... 207 17.5 SELF-EMPLOYED WORKERS......................................................................................................................... 207 17.5.1 Legal definition of self-employed workers .................................................................................. 207 17.5.2 Social law applicable to self-employed workers ......................................................................... 208 17.5.3 Self-employed workers and the independent practice agreement ............................................. 208 17.5.4 Self-employed workers in the Group’s staff ................................................................................ 209 CHAPTER 18 PRINCIPAL SHAREHOLDERS...................................................................................................... 210 18.1 IDENTIFICATION OF SHAREHOLDERS ............................................................................................................. 210 18.1.1 Ownership of share capital and voting rights ............................................................................. 210 18.1.2 Changes in ownership of the share capital and voting rights in the last three financial years ... 210 18.2 VOTING RIGHTS ....................................................................................................................................... 211 18.3 SHAREHOLDERS PACT, OWNERSHIP UNDERTAKINGS AND PARTIES ACTING IN CONCERT ........................................... 211 18.4 CONTROL OF THE COMPANY ...................................................................................................................... 211 18.5 AGREEMENTS THAT MAY CAUSE A CHANGE IN CONTROL OF THE COMPANY .......................................................... 211 CHAPTER 19 RELATED PARTY TRANSACTIONS.............................................................................................. 212 11 19.1 INTRAGROUP AGREEMENTS OR AGREEMENTS WITH RELATED PARTIES ................................................................. 212 19.1.1 Guarantees ................................................................................................................................. 212 19.1.2 Financing agreements................................................................................................................. 213 19.1.3 Settlement agreements............................................................................................................... 213 19.1.4 Executive Administrator Agreement and other agreements with Andreas Gaddum ................. 214 19.1.5 Authorization of the reorganization of the legal structure ......................................................... 214 19.1.6 Agreements with the company Immobilière de Laboratoires ..................................................... 214 19.2 STATUTORY AUDITORS’ SPECIAL REPORTS ON REGULATED AGREEMENTS ............................................................. 215 19.2.1 Statutory auditors’ special report on regulated agreements for the financial year ended December 31, 2012 ..................................................................................................................................... 215 19.2.2 Statutory auditors’ special report on regulated agreements for the financial year ended December 31, 2013 ..................................................................................................................................... 222 19.2.3 Statutory auditors’ special report on regulated agreements for the financial year ended December 31, 2014 ..................................................................................................................................... 231 CHAPTER 20 FINANCIAL INFORMATION CONCERNING THE COMPANY’S ASSETS AND LIABILITIES, FINANCIAL POSITION AND RESULTS............................................................................................................................... 240 20.1 IFRS FINANCIAL REPORTING ....................................................................................................................... 240 20.1.1 IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014 .................................................................................................................................................... 240 20.1.2 Statutory Auditor’s Report on the accounts established in accordance with IFRS principles for the financial years ended December 31, 2012, 2013 and 2014 ........................................................................ 495 20.1.3 Pro Forma Financial Information for the financial years ended December 31, 2013 and December 31, 2014 .................................................................................................................................................... 501 20.1.4 Statutory auditors’ report on pro forma financial information for the financial years ended December 31, 2013 and December 31, 2014 .............................................................................................. 509 20.2 DIVIDENDS ............................................................................................................................................. 511 20.2.1 Dividends paid in the last six financial years ............................................................................... 511 20.2.2 Dividends policy .......................................................................................................................... 511 20.2.3 Timeframe for claiming dividends .............................................................................................. 511 20.3 CONTENTIOUS PROCEEDINGS ..................................................................................................................... 511 20.3.1 Ordre des pharmaciens and Ordre des médecins ....................................................................... 511 20.3.2 French disputes ........................................................................................................................... 513 20.3.3 Portuguese disputes .................................................................................................................... 513 20.3.4 German disputes ......................................................................................................................... 514 20.4 SIGNIFICANT CHANGE IN THE FINANCIAL OR TRADING POSITION ......................................................................... 514 20.5 FEES PAID BY THE GROUP TO THE STATUTORY AUDITORS AND MEMBERS OF THEIR NETWORKS ................................. 515 CHAPTER 21 ADDITIONAL INFORMATION .................................................................................................... 516 21.1 SHARE CAPITAL ........................................................................................................................................ 516 21.1.1 The amount of the share capital ................................................................................................. 516 21.1.2 Securities not representing share capital .................................................................................... 516 21.1.3 Company’s self-controlling and self-held shares and acquisition of its own shares ................... 516 21.1.4 Potential capital .......................................................................................................................... 516 21.1.4.1 21.1.4.2 21.1.4.3 “Manager” Warrants .......................................................................................................................... 517 “Investor” Warrants............................................................................................................................ 518 “Mezzanine” Warrants ....................................................................................................................... 521 21.1.5 Unissued authorized share capital, capital increase commitments ............................................ 523 21.1.6 Information on the share capital of the Company or of its subsidiaries that is the subject of an option or a conditional or unconditional agreement providing for it to be made subject to an option and details of such options (including the identity of the persons to whom they relate) .................................. 525 21.1.7 Changes in the share capital over the past three financial years ............................................... 525 21.2 ARTICLES OF ASSOCIATION......................................................................................................................... 526 21.2.1 Corporate purpose (Article 3 of the Articles of Association) ....................................................... 526 21.2.2 Administrative, managerial and supervisory bodies ................................................................... 526 21.2.3 Rights, privileges, restrictions and duties attached to the shares .............................................. 526 21.2.3.1 Property rights and duties attached to the shares (Article 12 of the Articles of Association) ............ 526 12 21.2.3.2 Voting and communication rights attached to the shares (Article 12 of the Articles of Association) 527 21.2.3.3 Exercise of voting rights in the event of dismemberment of ownership of the shares, and indivisibility of the shares (Article 10 of the Articles of Association) ............................................................................................ 527 21.2.3.4 Distribution of profits according to the Articles of Association (Article 38 of the Articles of Association) ............................................................................................................................................................ 527 21.2.3.5 The form of securities issued by the Company (Articles 9 and 11 of the Articles of Association) ...... 527 21.2.3.6 Double voting rights (Article 31 of the Articles of Association) .......................................................... 528 21.2.3.7 Limitations on voting rights (Article 31 of the Articles of Association) ............................................... 528 21.2.4 21.2.5 Amendment of shareholders’ rights ........................................................................................... 528 General Meetings (Section IV of the Articles of Association) ...................................................... 528 21.2.5.1 21.2.5.2 21.2.5.3 Association) 21.2.5.4 21.2.5.5 Ordinary General Meetings (Article 33 of the Articles of Association) ............................................... 528 Extraordinary General Meetings (Article 35 of the Articles of Association) ....................................... 529 Convening, holding and conduct of General Meetings (Articles 28 and 31 of the Articles of ............................................................................................................................................................ 529 Participation in meetings (Article 30 of the Articles of Association)................................................... 529 Quorum and majority ......................................................................................................................... 530 21.2.6 Provisions of the Articles of Association capable of having an impact on the occurrence of a change of control ........................................................................................................................................ 530 21.2.7 Share ownership thresholds (Article 13 of the Articles of Association) ....................................... 530 21.2.8 Identification of the holders of securities (Article 9 of the Articles of Association) ..................... 531 21.2.9 Special provisions governing alterations of the share capital (Article 7 of the Articles of Association) ................................................................................................................................................ 531 21.2.10 Financial year (Article 36 of the Articles of Association) ......................................................... 531 CHAPTER 22 MATERIAL CONTRACTS ............................................................................................................ 532 22.1 CONTRACTS WITH TAUNTON AND SOMERSET NHS FOUNDATION TRUST AND YEOVIL DISTRICT HOSPITAL NHS FOUNDATION TRUST ............................................................................................................................................ 532 22.2 CONTRACTS WITH BASILDON AND THURROCK UNIVERSITY HOSPITALS NHS FOUNDATION TRUST AND SOUTHEND UNIVERSITY HOSPITAL NHS FOUNDATION TRUST ....................................................................................................... 532 CHAPTER 23 INFORMATION DERIVED FROM THIRD PARTIES, EXPERTS’ STATEMENTS AND DECLARATIONS OF INTEREST ..................................................................................................................................................... 533 CHAPTER 24 DOCUMENTS AVAILABLE TO THE PUBLIC ................................................................................. 535 CHAPTER 25 INFORMATION ON SHAREHOLDINGS ....................................................................................... 536 13 CHAPTER 1 PERSONS RESPONSIBLE FOR THE DOCUMENT DE BASE 1.1 PERSON RESPONSIBLE FOR THE DOCUMENT DE BASE Philippe Charrier, Chief Executive Officer. 1.2 STATEMENT BY THE PERSON RESPONSIBLE FOR THE DOCUMENT DE BASE “I hereby declare, after taking every reasonable measure to ensure such is the case, that the information contained in this document de base is, to the best of my knowledge, a true reflection of the facts and does not contain any omissions liable to alter the scope thereof. I have received an assignment completion letter (lettre de fin de travaux) from the statutory auditors, in which they state that they have verified information related to the financial position and to the financial statements provided in this document de base and have read through this entire document de base. The statutory auditors have drafted reports on the financial information prepared under IFRS, the projected financial information and the pro forma financial information presented in this document de base. These reports are provided in sections 20.1.2. “Statutory auditors’ report on the accounts established in accordance with IFRS principles for the financial years ended December 31, 2012, 2013 and 2014”, 20.1.4. “Statutory auditors’ report on pro forma financial information for the financial years ended December 31, 2013 and December 31, 2014”, and 13.2. “Statutory auditors’ report on the profit forecasts” of this document. These reports do not contain any observation or objection.” Philippe Charrier, Chief Executive Officer 1.3 PERSON RESPONSIBLE FOR THE FINANCIAL DISCLOSURE Vincent Marcel Chief Financial Officer 60-62, rue d’Hauteville – 75010 Paris www.labco.eu 14 CHAPTER 2 STATUTORY AUDITORS 2.1 PRINCIPAL STATUTORY AUDITORS “Deloitte et Associés” Represented by Gérard Badin Member of the Association of Statutory Auditors of Versailles (Compagnie régionale des Commissaires aux Comptes de Versailles) 185 avenue Charles de Gaulle 92200 Neuilly-sur-Seine 572 028 041 R.C.S. Nanterre Appointment by the general meeting of June 21, 2010 for a period of six financial years expiring at the end of the general meeting which will be convened to approve the financial statements for the financial year ending on December 31, 2015. “Aplitec” Represented by Pierre Laot Member of the Association of Statutory Auditors of Paris (Compagnie régionale des Commissaires aux Comptes de Paris) 4-14 rue Ferrus 75014 Paris 702 034 802 R.C.S. Paris Appointment by the general meeting of November 15, 2013 for the remaining term of the resigning principal statutory auditor, that is until the end of the general meeting which will be convened to approve the financial statements for the financial year ending on December 31, 2015. Pierre-Henri Scacchi et Associés was a statutory auditor of the Company, following his appointment by the general meeting of June 21, 2010 and until his resignation at the general meeting which approved the financial statements of the financial year ending on December 31, 2012. Pierre-Henri Scacchi et Associés resigned following its acquisition by Deloitte & Associés. 15 2.2 ALTERNATE STATUTORY AUDITORS “Beas” Member of the Association of Statutory Auditors of Versailles (Compagnie régionale des Commissaires aux Comptes de Versailles) Represented by Mireille Roux 195 avenue Charles de Gaulle 92200 Neuilly-sur-Seine 315 172 445 R.C.S. Nanterre Appointment by the General Meeting of June 21, 2010 for a period of six financial years expiring at the end of the general meeting which will be convened to approve the financial statements for the financial year ending on December 31, 2015. Mister Bruno Dechancé Member of the Association of Statutory Auditors of Paris (Compagnie régionale des Commissaires aux Comptes de Paris) 4-14 rue Ferrus 75014 Paris Appointment by the General Meeting of November 15, 2013 for the remaining term of the resigning alternate statutory auditor, that is until the end of the general meeting which will be convened to approve the financial statements for the financial year ending on December 31, 2015. Mr. Pierre-François was an alternate statutory auditor of the Company, following his appointment by the general meeting of June 21, 2010 and until his resignation at the general meeting which approved the financial statements of the financial year ending on December 31, 2012. 16 CHAPTER 3 SELECTED FINANCIAL INFORMATION The following tables present selected financial information at the dates and for the periods stated below. The financial information shown below is taken from the Group’s consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014 prepared under IFRS as adopted by the European Union, contained in section 20.1.1 – “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” of this document de base and the pro forma information contained in section 20.1.3 – “Pro forma financial information for the financial years ended December 31, 2013 and December 31, 2014” of this document de base. The consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014 have been audited by the Company’s statutory auditors. The statutory auditors’ reports on the Company’s consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014 are presented in section 20.1.2 – “Statutory auditors’ report on the accounts established in accordance with IFRS principles for the financial years ended December 31, 2012, 2013 and 2014” of this document de base. The pro forma information for the financial years ended December 31, 2013 and 2014 has been examined by the Company’s statutory auditors. The statutory auditors’ reports on the Company’s pro forma information for the financial years ended December 31, 2013 and 2014 are presented in section 20.1.4 – “Statutory auditors’ reports on the pro forma financial information for the financial years ended December 31, 2013 and December 31, 2014” of this document de base. The summary of the selected financial information below should be read in conjunction with (i) the Group’s audited consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014 and the pro forma information for the financial years ended December 31, 2013 and 2014, (ii) the discussion of the Group’s financial position and results of operations presented in Chapter 9 “Operating and financial review of the Group” of this document de base and (iii) the section relating to the liquidity and capital position presented in Chapter 10 - “Capital resources” of this document de base. Some quantitative data (including data in thousands, millions or billions) and percentages shown in this document de base have been rounded up. Some financial data contained in this document de base are taken from the consolidated financial statements of the Group or from pro forma financial data which were prepared in Euros and shown by rounding to the nearest thousand, which can explain small differences between the totals and the sums of the amounts due to rounding. Financial data shown in this document de base was rounded to the nearest million, the totals shown can slightly differ from the sums of the amounts due to rounding. 17 SELECTED FINANCIAL INFORMATION For financial years ended December 31, Income statement data 2012 2012 Restated in line with IFRS 51 2013 2013 Pro forma2 Revenue 568.1 514.2 547.3 594.7 Revenue growth 11.7% 6.4% 12.5% Organic4 Revenue growth 0.4% 1.8% 3.4% EBITDA 110.2 103.4 106.3 128.7 113.2 131.3 19.4% 20.1% 19.4% 21.6% 18.4% 20.2% 90.0 85.8 87.3 106.8 89.1 107.5 (28.1) (28.1) 12.8 14.1 (14.6) (7.1) (in millions of euros) 5 EBITDA margin Recurring operating profit Net profit 1 2 3 4 5 2014 2014 Pro forma3 615.6 649.6 The Group having sold its business activities in Germany to Sonic Healthcare on December 2, 2013, the cashgenerating unit sold was restated under discontinued operations in accordance with IFRS 5 (see Section 9.3.1 – “Overview” of this document de base). The Group’s pro forma Revenue and pro forma EBITDA for the financial years ended December 31, 2013 and 2014 were calculated using the Group’s Revenue and EBITDA in the same periods adjusted to reflect the acquisition in Italy of the SDN group on July 30, 2014 and included in the Southern Europe segment as if the SDN group had been part of the Group’s scope of consolidation on January 1, 2013. Financial data regarding the SDN group used to calculate pro forma Revenue and pro forma EBITDA are presented in Section 20.1.3 – “Pro forma financial information for the financial years ended December 31, 2013 and December 31, 2014” of this document de base. Idem As this term is defined in Chapter 9 “Operating and financial review of the Group” of this document de base. The EBITDA margin stated as a percentage represents EBITDA divided by Revenue. For financial years ended December 31, 2012 2013 BALANCE SHEET DATA (in millions of euros) Total assets Goodwill Cash Equity Borrowings and other financial liabilities Net debt 888.6 620.6 56.6 166.4 580.6 524.0 18 968.0 581.5 167.8 179.2 649.7 481.9 2014 1,046.8 702.4 74.1 143.0 724.5 650.4 CONSOLIDATED CASH FLOW STATEMENT DATA (in millions of euros) For financial years ended December 31, 2012 2013 Restated for the Restated for the German German business business activities activities Cash flow from (used in) operating activities Cash flow from (used in) investing activities Cash flow from (used in) financing activities Net increase/(decrease) in cash and cash equivalents1 1 2014 83.7 84.9 85.7 (58.6) 34.2 (163.1) (40.1) (7.8) (16.0) (11.5) 111.3 (93.5) The net increase/(decrease) in cash and cash equivalents restated for the German activities (financial years ended December 31, 2012 and December 31, 2013), which were sold in December 2013, is not the same as the sum of the cash flows presented above because the net increase/(decrease) in cash and cash equivalents includes the net increase/(decrease) in cash of the German activities, while the cash presented has been restated for the cash of the German activities. OTHER FINANCIAL DATA BY COUNTRY For financial years ended December 31, 2012 2013 2013 2014 Restated in line with IFRS 5 Pro forma1 514.2 547.3 594.7 615.6 (in millions of euros) 2012 Revenue 568.1 Northern Europe Southern Europe 382.8 185.3 328.9 185.3 357.9 189.4 357.9 236.8 401.3 214.3 401.3 248.2 EBITDA 110.2 103.4 106.3 128.7 113.2 131.3 Northern Europe Southern Europe 78.3 32.0 71.7 31.7 75.5 30.8 75.5 53.2 79.2 34.0 79.2 52.1 EBITDA margin3 19.4% 20.1% 19.4% 21.6% 18.4% 20.2% Northern Europe Southern Europe 20.5% 17.2% 21.7% 17.2% 21.1% 16.3% 21.0% 22.5% 19.7% 15.9% 19.7% 21.0% 1 2 3 2014 Pro forma2 649.6 The Group’s pro forma revenue and pro forma EBITDA for the financial years ended December 31, 2013 and 2014 were calculated using the Group’s Revenue and EBITDA in the same periods adjusted to reflect the acquisition in Italy of the SDN group on July 30, 2014 and included in the Southern Europe segment as if the SDN group had been part of the Group’s scope of consolidation on January 1, 2013. Financial data regarding the SDN group used to calculate pro forma Revenue and pro forma EBITDA are presented in Section 20.1.3 – “Pro forma financial information for the financial years ended December 31, 2013 and December 31, 2014” of this document de base. Idem The EBITDA margin stated as a percentage represents EBITDA divided by Revenue. 19 CHAPTER 4 RISK FACTORS Before buying shares in the Company, investors should carefully consider each of the risks described below, as well as other information contained in this document de base. The Company carefully reviewed the risks that could have a material adverse impact on the Company or its subsidiaries, their activities, their financial conditions, their cash conditions, their results or their outlook, and considers that, to the best of its knowledge, there does not exist any other material risks than those described below. The Company draws investors’ attention to the fact that the risks and uncertainties presented below are however not the only ones the Group faces. Other risks and uncertainties that are not currently known to the Company or that the Company deems immaterial as of the date of this document de base, could also have a material adverse impact on the Group’s activities, financial condition, cash condition, results or outlook. To further analyze efforts undertaken by the Group to manage its risks, see section 4.6.1 “Risk management” in this document de base. 4.1 RISKS RELATED TO THE BUSINESS SECTORS AND MARKETS IN WHICH THE GROUP OPERATES The Group operates in a highly regulated sector. Compliance with regulations applicable to the Group’s activities may increase its costs or restrict its activities. Failure to comply with such regulations may lead to penalties of various types. Future alterations to regulations applicable to the Group may have a material adverse impact on its activities. The medical diagnostics industry (including clinical laboratory testing) is subject to extensive regulations and controls by various regulatory authorities in each of the countries in which the Group operates. Those regulations and controls have a major influence on the way the Group carries out its activities. For clinical laboratories, those regulations mainly pertain to operating requirements, professional qualifications of laboratory personnel, ownership and corporate governance constraints on companies that operate laboratories (which are especially strict in France), and the pricing and reimbursement levels of clinical tests. The Group’s activities are also subject to numerous other laws and regulations, particularly as regards the handling and storing of certain chemicals and reagents, the disposal of biological waste (waste from care activities that carry a risk of infection), the handling and storage of personal data (including patients’ medical records) and the prevention of fraud to social security systems. The Group’s compliance with such regulations is monitored by the relevant administrative authorities in the countries and regions in which the Group operates, as well as by the competent professional associations such as the French Ordre des médecins and the French Ordre des pharmaciens, which are self-regulatory bodies holding disciplinary powers over the Group’s SELs and the Group’s laboratory doctors in France, and which also maintain the registries of companies which operate clinical laboratories and laboratory doctors allowed to exercise in France. Compliance with current or future laws and regulations may cause an increase in the Group’s administrative, legal and operational expenditure, force it to alter its commercial practices, its legal organization, the ownership structure and corporate governance of its subsidiaries or, more generally, reduce or limit its revenue. Those laws and regulations have a broad scope of application and their interpretation by the competent administrative authorities or professional associations is subject to change. Potential efforts to bring the Group in compliance with existing laws and regulations, with new interpretations of such laws and regulations, or with new laws and regulations may generate substantial new costs for the Group. Failure to comply with such regulations may also lead to sanctions of various kinds for the Group and potentially for laboratory doctors working within the Group. Sanctions may be administrative (fines, periodic penalty payments, temporary or permanent closures of laboratories), disciplinary (temporary or permanent removal of the right to practice), civil (damages), or criminal (ban on operating a clinical laboratory or imprisonment). For example, France has introduced minimum accreditation standards with which laboratories have to comply between 2016 and 2020. The implementation of these standards is proving costly and time-consuming for the Group. The laboratory accreditation process is likely to require the preparation of a written application, the undertaking of site studies, assessment of the extent of changes required to comply with the new standards, the appointment of external qualified experts, the participation of the Group’s staff in this process in addition to their usual workload, the payment of certain administrative fees and the implementation of new quality-control software. Accreditation may be delayed due to many factors, including the number of sites operated by a clinical laboratory and the responsiveness of the accreditation body, COFRAC (see section 6.5.1 – “Regulation – 20 France” of this document de base). The non-compliance by the Group’s laboratories with the accreditation standards may force the non-complying laboratories to abandon the activities for which they were not accredited. In addition, as discussed in section 6.5.1. “Regulation – France” of this document de base, the French regulation imposes stringent restrictions on the legal structure and ownership of SELs, particularly those operating clinical laboratories. In particular, the competent administrative authorities (the ARS) and the competent professional associations (Ordres Professionnels) may challenge the legal structure and more specifically the corporate governance arrangements of the SELs that the Group has selected to carry out its activities in France. Such challenge, if successful, could have a material adverse effect on the Group’s financial condition and operating results. The French Ordre des médecins and the French Ordre des pharmaciens have already claimed that the Group’s organization and legal structure contravened the fundamental principle of independence applying to laboratory doctors. The French Ordre des pharmaciens has punished certain SELs, which are subsidiaries of the Company, along with certain laboratory doctors working within these subsidiaries, for alleged violations of the applicable regulation. Some of those proceedings are still ongoing, although the French Ordre des pharmaciens has in general refrained from carrying out investigations in relation to them, in order to comply with the decision of the European Commission, recently confirmed by the European General Court, which found against it (see below and section 20.3 – “Contentious proceedings” of this document de base). In 2007, the Group filed a complaint against the French Ordre des pharmaciens before the European Commission on the grounds that the French Ordre des pharmaciens had inappropriately used the powers granted to it by impeding the development of free competition and the creation of groups of laboratories on the French clinical laboratory services market. After an investigation, in 2010, the European Commission ordered the French Ordre des pharmaciens to pay a €5 million fine for restrictions on competition. The Ordre des pharmaciens appealed to the European General Court, which, on December 10, 2014, confirmed that the French Ordre des pharmaciens had restrained competition on the clinical laboratory services market. Even though the Court confirmed the decision of the Commission, it reduced the fine imposed on the French Ordre des pharmaciens from €5 million to €4.75 million. Through a press release dated February 23, 2015, the Ordre des pharmaciens confirmed that it would not appeal the decision of the European General Court before the Court of Justice of the European Union. The French Ordre des pharmaciens has generally refrained from carrying out investigations in relation to new or existing disciplinary proceedings against the Group’s laboratory doctors or SELs, in order to comply with the European Commission’s decision. On March 5, 2015, the Conseil Central de la Section G, which is the body of the Ordre des pharmaciens competent for clinical testing, closed, during an administrative session, several disciplinary cases without taking any action. These case closings without any action taken result from a withdrawal of the complaints filed by its president against the Group’s laboratory doctors and SELs. Even though these withdrawals are not motivated, they are probably the result of the decision of the Ordre des Pharmaciens to not appeal its conviction by the European General Court. However, the Group cannot guarantee that the Ordre des pharmaciens will close its existing disciplinary proceedings without taking any action, will continue to refrain from carrying out investigations or engage new proceedings against the Group’s laboratory doctors or SELs, in order to comply with the decisions of the Commission and the Court. As a result, the Group could be subject to new disciplinary measures or other measures taken by the Ordre des pharmaciens, and the Ordre des pharmaciens may also, at the same time, resume proceedings that are dormant at the date of this document de base. The French Ordre des pharmaciens may impose disciplinary sanctions on laboratory doctors or SELs that are registered with the Ordre des pharmaciens, and the Ordre des médecins may do likewise to laboratory doctors or SELs that are registered with the French Ordre des médecins, including warnings, temporary suspensions or removal from the register, which may lead to withdrawals of the prefectoral agreements of the relevant SELs as well as withdrawals of the administrative authorizations of the laboratories they operate, which would disrupt the Group’s activities. In addition, if the competent administrative authorities (ARS) take the view that the Group’s organizational and legal structure breaches applicable statutory or regulatory requirements, they could suspend or withdraw the prefectoral agreements or administrative authorizations granted to the Group’s SELs and laboratories in France. In this regard, article L. 6223-5 of the French Public Health Code forbids various categories of persons, described in Section 6.5.1 “Regulation – France” of this document de base, from making any direct or indirect investment in the share capital of a company operating a French clinical laboratory, on the basis of their 21 activities or relations with certain activities in the medical or paramedical sector (hereinafter the “prohibited investors”). A breach of this prohibition, which cannot be remedied, would expose the Group’s French laboratories to periodic financial penalties and fines of up to €2 million per SEL, i.e. up to €82 million for all of the Group’s SELs at the date of this document de base. Such a situation, if it were to happen, could constitute a Material Adverse Effect within the meaning of the Amended RCF and could consequently lead, if the Lenders under the Amended RCF decided it, to the early repayment of the sums owed under the Amended RCF, as well as to its early termination. The French Public Health Code would also impose a penalty, if the Group’s interpretation of the aforementioned legal provisions were not borne out, on any investor, natural or legal person, acquiring any stake in the Company’s capital if that investor is a prohibited investor. The offending party (i.e. the prohibited investor holding a stake in the Company) would face a fine of up to €2 million for a legal entity and €500,000 for a natural person per Group SEL, i.e. up to a maximum cumulated amount of €82 million for a legal entity and of €20.5 million for a natural person given the number of the Group’s SELs at the date of this document de base. However, the Company and its legal advisors, whose interpretation has been confirmed by various academic professors, believe that “indirect ownership” as mentioned by article L. 6223-5 of the French Public Health Code, must be interpreted in the light of the rules set out by the French Commercial Code and that, accordingly, the penalties provided for by the relevant texts can only apply to the Group’s SELs and the concerned prohibited investor if a prohibited investor takes control of the Company. The Group’s representatives have consulted the French Ministry of Social Affairs, Health and Women’s Rights (which oversees the ARS), which did not put forward, at the date of this document de base, any different interpretation of the related legal provisions. Although the Company and its advisors consider that the risk of a different administrative interpretation is minor, and in order to avoid any risk of change of control of the Company, the voting rights that can be exercised by the same shareholder is limited under the Company’s articles of association (statuts) (see Section 21.2.3.7. “Limitation on voting rights (Article 31 of the articles of association)” of this document de base), it should be emphasized that failure to comply with the aforementioned legal provisions may lead to major financial sanctions if the Company’s interpretation of “indirect ownership” was not shared by the administrative authorities in charge of ensuring the application of the text. Such a challenge of the Company’s interpretation, although unlikely, as well as a change in these legal provisions, could thus have a material adverse impact on the Group’s activities in France, its operating income, its financial condition and its outlook, as well as on prohibited investors, which may also be subject to personal sanctions. Finally, failure to comply with these provisions may give rise to disciplinary sanctions or adverse administrative decisions by professional Associations (against the Group’s SELs and the laboratory doctors working within them), or to the suspension or revocation of prefectoral agreements or administrative authorizations held by the Group’s SELs and laboratories in France. Article R. 4113-13 of the French Public Health Code forbids various categories of persons from making any direct or indirect investment in the share capital of SELs of French doctors, on the basis of their activities or relations with certain activities in the medical or paramedical sector (see section 6.5.1. “Regulation – France” of this document de base). This provision will be applicable to SELs of anatomy and pathology doctors that the Group is considering acquiring. The Company and its legal advisors believe that “indirect ownership” within the meaning of article R. 4113-13 of the French Public Health Code must be interpreted in the same way as for article L. 6223-5 of the same Code. If the Company’s interpretation were challenged, this could give rise to disciplinary sanctions and adverse administrative decisions by the French Ordre des médecins (against the anatomical and pathology SELs that could be acquired by the Group and the doctors who work within them). The Group’s activities in France represented respectively 59% and 56% of the Group’s Revenue for the financial years ended December 31, 2013 and December 31, 2014. The French market is an important market for the Group’s growth strategy. As a result, any of the aforementioned events may cause material disruption to the Group’s activities and may have a material adverse effect on its financial condition and operating results. In general, if the Group fails to comply with applicable regulations, if they change or are interpreted in a manner adverse to the Group or if the Group cannot maintain, renew or secure required permits, licenses, accreditations, agreements or other necessary administrative authorizations, the Group may be unable to pursue its activities or market its services in the relevant jurisdictions, be excluded from participating in public healthcare programs, no 22 longer be able to enter into contracts with third-party payers, suffer penalties or civil and criminal fines, or be subject to complaints by third parties, with the financial consequences that may result. Changes affecting certain regulations or government programs that are not directly connected with the medical diagnostics sector, and in particular regulations relating to prescription control, co-payment, doctors, health insurers and hospitals, may also affect the Group and impair its operating results and its ability to expand its activities. For example, the 2010 amendment of the Portuguese regulation on doctors’ pension plans caused a significant number of practicing doctors to retire before the amendment came into effect, reducing the number of practicing doctors and therefore the amount of medical prescriptions the Group received. In all of the aforementioned cases, the Group’s reputation could be damaged, important relationships with government regulators or third parties could be adversely affected, resulting in a material adverse effect on its activities, development, operating results, financial condition and outlook. Current or future regulatory changes in France, and disputes initiated by the competent administrative authorities or Professional Associations, may affect the Group’s ability to develop its network of French laboratories through acquisitions, make it more dependent on laboratory doctors to check operations carried out by SELs, and call into question the Group’s organizational and legal structure. As explained in section 6.5.1. “Regulation – France” of this document de base, the Group is subject, in France, to regulatory constraints that particularly restrict the ownership of the share capital and voting rights of SELs by persons other than the laboratory doctors operating within such SELs. In order to comply with this regulation, the Group has established a legal structure under which it directly and indirectly holds shares representing around 99.9% of the share capital of its SELs, while certain laboratory doctors operating in such SELs hold the remainder of the shares. This structure can, as a general matter, no longer be used for SELs of laboratory doctors acquired since May 31, 2013, which is when the May 30, 2013 Law was enacted. This law requires that more than 50% of the share capital (in addition to the 50% of the voting rights) of a SEL of laboratory doctors be held by laboratory doctors practicing within that SEL. The law also provides an exemption for existing SELs of laboratory doctors that operated under a different share capital ownership structure on the date the law was promulgated, enabling them to continue operating under their existing structure and have the majority of their share capital held by companies operating laboratories. Laboratory doctors practicing in the SELs benefiting from this exemption have preemption rights under this law in the event of a transfer of the SELs’ shares. SELs of laboratory doctors in which the Group held a majority of the share capital when the law was promulgated benefit from a grandfathering exception. For SELs of laboratory doctors that joined the Group after that date, the law has considerably limited the Group’s ability to use the same previous structure. The new ownership and corporate governance structure, described in section 6.5.1. “Regulation – France” of this document de base, which allows the Group to own most of the SELs of laboratory doctors acquired since the law was promulgated, or that the Group may acquire in the future under arrangements that comply with the new regulations, may require the adoption of more complex legal structures than those used before the May 30, 2013 Law came into force (see the description of the “Operating Rules” in section 6.5.1. “Regulation – France” of this document de base). The May 30, 2013 Law may also limit the Group’s ability to sell or transfer shares in SELs of laboratory doctors that it holds at the date of this document de base or that it may acquire in the future, and render more complex any restructuring it might consider for its subsidiaries. The competent administrative authorities could interpret this new regime as preventing some forms of restructuring, such as those involving a universal transfer of all assets where the acquired SEL is not covered by the grandfathering exception. Such interpretation, which seems to be the one adopted by the French Ministry of Social Affairs, Health and Women’s Rights, may make some forms of restructuring of SELs of laboratory doctors within the Group more complex or prevent them outright. Even though the competent administrative authorities (ARSs) are not bound by the Ministry’s interpretation and may have already adopted different positions authorizing restructuring operations involving SELs that are not covered by the grandfathering exception, it is nonetheless probable that they will adopt such an interpretation in the future. Furthermore, the possible adoption in the future of any new law or regulation aiming to reduce again the proportion of SELs’ share capital, or the number of SELs, that may be held directly or indirectly by the same physical or legal person (working as a professional or as a third party to the profession) would require the Group to make further changes to the structure of its French activities, in order to comply with the new legal or 23 regulatory provisions. Even though some restructurings are already enacted in principle by the Operating rules in force in some of the SELs of the Group (see section 6.5.1. “Regulation – France” of this document de base), the Group can give no guarantee that the existing agreements cover all the potential cases of changes of the legislation or the regulation and will permit to the Group to comply with the new legal or regulatory provisions. In general, any adjustment to French regulations applicable to the Group’s SELs in the future, any adverse interpretation of existing regulations, and any introduction of new rules may affect or further limit the Group’s ability to own or control its subsidiaries in France. Although at the date of this document the Group was not required to carry out any restructuring (because of the grandfathering exception set out by the May 30, 2013 Law) and although the May 30, 2013 Law is unlikely to prevent the Group from continuing its development and fully consolidating acquired SELs, if the Group were to alter aspects of its structure in response to regulatory changes or a challenge to its current organizational and legal structure, it may no longer be able to fully consolidate its French activities in its financial statements, while its ability to centrally manage cash generated by French SELs or distribute dividends may be affected. Any new structure the Group may have to implement to comply with challenges or new requirements outlined above may result in the Group owning a smaller stake in its existing SELs and make it a minority shareholder, and would make the Group more dependent on the contractual, corporate governance and other mechanisms the Group uses, at the date of this document, to control its French SELs. Finally, the Group may also have to reduce its control over certain aspects relating to the activities of its French SELs or to the integration of the French SELs or the businesses they operate within its network. In addition, the competent administrative authorities (ARS) or the Professional Associations (which have administrative and disciplinary powers over laboratory doctors, doctors and SELs) may challenge the current organizational and legal structure of the Group (and especially the corporate governance framework established in its French SELs, laboratories and medical practices) described in section 6.5.1 – “Regulation – France” and force the Group to adopt modifications to such structure. Taking into consideration the French legal and regulatory framework, the Group has established a corporate governance, contractual and organizational structure that enables it to exercise control over its SELs. However, the efficiency of such structure is limited by French regulatory and ethical constraints regarding the independence of laboratory doctors, medical doctors and pharmacists who work in such structure, and such structure does not confer on the Group powers as absolute as the Group would have if it held all or a majority of the voting rights. The Group’s model aggregates laboratory doctors, medical doctors and pharmacists who join its network but they retain some autonomy over the day-to-day management of the laboratories and practices for which they are responsible. This decentralized and independent management and responsibility model is required by the regulatory framework applicable in some of the countries in which the Group operates, including France. Although that model includes various corporate governance mechanisms and other contractual and organizational arrangements entered into with laboratory doctors practicing in the SELs, relating mainly to the exercise of their voting rights and to the management of the SELs (for a description of these mechanisms, see section 6.5.1. “Regulation – France” of this document de base), as well as various incentives based on the performances of the SELs and the Group as a whole, in order to align their interests with those of the Group, the Group does not have total control over day-to-day management of the SELs. As a result, with respect to certain matters, the Group should involve, with regards to its decisions, the laboratory doctors who hold the majority of the voting rights in its SELs. It cannot be completely ruled out that these laboratory doctors may not share the Group’s views on the way the SELs should be managed, may not respect the various corporate governance mechanisms and other contractual and organizational arrangements they had nevertheless knowingly accepted, and may exercise their voting rights in a manner adverse to the Group’s interests. Even though the Group is convinced of the reality of its control of the concerned SELs, it can provide no absolute assurance that the existence of the various corporate governance mechanisms and other contractual and organizational arrangements, as well as of the incentivizing measures that have been enacted, will ensure that these laboratory doctors will manage their SELs in an economically satisfactory manner or in a manner consistent with the Group’s interests. The Group can neither give no absolute assurance that these laboratory doctors will comply with all the requirements of the SELs, in particular regarding internal control, reporting and accounting requirements. Even though the Group considers it to be a minor possibility, it cannot be ruled out that a problems in these areas, or the period required to implement the various mechanisms and other arrangements, could disrupt the operation of the Group’s French SELs and divert the Group’s managers’ time 24 and attention from their other activities. In addition, in the case of a failure or a non-application of the above palliative mechanisms, the Group could be unable to consolidate by full integration of its French activities in its financial statements, whereas his capacity to ensure a centralized management of the cash generated by its French SELs or the distribution of dividends could be affected. Regulation in force in certain markets in which the Group operates is undergoing reform and the Group may not be able to respond to such reforms effectively. Some markets in which the Group operates are reviewing their regulatory framework at the date of this document. These potential reforms may lead to increased competition and consolidation on such markets. In addition, the Group is subject to specific regulation in Belgium relating to the operation of clinical laboratories. In particular, Belgium’s Royal Decree of April 26, 2007 lists the legal forms that companies that operate clinical laboratories should take in order to be eligible for reimbursement of their services by the Belgian public health insurance system. The duration of this Royal Decree, which was initially due to expire on December 31, 2009, was extended retroactively until December 31, 2012 by a Decree of January 27, 2010. At the date of this document, no new extension had been enacted but the Group has received informal information that the Belgian government is preparing, at the date of this document, a second retroactive extension or another solution that would remedy this legal vacuum with retroactive effect as of January 1, 2013. The relevant Belgian authorities have given no indication that reimbursements would cease. However, the absence of any extension of the duration of the Royal Decree of April 26, 2007 would have a significant impact on all Belgian laboratories run by the Group in this legal form. Although the Group could develop a strategy to anticipate such regulatory changes, the Group’s industry and operating environment may not respond to those changes in the way the Group expects, and the Group may, as a result, be unable to maintain its markets’ position or apply its strategy on such markets. In addition, the Group’s competitors with greater financial resources or stronger market positions than the Group may be able to better respond to such regulatory reforms. If the Group cannot respond effectively to regulatory and market changes, the Group’s outlook and operating results could be adversely affected. The prices the Group may charge in certain markets are set by government-enforced rates that are often decreasing. In many countries, the Group’s activities are subject to regulated rates (particularly for clinical services), since its services are provided under public health programs funded partly or entirely by governments. As a result, some or all of the clinical testing services provided by the Group are subject to prices or required ratedetermination methods that are generally set by governmental authorities, and the Group has only limited influence on them. Rates may be revised at any time and recent revisions have involved reductions in rates. The recent financial crisis caused significant adverse changes to the prices of sovereign debt and increased financing costs for certain European countries such as Spain and Portugal. In order to address market concerns regarding their budgetary imbalances, those countries’ governments have adopted particularly harsh austerity measures that have included reductions in healthcare spending. Other European countries, including France, Italy and the United Kingdom, have also announced austerity measures aiming at curbing public healthcare expenditure. The Group must deal with such measures aiming at reducing healthcare spending in general, which affects services provided by the Group in particular. Governments typically control healthcare expenditure by reducing rates or reimbursement levels, seeking to reduce the number of tests prescribed by doctors, and limiting the testing services covered by their health, welfare or social security programs. In particular, the French government encourages healthcare professionals to limit the number of tests prescribed. Governments’ reimbursement schemes often limit the range of tests that are covered, and certain pre-existing or innovative tests that provided higher margins to the Group may be excluded from such coverage. In countries such as Spain, where prices are not currently regulated, government price containment measures may impact the Group’s activities, since the public healthcare system covers most of the population. In Portugal, the “Memorandum of Understanding on Specific Economic Policy Conditionality” between the European Commission, the International Monetary Fund and the Central Bank in 2011 was enacted in response to the economic bailout, and resulted in financial aid of €78 billion on the basis of a three-year policy program until mid-2014. The Group estimates that this program, which included the aim of reducing national spending on healthcare by 10% in both 2011 and 2012, has resulted in price reductions for certain tests in Portugal since 25 October 2011. According to the latest data available from the Portuguese national statistics institute (Instituto Nacional de Estatistica), public healthcare spending fell 1.1% in 2013 and 9.9% in 2012. In addition, two public healthcare insurance funds harmonized their rates in Portugal in 2012, which the Group believes has resulted in price reductions for certain tests in Portugal since August 2012. In Italy, a new national pricing program (nomenclatore tariffario) was decided in January 2013 and provides regions with non-mandatory recommendations regarding the rates they adopt. The Liguria region adopted these new rates in October 2013 and the Campania region in March 2013. The adoption of these measures led to a reduction in the Group’s Revenue relative to 2012. At the date of this document, Lombardy is launching a consultation about reforms to its healthcare system, and this could prompt it to adopt those rates as well in future, with the risk of a material reduction in Revenue for laboratories operated by the Group in the region. The Italian government has also set up a specialist committee to review national rates, which are due to be updated in 2015. This could lead to additional rate cuts, depending on whether the various regions adopt the rates. Efforts to reduce public healthcare expenditure are also being made in Belgium. In France, an industry agreement was signed on October 10, 2013 by the main French professional biologists unions and UNCAM following the government’s announcement of its plan to cut healthcare spending on French clinical laboratories by at least €110 million in 2012. The agreement aims to map out the business prospects of clinical laboratories for a three-year period while continuing to control healthcare spending. It aims to limit annual growth in clinical spending to 0.25% between 2014 and 2016, through moderate, gradual rate reductions and control over prescriptions, in order to offset natural volume growth. Professional unions meet with Healthcare Insurance Funds every six months to measure the impact of enacted rate changes and to determine the upcoming changes that may need to be made to achieve the annual growth target. In late January 2015, CNAM held discussions with professional unions to reiterate its commitment to the three-year agreement and to propose a rate revision commensurate with volume growth projected for 2014 and expected for 2015. The Company considers that the proposed rate revision will have a gross adverse effect on Revenue of around 1.50%, while the 2014 revision had a gross adverse effect of 2.51% and a net adverse effect of around 1.20% (reductions are estimates based on annual test volumes). According to CNAM’s projections, volume growth in 2015 will make up for the decline and result in the 0.25% target for annual clinical expenditure growth set by the agreement being attained. Definitive 2014 figures will be available in late June 2015, as will information about the trend in early 2015. On that basis, a decision will be made regarding a revision to the nomenclature in September 2015, in order to get as close as possible to the 0.25% target regarding growth in annual clinical expenditure. Decreases in regulated rates reduce the Group’s margins and may affect its Revenue from clinical testing services, its operating results or even the feasibility of providing certain testing services by some or all of its laboratories. Third-party payers and private health insurance companies have taken steps to control the use and reimbursement of healthcare services, including clinical laboratory testing services, which may adversely affect the Group’s activities. The Group must face efforts by non-governmental third-party payers – mainly private health insurers – to reduce utilization and reimbursement of medical and diagnostic work, such as clinical laboratory testing services. In certain markets (such as Spain), the Group receives payment for its services from private health insurers that have gained significant bargaining power allowing them only to reimburse healthcare services if such services are provided by pre-selected providers. Private health insurers negotiate fee structures with healthcare providers, including clinical laboratories, and some of them have already insisted on discounted fee structures as a condition for pre-selecting the Group in the past and may insist on further discounted fee structures in the future. If the Group is not pre-selected by private health insurers, or is required to accept unfavorable terms to secure such pre-selection, its operating results may be adversely affected. For example, four private health insurers in Spain accounted for a significant portion of the Group’s Revenue in Spain in 2013. A major Spanish private health insurer, for which the Group serves as a pre-selected provider, implemented significant price decreases in the first half of 2012. Another major Spanish private health insurer also implemented significant price reductions effective January 2013. A third major Spanish private health insurer introduced a per capita model for pricing in Madrid in 2008 and expanded the model to other regions in 2009, which caused a very significant 26 decrease in prices. Since the model is based on individual payments, the insurer pays a set annual fee per patient in return for which the laboratory network provides all testing services specific to that patient during the relevant year up to a pre-set limit. Costs associated with testing services required by such patients above the specified limit are borne by the laboratory and so some of the risks associated with changes in the volume of clinical testing services utilized by patients are transferred from the private health insurer to the Group, even if the Group sets up mechanisms (based on algorithms) to control the volume of prescriptions and reduce that risk. Pressure from private health insurers may also affect the Group indirectly. Private health insurers have exerted pricing pressure on private hospitals which, in turn, have exerted pricing pressure on the Group. For example, two large Spanish private hospitals that are customers of the Group negotiated significant reductions in the average price per test from the first quarter of 2013, primarily due to their merger. This resulted in a total revenue reduction of around €2.5 million. As a result, pricing pressure on private hospitals and the Group’s other customers are reducing those customers’ margins and may continue to cause customers to default on their obligations to the Group. In markets where private insurance supplements the public healthcare system (such as France), private insurers may seek to control their costs by reducing levels of reimbursement under their insurance plans, requiring the patient to pay all or a larger proportion of the shortfall. Such efforts by third-party payers to reduce the utilization of clinical laboratory testing services, or their exposure to risks associated with such utilization, and reimbursement levels on the services the Group provides, may have a material adverse effect on its operating results. Vitamin D tests, demand for which has grown very strongly since 2007, have seen repeated rate reductions in France: rates were cut by 25% in April 2013 and by 14% in April 2014. Continued weakness in economic conditions may have an adverse effect on the Group’s activities. The economic downturn and volatility in connection with the recent financial crisis has increased the risks associated with conducting the Group’s activities in certain countries where it has significant operations, especially in Spain and Portugal. Such risks include the risk of default by customers on their payment obligations to the Group. In Spain, significant payment delays by public payers are common. Economic difficulties have also resulted in reduced levels of activities and higher unemployment and have led governments, private insurers and other third parties to reduce their healthcare spending, which may affect the Group’s Revenue or margins. The Group’s customers include large companies to which it provides clinical laboratory testing services for their employees. Under labor laws in Spain and Portugal, employees are entitled to a regular check-up paid for by their employer. However, current economic conditions in those countries are leading to bankruptcies, headcount reductions, hiring freezes and financial difficulties for certain of these corporate customers, prompting them to reduce testing services volumes. In addition to volume reductions or payment defaults, this economic climate has resulted in downward pressure on prices and therefore on margins. Where patients, directly or indirectly (such as through private health insurance premiums) are responsible for all or part of the cost of medical tests, individual decisions to reduce healthcare expenditures may result in a reduction of demand for the Group’s services. More generally, a decrease in household disposable incomes, or merely the perception thereof, in times of economic downturn can lead to a reduction in individuals’ healthcare expenditure, including private insurance coverage and the level of such coverage, regardless of the level of reimbursement by public social security systems. 4.2 RISKS RELATED TO THE GROUP’S TECHNOLOGY AND INTELLECTUAL PROPERTY RIGHTS Failure to be supplied with new tests, technologies and services may negatively impact the Group’s testing volume and Revenue. The clinical laboratory industry faces challenges from regularly changing technology and new product introductions. The Group does not develop its own tests or technologies, but relies on equipment suppliers and test developers for the introduction of new tests. Other players, including the Group’s competitors, may obtain 27 patents, licenses or other rights that may prevent, limit or interfere with the Group’s ability to provide particular tests or that may increase its costs. In addition, the increasing development of “point of care”-type tests has affected the activity level of Group laboratories regarding the analysis covered by these standard tests. Some providers are developing “point of care” tests, which are performed outside the laboratory, by the patient’s bedside or even at the patient’s home. Those tests are used for urgent diagnostic work or routine monitoring. However, they must comply with professional laboratory quality standards. Integrated genetic diagnostic testing services, offered by certain professional websites and hardware suppliers like Affymetrix, may also reduce activity levels for Group laboratories. In addition, in markets where laboratory testing prices are unregulated (such as Spain), some of the Group’s competitors could introduce new, less expensive tests that may cause a decrease in the demand for its tests. The Group’s success in continuing to offer new tests, technology and services depends on its ability to contract with equipment suppliers and test developers on favorable terms. If the Group is unable to license new tests, technology and services to expand its specialty testing activity, its testing methods may become outdated and its testing volumes and Revenue may be adversely affected. Failures of the Group’s information technology systems, including failures resulting from systems conversions, may disrupt its operations and cause the loss of customers or business opportunities. Information technology systems are used extensively in all aspects of the Group’s business, including clinical testing, test reporting, billing, customer service, logistics and the management of personal data (particularly patients’ medical records). The Group’s activities depend on the continued and uninterrupted performance of its information technology systems. Information technology systems are vulnerable because of exposure to damage from a variety of sources, including telecommunications or other network failures, individual acts and natural disasters. Moreover, despite the security measures the Group has implemented, its information technology systems may be subject to physical or electronic attacks, computer viruses and similar disruptive problems that may affect its ability to function. Information technology problems may impact the Group’s ability to carry out tests, deliver test results or bill for tests in due time. For example, the laboratory that the Group operated in Duisburg suffered a breakdown of its telecoms system for several weeks in 2009, which led to a loss of clients and an adverse impact on the laboratory’s Revenue for that year. If the Group were to experience major or recurring information technology systems problems, including with the implementation of IT management systems for tests and billing, its activities would be disrupted. If its activities were so disrupted, that may adversely affect the Group’s reputation and result in a loss of customers and patients and reduce its Revenue. In addition, IT systems are also vital from the financial and accounting point of view. Given the number of tests that the Group manages (several million patients per year), an IT systems failure at one or more of its subsidiaries could affect the reliability of their financial statements. For example, the Group recently identified an error in the programming of the IT system used for most of its French SELs, which generated a Revenue figure in one SEL’s financial statements that was higher than the actual figure, and a Revenue figure in another SEL’s financial statements that was lower than the actual figure, whereas the corresponding invoices had been correctly prepared and the incoming payments had been correctly made. The Group has standardized some of its systems and is rolling out standard laboratory information and invoicing systems in all its operations, including those of recently acquired companies. However, the Group sometimes continues to use non-standard IT systems for billing and laboratory operations, as well as central information systems for certain recently acquired companies. The Group expects that the implementation of standardized practices and systems across its network will take several years to complete, and until such completion, there is a risk that its activities may be disrupted due to any incompatibility among some of the information technology systems it uses. This could have an adverse effect on the Group’s activities and operating results. The Group’s trademarks may not be protected uniformly across all countries in which the Group operates or in countries in which the Group may establish operations. Ten trademarks, including the trademark Labco (semi-figurative), the trademark Labco NOÛS Advanced Special Diagnostics (semi-figurative) and the trademark SDN (semi-figurative) have been filed with the Office for Harmonization in the Internal Market of the European Community, and are thus protected in the 28 countries of the European Union, including France (See section 4.2 “Risks related to the Group’s Technology and Intellectual Property rights” of this document de base). The trademark Labco (semi-figurative) was also registered in Switzerland, and other trademarks were also filed in Spain, Portugal and Italy. 28 However, the Group cannot be certain that steps taken in France and abroad to protect its trademarks will be successful or effective, or that third parties will not infringe or make unlawful use of its trademarks. Such unauthorized use of the Group’s trademarks may damage the Group’s competitive advantage and have a material adverse impact on its activities and operating results. Given its acquisition strategy (see section 6.4.4 “External growth strategy” of this document de base) and the importance that the Group places on developing a strong identity and strong brands (see section 6.3 “Group strategy” of this document de base), the Group is exposed to the risk of being unable to use its trademarks in jurisdictions in which they are not protected, for example where a competitor has made a previous application or where the local authorities have refused to protect the trademark. That situation could have an adverse impact on the Group’s activities or operating results. 4.3 RISKS RELATED TO THE GROUP AND ITS COMMERCIAL ACTIVITIES The Group’s debt position may affect its ability to finance its activities and support its growth, and have a material adverse impact on its financial position. At the date of this document de base, the Group has significant debt. As of December 31, 2014, the Group’s net debt was €650.4 million (see section 10.4. “Equity and financial debt” of this document de base). The Group intends to carry out a partial or total refinancing in the months following the Company’s Initial Public Offering (IPO). It intends to use a large proportion or all of the net proceeds from the capital increase taking place as part of the IPO to reduce its debt. However, the Group’s debt will remain significant after the IPO and after the aforementioned refinancing (see section 13.1.2. “Group forecasts for the financial year ending December 31, 2015” and section 10.4. “Equity and financial debt” of this document de base). The Group’s considerable debt levels may have adverse consequences. For example, they may: force the Group to use a material portion of its cash flow from (used in) operating activities to pay interest and repay debt, reducing the Group’s ability to use free cash flow to finance organic growth, carry out investments or meet the Group’s other requirements; make the Group more vulnerable to a slowdown in its activities and a deterioration in economic conditions; put the Group at a disadvantage relative to less indebted competitors; limit the Group’s flexibility in responding to changes in its business or in the sectors in which it operates; and limit the ability of the Group and its subsidiaries to borrow additional funds, raise capital in the future and seize acquisition opportunities, as well as increasing the cost of this additional financing. In addition, the Group’s ability to meet its obligations, pay interest on its borrowings as well as to refinance or repay its borrowings according to the agreed terms, will depend on its future operating performance and may be affected by numerous factors – such as the economic situation, debt market conditions and regulatory changes – over which the Group has no control. If the Group does not have sufficient liquidity to service its debts, it may be forced to reduce or postpone acquisitions or investments, sell assets, refinance debts or seek additional financing on unfavorable terms, and this could have an adverse impact on its activities or financial position. The Group may not be able to refinance its debt or obtain additional financing on satisfactory terms. Although the Group intends to reduce its debt significantly in the months following the Company’s planned IPO, the realization of the aforementioned risks could have a material adverse impact on the Group’s activities, results, financial position or outlook. The Group is also exposed to interest-rate risk, which mainly consists of the risk of changes in interest rates (see section 4.5.3. “Interest-rate risk” of this document de base). 29 Notwithstanding the Group’s ability to service its debt, certain contractual provisions included in the financing agreements to which certain Group Companies are party place restrictions on the Group’s ability to conduct its activities. The Amended RCF requires the Group to comply with financial undertakings and specific debt ratios (“covenants” or “financial undertakings”). Similarly, the terms of the High-Yield Bonds issued by the Company contain restrictive financial undertakings (for an analysis of all these financial undertakings, see Chapter 10 – “Capital resources” of this document de base). Together, those financial undertakings particularly restrict the Group’s ability to: issue shares, preferred shares or any security maturing less than six months after the Amended RCF’s contractual expiry date; pay dividends or make other distributions if the ratio conditions are not met, and in any event in excess of specified limits; carry out capital decreases; make certain payments or investments; grant security interests and guarantees on Group assets; carry out mergers or any other operations, including within the Group; change the nature of the Group’s activities (except for complementary activities); carry out certain transfers or disposals of assets; arrange debt other than the Amended RCF and the High-Yield Bonds, subject to limited exceptions and compliance with ratios; or make acquisitions other than those authorized or to the extent permitted by the Amended RCF and subject to compliance with the financial ratios set out by the Amended RCF and the terms of the HighYield Bonds. The restrictions arising from the Amended RCF and High-Yield Bond terms could affect the Group’s ability to pursue its activities and limit its ability to respond to market conditions or seize any commercial or acquisition opportunities that may arise. For example, the restrictions may affect the Group’s ability to finance investments in its activities, carry out strategic acquisitions, investments or alliances, restructure its organization or finance its capital needs. In addition, the Group’s ability to comply with those financial undertakings and restrictive covenants could be affected by events outside of its control, such as changes in economic, financial and industrial conditions. If the Group fails to meet its commitments or comply with those restrictions, it may cause the Group to breach the terms of the aforementioned financing arrangements, thereby allowing creditors to ask for early repayment of sums owed under financing arrangements. The terms of the High-Yield Bonds contain undertakings that are more restrictive than those of the Amended RCF in some respects. Until the High-Yield Bonds have been refinanced and the undertakings renegotiated, the Group will be bound by those undertakings and will not be able to benefit from any more flexible provisions of the Amended RCF. This kind of event may have an extremely adverse impact on the Group, and could even cause the Company to suspend payments. 30 The Group has used various assets as collateral, including shares in certain Group Companies. In the event of a payment default, that collateral may be claimed by creditors, which may have a material adverse impact on the Group’s activities. Under the Indentures relating to the High-Yield Bonds and the Amended RCF, some Group Companies have granted security interests over part of the Group assets. In the event of a payment default on the High-Yield Bonds or the Amended RCF, the collateral agent, acting on behalf of the creditors concerned, may claim one or more of those assets pledged as collateral, particularly the shares in Group Companies (see Chapter 10 “Treasury and capital” of this document de base). This kind of event may have a material adverse impact on the Group’s activities, strategy, results, financial position or outlook. The Group faces risks associated with its strategy of acquiring companies. The Group’s growth strategy includes acquiring small and medium-sized laboratories and integrating them into its network. In the financial years ended December 31, 2011 and 2012, the Group completed thirty-five and sixteen acquisitions, respectively. In the financial year ended December 31, 2013, the Group carried out eleven acquisitions and in the financial year ended December 31, 2014 it carried out sixteen acquisitions. The success of the Group’s strategy is dependent upon its ability to identify suitable acquisition targets, conduct appropriate due diligence, negotiate transactions on terms that are favorable for the Group, complete such transactions and integrate the acquired businesses into the Group. The Group’s objective of acquiring further companies in the future depends on the existence of suitable acquisition targets and its ability to finance their acquisition. Continued consolidation of the European medical diagnostics market, including clinical laboratory testing services, may limit opportunities for further acquisitions. For example, the continued consolidation of the French clinical laboratory testing market, as well as the restrictions on both regional market share and outsourcing, may reduce the opportunities for acquisitions. French regulations still impose controls on, or forbid, acquisitions and restructurings of laboratories (or the SELs that operate them), particularly where those operations would cause, in a given healthcare region, the market share of the laboratory resulting from the acquisition or merger to exceed certain levels in terms of the number of clinical tests performed (see section 6.5.1. “Regulation – France” of this document de base). Those regulations could limit the Group’s ability to pursue its strategy of developing regional laboratory hubs that combine several smaller local laboratories into a larger regional entity. Furthermore, French tax reforms could limit laboratory doctors’ willingness to sell their laboratory to a potential acquirer, particularly in the event of an increase in capital gains tax. The Group’s competitors, such as Biomnis, Cerba (which has recently announced the acquisition of Novescia), Sonic Healthcare, Synlab GmbH & Co. KG, Unilabs Group Ltd and Amedes Holding A.G., are following similar acquisition strategies to those of the Group. Other operators, such as Quest Diagnostics Inc. and Laboratory Corporation of America Holdings, while not yet significantly active in Europe, may choose to commence operations in Europe. Those competitors, and certain financial investors wishing to enter the markets in which the Group operates or may wish to enter, have greater financial resources than the Group or could be able to accept less favorable terms than the Group can accept, which may prevent the Group from acquiring the companies that it wants and reduce the number of potential acquisition targets. In addition, through such acquisitions, some of the Group’s competitors already have or may in the future gain a major presence in a particular country or, more generally, in Europe, making them attractive acquirers of potential targets seeking to join a network, the size of which would provide greater development prospects. If the Group carries out acquisitions, there can be no assurance that it will be able to retain all the customers and patients of the companies it acquires, generate expected margins or cash flows, or realize the anticipated benefits of such acquisitions, including expected growth or synergies. Although the Group analyzes acquisition targets on an ongoing basis, those assessments are subject to a number of assumptions concerning profitability, growth, interest rates and company valuations. There can be no assurance that the Group’s assessments and assumptions regarding acquisition targets will prove to be correct, and actual developments may differ significantly from its expectations. In most cases, acquisitions involve the integration of a company that was previously operated independently with systems and processes that differ from those used by the Group. The Group may not be able to integrate certain acquired companies successfully, or the integration may require more time and investment than expected, and the Group could bear or assume unknown or unexpected liabilities or risks related to customers, employees, suppliers, competent administrative authorities, professional associations (“Ordres 31 Professionnels” in France), public health programs, private health insurers or other persons, that could affect the Group’s operating results. The process of integrating acquired companies may disrupt the Group’s activities and cause slower growth in those companies’ activities or a decrease in the Group’s operating income as a result of difficulties or risks including, in particular: unforeseen legal, regulatory, contractual and other issues; the loss of customers, patients or key employees; difficulty in standardizing existing information and systems; difficulty in consolidating facilities and infrastructure; difficulty in realizing operating synergies; failure to maintain the quality or timeliness of services that the Group has historically provided; added costs caused by dealing with such disruptions; unforeseen challenges from operating in new geographic areas; and the diversion of management’s attention from the Group’s day-to-day management as a result of the need to deal with the aforementioned disruptions and difficulties. Furthermore, the Group operates and acquires companies in different countries, with different regulatory and corporate cultures, which may exacerbate the risks described above. If the Group were unable to implement its acquisition strategy or integrate acquired companies successfully, its activities and its growth may be affected. Increased quality and price competition could have a material adverse impact on the Group’s Revenue and profitability. The medical diagnostics market, including clinical testing services, is intensely competitive in each of the countries in which the Group operates. In markets in which fee structures are regulated, competition is based mostly on the quality of services provided, including reporting and other information technology systems offered and the skills of laboratory personnel. Reputation in the medical community is a key factor affecting the volume of testing services the Group provides in such markets, since healthcare professionals are an important source of patient referrals to its laboratories. The Group faces price competition in liberalized markets such as Spain, where price is often the determining factor for healthcare providers and third-party payers in the selection of a laboratory. Pricing is also a key driver in the outsourcing decisions of hospital laboratories, for which the main objective of the Group’s potential customers is cost reduction. Should the Group’s clients in Spain decide to regroup, they would reinforce their decision-making powers, and this could influence their decision to outsource or could lead them to re-internalize their clinical services. The ongoing consolidation of the European clinical laboratory industry is expected to enable larger groups to offer lower prices as they adopt large-scale automated testing allowing them to reduce their costs. Resulting testing procedures are particularly likely to induce increased cost reductions which allow companies to lower their prices. As a result of the size and structure of its network, the Group may be unable to achieve competitive levels of efficiency and may lose customers or tenders as a result. This may negatively impact its operating results and cash flows. Group competitors with greater financial resources and stronger market positions than its own may reduce their prices further than the Group might be able to in order to increase their market share, offer bigger operational resources and broader geographical reach, or conduct more effective marketing programs. In some markets where the Group operates, such as Spain, scale and geographic reach provide competitive advantages because private health insurers prefer negotiating national contracts with networks that have a substantial geographic footprint and offering them more favorable terms. The Group’s ability to compete effectively may be adversely affected if it did not have an extensive enough network in some of the markets in which it operates. 32 The Group has recognized a significant amount of goodwill and may never realize the full value of that goodwill. The Group has recognized a significant amount of goodwill. Goodwill represents the excess of acquisition cost over the fair value of the net assets of companies acquired, and totaled €581.5 million as of December 31, 2013 and €702.4 million as of December 31, 2014, equal to 60.1% and 67.1% of the Group’s total assets respectively. Under IFRS, goodwill is not amortized but is tested for impairment annually and whenever there is any indication of impairment. Impairment may result from, among other things, a deterioration in the Group’s performance, a decline in expected future cash flows, adverse market conditions, adverse changes in applicable laws and regulations (including changes that restrict the activities of, or affect the services provided by, the Group’s laboratories) and various other factors. The amount of any impairment must be reported immediately as a charge to its income statement and cannot be reversed. As an illustration, due to the challenging market conditions prevailing in Spain and Portugal as a result of the global economic downturn, the Group recognized a goodwill impairment charge of €95 million on December 31, 2011 with respect to its cash generating unit in the Iberian Peninsula. The Group also booked a €36 million goodwill impairment charge on its cash generating unit in Germany on December 31, 2012. Any further future impairment of goodwill may result in material reductions in the Group’s net income and equity under IFRS. In the UK, the Group operates almost exclusively through a partnership with NHS trusts. As a result, the Group’s UK activities are highly dependent on the NHS. If the Group’s relations with the NHS were to deteriorate, its UK activities may be in jeopardy. The clinical laboratory services market in the United Kingdom is dominated by the public sector, via hospital laboratories. The NHS accounted for 82% of UK healthcare spending in 2013 and so has a near-monopoly in the UK market. It provides clinical testing services to both inpatients and outpatients. In 2013, 95% of spending on clinical laboratory services took place in public-sector hospitals. (Source: L.E.K.). The Group has operated in the UK since 2010 via its Integrated Pathology Partnerships (iPP) joint venture with Sodexo. At the date of this document de base, the Group owns 90% of iPP, which has several partnerships with the NHS. On June 1, 2012, iPP started to cooperate with Taunton and Somerset NHS Foundation Trust and Yeovil District Hospital NHS Foundation Trust through a partnership. Under the partnership agreement, iPP delivers the full range of laboratory services while the clinical interpretation and clinical advice functions continue to be provided by those Trusts’ medical staff, who remain employed by the NHS. The partnership, which has an initial term of 20 years renewable for an additional 5-year period, was structured in a way that allows other medical trusts in the region to join. In May 2014, the Group – through iPP Facilities Ltd and iPP Analytics Ltd, two new entities created in the first half of 2014 – signed two partnership agreements with Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust to provide laboratory services. The contract has an initial term of ten years and may be renewed for a further five-year period (see section 6.4.3.1. “Presentation of Northern Europan Market – United Kingdom” of this document de base). As a result, if the Group’s relationships with the NHS or the trusts with which these joint arrangements have been formed were to deteriorate in the future, the Group’s ongoing activities in the UK may be jeopardized, given the Group’s high level of dependency on the NHS, and the Group’s presence in the UK may then be compromised. The Group may be unable to retain or recruit experienced laboratory doctors, which may weaken its relationships with local medical communities and adversely affect its operating results. The success of the Group’s clinical laboratories depends on employing and retaining qualified, skilled and experienced laboratory doctors who can maintain and enhance its reputation by providing testing services in accordance with its standards. If competition for the services of these professionals were to increase in the future, the Group may not be able to continue to attract and retain such laboratory doctors. The Group’s business depends partly on personal relationships and the professional reputation of its laboratory doctors with patients and with the customers that refer patients to its laboratories, such as general practitioners and private hospitals. Departing laboratory doctors who have close relationships with their local medical 33 community may draw some business away from the Group. For example, the Group’s operating results in Germany in 2009 and 2010 were adversely affected by the 2009 departure of a laboratory doctor operating in the Group’s laboratory in Duisburg, which led to the loss of a significant contract with a blood bank. Former executives, including the Chief Medical Officer, along with other staff members of the laboratory operated by the Group in Karlsruhe joined a competitor in 2011, and the subsequent reduction in the laboratory’s revenue affected the Group’s results and operations in Germany in 2011 and 2012. If the Group loses, or fails to attract and retain, qualified laboratory doctors who have positive relationships with their respective local medical communities, its Revenue and earnings may be adversely affected. If the Group loses the services of members of its senior management team, its activities and operating results may be seriously harmed. The execution of the Group’s strategy and its continued success depend in part on its ability to benefit from the continued skills, efforts and motivation of its senior management team, both at the Group corporate level and in each of the countries in which it operates. The Group’s strategy for organic growth and improved operating efficiency depends particularly on its senior management having deep knowledge of its activities. Its external growth strategy requires knowledge of the dynamics and major players in the various markets in which the Group operates. The departure of key members of its senior management or experienced personnel may disrupt the pursuit of the Group’s strategy. If one or more members of the Group’s senior management team or experienced personnel were unable or unwilling to continue in their present positions, including for health, family or other personal reasons, the Group may not be able to replace them easily or at all. An inability to attract and retain qualified members or key personnel in due time may could have a material adverse effect on the Group’s business, prospects, operating results and financial position. The development of new, more cost-effective tests that can be performed directly by the Group’s customers or patients, or a move by hospitals or doctors to carry out in-house testing, may negatively impact the Group’s testing volumes and Revenue. Advances in technology may lead to the development of more cost-effective tests that can be performed outside a commercial clinical laboratory, such as specialty tests that can be performed by hospitals in their own laboratories, point-of-care tests that can be performed by doctors in their surgeries, or home-testing that can be performed by patients or other non-medical professionals (such as test kits that already exist for HIV testing). Manufacturers of laboratory equipment and test kits may seek to increase their sales by marketing tests that can be performed in surgeries or directly by patients. The development of such technology and its use by the Group’s customers or patients would reduce the demand for the Group’s services and negatively impact its Revenue. Some of the Group’s customers or patients, including hospitals and doctors, may choose to perform themselves tests that the Group performs at the date of this document de base. If such customers or patients were to perform such tests themselves, and if the Group did not offer new or alternative tests attractive to its customers and patients, the demand for its testing services would be reduced and its Revenue would be materially adversely impacted. Failure to bill quickly or accurately for its services may have a material adverse effect on the Group’s activities. The Group invoices various customers for its services: patients, insurance companies, social-security organizations, medical doctors, hospitals and employers. Changes in laws and regulations, contractual terms agreed with payers or payment policies of payers may increase the complexity and cost of the Group’s billing process. Additionally, checking compliance with applicable regulations as well as internal compliance policies and procedures further increase the costs and complexity of the billing process. For example, billing arrangements in Spain for clinical testing services are subject to contractual arrangements that require compliance with significant administrative constraints. As a result, the billing systems for the Group’s laboratories in Spain are complex and require significant and regular investments in technology in order to maintain technology at the required level. In France, one of the Group’s SELs incorrectly invoiced certain tests for several years, due to an error inputting rates into its IT system. As a result, the primary health insurers concerned requested that the €2 million incorrectly invoiced be reimbursed to them, and the SEL concerned 34 made the reimbursement. Failure to bill quickly or accurately for the Group’s services or increased complexity in billing arrangements and procedures may result in delayed payments, increase its working capital requirements and adversely affect its operating results. Failure to comply with environmental, health and safety laws and regulations may result in fines, penalties and other costs as well as the loss of the Group’s licenses and authorizations, which could have a material adverse effect on its activities. The Group’s activities are subject to various licenses, authorizations and regulations under EU, national and local laws and regulations relating to the protection of the environment, human health and occupational health and safety, including those governing the handling, transportation and disposal of medical samples and biological, infectious and hazardous waste, as well as regulations relating to the health and safety of laboratory employees. The Group must meet strict requirements in all jurisdictions in which it operates for the disposal of laboratory samples at authorized facilities, such as those arising from French regulations relating to “waste from care activities that carry a risk of infection”. In order to organize its waste management policy, including the disposal of samples or other waste that may qualify as waste from care activities that carry a risk of infection, the Group’s companies may use services from external providers. If those providers failed to carry out their activities in line with legal and regulatory requirements, the Group Companies concerned may be held liable. In addition, the Group must meet a large number of requirements relating to workplace safety for employees in clinical laboratories, who may be exposed to various biological risks such as blood-borne pathogens (including HIV and the Hepatitis B virus). Medical and molecular imaging present specific risks arising from the possible exposure of Group staff to radiation. Requirements relate to work practice controls, the wearing of protective clothing and protective equipment implementation, training, medical follow-up, vaccinations and other measures designed to minimize exposure to, and the transmission of, blood-borne pathogens or pathogens borne by other medical samples. Environmental, health and safety regulations are likely to become even more stringent over time, and the costs incurred by the Group to comply with these requirements are likely to increase. Moreover, the Group may be sanctioned and incur substantial costs, including civil and criminal fines and penalties, enforcement actions, or the suspension or termination of its operating licenses and authorizations as a result of failure to comply with its obligations under those laws and regulations, which may have a material adverse effect on its activities. For example, in 2009, one of the Group’s small French laboratories was closed for three weeks as a result of disciplinary sanctions by the Ordre des pharmaciens due to a failure to maintain adequate safety and quality standards. The Group may also become subject to claims from employees or other persons claiming to be victims of injury or illness resulting from exposure to the samples or waste they have handled. The soil and groundwater at some of the sites the Group owns or leases may be contaminated with hazardous materials from industrial activities that may have occurred in the past. Under certain environmental, hygiene and safety laws and regulations, the Group may be required to investigate or remediate contamination at properties it owns or occupies, even if the contamination was caused by persons unrelated to the Group. While the Group is not aware, at the date of this document, of any significant soil or groundwater contamination on its sites, the discovery of previously unknown contamination or the imposition of new obligations to investigate contamination at these or other sites in the future may result in substantial unanticipated costs for the Group. Failure to comply with and establish appropriate quality standards as part of the Group’s testing services may adversely impact its reputation and operating results. The Group’s clinical testing services are intended to supply healthcare professionals with information to help them establish or support diagnoses and prescribe medical or other treatment for their patients. Inaccuracies or negligence in providing its clinical testing services may lead to inaccurate diagnoses by healthcare professionals, prescriptions of inappropriate treatments or decisions not to prescribe treatment when treatment is required, which may have serious consequences for patients (such as illness, harm, death or other adverse effects). Errors such as misidentifying or inaccurately labeling samples or compromising the integrity of samples, as well as errors caused by testing machines or reagents used for testing, may occur. The Group has been the subject of legal proceedings for alleged acts or omissions of its laboratory personnel and other employees in the past. If the Group were involved in such proceedings, and even if the Group were successful in its defense, the proceedings 35 would be costly and might result in substantial damage to its reputation in the medical community and with patients (see section 4.6.2. “Policy on insurance” of this document de base for a description of the Group’s policy on insurance in the civil liability field). Disruption, failure or the unsuitable performance of sample transportation services may adversely affect the Group’s activities and financial results. The proper handling of samples during collection and transportation is essential for maintaining their integrity, ensuring the quality of tests and guaranteeing safety from accidental exposure to potentially infectious microorganisms. The vehicles used to transport samples must satisfy applicable legal, practical and technical requirements, which vary depending on the type of samples transported. These requirements govern, for example, the use of appropriate containers and packaging, the labeling of containers, the manner in which samples and containers are stored in the vehicle, the temperature at which samples must be transported and the duration of the journey. Drivers employed to transport samples must be trained to handle them in accordance with best practice and applicable laws and regulations. Mishandling of the sample in the collection and transportation process may increase the likelihood of errors in laboratory testing. Disruption to the transportation of samples, failure to comply with requirements relating to samples or the inadequate performance of the transportation service may damage the Group’s reputation, lead to claims against it and the loss of customers and patients, which would adversely affect its operating results, financial position and outlook (see section 4.6.2. “Policy on insurance” of this document de base for a description of the Group’s policy on insurance in the civil liability field). In addition, in certain of the countries in which the Group operates, it has entered into outsourcing arrangements with third parties for the transportation of medical samples from specified sampling points (such as hospital sites, doctors’ surgeries and Sampling Centers) to its clinical laboratories. The Group does not control the facilities or operations of such third-party transport operators and therefore depends on the quality of their transportation services in order to maintain the integrity of samples. Any interruption in their services or failure to meet their contractual obligations may damage the Group’s reputation and result in claims against the Group and the loss of customers or patients, which would adversely affect its operating results, financial position and outlook. Extreme weather conditions may affect the Group’s activity levels and, consequently, its Revenue. A significant portion of the Group’s activities depends on the ability of patients - who are often ill, aged, pregnant or have limited mobility - to travel to see a doctor or to a laboratory. Accordingly, unusual or inclement climatic conditions, particularly those affecting ground transportation conditions, have already in the past caused, and may in the future cause, a decrease in demand for the Group’s testing services. The Group also maintains a logistics network to transport test samples from sampling points to laboratories and between laboratories. Its logistics network depends significantly on ground transportation, which may be disrupted by factors including snow and other adverse weather conditions. Disruptions to the Group’s logistics network restrict its ability to provide its services in affected areas and reduce its Revenue. For example, because of heavy snowfall, the Group’s operations in Monza (Italy) and Southwest France were significantly affected in December 2011 and February 2012, respectively. Similarly, heavy snowfall in Northern France in January and February 2013 also disrupted the Group’s operations and led to a reduction in Revenue during that period. 4.4 LEGAL, TAX AND INSURANCE RISKS 4.4.1 Risks related to disputes and litigation In the ordinary course of its business, the Group is involved or may in future be involved in certain contentious proceedings (including administrative, court, arbitration and disciplinary proceedings), relating to matters concerning the professional liability of its laboratories, disputes with laboratory doctors, medical doctors and employees and regulatory issues, as well as enquiries initiated by regulatory authorities, professional associations and health insurers, regarding, among other things, billing matters. The most significant current and potential disputes and the amount of provisions set aside by the Company with respect to proceedings underway are described in detail in section 20.3. “Contentious proceedings” of this document de base. 36 Some of the proceedings initiated by or against the Group may involve claims for material amounts and could divert management’s attention and time from day-to-day business operations to address such issues. Proceedings may result in substantial monetary damages, adversely affect the Group’s customer base and reputation, and reduce demand for its services. The final outcome of those proceedings or claims might have an adverse impact on the Group’s financial position. In some proceedings in which the Group is involved or could be involved, material amounts are claimed, or could potentially be claimed, from Group Companies. Any provisions set aside in this respect by the Group Companies concerned in their financial statements may prove insufficient if they were found liable, and this could have material adverse consequences on the Group’s activities and financial position (particularly on its operating results and cash flow) regardless of whether or not the underlying claim is well founded. In general, it is possible that, in the future, new proceedings, whether or not connected with those underway at the date of this document de base, may be initiated against the Company, its subsidiaries or their laboratory doctors, medical doctors or employees. Since such proceedings may be lengthy and costly, they could also, regardless of their outcome, have adverse consequences on the Group’s activities, financial position (particularly its results and cash position) and outlook. 4.4.2 Risks related to the protection of personal data and information security The Group is subject to legal obligations relating to respect for and protection of personal data – particularly patients’ medical records – and strict policies relating to information security. The Group receives, generates and stores significant volumes of personal and sensitive information, such as patient medical information, and must therefore comply with privacy and data protection regulations with respect to the use and disclosure of protected health information intended to ensure the confidentiality, integrity and availability of such information. Such regulations establish a complex regulatory framework on a variety of subjects, including: the circumstances under which the use or disclosure of protected medical health information is permitted or required without specific authorization by the patient; patients’ rights to access, ask for amendments to and receive, protected information contained in their medical records; requirements to notify patients of privacy measures to maintain the confidentiality of protected medical information; administrative, technical and physical backups required of entities that use or receive protected medical information; and the protection of computing systems that store protected medical information. If the Group does not adequately maintain confidentiality of patient data or other protected health information, or if such information or data is wrongfully used by the Group or disclosed to an unauthorized person or entity, the Group’s reputation could suffer and it could be subject to fines, penalties and administrative, legal or disciplinary proceedings. For example, in 2012, the Group entered into a settlement agreement with one of its patients in France following the involuntary disclosure of certain confidential medical information. 4.4.3 Risks related to taxation The Group is exposed to risks related to taxation in the various countries in which it operates. The Group organizes its commercial and financial activities on the basis of various and complex legal and regulatory requirements in the various countries in which it operates, particularly as regards taxation. Changes in regulations or their interpretation in the various countries in which the Group operates could affect the calculation of the Group’s overall tax burden (income tax, social security contributions and other taxes), along with its financial position, liquidity and results. In addition, the Group must interpret French and local regulations, international tax agreements, legal opinions and administrative practice in each of the jurisdictions in which it operates. The Group cannot guarantee that its application and interpretation of such provisions will not be challenged by the authorities concerned. In general, any breach of tax laws or regulations applicable in 37 the countries in which the Group operates could lead to tax adjustments, late-payment interest, fines and penalties. The Group’s activities, results, financial position, liquidity and outlook could be materially affected if one or more of the aforementioned risks materialized. 4.4.4 Risks related to tax rules regarding the tax-deductibility of interests In France, articles 212 bis and 223 B of France’s General Tax Code limit the portion of net financial expenses that can be deducted from income subject to corporate income tax, where those expenses exceed €3 million and subject to certain conditions and exceptions, to 75% for accounting periods ending on or after January 1, 2014. In addition, under French rules regarding under-capitalization, the tax-deductibility of interests paid on loans granted by a related party, or guaranteed by a related party, is authorized subject to certain conditions but limited, in accordance with rules set out in article 212 of France’s General Tax Code. There are similar rules in most countries in which the Group operates. The impact of those rules, or any adverse change in them, on the Group’s ability to deduct interest payments on its borrowings from taxable income could increase the tax burden on the Group and therefore have a material adverse impact on its results and financial position. 4.4.5 Risks related to VAT and French payroll tax Most of the Group’s activities are exempt from VAT. The Group cannot recover VAT applicable to charges and expenses relating to those VAT-exempt activities. As a result, any increase in the VAT rate on those charges and expenses (such as the increase in the Italian VAT rate from 21% to 22%, which came into force in October 2013, or the application in Spain of VAT at the standard rate of 21% from January 1, 2015 instead of the reduced rate of 10% previously applied to reagents and medical equipment) would represent an additional cost for the Group. The Group would not necessarily be able to pass on this additional cost in the prices it charges to its customers. Some of the Group’s existing or future activities are subject to VAT, which allows the Group to deduct VAT levied on the related charges and expenses. In the UK, the tax authorities have confirmed, after a long debate, that VAT is applicable to activities such as the management and provision of the Group’s assets and equipment. However, it is possible that those tax arrangements will be challenged by the tax authorities in the future. If that happens, the Group may not be able to pass on the resulting increase in costs to its customers. Similarly, given that most of the Group’s activities are VAT-exempt, the Group is subject to payroll tax in France. As a result, any change in the regulations applicable to the French payroll tax may have an adverse impact on the Group’s results and financial position. 4.5 FINANCIAL RISKS 4.5.1 Credit or counterparty risk Credit or counterparty risk is the risk that a party to a contract with the Group fails to meet its contractual obligations, leading to a financial loss for the Group. The financial instruments that could expose the Group to counterparty risks mainly consist of trade receivables, cash and cash equivalents, investments and derivative financial instruments. Overall, the carrying amount of financial assets in the Group’s consolidated financial statements for the financial year ended December 31, 2014, net of impairment, represents the Group’s maximum exposure to credit risk. At the date of this document de base, the Group considers that the risk of its counterparties defaulting on their obligations is low. The main counterparties are government bodies, parastatal entities, insurance companies, hospitals and leading banks. Concentration risk exists, particularly in respect of one customer that accounted for 7.2% of proforma Revenue in the Southern Europe segment for the financial year ended December 31, 2013 and 7.1% for the financial year ended December 31, 2014. 38 However, financial difficulties among certain Group customers or third-party payers may force the Group to accept less than the face value of its receivables. In countries where the Group directly contracts with private insurance companies, such as Spain, in countries where the third-party payers are private insurance companies, or in cases where hospital laboratories outsource clinical testing (in which case the Group acts as a subcontractor and is therefore paid by the outsourcing customer), the Group is exposed to credit risk in respect of those counterparties. In 2013, for example, the Group had to accept €0.7 million less than the face value of its receivables in respect of Clinica El Pilar, a Spanish private clinics operator that went bankrupt in October 2013. In addition, the Group lost €0.7 million in 2014 when Integra, a partner laboratory company in Spain, went bankrupt in September 2014. Significant or recurring incidents of bad debts would adversely impact the Group’s financial position and operating results. 4.5.2 Exchange-rate risk Almost all of the Group’s income and expenditure is denominated in euro, which is the Group’s functional currency. However, certain Group entities may be exposed to transaction risks relating to a purchase or sale transaction in a different currency. This is mainly the case with a subsidiary in the Iberian peninsula, which carries out tests from samples taken outside the Eurozone (mainly in Latin America). For those tests, customers are invoiced by the local trading subsidiaries in the relevant country’s currency. Accordingly, the Group generates revenue in Brazilian real and Colombian pesos. Similarly, some work, mainly related to genetic tests to detect Down’s syndrome, is outsourced from a US provider, resulting in expenditure in US dollars. In the UK, the Group’s income and expenditure are in sterling and do not give rise to any transaction risk for the Group. However, investments in UK subsidiaries and the financing of those subsidiaries may give rise to currency risk due to fluctuations in sterling against the euro. As regards Swiss subsidiary TEST SA, the Group has marginal exposure to risk relating to the Swiss franc exchange rate. The Group’s policy is to hedge that risk on a case-by-case basis. No hedging instrument was in place in respect of that risk as of December 31, 2014. Revenue denominated in currencies other than the euro accounted for 2.1% of total Group Revenue for the financial year ended December 31, 2013 and 6.0% for the financial year ended December 31, 2014, given the increasing activities of the Group in the United Kingdom. At the date of this document de base, the Company considers that the Group has minimal exposure to currency risk. 4.5.3 Interest-rate risk The Group is exposed to the risk of fluctuations in interest rates due to some of its borrowings, the interest rate on which is linked to EURIBOR or LIBOR. This concerns mainly the Amended RCF and, to a lesser extent, bank loans contracted by certain entities before they were acquired by the Group. At the date of this document de base, the Group is using the Amended RCF to finance acquisitions (for example, the Group drew €95 million on the RCF before it was amended to acquire SDN in July 2014 and is continuing to use regularly the Amended RCF to finance its acquisitions and cash requirements). Outstanding bank loans amounted to €13.6 million as of December 31, 2014, including €1.5 million at floating rates. €100 million due under the Amended RCF has been repaid, partly through the use of proceeds from the additional bond issue on February 11, 2015. €120.25 million is available under the credit facility at the date of this document de base, subject to compliance with covenants and financial undertakings and other standard undertakings (see section 10.4 – “Equity and financial debt” of this document de base). As a result, any increase in EURIBOR or LIBOR would lead to higher interest charges for the Group, reducing available cash for investment and limiting its ability to reduce the volume of its debts. The Group’s financing 39 agreements in force at the date of this document de base do not generally contain any clause requiring it to hedge some or all of its interest-rate risk exposure in respect of some or all of its debt. Liquidity consists partly of cash equivalents totaling €15.3 million, including floating-rate instruments, mainly units in open-ended investment companies (SICAVs), as of December 31, 2014. The rest of the Group’s cash position as of December 31, 2014 consisted of balances in bank current accounts and cash accounts, totaling €58.8 million. The Group used part of its cash to acquire the SDN group at the end of July 2014, reducing its liquidity. The Group’s cash is invested in money-market SICAVs, which are floating-rate instruments. Because of its structurally limited exposure to interest-rate risk, the Group has not taken out any interest-rate hedging contracts, interest-rate caps agreements or future rate agreements (FRA), except for a €1.2 million interest-rate hedge expiring in 2017. Net exposure, defined as financial assets minus financial liabilities, to interest-rate risk as of December 31, 2014 is as follows: December 31, 2014 (in millions of euros) Debt1 Financial assets Net exposure Fixed rate Floating rate Fixed rate Floating rate Fixed Rate Floating rate Less than 1 year 0 74.1 32.6 0.6 (32.6) 73.5 From 1 to 5 years 0 0 619.3 76.0 (619.3) (76.0) More than 5 years 0 0 3.8 0 (3.8) 0.0 Total 0 74.1 655.7 76.6 (655.7) (2.5) 1 nominal amount which does not include capitalized issuance costs in accordance with IAS 39 To the extent that the revolving credit facility is undrawn, the Group’s exposure to interest-rate risk on floatingrate financial liabilities is extremely limited. Given the respective proportions of fixed-rate and floating rate debt within the Group, the sensitivity of its financial expenses to increases in interest rates is very limited. If the Amended RCF were undrawn, the commitment fee invoiced would be calculated on the basis of a fixed rate. If the Amended RCF were drawn to the maximum amount of €128.25 million, the Group’s exposure to interest-rate risk on its financial liabilities would be a maximum of €1.3 million if floating interest rates increased by 100 basis points, minus the positive effect on floating-rate financial assets. This analysis assumes that all other variables remain unchanged. Please see Note 29 to the consolidated financial statements for the financial year ended December 31, 2014 included in section 20.1.1. “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” of this document de base. 4.5.4 Liquidity risk The Group manages liquidity risk by making forecasts regarding its cash position, analyzing differences between forecasts and actual outcomes, and seeking to align the maturity profiles of its financial assets and liabilities as closely as possible. In this way, the Group ensures that it has enough cash to meet its obligations. (see Chapter 10 “Capital Resources” of this document de base) At the date of this document de base, the Group also has a revolving credit facility totaling €128.25 million, all of which may be used to finance acquisitions (see Section 5.2 “Investments” of this document de base) and to cover its general financing needs. On the date of this document de base, the remaining amount under the revolving credit facility amounted to €120.25 million. The availability of that revolving credit facility is subject to covenants and financial undertakings and to other standard undertakings (see section 10.4 – “Equity and financial debt” of this document de base). 40 The Company also issued bonds in a principal amount of €500 million on January 14, 2011, fungible bonds with the same characteristics in a principal amount of €100 million on February 13, 2013 and fungible bonds with the same characteristics in a principal amount of €100 million on February 11, 2015 (together the “High-Yield Bonds”). The aim of the first issue was to repay historical debts previously arranged by the Group (mezzanine borrowings and syndicated loans), the aim of the second was to repay all drawings on the RCF (i.e. €67 million), and the aim of the third was to repay €100 million of the Amended RCF. The table below breaks down non-derivative financial liabilities as of December 31, 2014 by contractual maturity date: (in millions of euros) Less than one year Covered bonds with an effective interest rate of 8.5% 1 Syndicated Amended RCF at the effective interest rate 2 December 31, 2014 More than 5 1-5 years years 0 600.0 Total 0 600.0 0 75.0 0 75.0 Guaranteed bank loans at the effective interest rate 3.3 9.8 0.5 13.6 Accrued interest on the covered bonds 23.5 0 0 23.5 Debt relating to finance leases 6.0 10.0 3.3 19.4 Other debts 0.4 0.4 0 0.8 Total financial liabilities 33.2 695.3 3.8 732.3 1 2 Nominal amount which does not include capitalized issuance costs in accordance with IAS 39 Idem Please see Notes 23 and 29 to the consolidated financial statements for the financial year ended December 31, 2014 included in section 20.1.1 – “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” of this document de base. 4.5.5 Equity risk At the date of this document de base, the Group does not own any financial securities other than shares in companies accounted for under the equity method and shares in non-consolidated companies. As a result, the Group believes that it does not have any exposure to material market risk relating to equities or other financial instruments. 4.6 RISK MANAGEMENT POLICY AND POLICY ON INSURANCE 4.6.1 Risk management In 2014, as part of a project to set up a structured, formal internal control system, the Group mapped the risks to which it may be exposed, assessed them and defined actions to be taken in order to mitigate or manage them. Those risks were analyzed through interviews with members of the Group’s executive committee. For each risk identified, the Group assessed its exposure and the potential impact on its activities. Actions to be taken to mitigate or control those risks are currently being defined. For example, the main risks identified, in terms of potential severity, relate to regulations and difficulties in predicting movements in prices set by the competent authorities or major customers. There is no guarantee that the Company has correctly identified all risks to which the Group may be exposed, or correctly assessed its exposure to the risks of which it is aware. There is no guarantee that actions that have been or will be taken by the Company have reduced or will reduce the harm the Group may suffer if those risks were realized. There is also no guarantee that business continuity and business recovery plans will operate correctly or enable the Group to recover effectively following an incident and continue its activities. Regardless of whether or not those plans work, the realization of any risk identified by the Company or the occurrence of an incident could materially affect the Company’s financial results, cash position, activities, outlook and reputation. 41 If the Company does not regularly update its risk map, the Company may be unable to know or address additional risks to which the Group may be exposed since the risk-mapping exercise conducted at the beginning of 2014. In addition, there is no guarantee that any updated risk map would not show the same weaknesses described above for the current map. 4.6.2 Policy on insurance The Group and its subsidiaries have taken out third-party damage insurance policies aimed at covering, in addition to their liability as employers, occupation risks and risks associated with the performance of their professional activities. The Group and its subsidiaries have also taken out property/casualty policies to cover their movable property and real estate and to cover any loss of business resulting from an insured loss event. The Group does not take out insurance against certain operational risks for which no insurance exists or that can only be insured against on terms that seem unreasonable to the Group considering the covered risk. As an illustration, the Group has decided to auto-insure against risks relating to the recovery of money receivable from customers and loss of business in certain countries like Italy and, to a lesser extent, Spain. The Group has insurance policies covering other risks such as directors’ and officers’ civil liability, and motor insurance policies. The Group believes that existing insurance cover, as regards both the amounts covered and the insurance terms negotiated, provide the Group with sufficient protection against the risks the Group deems insurable and those he deems itself exposed to, at the date of this document de base, in the countries in which it operates. While the Group seeks to maintain appropriate insurance policies, certain risks to which the Group is or may be exposed are insurable or would be insurable at conditions the Group deems unreasonable considering the covered risk and there can be no assurance that the Group will not experience major incidents that fall outside the scope of its insurance policies. In addition, the insurance policies taken out by the Group feature guaranties caps and fees, which means that even when the considered risk is covered by the insurance policy, the Group cannot exclude that the indemnification paid by the insurer be only partial. In the event of exceptional loss events or substantial increases in premiums in the insurance market in general, the Group’s insurance costs may increase over time. The Group cannot guarantee that it will be able to maintain its rate of insurance coverage at the date of this document or continue to do so on satisfactory terms. The Group cannot guarantee that it will suffer no loss or that no legal proceedings will be initiated against it or any of its laboratories that fall outside the scope of coverage provided by existing insurance policies. The total insurance costs borne by the Group amounted to €2.2 million in the financial year ended December 31, 2014. Civil liability insurance The Group has civil liability insurance policies in the various countries in which it operates, that aim to cover it against the financial consequences of any liability for bodily injury, material or immaterial damage or consequential damage caused to third parties, subject to the application of guaranties caps and applicable fees. A group civil liability insurance policy was arranged in France in 2012 with the assistance of an insurance broker. The aim of this was to meet the Group’s needs more effectively by enabling laboratories to benefit from centralized management in France and consistent cover. Some risks are expressly excluded from the insurance policy and so are not covered. Directors and officers liability insurance Group managers are also covered by a “directors and officers liability” policy that aims to cover them – subject to certain coverage limits, conditions and exclusions – against the pecuniary consequences of any civil claim made against them in which they are held liable and that is attributable to any failure by a manager to fulfil his/her obligations arising from laws, regulations or the articles of association (statuts), to any mismanagement arising through recklessness, negligence, omission, error, inaccurate declaration or violation of regulations applicable to labor relations, and in general to any actual or alleged wrongdoing by that manager for which he/she may be liable solely through his/her managerial functions (excluding, for example, willful or malicious wrongdoing and any fine or criminal, tax or customs penalty). 42 The directors and officers liability policy also excludes losses arising from any claim initiated or conducted in any jurisdiction in the USA, or based on US law, and that originates from a market transaction, including market transactions carried out in the USA. However, that policy does cover claims based on or originating from the issuance or placement of the HighYield Bonds in the USA. Property/casualty insurance The Group has multi-risk insurance policies in the various countries in which it operates, covering damage to its movable property and real estate, subject to exclusions stated expressly in the policy. A French Group multi-risk insurance policy was taken out in 2012 with the assistance of a broker. That French Group policy was renegotiated in 2014 in order to extend the scope of the corresponding coverage, given the development of technical platforms. 43 CHAPTER 5 INFORMATION ABOUT THE GROUP 5.1 HISTORY AND EVOLUTION OF THE GROUP 5.1.1 Business name The Company’s business name is LABCO. It operates under the trade name LABCO. 5.1.2 Company registration place and number The Company is registered with the registre du commerce et des sociétés de Paris under number 448 650 085. 5.1.3 Date of incorporation and term of existence The Company was incorporated on June 5, 2003 with a length of life of ninety-nine years, that is until June 5, 2102. 5.1.4 Headquarters, legal form and applicable legislation At the date of this document de base, the Company is a société anonyme with a board of directors registered under French Law and governed by the provisions of the French Commercial Code and by its articles of association (statuts). The Company’s registered office is located at 60-62, rue d’Hauteville, 75010 Paris. The registered office phone number is +33 (0)1 56 02 67 40. The Company was originally incorporated as a société par actions simplifiée and was converted into a société anonyme in January 2012. The financial year ends on December 31 of each year. Ahead of the admission to trading of the Company’s shares on the regulated market of Euronext Paris, the general meeting of the Company’s shareholders adopted on October 2, 2014, subject to the non-retroactive condition precedent of the settlement-delivery of the Company’s shares issued or sold in connection with the initial public offering, the articles of association (statuts) that will govern it following the satisfaction of this condition. The main provisions of these articles of association (statuts) are described in Chapters 14 “Administrative, management and supervisory bodies and general management”, 16 “Functioning of the Company’s administrative and management bodies” and 21 “Additional information” of this document de base. 5.1.5 Significant events in the development of the Group’s activities Labco is a French company that is primarily active in buying or acquiring direct or indirect stakes in the capital of clinical testing companies or clinical testing laboratories, anatomical cytopathology testing companies or anatomical cytopathology testing laboratories, and more generally any company whose object is to contribute directly or indirectly to the operation of medical diagnoses. Labco, the holding company of the Group, holds its French laboratory-operating subsidiaries (in the form of SELs) directly and indirectly (through an Italian laboratory company) and its foreign subsidiaries indirectly (through several national holding companies and other laboratory companies). Labco also has full ownership of Biopar (formerly Bioval), of Labco Corporate Assistance and of Labco Services (companies providing services to Group companies), 90% of iPP, 100% of iPP Facilities, 100% of iPP Analytics and 100% of Labco Diagnostics UK. Evolution in the business activities of the Company and the Group The original objective of Labco’s founders, Eric Souêtre and Stéphane Chassaing, was to consolidate through integration (initially in France then in Europe) clinical testing laboratories to enhance the cost-effectiveness of healthcare systems and to help delivering higher-quality healthcare. It is still pursuing the same goal today. 44 At the outset, the Group positioned itself in the diagnostic services market in France in 2003, then expanded across the country by making various acquisitions that established it as one of the two leaders in this market at the date of this document de base. Next the Group expanded into the Spanish market during 2007 by acquiring General Lab S.A., then became the leader in the Spanish clinical testing market during 2008 by acquiring Sampletest, S.A., which had operations in Spain and Portugal. At the same time, the Group began operating clinical testing laboratories in Portugal when it acquired three laboratories in Lisbon from Soprelab, then became the leader in the Portuguese clinical laboratory services market in 2008 following the acquisition of Sampletest, S.A.. The acquisition of Sampletest, S.A. in 2008 enabled the Group to offer to patients in Portugal services that are reimbursed by the SNS. The Group also entered into reimbursement agreements with private insurance companies for patients covered by private insurance. The Group provides services to a public hospital in Cascais through a public-private partnership, and has a small number of companies as customers in Portugal. In 2011, the Group acquired Macedo Dias, the leader in the Portuguese anatomical pathology testing market. The Group is the number one player in the Spanish and Portuguese markets. The Group also started to operate laboratories in Italy in 2007 through the acquisition of the Baluardo laboratory and a shareholding in C.A.M., and following the acquisition of the SDN group in July 2014, the Group became one of the two leaders in the Italian market as of December 31, 2014. The Group also established a foothold in Belgium in 2008 by acquiring the Roman Païs laboratory. In 2008, the Group moved into Germany by acquiring six laboratories. Since the competitive environment is characterized by a high level of consolidation, with a few major players of international stature (Sonic Healthcare, Limbach, Synlab), and mid-sized regional players, the small size of the Group’s operations in Germany rapidly came to be seen as a highly detrimental factor. Furthermore, the results of the Group’s German operations were severely affected by fractious relationships with the former owners of some of the laboratories acquired by the Group. In recognition of the Group’s much weaker market position in Germany, the Company’s board of directors decided to sell all of its operations in this country. This sale took place on December 2, 2013. Following the regulatory changes introduced in France in January 2010, which reduced the restrictions on the outsourcing of clinical testing services and authorized the use of technical platforms, the Group set up technical platforms (some performing specialty testing, also called tests and esoteric testing). Some of the Group’s routine testing laboratories and technical platforms are located at or near hospitals and offer their services in particular to public and private hospitals under outsourcing contracts. In 2010, the Group set up Integrated Pathology Partnerships (iPP) in the United Kingdom, a joint venture with Sodexo, a leading global provider of facilities management services to the healthcare market. As a result of the purchase of 46% of iPP’s shares from Sodexo on October 25, 2013, the Group owned, at the date of this document, 90% of iPP’s shares, a call option on the 3% held by Sodexo and a call option on the 7% granted to iPP’s two main managers through the UK Employee Shareholders Scheme (see section 10.5 “Off-balance sheet commitments” of this document de base). In June 2012, iPP began to operate as scheduled under the three-way partnership with Taunton and Somerset NHS Foundation Trust and Yeovil District Hospital NHS Foundation Trust. Under the partnership agreement, iPP delivers the full range of laboratory services while the clinical interpretation and clinical advice functions continue to be provided by those trusts’ medical staff, who are still employed by the NHS. In October 2014, the Group – via its iPP Facilities and iPP Analytics subsidiaries – started operations with Basildon and Thurrock University Hospital NHS Foundation Trust and Southend University Hospital NHS Foundation Trust to provide laboratory services. Following the 2011 acquisition of CIC (since renamed Labco NOÛS), a specialty testing laboratory based in Barcelona, the Group has also provided clinical testing services to customers in Latin America, Eastern Europe, North Africa and the Middle East. The Group has also entered into outsourcing contracts with private hospitals (such as USP Hospitales, Sanitas Hospitales and Hospital de Manises), pursuant to which it performs testing services either in one of its essential services laboratories or at one of its nearby routine testing facilities laboratories or technical platforms. The Group also performs specialty tests for other private clinical laboratories. 45 The Group also established a presence in Switzerland during April 2013 by joining forces with the laboratory doctors within the Test SA joint venture. At December 31, 2014, the Group operated 64 Laboratories in France, 9 Laboratories and Integrated Diagnostics Centers in Italy, 56 Laboratories in Spain, 25 Laboratories in Portugal, 4 Laboratories in Belgium, 6 Laboratories in the United Kingdom and 1 Laboratory in Switzerland. 5.2 INVESTMENTS The Group’s investments break down principally into the following categories: Acquisitions of groups of companies, companies and/or businesses for consideration, net of cash acquired and the payment in certain cases of fixed or conditional earn-outs, which can be sub-divided as follows: - bolt-on acquisitions made in regions where the existence of the Group’s technical platforms allows industrial synergies to be harnessed rapidly; - acquisitions that improve territorial coverage, primarily acquisitions of platforms in new territories providing a springboard for the strategy of consolidating smaller laboratories; - acquisitions of medical expertise designed to give the Group additional scientific and technological capacity. Acquisitions of tangible and intangible assets, chiefly consisting of: - laboratory materials and equipment; - information systems, i.e. equipment, software, licenses and IT developments; - fixtures and fittings for leased premises and, in a few cases, real estate transactions. The Group’s business model is characterized by a need for investment, excluding acquisitions, of limited capital intensity, primarily consisting of fixtures and fittings for leased facilities and the acquisition of advanced technology analytical equipment. The industry supplying chemical reagents used in clinical testing currently offers a model of providing devices or renting them under integrated equipment rental agreements, supplying chemical reagents and maintaining them to help curb the size of equipment purchases required. These investments are funded using the Group’s treasury and, where appropriate, by drawing on the RCF. For further information about the Group’s credit facilities, see Chapter 10 “Capital Resources” of this document de base. 5.2.1 Historical investments The following table shows a breakdown of the Group’s investments in the financial years ended December 31, 2012, 2013 and 2014: (in millions of euros) 20121 2013 2014 Acquisitions of property, plant and equipment and intangible assets, net of disposals 15.4 18.7 35.5 Acquisitions of groups of companies, companies and/or businesses (net of cash) 45.2 20.4 130.2 Change in company acquisition liabilities (in particular fixed or conditional earn-out payments) (0.7) (0.2) (4.9) 1 2012 data as shown in the Group’s consolidated financial statements for the financial year ended December 31, 2012, including German activities 46 5.2.1.1 Acquisitions and disposals of groups of companies, companies and/or businesses Acquisitions prior to 2008 During financial year 2004, the Group made 11 acquisitions, then made a further 3, 8 and 11 respectively in the financial years ended December 31, 2005, 2006 and 2007. Acquisitions between 2008 and 2011 During financial year 2008, the Group made 28 acquisitions including 15 that enabled it to be established in new regions or facilitated the roll-out of new technical platforms and 13 bolt-on acquisitions for existing platforms. During 2009, the Group acquired 5 units or businesses, 2 of which enabled the Group to move into a new area of convergence in France and into a new region of Germany in and around Karlsruhe. The other 3 purchases were bolt-on acquisitions for existing technical platforms. During the financial year 2010, the Group made 16 acquisitions, including the significant acquisition of the entire share capital of CAM in Italy, giving it control of this unit and enabling it to consolidate it fully, and of another unit in France, enabling it to move into another area of convergence. In addition, the Group acquired the laboratory GDPN with medical expertise in Portugal, thereby expanding its range of genetic prenatal diagnostic testing solutions and made 13 bolt-on acquisitions for the existing platforms in France and Spain. During financial year 2011, the Group made 35 acquisitions for a total amount of €97.2 million in France, Spain, Portugal, Belgium and Italy. Of these acquisitions, 29 were acquisitions that bolted on to existing technical platforms, 4 expanded the Group’s geographical coverage and two supplemented the Group’s clinical project. The Group generated Revenue under French GAAP of €236 million during the financial year ended December 31, 2008. The Group’s Revenue in the financial year ended December 31, 2009 stood at €424 million under French GAAP and €426 million under IFRSs. The Group generated Revenue under IFRSs of €455 million in the financial year ended December 31, 2010 and €508 million in the financial year ended December 31, 2011. Acquisitions in 2012 During 2012, the Group made sixteen acquisitions costing a total of €45.2 million in France and the United Kingdom. Of these acquisitions, fourteen were bolt-on acquisitions to existing technical platforms and two expanded the Group’s geographical coverage. With the addition of the Isolab laboratory, the Group acquired six facilities in the Charente region of western France. This new area of convergence subsequently enabled it to make bolt-on acquisitions in the region, such as the Seudre Biologie laboratory in Marennes, which it purchased in December 2012. During the first quarter of 2012, the Group gained a contract via its Labco Diagnostic UK Ltd subsidiary with the Fresenius Medical Care Ltd group to manage and operate a dialysis laboratory in northern England. This deal gave the Group an operational presence in the United Kingdom, which will facilitate the development of iPP, the joint venture with Sodexo. Acquisitions in 2013 During financial year 2013, the Group made eleven acquisitions costing a total of €20.4 million in France, Spain, and Germany. Of these acquisitions, ten were acquisitions that bolted on to existing technical platforms of the Group and the last one supplemented the Group’s clinical project. The acquisition of the Gemolab laboratory in Spain, which has pioneered cytogenetics and molecular biology testing, bolstered the expertise of the Madrid technical platform and the Group’s range of clinical services. During the summer of 2013, the Group completed a strategic review of its operations in Germany and decided to pull out of the German market to focus on national markets in which it has achieved or may rapidly achieve a 47 leadership position, a distinctive positioning or significant market share. As a result, the Group sold its German business to Sonic Healthcare for €76.0 million during the second half of 2013. Acquisitions in 2014 During the financial year 2014, the Group made sixteen acquisitions for a total amount of €130.2 million in France, Italy, Belgium and Spain. Of those 16 acquisitions, eight were acquisitions that bolted on to the Group’s existing technical platforms and eight, of which five formed part of the SDN group acquisition, supplemented the Group’s clinical project. Of the bolt-on acquisitions, the purchases of the Normandy and Centre laboratories in France were the largest. These two companies had the same majority shareholder, which bolstered the management teams in the Group’s French organization. The two laboratories, which are located across 11 sites, have over 150 employees handling around 1,000 files per day. These two acquisitions have been integrated with the Group’s existing convergence areas in the Normandy and Centre regions and major industrial synergies are expected to be harnessed rapidly. Following on from the acquisition of Gemolab in 2013, the Group made two further acquisitions of clinical expertise in Spain in 2014, buying the Sanilab Molecular and CIG-GM laboratories. These acquisitions have helped to enhance the Group’s clinical expertise in molecular biology and cytogenetic testing. In September 2014, the Group bought a stake in the Alpigène laboratory, which is authorized to carry out cytogenetic and postnatal human genetics work, and also prenatal activities relating to serum markers for Down’s syndrome in fetuses. The Group owns 55% of the economic rights, with the rest being owned by the founding biologist and chairman, and by a group specializing in environmental analysis services. As a result, the Group is able to offer leading-edge molecular biology services in the French market. In July 2014, the Group completed the highly strategic acquisition of the SDN group in Italy. The acquisition of the SDN group, which operates 5 Laboratories, including 3 Integrated Diagnostics Centers in the Naples region and has 255 employees, represented a major boost to the Group’s clinical project. The SDN group possesses unique know-how in integrated diagnostics encompassing clinical testing and the full range of medical imaging solutions, such as X-ray, MRI and the most sophisticated medical imaging technologies, such as PET scans combining molecular and nuclear imaging (see section 6.4.3.2 “Group’s operations by country – Overview of Southern European Market– Italy” of this document de base). Acquisitions in 2015 At the date of this document de base, the Group has made 3 acquisitions for a total amount in enterprise value of around €4 million, in France, Italy and Belgium. On March 2, 2015, the Group finalized the acquisition of the Laboratory CPG in the Brussels region for an enterprise value of €2.2 million. In addition, on April 3, 2015, the acquisitions of TMA and TMA Medica in Genoa for a global enterprise value of approximately €2 million reinforced the Group’s clinical imaging offer in this region. The Group expects in addition to make acquisitions of Unibionor and Unibio Laboratoire before the end of the first semester 2015 for a global enterprise value of approximately €45 million. Those companies own a group of laboratories that is one of the leading clinical testing players in the Lille region. The group operates 15 sites, including a technical platform, and treats around 1,700 patients per day. It has around 130 employees and generated revenue of €18 million in the financial year ended December 31, 2013. The acquisition will enable the Group to achieve broader and denser geographical coverage of the Group in France. It will lead to a structural reorganization in the region, since two of the Group’s laboratories will be merged with the acquired companies, eventually forming a new structure with revenue of €35 million across 23 sites, including a central technical platform. The Group expects at last to make, before the end of the first semester 2015, the acquisition for an enterprise value of approximately €10 million of the laboratory BBM, which would strengthen the Group’s position in the Aquitaine region. 48 5.2.1.2 Acquisitions of property, plant and equipment and intangible assets Acquisitions of property, plant and equipment and intangible assets represented approximately 2.7%, 3.4% and 5.8% of the Group’s Revenue for the financial years ended December 31, 2012, 2013 and 2014, respectively. For the financial year ended December 31, 2014, exceptional acquisitions of intangible assets, totaling €15.3 million and relating to the real-estate projects described below, were made. Laboratory equipment and fittings Aside from acquisitions, the Group’s main investments are in fixtures and fittings for leased premises, the renewal of laboratory equipment including vehicles, and the acquisition of high-tech tools. Investments in equipment, excluding vehicules, fixtures and fittings for premises, amounted respectively to €6.2 million, €5.9 million and €5.2 million for the financial years ended December 31, 2012, 2013 and 2014, and represented around 1% of the Group’s revenue on December 31, 2014. Information systems In 2012, the Company selected an asset and procurement management system. The system is made up of two modules. The first provides a unified, standardized and centralized view of all Group assets. In particular, it makes it easier to exchange and transfer equipment between Laboratories. The second relates specifically to procurement management and creates a semi-centralized, unified supply chain. It automates purchasing, listing, procurement, inventory, order/reception and dispute management operations. The module connects with local accounting systems and significantly improves order monitoring. It is also facilitating the gradual implementation of data exchanges, making it easier to automate exchanges with suppliers. At the date of this document de base, the asset management module had been rolled out across the whole Group, while the procurement management module had been deployed in Spain, Portugal, the United Kingdom, Italy and Switzerland. The total investment cost is estimated at €1.5 million over the financial years 2012, 2013, 2014 and 2015. In 2013, the Group set up a database that centralizes information relating to all laboratories’ operational transactions and sent daily by almost all IT systems belonging to laboratories within the network. The information includes revenue, the number of files, the number of tests, information relating to patients (who are anonymized), information relating to employees in France, inventories and purchases. The data enables the Group to carry out unified supervision of its business units. Roll-out of this project was completed in late 2014. Its development and deployment represented an investment estimated at €2.5 million. In 2014, the Group acquired the “iLab” mobile application enabling health professionals and partners of the Group’s laboratories to view the testing catalogues and sampling handbooks and to keep themselves up to date with any changes. New functions for healthcare professionals (nurses, medical doctors and laboratory doctors in particular) and patients are being added to the application on an ongoing basis. Numerous updates are planned for 2015 and 2016. This acquisition represented an outlay of less than €0.3 million. Real estate investment The Group did not make any major real estate investments during the financial years 2012 or 2013. Three investments were carried out in 2014: the Group has started the project of establishing a new technical platform completed two new technical platforms in Barcelona, Spain for a total cost of €15.5 million, including €12 million which were spent in the financial year 2014. The Group has also started the project of establishing a new technical platform in Basildon in the United Kingdom for a total cost of €7.6 million, including €1.6 million which were spent in the financial year 2014 (see section 8.1.1. “Real estate properties” in this document de base”). Finally, the Group acquired a building in Brussels as part of the acquisition of a Laboratory on July 1, 2014, for the total amount of €1.7 million (see section 5.2.1.1. “Acquisitions and disposals of groups of companies, companies and/or businesses - Acquisitions in 2014” of this document de base). 49 5.2.2 Current investments Only investments in excess of €1 million are presented in this section. 5.2.2.1 Acquisitions of groups of companies, companies and/or businesses In addition to the on-going acquisitions described in the present Section 5.2.1.1. “Acquisitions of groups of companies, companies and/or businesses” of this document de base, the Group has sent out letters of intent to owners of several laboratories with a view to potentially acquiring those laboratories for a total, in enterprise value, of around €16 million, of which €9 million relate to acquisitions intended to bolster the Group’s medical offering in the anatomy and pathology area. Should these letters of intent lead to legally enforceable agreements being entered into, the corresponding investments would be described in an update to this document de base or in an offering circular concerning the Company’s IPO. 5.2.2.2 Acquisitions of property, plant and equipment and intangible assets Information systems In 2014, the Group launched a plan to harmonize the laboratory information systems of all the network members by 2020. The aim of this “EuroLIS” project is to replace all information systems currently in service in network laboratories in order to achieve a significant increase in flexibility and productivity. This LIS will initially be installed in 2015 in a pilot laboratory in France, before being rolled out across all French laboratories. Subsequently, EuroLIS will be rolled out across Europe. The investment required to complete the development of EuroLIS is estimated at around €10 million between 2015 and 2017. 5.2.3 Future investments The Group has a portfolio of potential acquisitions enabling it to continue its expansion drive to bolster its geographical coverage and bulk up its network of clinical laboratories. Although the Group has not given any firm commitments other than those described in section 5.2.2. “Current investments” of this document de base, the Group plans to continue making investments in markets in which it is present or in markets in which it is looking to expand for potentially significant amounts. The investments to be made in pursuit of the Group’s investment strategy may help it establish itself in countries where it has little or no presence. The Group also intends to pursue further expansion in specialty clinical testing, such as molecular biology and genetic testing and other clinical diagnostics including anatomical pathology testing and medical imaging, to produce integrated diagnoses, by making selective acquisitions or forging commercial partnerships with biotechnology companies. 50 CHAPTER 6 OVERVIEW OF GROUP BUSINESSES This chapter 6 “Overview of Group Businesses” describes the business sectors and business activities of the Group. It contains information relating to the markets in which the Group operates, the business segments in which the Group is active and the Group’s competitive position. In addition to estimates produced by the Group, certain elements on which the Group’s statements are founded are drawn from information received from third parties and in particular from L.E.K. M&A SAS which established a report dated as of February 23, 2015, commissioned by the Group (the “L.E.K. Report”) and from other supplementary sources (see chapter 23 “Information derived from third parties, experts’ statements and declarations of interest” of this document de base). Some information contained in this document de base is information available to the public that the Company considers to be reliable but which has not been verified by an independent expert. 6.1 OVERVIEW OF THE GROUP The Group is one of the leading groups in the European clinical laboratory services market. It is the market leader in Spain and Portugal based on the revenue 2013. It is one of the top two leaders in France and in Italy, where it has a strong presence in medical imaging and ambulatory services. The Group also has a significant base in Belgium, where it is one of the market leaders, particularly in Brussels and in Wallonia. In addition, the Group has been present in the United Kingdom since 2010, where it provides laboratory outsourcing services to hospitals and other healthcare organizations, ranking number three in this market. The Group has also established a presence in Switzerland in 2013, by acquiring a stake in the Test SA joint venture. As of December 31, 2014, the Group provided services through its network of approximately 165 laboratories in seven European countries and approximately 1000 Sampling Centers. It also provides clinical laboratory testing services in Eastern Europe, Latin America, the Middle East and North Africa. The map and the graph below provides information setting out the number of Laboratories held by the Group as of December 31, 2014 and revenue for the financial year ended December 31, 2014 with the acquisition of the SDN group recognized on a pro forma basis. 2014 revenue €650 million 51 Southern Europe segment Northern Europe segment France Total Revenue Revenue contribution Number of Laboratories* €342.3 million 52.7% 64 Portugal Total Revenue Revenue contribution Number of laboratories* (Portugal) €43.1 million 6.6% 25 Belgium Total Revenue Revenue contribution Number of Laboratories* €30.0 million 4.6% 4 Spain Total Revenue Revenue contribution Number of laboratories* (Portugal) €115.6 million 17.8% 56 United Kingdom Total Revenue Revenue contribution Number of Laboratories* €27.0 million 4.2% 6 Switzerland Total Revenue Revenue contribution Number of Laboratories* €2.0 million 0.3% 1 Italy Total Revenue Revenue contribution Number of laboratories* €89.5 million 13.8% 9 * Excluding Sampling Centers 6.1.1 Group businesses The Group is a private actor in the healthcare sector, active in the field of medical diagnostics, mainly clinical laboratory services. The Group offers a wide range of analysis and diagnostic testing services, notably including: In the field of clinical testing, plus 5000 routine and specialist tests (including molecular biology and nutritional biology); anatomical pathology testing of both histological and cytological samples; diagnostic imaging using medical and molecular imaging technologies. The Group also offers MAR services in some of its laboratories in France. Healthcare systems differ from one country to the other in terms of the level of coverage of diagnostic and analysis costs by the public healthcare organizations. Although the final client is always a patient, services may be paid for by private health insurers, employers, hospitals, other laboratories or patients themselves. As of December 31, 2014, the Group had almost 6,000 employees and medical staff and more than 600 people qualified to run a clinical laboratory. Since it was founded in 2003, the Group has mainly developed its network through selective acquisitions of small and medium-sized clinical laboratories. Between 2007 and 2011, the Group extended its footprint notably through the acquisitions of Roman Païs in Belgium, CAM and Baluardo in Italy, and General Lab and Sampletest in Spain and Portugal. In 2012, 2013 and 2014, the Group completed sixteen, eleven and sixteen acquisitions respectively, in France, Belgium, Spain, the United Kingdom and Italy (see section 5.2 “Investments” and, in particular, section 5.2.1.1 “Acquisitions and disposals of groups of companies, companies and/or businesses – Acquisitions in 2014” of this document de base). 52 In 2013, the Group decided to withdraw from the German market, taking the view that it did not enjoy critical mass there, and concentrated on strategic markets where it could attain or strengthen market-leading positions and generate significant economies of scale. This transaction also provided the Group with the resources necessary to make further acquisitions whilst at the same time reducing debt. For the financial years ended December 31, 2013 and December 31, 2014, the Group recognized a revenue pro forma for the acquisition of the SDN group of €594.7 million and €649.6 million, respectively, and generated an EBITDA pro forma for the acquisition of the SDN group of €128.7 million and €131.3 million, respectively (See Chapter 3 “Selected Financial Information” of this document de base). 6.1.2 1. The Group’s economic advantages A market combining long-term growth and resilience The European clinical laboratory services market is characterized by regular growth and high barriers to entry, which has allowed the Group to withstand the effects of economic cycles. The key factors driving this growth are: 2. demographic factors, particularly the ageing population in the Group’s markets and the increased prevalence of chronic and long-term diseases the management of which requires regular and substantial use of diagnostic services; the growing use by public healthcare organizations of the private sector companies for the operation of their diagnostic services through outsourcing contracts; technological advances producing a considerable broadening of the scope of use of diagnostics, particularly as part of the development of so-called “4P” medicine (see section 6.2.2.3. “Presentation of sectors in which the Group is active – Sector trends – Sub-contracting and outsourcing” of this document de base); and the growing responsibility taken by individuals for the management of their own health, leading them to decide independently to seek clinical analysis that is not necessarily covered by public healthcare systems (nutritional analysis, Down’s Syndrome testing, etc.). A pan-European presence widely deployed Since 2007, the Group has significantly expanded its operations and now benefits from a pan-European network of clinical laboratories in seven countries. It is the market leader in Spain and Portugal and one of the top two leaders in France and in Italy based on pro forma revenue on December 31, 2013. Moreover, in the United Kingdom, the Group ranks third in the market for sub-contracted and outsourced clinical analysis services for NHS hospitals. The professionalism of its management systems and operational management, coupled with its extensive laboratory network allows the Group to combine economies of scale at a European level with a detailed knowledge of the local environment. 3. A medical project enabling the Group to build leading positions in cutting-edge medical specialties The Group is a leader in clinical diagnostics, offering a full range of standard and specialist analysis, anatomical pathology and medical imaging services. Thanks to this full range of services and to its established expertise, the Group is ideally placed to take full advantage of the transition from curative medicine to so-called “P4” medicine (see section 6.2.2.4 “Presentation of sectors in which the Group is active – Sector trends – Medicine and new technologies” of this document de base). 4. Proven experience as a consolidating force in a market that is still too fragmented A pioneer in consolidation, the Group has developed a structured methodology for the management of the whole acquisition process, from the initial phase of identifying targets through to the integration of the entities acquired, via all intermediate stages of due diligence and negotiation. Since its creation in 2003, the Group has 53 made and integrated more than 160 acquisitions in 7 European countries. The sector remains highly fragmented and the Group is ideally placed to be a major player in its future consolidation. 5. An industrial approach to the structural challenges of the sector In Europe, the highly fragmented sector has not taken full advantage of substantial potential economies of scale. The Group is a leader in identifying and developing sources of improved productivity that enable the concentration and automation of testing and the standardization of processes. 6.1.3 The Group’s strategy The Group’s ambition is to become the major pan-European reference in medical diagnostics, similarly to major international groups in the sector (Sonic Healthcare in Australia, Quest Diagnostics Inc. and Laboratory Corporation of America Holdings in the USA), by drawing on the relevance of its strategy and the expertise of its teams. The Group’s strategy has 4 key planks: The medical project: anticipate future developments in order to seize new opportunities for growth: - Maintain medical excellence to capitalize on the substantial scientific and technological progress made in the field of clinical laboratory services, and drive innovation to be part of the development of a wider range of new tests and services at a pan-European level; Develop a strong identity and strong brands alongside a structured marketing approach, allowing new sources of potential demand to be identified and addressed. Deploy a strategy of growth through acquisitions focused on the creation of shareholder value, building on the Group’s strong ability to integrate acquisitions and a cautious approach to valuations; Establish the Group as a preferred partner of public organizations seeking to outsource their medical diagnostic services; and Build on operational efficiency and economies of scale in order to continue to drive growth in the Group’s cash flow. 6.2 PRESENTATION OF SECTORS IN WHICH THE GROUP IS ACTIVE This section 6.2. “Presentation of sectors in which the Group is active” of this document de base sets out information on the countries, sectors and segments in which the Group is active. 6.2.1 Background The Group, a major player in public health, conducts its business in Europe in the private healthcare sector, and more precisely in the field of clinical diagnostics. The clinical diagnostics market offers, through companies, Diagnostics Centers and Laboratories, analysis and diagnostics services, which notably include the following services: clinical biological testing, including standard and specialist tests (molecular biology, nutritional biology); anatomical pathology testing of both histological and cytological samples; diagnostic imaging using medical and molecular imaging technologies. It is estimated that diagnostic services are used in approximately two-thirds of medical decisions, but they are generally considered to account for approximately 3% of health spending in France in 2012 (source: L.E.K. Report). It is widely agreed that technological innovation, coupled with the quest for greater clinical and financial efficiency in the health sector, is likely to drive an increase in the share of total health spending that goes on diagnostic services. 54 Operational models can differ significantly depending on (i) the structure of the local healthcare system and the nature of payers, (ii) the nature of prescribers, (iii) regulation and (iv) the approach to patient care. In reality: 6.2.2 healthcare systems differ from one market to the other, with varying levels of coverage by the public healthcare organizations. Other groups of payers include private insurance companies, employers, hospitals, other laboratories or patients themselves; regarding prescribers, the healthcare professional prescribing to the patient the analysis necessary for a good clinical diagnosis may have a more or less direct effect on the choice of the laboratory. In some markets, healthcare professionals may themselves be able to offer a limited range of analysis. In addition, for certain types of analysis, patients sometimes decide to avail themselves of laboratory services; regulation relating to the qualification of laboratory doctors and the laboratory personnel, technical conditions for testing, professional independence of laboratory doctors and conditions for owning, establishing and operating clinical laboratories vary from one country to another (see section 6.5 “Regulation” of this document de base); lastly, there is a distinction to be drawn between the in-patient sector and the out-patient or “ambulatory” sector. In the in-patient market, clinical laboratories increasingly compete with for hospital laboratory sub-contracting and outsourcing contracts, from both public and private hospitals, mainly on the basis of price and quality of service. Sector trends The Group expects a number of key trends to affect the clinical laboratory services market in Europe generally, as well as the Group’s business. The current economic slowdown has reduced industry growth rates. However, because clinical testing is an essential healthcare service and because of the key trends discussed below, the Group believes that the industry will continue to grow over the medium and long terms. 6.2.2.1 Demographics Demographic factors, particularly the ageing of Europe’s population and the increase in the number of chronic and long-term illnesses around the world, will have a positive effect on the future volume of clinical testing. In France, for example, the percentage of the population aged over 64 is expected to rise from 16% in 2008 to 20% in 2018. This proportion is likely to change in a similar way than in the other countries where the Group is active: from 17% to 19% in Spain; from 18% to 21% in Portugal; from 20% to 23% in Italy; from 17% to 19% in Belgium and from 16% to 18% in the United Kingdom (source: Oxford Economics). In France, a 2009 study showed that persons aged over 70 spent 11 times more on average on clinical tests than persons aged under 20 (source: L.E.K. Report). Similarly, a large number of studies show that chronic and long-term illnesses, notably cancer and diabetes, are increasing in number and that they are likely to continue to increase through 2035. In France, for instance, the number of people with long-term conditions (Affections Longue Durée) increased on average by 5% each year, rising from 6.6 million in 2004 to 9.5 million in 2012 and is likely to reach 10.5 million in 2015 (source: Xerfi). 6.2.2.2 Pricing conditions The market for clinical laboratory services has suffered prices’ decreases in most of the countries where the Group is active. Pressure on government budgets has produced reductions, sometimes repeatedly, in the level of reimbursement by public healthcare organizations. For example, according to estimations, prices have fallen in France for each of the past seven years by an average of 2% to 3% per year (source: L.E.K. Report). The French government announced its intention of requiring at least €110 million in reductions in spending on clinical laboratory costs in 2012; an agreement was 55 finally reached on October 10, 2013 between the main French biologists’ trade unions and UNCAM. The purpose of this agreement is to give clinical laboratories visibility on their financial prospects over a three-year period, whilst also exerting control over healthcare spending. This resulted in the determination of an annual rate of growth for spending on clinical laboratory services of 0.25% between 2014 and 2016. This target will be reached by modest reductions in prices to be spread over the period, and by a control of prescriptions, in order to tackle the natural growth in volumes. Trade unions meet with the health insurance funds every six months, in order to assess the impact of changes in prices and determine what future changes may be necessary to meet the annual growth target. In late January 2015, CNAM held discussions with professional unions to reiterate its commitment to the three-year agreement and to propose a rate revision commensurate with volume growth projected for 2014 and expected for 2015. The Company estimates that the proposed rate revision will have a gross adverse effect on revenue of around 1.50%, while the 2014 revision had a gross adverse effect of 2.51% and a net adverse effect of around 1.20% (reductions are estimates based on annual test volumes). According to CNAM’s projections, volume growth in 2015 will make up for the decline and result in the 0.25% target for annual clinical expenditure growth set by the agreement being attained. Definitive 2014 figures will be available in late June 2015, as will information about the trend in early 2015. On that basis, a decision will be made regarding a revision to the nomenclature in September 2015, in order to get as close as possible to the 0.25% target regarding growth in annual clinical expenditure. 6.2.2.3 Sub-contracting and outsourcing Sub-contracting and outsourcing by public and private hospital laboratories to the benefit of private organizations is another trend observed in the European clinical laboratory services market over the last few years, driven in particular by the productivity gains that it brings to hospital operators. The Group believes that sub-contracting and outsourcing will potentially represent a growing source of income and will give the Group greater visibility on future income. This trend is not at the same stage of maturity in all European countries. It is at the date of this document de base most prevalent in Spain, especially in the private hospital sector. In Portugal, most public hospitals supply clinical laboratory services internally, whilst private hospitals tend to sub-contract or outsource such services to private laboratories. For example, the hospitals of Cascais and Laures have outsourced their services to the Group. In the United Kingdom, NHS hospitals, facing tight budget restrictions, are increasingly outsourcing their clinical laboratory services to private groups (see section 6.4.3.1 “Group operations by country – Overview of Northern Europan segment – United Kingdom” of this document de base). The Group believes that these trends in the United Kingdom, French, Portuguese and Belgian markets encompass growth potential in the near future. 6.2.2.4 Medicine and new technologies It is widely acknowledged that medical practice has entered a period of profound change, moving from curative medicine to so-called “4P” medicine: Personalized, Predictive, Preventative and Participatory. Clinical laboratory testing will have a key role to play in the development of so-called “4P” medicine, the growth of which will be accompanied by strong growth in the most advanced segments of medical biology, especially molecular biology. Medicine will become Personalized in the sense that it has been established that some treatments may work with one patient but not with another and a simple test can be carried out to identify those for whom it will be effective. Thus Personalized medicine could see the development of treatments suited for specific groups of patients with shared medical and genetic characteristics, ensuring greater effectiveness. Most cancer treatments brought on to the market are accompanied by pharmaco-genetic tests (or “companion tests”) which, according to the patient’s genetic profile, help identify the right product and dose to optimize the response and reduce secondary effects. Molecular biology tests (or genetic analysis), and in particular monitoring of biomarkers indicating the state of health of individuals, help identify the risk of certain diseases, allowing for the early detection of potential problems before the emergence of clinical symptoms. This is Predictive medicine. Certain genetic tests can assess the risk for an individual of developing certain types of cancer, such as breast or colon cancer. Predictive medicine creates the conditions for a massive preventative program through the organization of screening campaigns for certain diseases. This increased involvement of medicine in the lives of 56 populations is termed Preventative medicine (i.e. early diagnosis of chronic diseases such as diabetes and high cholesterol). Lastly, an approach which sees individuals take more responsibility for their health and treatment is becoming more widespread. This is Participatory medicine. The development of epigenetics – the interaction between the environment and the expression of genes – shows that patients can, through their behaviors, influence the onset or the evolution of diseases: the nutritional tests offered by the Group, for example, help guide changes in dietary habits and thus reduce the risk of contracting certain diseases. However, increased individual responsibility for health can only come through offering patients a broader range of more sophisticated medical analysis tools. For example, the Group is developing an adjustment for the European market of an american application for health monitoring. This platform, accesible for both the patient and his doctor, contains medical and nutritional information, information related to the patient’s physical activity as well as regular medical diagnostics in order to anticipate or avoid certain diseases. All in all, the Group expects that the components of so-called “4P” medicine, combined with the most sophisticated diagnostic techniques, will produce significant gains in therapeutic and economic effectiveness. In particular, new opportunities in prevention and in efficient targeting of treatments should allow significant cost savings for healthcare systems, on a scale that would affect the selection of policies that accelerate the expansion of diagnostic activities. The acceleration in the development of so-called “4P” medicine has been made possible thanks to profound changes in biological and medical research that has opened the way to a considerable expansion of the application of clinical testing in diagnostics. This transformation has been driven by the development of new techniques which give access to a substantial amount of data on the individual. Progress in DNA sequencing has had a particularly decisive impact and has become the main engine driving the development of molecular diagnostics. There has been a diversification in the methods available, with polymerase chain reaction (PCR) and high-speed next-generation sequencing (NGS), allowing for more targeted testing and analysis and even the sequencing of an entire genome, which is now considerably quicker and cheaper to achieve. The cost of sequencing a human genome has fallen significantly in the past few years. It was less than $5,000 in 2014 (source: L.E.K. Report) and is likely to continue to fall. We are therefore likely to witness a real explosion in demand for and the use of sequencing in medical diagnostics. Technical progress in sequencing is moving hand-in-hand with ever better understanding of biological mechanisms. For instance, based on the observation that fragments of fetal DNA circulate freely in maternal blood during pregnancy, progress in sequencing has made it possible to develop non-invasive prenatal tests for Down’s Syndrome using maternal blood. It is easy to imagine that molecular biology will soon make it possible to detect a number of other disorders – such as cystic fibrosis and spinal muscular atrophy – from a sample of maternal blood. Similarly, it is now possible to identify the genetic changes that give tumors their malignant nature, through analysis of the tumor genome, allowing for a greater role for clinical testing in the field of oncology. More generally, these developments in molecular biology, combined with the identification of new biomarkers, for all sorts of diseases, as well as new tests for allergies and in nutritional biology, are likely to drive a phase of significant expansion in the portfolio of tests carried out in the most sophisticated laboratories. 6.2.2.5 Quality Standards Changes in quality standards are also shaping the clinical laboratory services market. The Group believes that the quality standards applicable to the sector, although varying from 1 country to the next, are likely to become increasingly restrictive over the next few years. For example, French legislation now requires that all clinical laboratories should have undertaken a quality accreditation process (see section 6.5.1. “Regulation – France” of this document de base) and it is likely that other European countries will follow suit. In view of the resources required to implement these quality standards, many small and medium-sized independent laboratories could be tempted to join a network offering a quality department and dedicated teams. 57 6.2.2.6 A personalized direct offering The growing responsibility taken by individuals for the management of their own health is leading them to decide independently to seek clinical analysis that may not be covered by public healthcare systems. Strong growth in nutritional medicines in Belgium or the rapid increase in the number of non-invasive tests in Spain are good examples of this (see sections 6.4.3.1. “Group operations by country – Overview of Northern European market – Belgium” and 6.4.3.2. “Group operationss by country – Overview of Southern Europe market – Spain” of this document de base). The Group believes that this trend is likely to continue and could even accelerate with the development of further new tests. These are a few examples of the innovative tests offered in the Group’s catalogue: “A200”, a test developed especially for the Group that detects, from a blood sample, a patient’s intolerances to more than 200 kinds of food; “Septin9”, a non-invasive test to detect early-stage colon cancer, avoiding the need for colonoscopy; “Breast cancer genes panel”, using the new sequencing generation (NGS), this testing profile detects the presence of each of the 21 genes involved in the hereditary risk of developing breast cancer; “Prosigna”, provides reliable identification of the 10-year risk of future recurrence and sub-type of breast cancer, enabling oncologists to decide whether or not to prescribe chemotherapy; “TDAGen+”, a genetic test for patients with Attention Deficit Hyperactivity Disorder (ADHD) which collects personalized information on the main genetic factors involved and allows predictive treatment in response; “Life Length”, measures the percentage of short telomeres in individual cells taken from a blood and/or tissue samples, providing an accurate indicator of telomere dysfunction and cellular ageing. “HPV OncoTect”, a test allowing an early detection of cervical cancer; “Gut Microbiome”, a functional biology genetic test aiming at analyzing genomes of normal living microorganisms of the human being (microbiota); “Recombine”, a non-invasive test to detect the risk of transmitting a genetic disease to one’s child; “Migratest”, a test helping to evaluate the potential causes of migraine headaches in order to determine the best treatment; “Liquid Biopsy de Pangaea Biotech”, a test revealing the potential mutations of EGFR, BRAF and KRAS genes (usually related to lungs cancer) and which facilitates the determination of the best treatment for the patient. 6.2.2.7 Consolidation The Group believes that a combination of factors, including pricing pressure, changing quality standards, the increasing complexity and technical demands of tests and the on-going industrialization of processes seeking to generate economies of scale and cost reductions, are likely to lead to the consolidation of the portion of the European clinical laboratory services markets that remains fragmented. Consolidation could increase the market share of medium- and large-scale laboratory groups with a pre-existing footprint and level of expertise and could accelerate the entrance of large competitors in the European market. 6.2.3 Competitive features The European clinical laboratory services market is highly competitive. The Group believes that as of the date of this document de base there are very few truly pan-European players in the market. However, the Group expects further cross-border consolidation among certain of its competitors as well as possible increased 58 penetration of the European clinical laboratory services market by some of the major non-European laboratory groups. The Group continues to implement strategies designed to improve its competitive position. Due to the regulated price structure of most European clinical laboratory services markets, the Group competes mostly on the basis of the quality of the services provided. However, in liberalized markets such as Spain, healthcare providers and third-party payers often select a clinical laboratory on the basis of price. The Group believes that patients who are free to choose the clinical laboratories where they are tested usually base their choice on a laboratory’s proximity to their home or workplace, and that other clients of clinical laboratories (mostly doctors, hospitals and other healthcare providers) consider the following factors, among others, in selecting a clinical laboratory: accuracy, timeliness and consistency in reporting test results; the reputation of the clinical laboratory in the medical community or field of specialty; the number and type of tests performed; the method of delivering/publishing results; and the tools available for interpreting results. In addition, price is a key factor in the hospital outsourcing market. On an individual basis, the Group’s clinical laboratories compete with independent clinical laboratories, hospital-based laboratories and doctor-office laboratories. At a group level, the laboratories compete with other national, European and international groups. Regional and national clinical laboratory groups. Certain national champions emerged between 1995 and 2005, such as Cerba (which recently announced the acquisition of the Novescia group) and Biomnis in France, Unilabs in Switzerland, and General Lab and Echevarne in Spain. Since 2006, these groups began consolidating into European groups. However, a number of leading independent national groups remain in Europe, such as Medina in Belgium, Viollier and Medisupport in Switzerland, Bio-Access in France and Echevarne in Spain. In addition, in a number of countries, and in particular in France, there has been an emergence in the recent past of groups organized at a regional level, which are amongst the main competitors of the Group in their particular regional markets. European clinical laboratory groups. In the last few years, investment funds have shown an increased interest in the healthcare industry, and more particularly in the clinical laboratory services market. This trend is likely to accelerate market consolidation and the integration of pan-European groups. For example, Unilabs, in which Apax and Nordic Capital invested in 2007, operates in eleven European countries including France, Switzerland, Spain, Portugal and the United Kingdom, and Synlab, in which BC Partners invested in 2009, operates in about twenty five countries including Italy, Belgium, the United Kingdom and Switzerland. The consolidation in western Europe has been echoed further east with the formation of pan-European groups such as Synevo, which is present in ten countries, and Medicover (Alpha Medical and Diagnostyka), which is one of the leaders in Poland, the Czech Republic and Slovakia. 59 Non-European clinical laboratory groups. The major international players are notably: Sonic Healthcare, an Australian group, the only truly international player in the clinical laboratory services market, with operations in Australia, New Zealand, the United States of America, Belgium, Germany, Switzerland, Ireland and the United Kingdom. Quest Diagnostics and Laboratory Corporation of America are both American companies and are by far the two largest clinical laboratory groups in the world but operate predominantly in the United States; and Diagnosticos da America and Fleury Medicina e Saúde, Brazilian groups which operate exclusively in Latin America. 6.2.4 Clinical Laboratory Services Market in Europe 6.2.4.1 Overview The European clinical laboratory services market is highly fragmented. In the countries where the Group is present, namely France, the United Kingdom, Belgium, Switzerland, Portugal and Italy, there were in 2013 around 9,000 working private laboratories (source: L.E.K. Report). There are generally three main types of clinical laboratory service providers: hospital-based laboratories, doctor office laboratories and independent laboratories. In 2013, the clinical laboratory services market in countries where the Group operates generated revenues estimated at approximately €22 billion (of which approximately €8.7 million in the private sector), representing around 70% of the revenue generated in the overall clinical laboratory services market in Europe, estimated at approximately €30 billion (source: L.E.K. Report and GIA). From 2015 to 2020, the european market is expected to grow by about 3.3% a year to reach approximately €40 billion (source: GIA). The diversity of healthcare systems in Europe also means that the provision of clinical laboratory services varies from country to country, particularly in relation to the following aspects: in 2013 the number and the density of private clinical laboratories: the density of private laboratories is estimated to amount to 6 laboratories per 100,000 inhabitants in France and 1 laboratory per 100,000 inhabitants in Belgium (source: L.E.K. Report); regulation relating to the qualification of laboratory doctors and laboratory personnel, technical conditions for testing, professional independence of laboratory doctors and conditions for owning, establishing and operating clinical laboratories vary by country (see section 6.5 “Regulation” of this document de base); quality standards: in certain countries, national regulation requires, or will soon require, the accreditation of all clinical laboratories, while other countries accept internal quality management standards. For example, in France, starting November 2016, accreditation for clinical laboratories will gradually become compulsory; pricing of clinical laboratory tests: the prices in most clinical laboratory services markets in Europe are, to a large extent, regulated. Significant disparities in prices persist, with higher prices per test in France, Portugal and Italy than in Belgium. In some countries, such as Spain, regulated prices in private clinical laboratory services have been replaced by contractually negotiated prices with private third-party payers; choice of laboratory: depending on the country, the choice of clinical laboratory for a patient’s tests is made by the patient, by his or her doctor or by his or her health insurance provider. 6.2.4.2 Northern Europe segment France In 2013, the French clinical laboratory services market generated revenues of approximately €7 billion, with private laboratories representing approximately 66% of these revenues (sources: BIOLAM, Rapport de la Cour 60 des comptes pour la biologie médicale en date du 18 juillet 2013 and L.E.K. Report)). In 2011, 2012 and 2013, outpatient clinical laboratory services in private laboratories generated fairly stable revenue levels of approximately €4.6 billion, €4.5 billion and €4.6 billion respectively (sources: BIOLAM and L.E.K. Report). France is the biggest market in Europe, ahead of Germany, Italy, the United Kingdom and Spain (source: GIA). In 2013, there were approximately 3,900 private laboratories out of a total estimated at 4,700 laboratories operating in France (source: L.E.K. Report). The number of qualified clinical pharmacists on December 31, 2013 was about 7,600 compared with approximately 8,000 on December 31, 2009 (source: Xerfi). The French clinical laboratory services market is highly regulated. It has some of the highest prices in Europe. In 2012, the French health system was, according to estimations, around 76% funded by the Social Security system, with private health insurers contributing to 14% approximately and patients themselves to 10% (sources: HCAAM and L.E.K. Report). In France, medical doctors prescribe clinical tests for their non-hospitalized patients, who are free to choose their laboratory. Patients typically choose a laboratory based on proximity to their home or workplace. Accordingly, the choice of a high-traffic location and a good reputation for quality of services are key factors. Prices for clinical tests are set by a commission consisting of representatives of the Ministry for Social Affairs, Health and Women’s Rights, of the CNAM and of professional bodies. Tests for which reimbursement is authorized are included in the nomenclature of clinical tests and quantified by the letter B, which is worth €0.27 (sources: BIOLAM and L.E.K. Report). Generally, more specialized and newer tests are not reimbursed and are thus not included in the nomenclature, at least initially. The pre-requisite for inclusion of a test in the nomenclature is CE labelling of the In Vitro Medical Diagnostic Device(s) necessary for the test to be carried out. Depending on the uptake of a test or of its therapeutic advantages, UNCAM may seek an opinion from the Haute Autorité de Santé (HAS) and UNCAM regarding the inclusion of the test in the nomenclature. HAS will base its opinion on the following criteria: the indications for which the proposed service is assessed and those for which HAS believes inclusion is warranted, identifying, where appropriate, the population groups affected; a description of the role of the act or service in therapeutic strategy; an assessment of the improvement offered by the proposed service compared to alternative standard therapeutic approaches based on current scientific data, notably with regard to the comparative effectiveness of these treatments. The improvement offered by the proposed service is evaluated for each indication and, where appropriate, by population group. With or without this advice, a commission for the evaluation of clinical laboratory acts and services approves or rejects the inclusion of the act in the nomenclature and the price that has been determined by UNCAM. This commission consists of 6 biologists representing the profession (trade unions), 3 UNCAM representatives, each holding 2 votes, and a chairman, elected by the commission, who holds a single vote. However, UNCAM has the final decision on proposing the inclusion of a test in the nomenclature and submitting its proposal to the Minister. If the Minister accepts the proposal, the test is included in the nomenclature by advertisement in the Official Journal. The government had announced its intention of better controlling healthcare spending, particularly spending on clinical testing by French clinical laboratories. An agreement was signed on October 10, 2013 by the main French biologists’ trade unions and UNCAM. The purpose of this agreement is to give clinical laboratories visibility on their financial prospects over a three-year period, whilst also exerting control over healthcare spending. This led to a three-year agreement setting annual growth in clinical laboratory spending at 0.25% for the period 2014-2016 (source: Social Security accounts, June 2014). This target will be reached by modest reductions in prices to be spread over the period, and control of prescriptions, in order to offset the natural growth in volumes. Trade unions meet with the Health Insurance Funds (Caisses d’Assurance Maladie) every six months, in order to assess the impact of changes in prices and determine what future changes may be necessary to meet the annual growth target. In late January 2015, CNAM held discussions with professional unions to reiterate its commitment to the three-year agreement and to propose a rate revision commensurate with volume growth projected for 2014 and expected for 2015. The Company estimates that the proposed rate revision will have a gross adverse effect on revenue of around 1.50%, while the 2014 revision had a gross 61 adverse effect of 2.51% and a net adverse effect of around 1.20% (reductions are estimates based on annual test volumes). According to CNAM’s projections, volume growth in 2015 will make up for the decline and result in the 0.25% target for annual clinical expenditure growth set by the agreement being attained. Definitive 2014 figures will be available in late June 2015, as will information about the trend in early 2015. On that basis, a decision will be made regarding a revision to the nomenclature in September 2015, in order to get as close as possible to the 0.25% target regarding growth in annual clinical expenditure. France has the highest number of clinical laboratories and laboratory doctors per capita of all the countries in which the Group operates. There are therefore significant consolidation opportunities in the French clinical laboratory services market. Several factors drive this consolidation, including: the high number of small-size laboratories in France, where levels of automation are still low; regulation of spending by cuts in prices to offset rising volumes, creating an erosion of margins which can only be offset by the use of increasingly larger structures; since November 2013, French legislation has required all clinical laboratories to provide evidence that they have started the accreditation process (decree no. 2010-49 of January 13, 2010, article 8, paragraph V); as of November 1, 2016, all clinical laboratories will have to be accredited for 50% of the clinical tests they carry out. This level will increase to 70% starting November 1, 2018 and to 100% starting November 1, 2020 (see section 6.5.1. “Regulation – France” of this document de base). The heightened accreditation standards may force a number of the small clinical laboratories to merge or to be acquired by larger laboratory groups (source: HPST Law and Ballereau Decree no. 2010-49); the Group’s laboratories’ failure to comply with these thresholds by the requisite date could force the laboratories concerned to discontinue the activities for which they are not accredited (see Section 6.4.5. “Quality Standards” of this document de base); regulatory constraints that limited regrouping are easing: the restrictions on the number of clinical laboratories that may be owned and operated by the same laboratory company, or of which a laboratory doctor can be a shareholder, and the restrictions on the outsourcing of tests between clinical laboratories and technical platforms operated by the same SEL have been eased; at the time of this document de base, there is a limited level of outsourcing by public hospitals in France. These different factors driving consolidation are reflected in the figure for “average number of laboratories per SEL”, which went from 1.1% in 2006 to 1.15 in 2009 to reach 1.55 in 2012 (source: Xerfi). Prior to decree n°2010-49 of January 13, 2010, laboratories were located on a single site and provided the entirety of the testing process: pre-analysis, analysis and post-analysis in their specialty area (immunology, bacteriology, etc.). Since the publication of this decree, several laboratories have merged, creating multi-site clinical laboratories, pooling most of their testing on a single technical platform. Some local laboratories have thus become simple Collection Centers. The effects of scale are clearly reflected in the operating performances of clinical laboratories. Average EBITDA margins for small structures (those with an annual revenue of less than €2 million) and for medium structures (those with an annual revenue between €2 million and €5 million) was indeed respectively 10% and 11% in 2013, whilst the figure reached 18% for larger structures (with an annual revenue of over €5 million) (source: Xerfi). Including the merger between Cerba and Novescia, the four largest clinical laboratory groups (routine and specialty tests combined) represented approximately 23% of the market based on available 2013 revenue figures. These four groups were Cerba-Novescia, Labco, Biomnis and Unilabs (source: L.E.K. Report) with market shares of approximately 8.5%, 7.1%, 4.6% and 2.8%, respectively. 62 United Kingdom In 2013, public health spending on clinical laboratory services and anatomical pathology in the United Kingdom was around €5.2 billion, of which it is estimated that approximately €0.3 billion corresponded to sub-contracting of services to approximately 40 private laboratories (source: L.E.K. Report). The market for clinical laboratory services is dominated by the public sector, through hospital laboratories. The NHS, which accounted for 82% of health spending in 2013, provides testing services for both in-patients and out-patients (sources: OMS, BMI Healthcare). However, contracts are awarded by the distinct entities responsible for each region: NHS England, NHS Scotland, NHS Wales and Northern Ireland. In 2013, nearly 95% of spending on clinical laboratory services was dedicated to public hospitals (source: L.E.K. Report). In addition, the investments were necessary to keep or obtain the “CPA” quality accreditation from the UKAS (see Section 6.4.5. “Quality standards” of this document de base). Medical services provided to patients outside NHS hospital structures are commonly referred to as “out-patient” or “primary care services”. The services are provided by medical doctors, generalists and specialists, gathered under Clinical Commissioning Groups (CCGs) and Local Area Teams (LATs). In the specific case of clinical services, CCGs and LATs negotiate contracts with NHS hospitals for the testing of their samples. These contracts are generally negotiated on the basis of the volume and typology of the previous year tests and can be amended annually or semi-annually. The remuneration attached to the contracts is a fixed annual lump-sum. Some groups of doctors have already subcontracted their clinical services to private operators. The acute care are branches of NHS hospitals with an autonomy relating to the providing of medical services and to the allocation of expenses. These acute care are usually managed in coordination with NHS hospitals and CCGs. Facing tight budget restrictions, NHS hospitals are increasingly outsourcing their clinical laboratory services to private groups, with a view to making them more economically efficient. This trend towards outsourcing began in London and south-east England, and is now spreading to the whole of the United Kingdom. Studies have shown that between 2008 and 2013, the value of clinical services outsourced to the private sector rose from less than €120 million to more than €270 million, i.e. an annual average growth of 18.5% over this period (source: L.E.K. Report). By 2018, it is estimated that this figure is likely to increase by more than 16.4% per year, taking it to €0.6 billion (source: L.E.K. Report). This trend, which is supported by the government, as reflected in the 2010 Carter Report, is likely to continue, given the cost savings and improved operational efficiency that this outsourcing brings. Outsourcing is subject to tender procedures which generally last between 12 and 18 months, resulting in long-term contracts, of more than seven years. Prices of tests are negotiated directly between the NHS Trust hospitals and private clinical laboratories. Prices are usually indexed to inflation over the life of the contract. During the financial year 2014, 16 trusts of the NHS outsourced their clinical services and 5 initiated tender procedures to this effect (source: L.E.K. Report). In 2014, the three main private sector clinical laboratory groups had a combined share of more than 90% of the clinical tests’ outsourcing private market in the United Kingdom. These four groups were The Doctors Laboratory (TDL), Quest Diagnostics, Viapath (formerly GSTS) and Labco, which holds approximately 13% of market’s shares (source: L.E.K. Report). Belgium In 2013, the Belgian clinical laboratory services market generated revenues of approximately €1.4 billion, with private laboratories representing approximately half of the total (source: L.E.K. Report). In 2013, there were approximately 100 private clinical laboratories operating in Belgium, including 64 Not-For-Profit Organizations (source: L.E.K. Report). Between 2004 and 2013, the market for private clinical laboratory services grew from €0.4 billion to €0.7 billion approximately (source: INAMI). This market grew rapidly between 2004 and 2009, before stabilizing at an average growth rate evaluated at 1.3% per year between 2009 and 2013 (source: INAMI). Prices of the large majority of laboratory tests are state-regulated and set annually by the INAMI. Reimbursement levels for tests included in the INAMI nomenclature are set by the Belgium health authorities. They typically provide for a co-payment by the patient. A small number of laboratory tests, however, are excluded from the INAMI’s price regulation and are set freely by individual laboratories. Most nutrition tests, 63 for example, are not reimbursed by the INAMI. As in other European countries, efforts are being made to control health spending more effectively. The SPF Santé Publique, the Belgian government organization responsible for health issues, is competent for accreditation of laboratories in Belgium, which gives them the right to collect samples and carry out analysis in specific areas. For accredited laboratories, reimbursement is made by the INAMI. The outpatient laboratory services market in Belgium is mostly structured on the basis of a business-to-business model: samples are collected at a medical doctor’s practice before being delivered for testing to a technical platform such as those operated by the Group. Certain laboratories, including the ones owned by the Group, also operate networks of Collection Centers for patients whose doctor does not take the sample. The Belgian market is now fairly highly concentrated following the consolidation that took place in the 1990s and 2000s. Market consolidation was triggered by the easing of rules on ownership of clinical laboratories by investors who are not laboratory doctors. It is also due to the increasing difficulty in obtaining authorizations to operate and to reductions in prices. In 2013, the two largest clinical laboratory groups represented nearly 25% of the private clinical laboratories’ market while the 8 largest represented about 40% (source: L.E.K. Report). The two leaders are Sonic Healthcare and CMA-Medina, representing about 12% of the market shares each (representing approximately €86 million and €83 million). Synlab, Olivier (Amedes Group), Cerba, Roman Pais (the Group’s laboratory), IBC and Nuytick each have a market share of 2 to 3%. The Group’s market share in Belgium does not include the nutritional biology’s revenue as some tests are carried out on behalf of foreign laboratories or patients and most of them are excluded from the nomenclature. Including nutritional biology, the Group is number 3 in the Belgian market based on the 2013 revenue behind Sonic Healthcare and CMA-Medina. Switzerland In 2013, the Swiss clinical laboratory services market generated revenues estimated at approximately €1.9 billion, with private laboratories representing approximately 30% of the total. In 2013, there were, according to estimations, approximately 50 private clinical laboratory groups in Switzerland operating more than 150 laboratories (source: L.E.K. Report). In Switzerland, clinical testing is carried out by general practitioners (45% approximately of total volumes for the 50 most common tests), private laboratories (around 30% of this volume) and hospitals (around 25% of this volume) (source: L.E.K. Report). Two-thirds of general practitioners have a clinical laboratory within their practice where they carry out the simpler tests, whilst sub-contracting more complex tasks to private laboratories. Studies show that the majority of small-size hospitals sub-contract clinical testing to private laboratories, whilst larger hospitals generally have their own laboratories, and sub-contract only specialty testing (source: L.E.K. Report). Prices for clinical tests are set by the DFI and the Decree on Health Insurance Services of September 29, 1995. The DFI defines a list of tests to be reimbursed under compulsory medical insurance and allocates a number of points per test, with each point worth CHF1. Created in 1994, the catalogue contained some 1,500 tests in 2014, including 55 tests that can be carried out in medical practices by general practitioners (source: L.E.K. Report). The last major revision took place in 2009 to reflect the increased automation of the most commonly used tests. A new revision, applicable as of January 1, 2015, introduces a significant increase in prices for some tests, for general practitioners only. This measure aims to increase the number of general practitioners setting up in Switzerland by offering them a source of additional income more attractive than before. The main factors behind the choice of a private clinical laboratory in Switzerland are proximity, the time needed to return results, pre-analysis costs charged by general practitioners and the relationship with the laboratory’s sales team. For specialty tests, the main selection criteria are the laboratory’s reputation and the qualifications and medical expertise of its staff. In 2013, the private clinical laboratory market in Switzerland was relatively highly concentrated, with 50 groups operating a total of more than 150 facilities, of which approximately 100 were operated by the 6 main groups: 64 Unilabs, Medisupport, Synlab, Laboratoire Viollier, Laboratoire du Dr. Risch and Meditest (source: L.E.K. Report). The market leaders are concentrated around the major cities; the rest of the country is covered by smaller laboratories, which generally operate one or two facilities focusing on a local client base. In 2013, the four largest clinical laboratory groups represented more than 50% of the private clinical laboratory market, according to the number of clinical laboratories (source: L.E.K. Report). These groups were Medisupport, Unilabs, Synlab and Laboratoire Viollier. 6.2.4.3 Southern Europe segment Spain In 2013, the Spanish clinical laboratory services market generated revenues of approximately €2.6 billion (source: DBK), with private laboratories representing approximately 35% of the total (source: DBK). In 2013, there were about 1,900 private laboratories operating in Spain. In Spain, all patients are covered by the public healthcare system and, in 2013, 22% of them (source: ICEA) had supplementary private medical insurance (this proportion is higher in the major urban areas). For example, in 2011, in the regions of Madrid and Catalonia, where the Group has a strong presence, respectively 31% and 27% of people (source: ICEA) were covered by supplementary private medical insurance. Almost all of the Group’s revenues come from the private system. Despite the current economic environment in Spain, the Spanish private medical market continues to grow as patients seek to insure themselves against an increasingly constrained national health system. A privately insured patient’s choice is limited to clinical laboratories that have entered into a reimbursement agreement with his or her private insurer. Generally, prices for tests performed by private clinical laboratories are negotiated at a regional level with private or mutual insurance companies and private hospitals. There are two basic fee structures: a per patient fixed fee (Capitation Model) and an activity-based model. Under the Capitation Model, the insurer pays a fixed price for each policyholder. The laboratory thus carries the risk of a volume of analysis services over and above a certain level. However, certain tests, such as molecular biology, are mostly not included in the fixed prices and are subject to specific invoicing. Under an activity-based fee structure, the insurer pays the laboratory for each test performed, in accordance with a schedule of agreed test prices. In the Spanish outpatient market, doctors, as well as general and specialized care centers may collect samples and send them to a clinical laboratory for testing. Spanish labor law requires employers to provide regular health checks for their employees. The services relating to these checks are generally provided by specialized companies which then sub-contract clinical testing to private laboratories such as the Group’s. Numerous private hospitals outsource their medical analysis to private laboratories. On the other hand,, outsourcing by public hospitals to private clinical laboratories, under public–private partnerships, is currently mostly limited to specialty testing. Spanish law requires public hospitals to conduct tender processes when choosing service providers. This market remains fairly fragmented but is quickly consolidating under the pressure of competitive pricing levels, as laboratories seek to enhance their bargaining power with suppliers and customers. The Spanish market is in the throes of consolidation. The number of laboratories has fallen from more than 3,600 in 2008 to about 2,600 in 2013, including some 670 hospitals’ laboratories and about 1,900 private laboratories (source: DBK). In 2013, the five largest clinical laboratory groups represented more than 30% of the private clinical laboratory market. These groups were Labco, Laboratorio Dr. Echevarne, Unilabs, Megalab and Reference Laboratory, with market shares, respectively, of 11.9%, 6.4%, 5.6%, 3.2% et 3.2% (source: L.E.K. Report). Portugal In 2013, the Portuguese clinical laboratory services market generated revenues of approximately €0.6 billion (source: L.E.K. Report), with private laboratories representing approximately €253 million (approximately 40% 65 of this market) (source: L.E.K. Report). It is estimated that in 2012, there were approximately 430 private laboratories. Healthcare spending in 2013 represented 9.3% of Portuguese GDP (sources: OMS and BMI Healthcare). In Portugal, there are three major categories of healthcare providers: the national health insurance (Serviço National de Saude); several subsystems in which healthcare is provided by public institutions or by contract with private or public providers (or in some cases, a combination of both); and private healthcare insurers. Each of these healthcare providers establishes its own table of prices and reimbursement levels of laboratory tests. Outpatients are generally free to choose a clinical laboratory from the public or the private sector for their medical testing. Patients with no private insurance can choose to go to public laboratories or to private laboratories that have agreements with the Serviço National de Saude (or a subsystem, if applicable). The prices for clinical laboratory services in the public sector are set and partially borne by the Serviço National de Saude (or the relevant subsystem). Typically, patients are required to make a co-payment. Patients with private insurance can choose to go to public or private laboratories that have entered into reimbursement agreements with their private insurers. The cost of clinical laboratory services in the private sector is not state-regulated and test prices are negotiated between private laboratories and private health insurance companies. At the date of this document de base, the Serviço National de Saude is not entering into new reimbursement agreements with clinical laboratories (except in specific situations where the reimbursement agreement is made on a case-by-case basis in the public interest), creating a significant barrier to entry for new players. At the date of this document de base, outsourcing by public hospitals to private laboratories remains limited in Portugal. Opportunities for such contracts mainly arise in connection with newly constructed public hospitals. However, private hospitals are making increased use of outsourcing for their clinical laboratory services. The Portuguese private clinical laboratory services market is relatively consolidated. Further consolidation opportunities remain however. In 2013, the 5 largest clinical laboratory groups represented approximately 50% of the private clinical laboratory market (source: L.E.K. Report). These five groups were Labco, Joaquim Chaves, Unilabs, BMAC and Beatriz Godinho, with market shares, respectively, of 17.1%, 10.2%, 8.9%, 7.3% and 3.3%. Italy In 2013, the Italian clinical laboratory services market generated revenues of approximately €3.4 billion (source: GIA and L.E.K. Report). The market grew by approximately 1.8% a year on average for the period 2010-2013 (source: L.E.K. Report) and this trend is expected to continue for the period 2013-2018, with growth estimated between 1.5% and 2% per year (source: GIA and L.E.K. Report). Studies show that the activity of private clinical laboratories accounted for approximately 45% of the market (source: GIA and L.E.K. Report). In 2011, there were approximately 2,600 private laboratories operating in Italy (sources: Italien Ministry of Health and L.E.K. Report). The market share of private laboratories, however, varies significantly by region due to Italy’s fragmented and decentralized healthcare system. Generally, prior to undergoing any test, a patient must pay a fee that corresponds to only part of the test’s actual cost. The amount of this prepayment varies by region and according to the patient’s social eligibility criteria. The remaining portion of the cost is covered by the national healthcare authority (Servizio Sanitario Nazionale) or by private healthcare insurers. The Servizio Sanitario Nazionale provides guidelines for prices applicable to accredited private clinical laboratories. However, regional authorities (the ASL) have the authority to set local prices above or below national guidelines. On an annual basis, each accredited private clinical laboratory must sign an agreement with local authorities that determines the specific reimbursement rules in terms of applicable prices and the total budget allocated to the laboratory for the year. Once the annual budget has been reached, which generally occurs in the final quarter of the year, ASLs will no longer, or only partly, reimburse the tests carried out by the laboratories. As a result, reimbursement levels vary widely from one region to the other. In the improbable case where the annual budgets allocated by the ASLs for a given year are not reached by the end of the year, the Group does not exclude a revision aiming to lower these budgets the next year. 66 In Italy, out-patients’ doctors prescribe clinical tests and patients are free to choose the clinical laboratory in which the tests are conducted. Patients typically choose a laboratory based on proximity to their home or workplace. The main diagnostic testing laboratories in Italy, such as Alliance Medical, Centro Diagnostico Italiano (CDI) and Labco, offer an Integrated Diagnostic Testing service, providing clinical testing, diagnostic imaging services, ambulatory care and other health care services. In Italy, the radiology market represented approximately 60 million scans in 2011 (sources: Italian Ministry of Health, L.E.K. Report). Studies suggest that from 2006 to 2011, the market grew by 1% a year on average in volume (sources: Italian Ministry of Health, L.E.K. Report). During that period, there was a balancing shift between basic and more complex scans, evidenced by the trend in equipment sales. From 2005 to 2012, equipment suppliers achieved average sales growth of 6.3% a year and are forecasting annual growth of approximately 8.5% for the period 2012-2020, with an above-average growth in CT-scanners (approximately 9.7%) and MRI scanners (approximately 11.8%), which are mainly used for high-value added scans (source: L.E.K. Report). As regards nuclear medicine, in 2012, the Italian market represented approximately 1.5 to 2 million scans, of which about one quarter were high value-added PET scans which cost some €1,000 per scan (source: L.E.K. Report). Equipment suppliers achieved average sales growth of approximately 3.5% a year from 2005 to 2012 and are expecting growth to accelerate at approximately 5.8% a year between 2012 to 2020 (source: Global Data and L.E.K. Report). The Group believes that there are significant consolidation opportunities in the Italian clinical laboratory services market as a result of the market’s high fragmentation, decreases in prices (following the recent decreases in national nomenclature prices), the lag in standardization and automation affecting small laboratories and the recent national and regional reductions in healthcare expenditure. At the date of this document de base, the four main groups offering Integrated Diagnostic Testing on a national scale are, in decreasing order of 2013 revenue, Labco (pro forma for the acquisition of the SDN group), CDI, Alliance Médical and Synlab. However, in terms of clinical laboratory business within these groups and based on 2013 revenue, the main companies operating in Italy in the same regions as the Group are Synlab, Labco (pro forma for the acquisition of the SDN group), Laboraf and CDI, with market shares, respectively, of 3.3%, 2.1%, 2.0% and 1.9% (source: L.E.K. Report). 6.2.4.4 Emerging markets These markets have particularly attractive features for a number of reasons. First, the rising living standards in these countries is generally accompanied by strong growth in demand for healthcare, due in particular to the rapid spread of chronic diseases and a growing desire for preventive treatment. In addition, the investments’ efforts made by these countries have in many cases focused on the creation of a hospital infrastructure, whilst the introduction of a full range of medical services has not necessarily kept pace. 6.3 GROUP STRATEGY The Group’s ambition is to become the major pan-European force, in the image of major international groups in the sector (e.g. Sonic Healthcare in Australia, and Quest Diagnostics Inc. or Laboratory Corporation of America Holdings in the USA) by drawing on a strategy adapted to sector specifics and, in particular, the necessary structural changes in the European sector. It therefore intends to demonstrate real leadership and become a driving force of the change process in European healthcare systems. In each of the countries where the Group is active, its aim is to be the leader on the market of clinical laboratory services or, failing this, in a specific high added value segment, such as nutritional biology in Belgium, genetic testing in Spain and integrated diagnostics in Italy. To the extent allowed by the local context, the Group’s strategy is applied in each of the countries where it is active around the following four key ideas: 67 The medical project: anticipate future developments in order to seize new opportunities for growth a. Maintaining medical excellence to capitalize on the substantial scientific and technological progress made in the field of medical biology and drive innovation so as to be part of the development of a richer range of new tests and services at a pan-European level: completing the enrichment of the offering in order to internalize all specialist tests at a European level on platforms such as the ones that already exist in France and Spain; developing and deploying innovative tests such as those already introduced by the Group in recent years (non-invasive genetic testing for Down’s Syndrome in fetuses, detection of markers for colon cancer without the need of a colonoscopy, genetic evaluation of the risk of developing breast or ovarian cancer, cystic fibrosis, etc.); increasing technological investment in new tools: high-speed sequencing equipment, broadband communications equipment for tele-pathology services, PET scanners, etc.; creating centers or expert networks for each specialty: - b. offering integrated diagnostics through technological convergence; the acquisition of the SDN group is a recent illustration of this, as its main attraction was the integration of medical imaging and clinical testing; functional medicine (such as nutritional biology, anti-ageing biology, etc.); anatomical pathology; oncology; gynecology and female biology; extracting value from the considerable volume of clinical and biological data gathered by Group establishments; strengthening the quality policy that has been introduced, and standardization on a European scale through the pooling of the best practices from each country. Develop a strong identity and strong brands alongside a structured marketing approach allowing new sources of potential demand to be identified and addressed: The Group intends to continue to develop its identity as well of that of its strong brands such as Labco NOÛS Advanced Special Diagnostics. In addition, over and above the existing market segments in which the Group operates, the diagnostics market has customer bases that are still relatively underdeveloped and which the group has undertaken to prospect in a systematic way, allocating marketing resources and creating dedicated sales teams. The main actions in this regard concern: the development of an individualized direct offering to patients (nutritional medicine, participatory medicine, preventive medicine, etc.); bidding for contracts with hospitals, clinics, retirement homes and medical care establishments (notably for sub-contracting and outsourcing contracts); building a position in the market for health checks offered by employers or insurance companies; building a position in the market for biological modelling as part of compulsory occupational healthcare. 68 Deploy a strategy of growth through acquisitions focused on the creation of shareholder value, building on the Group’s strong ability of integration and a cautious approach to valuations There are numerous potential acquisition targets, selected using clear and predetermined criteria, in order to strengthen the Group’s leadership in its various markets and maximize the return on investment. The acquisition strategy is focused on the Group’s main geographical markets in order to generate synergy between platforms and medical content under the following outline: incremental acquisitions made in regions where the existence of Group technical platforms allows synergy to be generated rapidly through the transfer of clinical testing to existing capacity. Such acquisitions are sometimes termed “buy and close”, and concern smaller structures which can quickly be brought up to margin levels in line with those of the Group as a whole; acquisitions that improve territorial coverage: primarily acquisitions of platforms in new territories from which the strategy of consolidating smaller laboratories can be deployed; acquisitions of medical content designed to bring the Group new technological capacity which can be used in all Group laboratories. These acquisitions will serve to accelerate the enrichment of the offering, which is necessary to drive organic revenue growth (molecular diagnostics – both biological and imaging – anatomical pathology, bio-IT, new high added value services, etc.). Establish the Group as a preferred partner for public organizations seeking to outsource their medical diagnostic services The outsourcing of clinical diagnostics is well developed in Spain and the United Kingdom and is gradually expanding in the other countries in which the Group is active. The Group wants to develop its expertise on a European level to seize these opportunities and thus improve visibility on future revenues. Sharing best practices on a European scale; Continuing to develop Group expertise in Public-Private Partnerships (PPP) such as those introduced in the United Kingdom and Portugal; Increasing the number of partnerships with university hospitals, along the lines of those with Erasmus MC and Massachusetts General Hospital. Building on preferred partnerships with hospitals, to anticipate future requirements of clinical teams and better understand the relevance of new approaches in the area of convergence and particularly integrated diagnostics. Build on operational efficiency and economies of scale in order to continue to drive growth in the Group’s cash flow The group was one of the pioneers in the clinical laboratory sector in introducing structured management approaches and IT solutions, as commonly used in industrial sectors and more mature service industries. It aims to continue to standardize and simplify its processes in all the countries where it is active, in order to offer services that combine medical excellence and low costs. Increase the Group’s competitive advantage through operational excellence and productivity gains through greater efficiency, by: procurement programs that aggregate order volumes at a European level, listing a limited number of suppliers for each purchasing category and centralizing decisions; optimizing production processes in laboratories through comparison and identification of best practices, standardization of the main tasks and rigorous monitoring of indicators of operating 69 performance (tests produced by ETP, capacity utilization rates for automated machines, percentage of automation, etc.); optimizing logistics processes; centralizing information through the use of Enterprise Resource Planning software, working with modules already used by the Group for the management of purchasing, inventories and assets; centralizing and optimizing support functions (quality, accounting, payroll, etc.). Meeting the challenge of the profusion and richness of medical and biological diagnostic information by deploying the most powerful IT systems Control over IT systems is a strategic and competitive advantage. They are essential for benefiting from the potential productivity gains generated by the concentration of resources and volumes on platforms of increasing sizes. These IT systems will also enable the Group to take advantage of the technological developments in the sector. The Group’s initiatives to strengthen these systems take several forms, such as: the Group has already approved significant investments in improvements to the efficiency of IT systems in laboratories for both management and medical aspects; specific development projects for new services based on IT systems are under development at the date of this document de base: LabMedica, tele-pathology (i.e. using a digital medium), mobile applications (tablets, smartphones, etc.); lastly the Group is working on adapting its IT systems to the challenges posed by manipulating the considerable volume of data generated by new clinical and biological diagnostic activities: data anonymity, complex data processing, bio-IT. The application of these key elements of strategy in each of the countries in which the Group is active is described in section 6.4 “Detailed presentation of the Group” of this document de base below. 6.4 DETAILED PRESENTATION OF THE GROUP 6.4.1 Services offered by the Group The Group offers a wide range of clinical laboratory tests, whether routine or specialty. The nature of these services varies from one country to the next and according to the type of establishment. Testing is generally organized in three phases: (i) the pre-analytical phase, which consists in collecting samples and delivering them to the clinical laboratory; (ii) the analytical phase, during which the test itself is carried out; and (iii) the post-analytical phase during which test results are sent to the prescribing doctor and the patient while the Group’s laboratory doctors provide help in terms of interpreting results. (i.) Pre-analytical phase. Before clinical testing is performed, samples are collected from the patient, identified and delivered to the Group’s analytical laboratories. Patient samples are labeled immediately with an identification number that is logged into an information technology system by the health practitioner who performed the extraction. Tests are subjected to quality control as well as technical and biological validation procedures. Samples are usually accompanied by a test request form (in electronic or paper format). It says which tests are to be performed and provides the necessary billing information. Collecting and analyzing samples taken from patients is often carried out at different locations, and samples therefore have to be moved from their collection point (hospital sites, doctors’ practices or Collection Centers) to the Group’s Laboratories. In France and Spain, some of the Group’s laboratories maintain their own fleets of vehicles and provide both transportation and logistics services with respect to shipping samples to Group Laboratories. In other countries, the Group outsources this service to 70 major transportation companies such as DHL, UPS, etc. The transportation of samples is subject to a number of legal requirements, with respect to sample integrity and data confidentiality, in particular. Maintaining its own logistics network allows the Group to tailor logistics solutions for the collection of the samples to the needs and requirements of the customers. (ii.) Analytical phase. Once the test request form has been entered into the Group’s information technology systems and the samples have been collected, the tests are performed, either automatically (in the case of most routine tests) or by the Group’s laboratory doctors or by technicians (in the case of most specialty tests). (iii.) Post-analytical phase. As soon as they are available, test results are inputted either manually or through an electronic data interchange system that is connected with the practitioner, clinic or hospital, depending upon the kind of tests carried out, the type of equipment used and the country in which the test is performed. Specialty - clinical tests Routine – clinical tests The following summary table presents the main tests and the main examinations offered by the Group: Category of tests Examples Descriptions Chemistry Urea, creatinine Chemical dosages of various components of blood and urine Bacteriology Cyto-bacteriological examination of urine Immunology Hormonal dosages, dosage of tumoral markers Test looks for pathogenic bacteria in samples: identifies them and assesses their resistance to antibiotics Dosages of hormones and tumoral markers that can be involved in a pathology Hematology Determination of blood formula Studies and diagnostics of blood, hematopoietic organs and blood diseases Hemostasis INR Study of coagulation factors; monitoring of anticoagulant treatment drug doses Allergology Specific IgE tests Tests look for and measure of antibodies involved in allergic diseases Auto-immunity Antinuclear antibodies, antitransglutaminase antibodies Specialized hormonology Thyroglobulin, anti-mullerian hormone Test looks for and quantifies antibodies that may be involved in autoimmune diseases Dosage of hormones involved in certain pathologies Specialized bacteriology Detection of Koch’s bacillus and antibiogram Test looks for bacteria involved in certain pathologies, nosocomial infection Molecular biology NIPD, breast cancer Locating and identifying genes involved in various pathologies Nutritional tests Proteinic profile (e.g. albumin), food intolerances, amino acids, metabolic profile, oxidative stress Determination of nutritional status via the analysis of certain kinds of food and evaluation of key metabolic zones 71 Anatomical pathology Category of tests Examples Descriptions Virology HIV, Ebola Study of viruses and associated infectious agents Histology Smear tests, biopsy Study of tissues put on to a glass slide Cytology Smear tests, biopsy Study of cells put on to a glass slide Imaging & Nuclear medicine Molecular pathology Tests provide diagnostic criteria, diagnoses and predictive factors with regard to the patient’s response to treatment in some diseases, via the analysis of proteins and nucleic acids Radiology Scanography, mammography, sonography, Doppler sonography, etc.. Nuclear medicine Positron emission tomography (PET), nuclear imaging MAR Artificial insemination In vitro fertilization 6.4.1.1 Clinical laboratory tests Routine tests The clinical laboratory tests offered by the Group are regularly used in general patient care. They allow health professionals to establish or confirm a diagnosis, to monitor treatment or to search for an otherwise undiagnosed condition. The most frequently requested types of test are the following: biochemistry, hematology, immunology and bacteriology. The Group performs these categories of routine tests in all its laboratories, including hospital laboratories when it operates under an outsourcing contract, as is the case in the United Kingdom. The Group performs most routine procedures, and generally reports their results within 24 hours, by using a variety of sophisticated and computerized laboratory testing instruments. Within the catalogue of tests offered by the Group, not all tests have comparable volumes. For instance, in 2012, approximately 2% of the tests found in the French nomenclature of clinical laboratory tests (BIOLAM) accounted for 66% of all prescribed tests and for 54% of reimbursements (source: BIOLAM). Specialty tests As specialty tests involve a higher level of complexity than routine tests, they are conducted by highly skilled biologists and generally use more sophisticated technology, equipment or materials than routine testing. Due to constraints related to costs or infrastructure shortcomings, hospital, routine or doctor-office laboratories develop and perform a broad range of specialty tests in-house. The Group’s Laboratories that provide specialty testing services specialize in particular in the following fields: allergology, autoimmunity, specialized hormonology, specialized bacteriology, molecular biology, nutritional biology and virology. The Group currently performs specialty tests in all the countries in which it operates. 72 The Group operates, together with Labco NOÛS and Roman Païs, two specialty laboratories with an international reputation. Labco NOÛS is a specialty laboratory offering, in particular, a catalogue of 5000 tests in part ISO 15 189 accredited, the possibility for its clients to integrate their results directly in their SIL, a medical and technical multilingual support and an expertise in the field of samples transportation. Through this know-how, Labco NOÛS can satisfy to the outsourcing needs of most laboratories around the world. Labco NOÛS clients are mainly located in Spain, Latin America, the Middle-East, Eastern Europe and North Africa. The Group plans to develop the marketing of the services of Labco NOÛS through its local subsidiaries, and in particular in Italy and the United Kingdom. As for Roman Païs Laboratory, it has a very specific offer in the field of nutritional biology and functional biology. This expertise has permitted it to develop an international client base representing in 2014 slightly more than half of its Revenue on this segment. In France, before 2010, the Group outsourced most specialty tests to clinical testing laboratories that specialize in performing specialty tests, such as Cerba, Biomnis or Institut Pasteur de Lille. Regulatory changes implemented in France in January 2010 led the Group to set up technical platforms and start carrying out itself a limited number of previously outsourced specialty tests. The Group intends to expand the range of specialty tests it performs in France when this option proves to be profitable from an economic viewpoint. To remain competitive in the clinical testing market, the Group intends to enhance further its testing capacity. The teams of the Group’s Chief Medical Officer monitor the scientific literature and trade press, and hold talks with test manufacturers and suppliers in order to identify new tests that become commercially available and, when appropriate, add them to the Group’s range of services. Introducing new tests requires giving players who can prescribe them (such as doctors and hospitals) information about these tests and in many instances thirdparty payers have to cover the reimbursement of said tests. The Group frequently uses initiatives of scientific information with the medical and pharmaceutical professionals aimed at providing prescribers with information about these new tests. The Group describes the range of routine and specialty tests offered by each of its laboratories in its “pathology handbook/test catalogue” that enables it to keep the main prescribers regularly informed about any change that may occur in the services the Group provides. Below are a few examples of innovative tests offered by the Group in its catalogue: “A200”, a test developed especially for the Group that detects, from a blood sample, a patient’s intolerances to more than 200 kinds of food; “Septin9”, a non-invasive test to detect prematurely colon cancer, avoiding the need for colonoscopy; “Breast cancer genes panel”, using the new sequencing generation (NGS), this testing profile detects the presence of each of the 21 genes involved in the hereditary risk of developing breast cancer; “Prosigna”, provides reliable identification of the 10-year risk of future recurrence and sub-type of breast cancer, enabling oncologists to decide whether or not to prescribe chemotherapy; “TDAGen+”, a genetic test for patients with Attention Deficit Hyperactivity Disorder (ADHD) which collects personalized information on the main genetic factors involved and allows predictive treatment in response; “Life Length”, measures the percentage of short telomeres in individual cells taken from a blood and/or tissue samples, providing an accurate indicator of telomere dysfunction and cellular ageing. “HPV OncoTect”, a test allowing an early detection of cervical cancer; “Gut Microbiome”, a functional biology genetic test aiming at analyzing genomes of normal living microorganisms of the human being (microbiota); “Recombine”, a non-invasive test to detect the risk of transmitting a genetic disease to one’s child; “Migratest”, a test helping to evaluate the potential causes of migraine headaches in order to determine the best treatment; 73 “Liquid Biopsy de Pangaea Biotech”, a test revealing the potential mutations of EGFR, BRAF and KRAS genes (usually related to lungs cancer) and which facilitates the determination of the best treatment for the patient. Moreover, in oncology, numerous treatments require companion tests that enable groups of patients to be detected among whom a drug will be efficient or not, well tolerated or not. Most of them are available in the Group’s catalogue of tests, as for example the HER2. 6.4.1.2 Anatomical pathology Anatomical pathology is, along with clinical laboratory testing and imaging, one of the principal diagnostics disciplines. This discipline is dedicated to the morphological study of macroscopic and microscopic anomalies of biological tissues and pathological cells removed from a living or dead human being. Anatomical pathology is widely used in oncology to detect and assess the efficiency of the ablation of tumors. Preparing samples is an important and highly technical stage during which a thin slice, only a few microns thick, is put on to a glass slide before being colored in order to be examined with a microscope. The preparation, examination and diagnostic of a sample cannot be automated, resulting in substantial labor costs. Anatomical pathology also encompasses cytopathology. Cytopathology studies cells smeared over a glass microscope slide and not cross-sections of cells. The cells are accordingly whole-cells and more easily observable. Among the most widespread cytologic analyses, we can mention lumbar or articular punctures, b1 marrow punctures and pap smears. In the last few years, the development of the so-called telepathology technology has paved the way for major progress in the discipline. Sample slides can now be digitalized and sent to a microscope that will automatically identify the zone to be diagnosed and the pathology. This technology, in addition to allowing the doctor’s diagnostic to be established more easily and more swiftly, enables the sample to be sent to specialized doctors anywhere in the world in case of a complex pathology. The Group offers anatomical pathology services in France, the United Kingdom, Italy, Belgium, Spain and Portugal. At the date of this document de base, the Group was holding talks aimed at acquiring an anatomical pathology group. If this acquisition were to be successfully completed, integrating this target might enable the Group to significantly increase its business in this field. 6.4.1.3 Medical imaging Medical imaging Medical imaging encompasses several disciplines such as radiology, scanography, mammography, orthopantomography (dental imaging), sonography, Doppler sonography, etc. Medical imaging services are mainly provided by the Group’s Italian subsidiaries. Scanning and mammography are the most widely used disciplines within the Group. Scanography is a medical imaging technology derived from conventional X-ray radiology. The extent to which X-rays are absorbed by tissues is measured by a transmitter and a receiver pivoting around the patient who can be standing or lying down. 2D or 3D images of anatomical structures are then digitally built. A mammogram uses X-rays to examine the human breasts and detect possible anomalies, and most frequently cases of breast cancer. A mammogram provides images of tissues within the breast from different angles in order to make a doctor’s diagnostic easier. Technical, technological and IT developments allow more sharply defined and more precise images to be obtained and, furthermore, more rapidly than in the past. This trend will likely continue to gather momentum. Moreover, systems already enable images to be digitalized and communicated so as to allow a diagnostic to be made remotely. 74 Nuclear medicine Molecular imaging is a medical imaging discipline that provides an in-depth view of what occurs within the human body, at the level of molecules and cells. While conventional medical imaging (X-rays, scanography and ultrasounds) generates an image of the patient’s physical structure, molecular imaging enables a doctor to observe the metabolic or molecular activity of the body or of organs by using a technique that is not significantly invasive. Molecular imaging offers a unique vision of the body that allows doctors, inter alia, to: access information that cannot be provided by other imaging technologies or that would require invasive methods such as biopsy or surgery; identify the pathology at an earlier stage while locating with a high degree of precision the contaminated zone, often before the symptoms actually appear or before anomalies can be detected by other diagnostic methods; determine the most appropriate therapy on the basis of the patient’s specific biological characteristics; study and monitor the patient’s reaction to a specific drug or treatment; determine precisely the treatment’s efficiency on the patient; adapt treatment rapidly in response to the change in cellular activity; gauge the progression of the disease. Molecular imaging is deemed to be part of nuclear medicine because it uses small amounts of radioactive material (radioactive marker) to diagnose a pathology. The method consists in injecting into the patient a tracer marked by a radioactive atom (carbon, fluorine, nitrogen, oxygen, etc.). The behavior and the biological properties of this tracer are known. The scanner will detect how the tracer moves within the living organism or the organ; this information will then be collected and processed by computers to highlight the presence, or the reaction, of a given number of molecules. The Group has a cyclotron in its SDN Integrated Diagnostics Center that enables it to produce, and potentially market, its own tracers. PET technology combined with scanography, as offered in the Group’s center of excellence in Italy, is a nuclear medicine test combining the two technologies and providing a three-dimensional view of how the organism operates. A PET-CT uses a small dose of radioactive tracer in order to show very precisely the difference between healthy tissues and contaminated tissues. Molecular imaging techniques are non-invasive, reliable and painless for the patient. They enable doctors to detect, inter alia, cases of cancer, of heart diseases, of nervous system diseases such as Alzheimer’s disease or Parkinson’s disease, b1 diseases, lung diseases, etc. Changes in molecular imaging will be shaped by the arrival of new tracers and new molecules that will give birth to numerous new applications. As for changes in equipment, the number of PET-CTs will likely grow further in the next few years thereby paving the way for additional progress in terms of rapidity, sensitivity and resolution. Medical imaging within the Group Nearly all imaging services are provided by the Group’s Italian subsidiaries following the 2007 and 2008 acquisitions of laboratories CAM and Baluardo. With the acquisition of the SDN group, the Group now boasts expertise and cutting-edge technology in the field of molecular imaging that it intends to use and deploy in its other subsidiaries. For the financial year 2014, in their pro forma version in order to take into account the acquisition of the SDN group, revenue generated by imaging services amounted to circa €40 million. 75 6.4.1.4 MAR MAR, in a broad sense, covers two main applications: the IUI, which consists in collecting a sample of sperm, preparing it (selection and concentration of the fastest moving sperm) before giving it to the male spouse so that he can rapidly go to his partner’s gynecologist, who will place this concentrate through natural means; the IVF generally takes place in a hospital and consists in taking sperm from the man and also oocytes from the woman through a light surgical intervention. Once they have been collected, the sperm and oocytes are put into contact, either naturally in a test tube, or via a micro-injection of sperm into the oocyte using a micro-needle. Before resorting to these protocols, there are two additional disciplines offered by most of the Group’s laboratories: spermiology, i.e. the study of the quality of sperm and hormonology, i.e. the study of a woman’s fertility. In France, to perform IVF and IUI, specifically qualified biologists are needed and they need special authorizations. Every local health authority appoints a very restricted number of public and private players in the region it covers. Still in France, the Group offers IVF in Nantes in two clinics as well as in a hospital located in Lens. The business generated by these three centers enables the Group to position itself as one of the leaders on the IVF market among private players. The Group offers IUI in these two cities and works with private gynecologists, and also offers IUI in Calais, Libourne, Nice, Orleans and Rodez. 6.4.2 The Group’s organizational models The fact that it operates in various countries has enabled the Group to put in place the three major organizational models found in this industry and to become an expert in them: clinical laboratories; sub-contracting and outsourcing operations on behalf of hospital laboratories; export services. 6.4.2.1 Collection centers and clinical Laboratories The Group’s Diagnostics Centers and Collection Centers can be categorized into six types: sampling centers, which are centers that do not perform tests. Samples are collected from patients then sent to routine testing laboratories, technical platforms or third parties’ laboratories where tests are carried out. The Group owns and manages Sampling Centers in all the countries in which it operates Laboratories; routine laboratories, which collect samples, carry out routine tests and usually communicate results to patients and prescribing healthcare professionals; hospital laboratories providing emergency services, which are routine testing laboratories located in hospitals that provide emergency diagnostic tests. These laboratories are open all day. They are smaller and have more limited facilities than the Group’s other types of laboratories; technical platforms, which are regional platforms that centralize the processing of all or some test samples from collection centers, laboratories and hospital laboratories of the Group. Technical platforms are equipped with sophisticated, highly automated material and can carry out all routine tests as well as, generally, some specialty tests. They also sometimes have on-site facilities for collecting samples; 76 laboratories and specialty platforms, which perform specialty tests for the Group’s laboratories or external laboratories. The Group has 12 laboratories dedicated exclusively to specialty tests, including one specialty platform in Spain following the acquisition of CIC, which has since changed its name to Labco NOÛS. The Group also has 4 technical platforms providing a broad range of specialty tests to members of the network in Barcelona, Cambrai, Madrid and Nice; Integrated Diagnostics Centers or Poly-Ambulatory Centers, which combine several diagnostic disciplines including clinical laboratory testing, anatomical pathology, ambulatory surgery, physiotherapy and medical imaging. Of the Group’s nine Laboratories in Italy, five are Integrated Diagnostics Centers. The type and size of Laboratories vary to a huge extent from one country to another because of differences between health systems and regulatory environments (see section 6.2. ”Presentation of sectors in which the Group is active” of this document de base). The number of Laboratories operated by the Group is in constant evolution, with the acquisitions and the transformation in Sampling Centers. Between 2012 and 2014, even though the group made 43 acquisitions, the number of Laboratories decreased as illustrated in the below chart: 6.4.2.2 Country France 2012 77 2013 64 2014 64 Belgium 4 3 4 United Kingdom 3 4 6 Spain 57 58 56 Portugal 27 24 25 Italy 4 3 9 Suisse - 1 1 Total 172 157 165 Sub-contracting and outsourcing laboratory services The Group offers public and private hospitals and clinics the possibility of sub-contracting and outsourcing their clinical laboratory services in France, in Spain, in Portugal and in the United Kingdom. The range of services it offers differs according to customers’ needs. Some customers want to outsource all their clinical laboratory services, while others prefer to keep some of these services in house such as, for instance, sampling, the logistics involved in collecting samples (in the United Kingdom, hospitals are in charge of the logistics involved in collecting samples from the general practitioners of their region who take such samples), the diagnostic of certain types of pathologies, medical validation or emergency services. The markets in which the Group offers to sub-contract and outsource clinical laboratory services are characterized by high entry barriers. In other words, the process followed in a call for tenders requires spending a lot of time before a company is selected as a joint subcontractor. Being selected demands undeniable technical expertise and substantial investment capacities. Finally, the reputation and experience of the bidders are key criteria if they are to be picked as a joint subcontractor. The Group has built up acknowledged know-how in terms of bidding in calls for tenders for the outsourcing or sub-contracting of clinical laboratory services. The most important elements taken into account in this regard relate to: whether existing technical platforms are used or not, the Group has technical platforms in most of the countries in which it operates. Accordingly, it can swiftly and efficiently cope with the additional tests resulting from a new contract. In the United Kingdom, the Group does not have as dense a network as in the other countries where it operates, and sometimes it needs to set up a laboratory on an ad hoc basis, as was the case in Taunton in the Southwest region and Basildon in the Essex region; operational reorganization, the operations of the existing laboratory(ies) within the hospital or the clinic are slashed to a strict minimum in order to manage the urgent needs of patients, while the 77 remaining tests are transferred to the Group’s technical platform. During this transition, the Group offers appropriate and adapted training courses to facilitate this reorganization; harmonization of equipment, to benefit from additional economies of scale. This possible change in equipment can lead to additional investments stemming from the need to terminate contracts with a non-referenced supplier; transfers and reorganization of human resources, transfers of employees is crucial, especially when it consists in a transfer from the public sector to the private sector. In the United Kingdom, the Group has benefited from Sodexo’s experience in this respect; harmonization of information systems, the Group must ensure the harmonization of the IT systems of the relevant hospitals and its own systems. The teams that submit bids to these calls for tenders build a specific financial model for every contract that takes into account all variables (investments, term, growth, etc.) in order to determine the net present value of the project’s return on investment and the time needed to reach said return on investment. These two key criteria play a crucial role in terms of determining the price offered to the potential future customer. Moreover, although EBITDA margins recognized in these calls for tenders are generally lower than in the other countries where the Group operates, these contracts offer excellent visibility on future income because prices are set for the entire duration of the cooperation and are even often reassessed in line with inflation indices. The valuation approach is therefore different from the one used in the Group’s conventional acquisitions. The Group has developed a transferable expertise in the fields of sub-contracting and outsourcing. For instance, the Group transmitted the know-how it developed in Spain and Portugal in the field of outsourcing to its subsidiary located in the United Kingdom. Since this initiative began, the Group has already won two contracts with four NHS hospitals. In 2015, according to the Group, at least three more NHS hospitals are expected to launch calls for tenders for outsourcing laboratory services. Some of these tenders some are located close to the Group’s Laboratories. This success is mainly due to the approach focused on the quality of services and the establishment of a partnership with NHS hospitals, for example in the form of a scientific committee composed mainly of doctors employed by hospitals and in charge at first of the surveillance of the transition and later on of the proper functioning of the service. In addition, the hospitals benefit from more attractive prices in the case of an increase in their volumes or in the case new hospitals joined the technical platform of their respective region. The Group is therefore seeking to enhance its expertise at a European level in order to seize future opportunities in sub-contracting and outsourcing that will arise and thus improve visibility on future income generated by such contracts. According to the Group, technical platforms of the regions of Southwest and Essex (under construction) allow to cover markets of approximately £100 million and £500 million, respectively. The Group thus undertakes to enhance his expertise on the European level to seize future opportunities in subcontracting and outsourcing and hence to improve his visibility on future income generated by these contracts. 6.4.2.3 Export services Developing business in emerging countries is one of the Group’s organic growth drivers. Integrated providers of diagnostic services such as the Group can contribute substantial value added to the healthcare sector in emerging countries. Via Labco NOÛS, its Spanish subsidiary, the Group has already established a promising bridgehead in some of these markets. This presence can take various forms such as: making capacity available for specific tests or providing capacity to cope with excess demand when volumes exceed installed capacity. The laboratory of Labco NOÛS in Barcelona is a central platform for the performance of specialty tests. This is a specialty laboratory that can carry out highly sophisticated tests when sub-contracting for other laboratories. Samples are sent by plane and results are e-mailed to prescribing doctors and patients. This business has been especially developed in Latin America, where prospecting for customers is carried out by local subsidiaries, controlled by the Group but with minority interests held by local partners. This is the case in Brazil, Colombia, Peru and Mexico. In other countries in Latin America (Ecuador, Uruguay and Chile), in Eastern Europe 78 (Romania and Poland), in North Africa or in the Middle East, the Group may use commercial agents, paid on a commission basis, in order to set up partnerships; 6.4.3 providing assistance for designing laboratories or providing laboratory management for local partners lacking this kind of expertise. The Group is working, at the date of this document de base, on preparing bids for several calls for tenders related to this kind of assistance in Latin America and the Middle East. Group’s operations by country 6.4.3.1 Overview of Northern European market France Calais Cambrai/Valenciennes Lens Fécamp Lannion Avesnes/Seda n St Quentin Rouen Paris Nancy Chartres Argentan Orléans Nantes Technical Platform Hospital Laboratory Laboratory Integrated Diagnostic Center Specialty Laboratory (incl. anatomopathology and MAP) Specialty & Technical Platform Paray/Montceau Clermont/Vichy Lyon Charente s Alpes Roussillon Aurillac Castillo n Montélima r Rodez Montpellier Auch Revel Nice Air-sur-Adour Aix/Marseille Elne The Group entered the diagnostic services market in France in 2003 and, at the date of this document de base, is one of the two French leaders. Its Laboratories are evenly distributed throughout France, and are mainly located in small towns or rural areas; nevertheless, the Group is also present in major cities such as Bordeaux, Clermont-Ferrand, Marseille, Montpellier, Nancy, Nantes, Nice, Orleans and Paris. The Group’s limited operations in large cities is due to its conviction that clinical laboratories can be bought at more attractive prices in small towns than in big cities, while the revenue and average profitability per laboratory in large cities is generally lower than the national average in France. As of December 31, 2014, the Group operated 255 facilities including 64 laboratories in France. Each of the Group’s Laboratories and Sampling Centers in France employs a medical doctor. The Group has set up its technical platforms (some of which provide specialty testing services) in the wake of the regulatory changes implemented in January 2010 that loosened restrictions on the outsourcing of testing and authorized the operation of technical platforms. Some of the Group’s routine testing laboratories and technical platforms are located in hospitals or near hospitals. They offer their services, in a non-exclusive manner, to public and private hospitals pursuant to outsourcing contracts, such as for example the Biofrance laboratory in northern France. In September 2014, the Group took over the Alpigène laboratory in Lyon and can now provide cutting-edge molecular biology services in the French market. Depending on the urgency of the demand and the test’s production cost, the Group decides to do the test internally (keep it) or to outsource it (sell it). In the case of an internal test, the Group determines the facility in 79 which the test will be taken out in order to strike the best balance between the results date and the production cost. This arbitration results in the transfer of samples to technical local, regional or national platforms offering specialty tests or specialty laboratories such as Labco NOÛS, Roman Païs or Alpigène. Generally speaking, samples collected by the Group’s Collection Centers and Laboratories are sent for processing at its technical platforms by using vehicles the Group owns or rents. The Group’s two specialty platforms also have the equipment required to perform specialty tests, but in some cases it may be more efficient and less expensive for the Group’s routine testing laboratories to outsource such tests to third parties (which generally transport samples from sampling points to their laboratories). As the Group expands further its network of technical platforms, it plans to transform gradually most of its routine testing laboratories into Sampling Centers and to consolidate, on its own technical platforms, its processing activities for routine tests as well as for specialty tests. From 2010 to 2014, the aggregate number facilities in France, including Laboratories and Sampling Centers, rose from 150 to 255. Over the same period, the proportion of Sampling Centers rose from 37% to 75% of the total. Moreover, the number of health territories (as defined by the ARS) served by Group facilities rose from 36 in 2010 to 47 in 2014, i.e., 44% of the 108 existing health territories. Over the same period, the average number of collection centers attached to a technical platform has risen from 2 to 7. Similarly, the average number of case files handled per Laboratory has risen from about 60,000 in 2010 to about 155,000 in 2014. During the period 2011-2014, the Group made respectively 31, 15, 8 and 6 acquisitions in France. Amongst these 60 acquisitions, 54 are bolt-on for already existing platforms. The Group’s revenue in France in the financial years ended December 31, 2013 and December 31, 2014 amounted to €325.3 million, or 54.7% of pro forma Group revenue, and €342.3 million, or 52.7% of pro forma Group revenue, respectively. The number of case files per financial year handled by the Group amounted to 8.42 million, 9.17 million and 9.82 million for the financial years 2012, 2013, and 2014, respectively. The following chart shows the geographical distribution of case files handled by the Group: 80 United Kingdom Notthingam Basildon Technical Platform Hospital Laboratory Laboratory Integrated Diagnostic Center Specialty Laboratory (incl. anatomopathology and MAP) Specialty & Technical Platform Taunton Southend-on-Sea Yeovil In 2010, the Group set up Integrated Pathology Partnerships, or ”iPP”, a joint venture with Sodexo, a leading global provider of facilities management services to the healthcare market. The Group holds a 90% equity interest in iPP and two call options over the 3% held by Sodexo and the 7% granted to the two main managers of iPP through a “UK Employee Shareholder Scheme” (see section 10.5. “Off-balance sheet commitments” of this document de base). On December 31, 2014, the Group operated 6 Laboratories in the United Kingdom and ranked number three in the market for outsourced and sub-contracted laboratory services. Since January 2012 and the signature of a 10-year contract, Labco Diagnostics UK operates the clinical diagnostic testing business of Fresenius Medical Care (dialysis). The business related to this contract is expected to generate cumulative revenue estimated to be worth at least £15 million over 10 years. In June 2012, iPP began to operate under the partnership set up with Taunton and Somerset NHS Foundation Trust and Yeovil District Hospital NHS Foundation Trust. Under the partnership agreement, iPP delivers the full range of clinical laboratory and anatomopathology laboratory services, while the clinical interpretation and clinical consulting functions continue to be provided by the medical staff of these trusts, who remain NHS employees. This partnership and related agreements have an initial term of twenty years, renewable for five years and should generate, over the initial period, an approximate revenue of £300 million. As of the date of this document de base, this partnership focused on providing services to Taunton and Somerset NHS Foundation and Yeovil District Hospital NHS Foundation, but it was structured in a way that allows other medical trusts in the region to join it. After two years of losses in 2012 and 2013, due to heavy investments and restructuring of the service, the contract has been profitable since 2014. This contract was amended on March 2015 and restructured into two separate contracts, one regarding site management and material (related to the provision of material, reagents, consumables and support functions), and the other relating to provision of staff (related to the provision of technical team). This amendment puts the commercial organization in line with that put in place for contracts signed in 2014 and described below. Taunton and Somerset NHS Foundation Trust, the Group’s current client, was designated as privileged interlocutor for the integration of Weston Area Health Trust. When the merger is effective, before the end of 2015, the Group will have to optimize the use of its technical platform by integrating the incremental volumes from the Weston hospital. The Group will also manage an additional hospital Laboratory. 81 Following the signature of this contract, iPP focused on developing its technical platform in Taunton. Once it became operational in 2013, iPP was able to start transforming the existing hospital laboratories into laboratories able to provide emergency services. During the first half of 2014, the Group set up 2 new business units, i.e. iPP Facilities Ltd. and iPP Analytics Ltd. Note that iPP Analytics was set up in order to integrate NHS staff under TUPE regulations (see section 6.5.2. “Regulation –United Kingdom” of this document de base) and provide testing results. iPP Facilities, in charge of managing the Group’s facilities and equipment in the United Kingdom, was also created to meet potential tenders to manage devices or provide point of care testing (POCT) devices. The Group, through these two companies, signed two partnership agreements in May 2014 with Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust to provide laboratory services. These partnerships will also allow new customers to be integrated. Operations began in October 2014 for an initial 10-year term with a possible 5-year extension. The business related to these contracts is expected to generate, over the initial period, a revenue estimated to be in excess of £240 million over 10 years. Under these new partnerships, iPP will manage the Sampling Centers of both hospitals as well as providing the full range of clinical laboratory and anatomical pathology laboratory services. In total, 560 persons were transferred from NHS to entities of the Group, respectively 164 under the agreements between Taunton and Somerset NHS Foundation and Yeovil District Hospital NHS Foundation and 396 under the agreements with Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust. In the United Kingdom, during the period 2011-2014, the Group made 1 acquisition in 2012 and 1 acquisition in 2013. Group revenue in the United Kingdom in the financial years ended December 31, 2013 and December 31, 2014 amounted to €4.4 million, or 0.7% of pro forma Group revenue and €27.0 million, or 4.2% of pro forma Group revenue, respectively. All Group revenue in the United Kingdom is generated by contracts signed with public or private hospitals. However, a significant proportion of the tests analyzed by the Group is collected from independent doctors, who themselves are partners of these hospitals. In the United Kingdom, the Group carried out a total of 0.39 million tests in 2012, 1.64 million in 2013 and 10.37 million in 2014. In 2014, 6.7 million tests were carried out under contracts with Taunton and Somerset NHS Foundation and Yeovil District Hospital NHS Foundation, 3.2 million under contracts with Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust and 0.5 million tests were carried out by Labco Diagnostics UK. 82 Belgium Brussels Nivelles Mont Saint Guibert Technical Platform Hospital Laboratory Laboratory Integrated Diagnostic Center Specialty Laboratory (incl. anatomopathology and MAP) Specialty & Technical Platform (Sources: Factiva C&E, companie’s websites, Société, Statbel) The Group moved into Belgium in 2008 after acquiring the Roman Païs biology company. On December 31, 2014, the Group operated four Laboratories in this country. Its facilities are concentrated in the region of Brussels and in Wallonia. The Group’s laboratories in Belgium mainly propose routine and specialty tests. Samples are collected in the Group’s Sampling Centers or are sent by healthcare professionals. In addition to conventional clinical laboratory tests, the Group’s Laboratories in Belgium perform nutritional tests, which are not usually subject to regulated prices. For the financial years ended December 31, 2013 and 2014, the nutritional biology business accounted respectively for €10.9 million and €12.2 million, amounting to 40% and 41% of the Group’s Revenue in Belgium. In 2014, a bit more than half of the samples of nutritional biology was transferred by laboratories and doctors based outside of Belgium. This activity has witnessed an average annual growth of approximately 15% since 2011. In Belgium, during the period 2011-2014, the Group made 1 acquisition in 2011 and 1 acquisition in 2014 Group revenue in Belgium in the financial years ended December 31, 2013 and December 31, 2014 amounted to €27.2 million, or 4.6% of pro forma Group revenue, and €30.0 million, or 4.6% of pro forma Group revenue, respectively. The Group revenue in Belgium on December 31, 2014 can be broken down as follows: poly-ambulatory business 53%, nutritional operations 41% and other tests (anatomical pathology, veterinary, etc.) 6%. The Group handled a total of 0.37 million case files in Belgium for the financial year 2014, 0.33 million for the financial year 2013 and 0.34 million for the financial year 2012. In 2014, the number of case files related to nutritional biology amounted to approximately 62 000. 83 Switzerland Technical Platform Hospital Laboratory Laboratory Integrated Diagnostic Center Specialty Laboratory (incl. anatomopathology and MAP) Specialty & Technical Platform Geneva As of the date of this document de base, the Group operates one Laboratory, TEST SA, in Switzerland since 2013. This laboratory, jointly owned with the two founders, began to operate in 2013. The Group holds 48% of issued capital and a call option at a set price on the remaining 52%. The Group’s main objective, via this transaction, is to improve its knowledge of the Swiss market and its specific features, in view of potential investments it could make in this market. This laboratory works mainly with independent healthcare professionals. It offers them an all-electronic solution that enables them to look at the catalogue of tests, select the tests to be conducted, input information about the patient, look up results, order additional tests, etc. The Group’s revenue in Switzerland in the financial years ended December 31, 2013 and December 31, 2014 totaled €0.9 million, or 0.2% of pro forma Group revenue, and €2.0 million, or 0.3% of pro forma Group revenue, respectively. 6.4.3.2 Spain Overview of Southern European market San Sebastian La Coruna Pontevedra León Vitoria-Gasteiz Manresa Zaragoza Zamora Technical Platform Hospital Laboratory Laboratory Integrated Diagnostic Center Specialty Laboratory (incl. anatomopathology and MAP) Specialty & Technical Platform Vic Barcelona Lleida Tarragona Madrid Manises Valencia Badajoz Torrevieja Sevilla Murcia Malaga Cádiz Marbella CANARY ISLANDS La Palma Gibraltrar Santa Ceuta Cruz Adeje The Group entered the Spanish market in 2007 when it acquired General Lab S.A., and it became the leader of the Spanish clinical laboratory services market in 2008 with the acquisition of Sampletest, S.A. On the date of this document de base, the Group was the leader in the Spanish market (on the basis of 2013 and 2014 revenues). 84 On December 31, 2014, the Group operated 56 Laboratories and a network of more than 500 Sampling Centers a majority of which is managed with partners (mainly medical cabinets and nurse cabinets) under collaboration agreements. Most of the Group’s facilities are concentrated in Catalonia, Madrid and Andalucia which are the three main population centers in Spain. In 2015, the Group plans to carry out a major restructuring in Spain following the 2014 acquisition of a building in Barcelona (see section 8.1.1. “Real estate properties” of this document de base), which will house all its regional operations. The restructuring will entail closing down five Laboratories, one warehouse and an administrative office, rationalizing the equipment currently used, insourcing some of the Group’s tests to the new Barcelona platform and splitting the support functions between Madrid and Barcelona. This restructuring should significantly improve the productivity of the Group’s operations, with some 100,000 tests performed daily at the new facility and a decrease in staff of about 50 employees nationwide. According to the Group, this restructuring should result in an estimated reduction in recurring costs of €0.85 million for the financial year 2015 and €1.20 million for the financial year 2016 across Spain as a whole. During 2014, the Group acquired and integrated three laboratories specialized in cytogenetics and in molecular biology in Madrid and thus built up its business in these fields. In early 2013, the Group added to its catalogue a non-invasive prenatal screening test. This test detects the most frequent chromosomal anomalies in a fetus from a blood sample taken from the mother. Said anomalies include, inter alia, Down syndrome (trisomy 21), Edwards syndrome (trisomy 18) and Patau syndrome (trisomy 13). This test has the advantage of not exposing neither the mother nor the fetus to any risk whatever while being more reliable than all the other tests aimed at detecting these chromosomal anomalies found on the market. After its successful introduction in Spain, this test was launched on the Portuguese and Italian markets. This high value-added test has since then been sold tens of thousands of times within the Group. The Group’s laboratories in Spain offer a range of routine and specialty tests (including anatomical pathology tests). The laboratory in Barcelona is the most technologically advanced in the Group’s network. The Group’s geographic footprint allows it to enter into nationwide agreements for private market clinical laboratory services with private health insurers, the main ones being with Adeslas, DKV, CASER, Medifiatc, Sanitas (Bupa Group), Assistancia Sanitaria, Colegial, Mapfre, Axa, Divina Pastora, CIGNA and Allianz. These agreements are mainly based on an activity-based fee structure, under which the Group is paid per test performed. For more than 10 years, the Group has managed outsourcing contracts with private hospitals such as Quiron Hospitales, Allançia (Capio group), Sanitas Hospitales and Hospital de Manises (since 2011). It carries out tests, either in one of its emergency services laboratories, or at nearby routine testing laboratories or technical platforms. The Group also performs specialty tests for other private clinical laboratories. On December 31, 2014, the Group had signed outsourcing contracts with 37 hospitals. The Group also has a portfolio of companies and occupational health service providers for which it provides clinical laboratory testing services in connection with regular check-ups for employees. In addition, the Group provides other services in Spain such as specialty tests for public hospitals and out-of-pocket services for patients. Following a ruling handed down by the Court of Justice of the European Union on January 17, 2013, Spain was forced to change its regulations relating to the VAT rate applicable to pharmaceutical products and medical equipment. The VAT Directive (in particular its Annex III) authorizes a Member State to apply a lower VAT rate to the following products: pharmaceutical products used for health care, prevention of illnesses and as treatment for medical and veterinary purposes, especially when used for contraception or sanitary protection purposes; medical equipment, aids and other appliances normally intended to alleviate or treat disability, for the exclusive personal use of the disabled, including the repair of such goods, and supply of children’s car seats. 85 The scope of Spanish VAT regulations has been deemed to exceed the VAT Directive and by consequence in need of changes. The reduced VAT rate of 10% is no longer applicable as of January 1, 2015 and the standard rate of 21% will have to be applied. The Group projects that this change will result in an additional annual expense that it estimates will range between €2 million and €3 million on purchases of reagents. Since the acquisition of CIC in 2011, renamed Labco NOÛS in 2013, a laboratory based in Barcelona that performs specialty tests, the Group has also offered clinical diagnostic testing services since Spain to customers in emerging countries (see section 6.4.3.3. “Emerging countries” of this document de base). In Spain, during the period 2011-2014, the Group made 1 acquisition in 2011, 2 acquisitions in 2013 and 3 acquisitions in 2014 The Group’s revenue in Spain for the financial years ended December 31, 2013 and December 31, 2014 totaled €108.0 million, or 18.2% of pro forma Group revenue, and €115.6 million, or 17.8% of Group revenue, respectively. The Group’s revenue in Spain for the financial year December 31, 2014 can be broken down as follows: polyambulatory business 36%, sub-contracting and outsourcing generated by hospitals 29%, specialty tests 12%, occupational healthcare 11%, anatomical pathology 6% and other income sources 6%. The Group handled a total of 5.90 million case files in Spain in the financial year 2014, 5.27 million in the financial year 2013 and 5.01 million in the financial year 2012. Portugal Porto Centro Technical Platform Hospital Laboratory Laboratory Integrated Diagnostic Center Specialty Laboratory (incl. anatomopathology and MAP) Specialty & Technical Platform Lisboa Evora Faro Madeira The Group started operating laboratories in Portugal in 2007 in the wake of the acquisition of General Lab S.A., and subsequently became the Portuguese clinical testing market leader in 2008 with the acquisition of Sampletest, S.A., which also operates in Spain. In 2011, the Group purchased Macedo Dias, the leader in the Portuguese anatomical pathology market. On December 31, 2014, the Group operated 25 laboratories in Portugal, including two technical platforms in Lisbon and one in Porto, which offer a range of routine and specialty tests. The Group has a network of more than 200 Sampling Centers half of which are managed with partners (mainly medical cabinets and nurse cabinets) through collaboration agreements. Most of the Group’s facilities are concentrated in the cities of Lisbon, Faro and Porto and in their suburbs. 86 The Group has signed reimbursement agreements with private insurance companies for patients covered by said private insurers. The Group provides, inter alia, services to hospitals in Cascais and Laures based on a model of a flat fee per patient (see section 6.2.4.3. “Southern Europe Segment – Spain” of this document de base). At December 31, 2014, the Group had signed outsourcing contracts with 9 hospitals, including 2 public hospitals. Furthermore, the Group has entered into contracts with a small number of companies to provide diagnostic services for their employees. In Portugal, during the period 2011-2014, the Group made 1 acquisition in 2011. The Group’s revenue in Portugal in the financial years ended December 31, 2013 and 2014 totaled €43.2 million, i.e. 7.3% of pro forma Group revenue, and €43.1 million, or 6.6% of pro forma Group revenue, respectively. The Group’s revenue in Portugal for the financial year ended December 31, 2014 can be broken down as follows: poly-ambulatory business 59%, sub-contracting and outsourcing generated by hospitals 30%, anatomical pathology 4%, specialty tests 1%, occupational healthcare 1% and other income sources 5%. The Group handled a total of 1.74 million case files in Portugal for the financial year 2014, 1.68 million for the financial year 2013 and 1.55 million for the financial year 2012. Italy Monza Binasco Genoa San Remo Technical Platform Hospital Laboratory Laboratory Integrated Diagnostic Center Specialty Laboratory (incl. anatomopathology and MAP) Specialty & Technical Platform Naples The Group started operating laboratories in Italy in 2007. On December 31, 2014, the Group operated 9 Laboratories including 5 Integrated Diagnostics Centers located in Lombardy, Campania and Liguria. In addition to clinical testing services, the Group also offers other diagnostic services, including medical imaging services, medical check-ups, a complete offer in occupational medicine, physiotherapy services and day surgery. In July 2014, the Group wrapped up its acquisition of the SDN group, which offers integrated diagnostic services including (routine and specialty) laboratory tests and the entire range of medical imaging: radiology, MRI but also the most sophisticated PET scanning medical imaging technologies, combining molecular imaging and nuclear medicine. Of all the medical imaging companies in Italy, the SDN group is the only one to hold the status of IRCCS (Istituto di Ricovero e Cura a Carattere Scientifico or a research hospital and treatment center), acknowledging its advanced level of technical expertise. Furthermore, SDN is the driving force of a not-for87 profit scientific foundation that conducts research aimed at improving nuclear diagnostic technologies. The foundation boasts unrivalled experience and has been granted substantial European subsidies. In 2013 and 2014, the SDN Foundation published 99 and 98 research articles respectively in specialized journals such as “the Journal of the American College of Cardiology”, the “European Heart Journal” and the “American Journal of Gastroenterology”. The SDN group holds substantial market share in Campania, particularly in molecular imaging, and has developed an efficient operational organization, thanks to its integrated production of contrasting radioactive products (so-called “tracers”) in a cyclotron located at the company’s main site. Apart from accreditation for all the group’s activities, the SDN group is also accredited by “The Joint Commission”, a US non-profit organization, certifying the quality of care provided to patients while continuing to improve care levels. Integration of the SDN group is proceeding according to plan. In 2014, the SDN group’s revenue increased by more than 1.5% compared with 2013. For instance, in the first two months of 2015, more than 600 “patient” case files were exchanged between the Group’s Laboratories through teleradiology. The acquisition of the SDN group fits in perfectly with the Group’s strategy. This acquisition will reinforce and complete the medical imaging and integrated diagnostics already offered in Genoa since 2008 by the laboratory of the Baluardo Group. The know-how and expertise of the SDN group in the field of integrated diagnostics can be used by other entities of the Group when the national markets where they are present mature. In Italy, during the period 2011-2014, the Group made 1 acquisition in 2011 and 6 acquisitions in 2014, including 5 during the acquisition of the SDN group. The Group’s revenue in Italy in the financial years ended December 31, 2013 and December 31, 2014 totaled €38.2 million, or 6.4% of Group revenue, and €55.6 million, or 9.0% of the Group’s revenue, respectively. Including the SDN group’s revenue in the same periods, pro forma Group revenue in Italy totaled €85.6 million, or 14.4% of pro forma Group revenue and €89.5 million, or 13.8% of pro forma Group revenue, respectively. Pro forma Group revenue in Italy in the financial year ended December 31, 2014 can be broken down as follows: clinical laboratory services 40% (including poly-ambulatory 30%, occupational healthcare 8% and specialty tests 2%), molecular imaging 23%, medical imaging (other than molecular) 22%, other medical services 13% and other revenue sources 2%. The Group handled a total of 0.71 million case files in Italy for the financial year 2012, 0.79 million for the financial year 2013 and 1.23 million for the financial year 2014 (pro forma for the acquisition of the SDN group). The number of clinical case files amounted to 878 000, the number of medical imaging case files to 152 000, the number of nuclear imaging case files to 35 000 and the number of other case files to 169 000. 6.4.3.3 Emerging countries The Group began to offer services in emerging countries after acquiring the CIC laboratory in 2011 (renamed Labco NOÛS in 2013), a laboratory located in Barcelona that performs specialty tests. At the time of the transaction, Labco NOÛS already had a sales office in Brazil, Colombia and the Middle East. Since then, it has opened two more in Peru and Mexico. The Group’s legal representations in Latin America are purely commercial subsidiaries that negotiate service contracts with local laboratory groups that allow them to send their specialty tests to the technical platform in Barcelona. Local representatives are also in charge of supervising customer relations, promoting new tests and ensuring the smooth running of operations. In addition to its sales offices, Labco NOÛS also has sales representatives who are Group employees as well as independent commercial agents whose mission is to negotiate new contracts in the regions where the Group is not yet present such as Latin America (Ecuador, Uruguay and Chile), Eastern Europe (Romania and Poland) and in North Africa. As tests for emerging countries are performed by Labco NOÛS in Barcelona, business from emerging countries is allocated to the Iberian Peninsula cash generating unit and therefore the corresponding revenue is recognized in Spain’s revenue. 88 6.4.3.4 Germany In 2008, the Group entered the German market by acquiring six laboratories located in the country’s four western Länders: Baden-Württemberg (Karlsruhe), Hesses (Dillenburg, Giessen and Marburg), Saar (St-Ingbert) and Rhineland-Palatinate (Duisburg). The competitive environment is characterized by the advanced level of market consolidation, with a few major players of an international size (Sonic Healthcare, Limbach, Synlab), and mid-sized regional players. In such a highly technical environment, the small size of the Group’s operations in Germany rapidly came to be seen as a highly detrimental factor. Furthermore, the results of the Group’s German operations were severely affected by contentious relations with the former owners of some of the laboratories acquired by the Group. Owing to the lack of non-compete clauses, they left the Group to set up directly competing businesses in the very same local markets. Lastly, in the case of the Dillenburg laboratory, the Group discovered that the selling shareholders had overcharged private insurance companies and the Hessen health insurance fund. As the new management team notified the relevant authorities of the fraud, this laboratory’s revenue has massively contracted and, in turn, this has slashed the overall results of this subsidiary. The Group sued the former owners of this laboratory and recovered compensation of €5.5 million. Although a new management team was recruited in 2011 and an ambitious operational efficiency program was implemented (consolidation of Hessen-located laboratories, internalization of logistics, pooling of support functions on the Frankfurt site), the turnaround in German operations remained insufficient in view of the Company’s profitability demands. In particular, this turnaround significantly slowed down because of sweeping price reductions implemented in 2012 at a regional level, and from 2013, onward at a federal level. Owing to the severe deterioration in results recognized in Germany, a €36 million goodwill impairment loss was recognized on the German business in the Group’s 2012 consolidated financial statements. Acknowledging the Group’s badly weakened market position in Germany, the Company’s Board of directors decided in the summer of 2013 to conduct a strategic review of its operations in this country. Shortly after launching this review, the Group received an indication of interest in these very same operations from Sonic Healthcare, an Australian competitor that already has significant operations in Germany. Talks were soon opened and swiftly enabled the main points of a sale agreement to be agreed upon, and the agreement was signed on September 26, 2013 to complete the transaction on December 2, 2013. German operations were sold for the price of €76 million, thereby showing a double-digit EBITDA multiple. The sale contract of German operations includes the usual liabilities guarantee clauses and provides that the Group remains exposed to possible residual eventual legal risks related to the specific situations relating to the Dillenburg and Duisburg laboratories. Finally, this sale contract does not include a non-compete clause, as the Group reserves the right to re-enter the German market should the conditions allowing it to acquire a leadership position on this market be met. 6.4.4 External growth strategy Since it was founded in 2003, the Group has implemented a selective external growth strategy aimed at developing its network of clinical laboratories and constantly reviews acquisition opportunities. The Group’s strategy with regard to acquisitions in particular consists in targeting three categories of laboratories or Medical Diagnostics Centers. First, the Group targets incremental acquisitions made in zones in which the Group already has technical platforms since this leads to the rapid implementation of industrial synergies. In addition, the Group is interested in acquisitions that enable it to improve its coverage of a given region and this means mainly acquiring large platforms in new markets from which it will be able to implement its strategy of consolidating smaller operators. Lastly, acquisitions with a high medical content will give the Group new scientific or technological capacities that can subsequently be deployed throughout the network. With respect to this last point, the Group is particularly interested in innovations that fit in with its medical project, especially anatomical pathology, molecular biology and molecular imaging. Generally speaking, the Group requires the laboratory doctors who have sold their business to the Group to continue to operate them. In addition, depending on the case, The Group may either offer to these laboratory 89 doctors the possibility to reinvest some of the proceedings from the sale in buying or subscribing Company’s shares, or impose such a reinvestment. In the case of substantial acquisitions, as a rule, the Group would like to ensure that the key managers of companies it has taken over, who know the region very well, join the Group’s management team. For instance, Luis Vieira, the current Executive Vice-President of Corporate Development in Europe, became a member of the Group’s management team following the acquisition of Sampletest, S.A. in 2009. The Group has developed a structured approach to acquisitions that enables it to complete these transactions under the best financial and legal terms and conditions, while carrying them out fast enough to be able to acquire, in a short time span, the most attractive opportunities. In other words, once the acquisition has been completed, the Group expects to achieve about half of the synergies expected from the transaction in the first year and then virtually all the remaining synergies during the second year. Three kinds of synergies can be generated: a reduction in the cost of sales, rightsizing the workforce and cutting other costs related to operations, mainly logistics costs and administrative expenses. In the large majority of cases, the Group expects to reach a multiple of acquisition after achieving synergies in the range of 5 times the EBITDA of the acquired laboratory. The acquisition of CIC (renamed Labco NOÛS in 2013) illustrates the Group’s know-how in terms of integrating acquisitions as well as its ability to implement swiftly operational and financial synergies. Operational management was enhanced thanks to the implementation of the following measures: broadening the range of laboratory services offered by Labco NOÛS in the field of specialty tests; expanding Labco NOÛS’ commercial presence in new emerging countries in Latin America, other than Brazil (such as Peru, Mexico, Ecuador, Uruguay and Chile), Eastern Europe (Romania and Poland), North Africa and the Middle East; improving infrastructures and logistics, in particular via the deployment of the Group’s LIS. Despite the highly adverse evolution of the exchange rate between the Brazilian real and the euro, going from 1 euro for 2.22 Brazilian reals at the end of December 2010 to 1 euro for 3.22 Brazilian reals at the end of December 2014 (source: BCE), the above initiatives have helped to improve the performance of Labco NOÛS since its acquisition in 2011: The revenue of Labco NOÛS grew by 43% between 2010 and 2014, climbing to €10.5 million in 2014 (versus €7.3 million in 2010). EBITDA rose from €1.3 million in 2010 to €2.5 million in 2014, and increase of more than 90%. The EBITDA margin improved by 6 percentage points to 23.5% from 17% in the year of the acquisition. Therefore, after factoring in operating gains, the acquisition multiple achieved by the Group on Labco NOÛS was 2.6 times 2014 EBITDA versus 4.9 times 2010 EBITDA. Labco NOÛS’ activity is recognized in the Iberian Peninsula’s cash-generating unit, the samples coming from Latin America are treated in Spain on the technical platform in Barcelona. In France, the Group’s strategy has consisted in unifying the analytical phase and transferring the pre-analytical phase to Sampling Centers. As a result, operational synergies and significant productivity gains were achieved thanks to the automation of tests, the rightsizing of the workforce, the existence of a common IT platform and the setting up of a shared services center. To illustrate the Group’s development strategy in France, the example of a platform built on the basis of an initial investment made in 2006 in an area selected from its high potential shows how the subsequent deployment by repeated incremental acquisitions can accelerate revenue growth and improve the business’ profitability. In this particular case, the test production business has been centralized on the main platform, leading to significant productivity gains by automating tests, rightsizing the workforce and providing access to shared service centers for the support functions. On a base of 100, the relevant entity’s revenue grew by more than 180% from 2008 to 2014, with the 6 acquisitions made by this platform over the period and the transfer of 90 a facility to the platform in 2013 contributing to 170% of this growth. Furthermore, the various productivity measures taken over the period have resulted in an increase in the entity’s EBITDA margin from 30% to 40%. 2008 – 2014 Revenue Build-up Revenue rebased to 100 in 2008 # of sites 5 6 8 12 13 14 (2 13 ) 2008 – 2014 EBITDA Based on revenue rebased to 100 in 2008 EBITDA Margin (%) 40% +1 0 pt s 30% (1) (2) Year on which acquired company joined platform One lab closed in 2013 The Group has set up a European development team dedicated to identifying potential acquisition targets. This team, which includes financial analysts, jurists and local representatives in charge of development, carries out market studies covering in particular regulatory and competitive environments of the countries in which the Group operates or in countries where the Group is considering the possibility of setting up operations. This team can draw upon, in every one of these geographical zones, a constantly updated database covering the potential acquisition targets. By the end of February 2015, the database contained more than 70 entities; were the acquisitions to take place, 10 of these entities would strengthen the Group’s geographical coverage and 10 of them would broaden its clinical service offering, while the other acquisitions would be integrated incrementally with the existing Laboratories. The 70 entities include those referred to in section 5.2.2.1 “Acquisitions of groups of companies, companies and/or businesses” of this document de base. Group representatives use this database, among other things, to decide whether to get in touch directly or indirectly with the legal entities or natural persons who hold controlling interests in the potential targets. These targets are also identified thanks to relations forged by Group laboratory doctors with their colleagues and proposals made by owners of laboratories who would like to join the Group’s network. When assessing potential targets, the Group studies and evaluates several financial, legal and strategic criteria that may vary according to the country where the targets are located. The financial criteria include the target’s EBITDA margin, return on capital employed (pre-tax operating income as a ratio of capital employed, defined as non-current assets plus working capital), potential synergies and compatibility of investments with the Group’s financial commitments. Amongst the legal criteria, are analyzed the legal structure of the target, the regulatory constraints applicable to this target, as well as the entirety of the commitments and events that can likely generate constraints or specific risks. The strategic criteria include the target’s margins, the extent to which the relevant market has already undergone concentration and its remaining consolidation potential, how close the target’s laboratories are located to the Group’s, as well as their size and profitability. Another major assessment criterion consists in the extent to which the considered target’s laboratories are strategically a fit, in particular with respect to the techniques used and the tests performed by these laboratories that could help the 91 Group build up some of its operations or widen its know-how to specific fields that would complete its range of expertise. Furthermore, when the people who could relinquish control of such a target consider the option of continuing to work subsequently for the Group, their ability to buy into the Group’s model and corporate culture is also a decisive criterion in terms of making the acquisition or not. As soon as a target is identified, the acquisition project is reviewed by a deal team composed of a project manager, the relevant country managing director and Labco’s President of Corporate Development. The project is then presented on the basis of a complete and duly documented dossier to the Group’s General, Financial and Legal Management Departments for an initial assessment of the main operational and legal aspects and related costs in order to ensure that they fit in with the Group’s strategy in a consistent manner and in line with the Group’s legal security procedures and comply with its financial capacities. When the investment under consideration is so large or of a nature that requires the prior approval from the board of directors, the board of directors decides whether to approve the project or not, after an examination of a complete presentation file and, when relevant, attributes the powers enabling the transaction to be completed to the Company’s General Management team so that it can prepare the plan on how to integrate the laboratory, or the entity holding said laboratory or laboratories, within the Group, negotiate the legal and financial terms and conditions of the contemplated acquisition and carry it out. When the amount of the investment under consideration does not require prior approval from the Company’s board of directors, the Chief Executive Officer of the Company, after the projected acquisition is assessed at a financial level by the Financial Management Department of the Group and, on the legal side, by the Legal Department of the Group, decides whether the acquisition is to be carried out or not and supervises the manner in which it is. The Chief Executive Officer periodically reports back to the board of directors of the Company on the way in which acquisitions made under his responsibility are carried out. Following the date of the IPO of the Company, in case the amount of the contemplated investment requires the approval of the Company’s board of directors, the project will be presented to the board of directors following an opinion by the Strategic and Major Projects Committee. In any case, no letter of intent or engagement letter can be sent to the potential seller of the target unless the consent procedure described above is followed. A “compliance certificate” is drawn up. It sums up the relevant acquisition in particular by showing the target’s key parameters, the performance indicators and key financial items so that these indicators can subsequently be monitored to assess the target’s performance. Acquisitions carried out less than one year earlier are reviewed to a limited extent every month while the performance of acquisitions made more than a year earlier is reviewed in an extensive manner every 6 months during three years. 6.4.5 Quality standards In every country where it operates, the Group is subject to the national legislation that defines the mandatory quality standards it must comply with in its operations. These regulatory requirements vary from one country to the next. As it seeks to harmonize quality standards throughout its network, the Group has set up a quality insurance program that also includes compliance with applicable accreditation or certification standards such as those laid down by the International Organization for Standardization (“ISO”), as well as internal quality insurance standards. The three ISO standards most commonly applied in the sector are ISO 9001, ISO 17025 and ISO 15189. The latter, which is extremely demanding, more specifically applies to clinical laboratories. Laboratory accreditations according to ISO 15189 are difficult to compare as they vary greatly in scope. Accreditation can cover: - the facility(ies): place(s) where the tests or samples are carried out; - test categories: biochemistry, hematology, coagulation, etc.; - the tests themselves within each of their respective categories: cholesterol, glucose, white blood cells, blood platelets, HIV serology, etc. 92 For example, an SEL with 5 facilities comprising two Laboratories and three Sampling Centers could be accredited as follows: - accreditation of one Laboratory for the “bacteriology” test category and for samples at that site; - accreditation of the other Laboratory for “biochemistry”, “hematology” and “coagulation” test categories; - accreditation of the three Sampling Centers for samples. All of such SEL’s facilities are therefore accredited but it would be wrong to say that the SEL is accredited for all the tests it performs. The Group is conducting, as of the date of this document de base, quality programs at a national level in all the countries where it operates. Thus, it has set up a European Quality Committee, composed of laboratory doctors and clinical pharmacists, which is in charge of these national quality programs. France has the strictest quality standards (see section 6.5.1. “Regulation – France” of this document de base). The Group has therefore set up a national committee composed of five laboratory doctors (or clinical pharmacists) and three internal quality experts, as well as a “Quality” Department that employs some 40 “Quality” experts. In addition, upon their integration within the Group, each SEL President undertakes to sign a charter setting out the internal quality policy. In France, the Group supplies every Laboratory in its network with a quality reference handbook along with an access to a documentary database, including ISO 9001, ISO 17025 and ISO 15189. The Laboratories are subject to an internal audit and an independent external audit (COFRAC). Accreditations are subject to periodic review every 12 months and then every 15 months. As of November 1, 2016, all clinical laboratories will have to be accredited for 50% of the clinical laboratory tests they perform. This threshold will be raised to 70% as of November 1, 2018 and then to 100% as of November 1, 2020 (see section 6.5.1 “Regulation – France” of this document de base). In order to achieve this target, the Group has already made significant progress toward complying with the minimum accreditation standards required under ISO 15189. On December 31, 2014, 90% of the Group’s SELs were ISO 15189 accredited by COFRAC, 5% were awaiting COFRAC’s decision and 5% were awaiting a COFRAC audit. For comparison purposes, according to COFRAC, the percentage of laboratories that have obtained accreditation was estimated at almost 30% on November 1, 2014. In the United Kingdom, laboratories have to be accredited by the United Kingdom Accreditation Service (“UKAS”), the national accreditation bureau or the Clinical Pathology Accreditation (“CPA”). In 2009, CPA became a subsidiary of UKAS pursuant to the modernization and development of clinical services in the United Kingdom. All the laboratories accredited CPA will have to be comply with the UKAS accreditation. UKAS accreditation standards are converging with those of ISO 15189 accreditation. In Belgium, the March 19, 2008 Royal Decree imposes on any laboratory wishing to offer molecular clinical services to get an ISO 15189 accreditation relating to the departments’ tests from the BELAC, the national bureau for Belgian accreditation. Of the 6 laboratories in the United Kingdom, 5 are CPA accredited and 1 is UKAS accredited. In the other countries where the Group operates, although accreditation is not a prerequisite, most of the Group’s laboratories have begun the accreditation process in order to obtain this international recognition of quality. 6.4.6 Group’s customers The Group provides testing services to a diverse range of customers. The Group considers any party that either directly gets in touch with it or refers a patient to one of its laboratories to be a “customer”. The Group considers any party from whom a sample is taken or a test is performed on to be a “patient”. Lastly, it considers any party 93 that pays for the tests performed to be “a payer”. In most cases and depending on the country, the Group’s customers, patients and payers are different persons or entities. The Group’s customer base varies considerably from country to country. The main categories of customers the Group provides its services to are as follows: Patients. In France, Italy and, albeit to a lesser extent, in Portugal, patients with a prescription for testing from their doctor may choose the clinical laboratory in which their tests will be performed. The main factor taken into consideration when choosing a laboratory is usually how close it is to their home or workplace. The Group has therefore positioned its network of laboratories in France and Italy mostly in small towns, city centers and suburbs. Independent healthcare professionals and partnerships of health professionals. In certain countries, healthcare professionals who require testing for their patients can recommend the Group’s laboratories. In Belgium and in Switzerland, healthcare professionals take samples themselves and send them to hospitals or private laboratories such the Group’s. By consequence, the relationship with healthcare professionals is a major commercial challenge. Hospitals. The Group provides hospitals with services ranging from routine and specialty testing to contract management services. The Group operates certain on-site clinical laboratories which hospitals generally maintain on-site to perform immediate testing. However, hospitals may also refer less time sensitive, less frequently needed and highly specialized procedures to outside facilities, including independent clinical laboratories such as the Group’s. The Group provides hospital outsourcing services in France, Spain, Portugal, Germany and the United Kingdom. It typically enters into mid- and long-term contracts with such clients. Laboratories. The Group performs specialty tests for other clinical testing laboratories via its Labco NOÛS subsidiary. These laboratories are found in Europe but also in Latin America, the Middle East and North Africa (see section 6.4.3.3. “Group’s Operations by country – Emerging Countries” of this document de base). Private Medical Insurance Companies. In certain countries such as Spain, private medical insurance companies typically require their insured patients to choose from a list of pre-selected laboratories with which these insurers have a reimbursement contract. The list varies depending on the patient’s private insurance scheme. Companies. In jurisdictions such as Spain, Portugal and Italy, employees are entitled to a regular medical check-up paid for by their companies. The services related to these check-ups are generally provided by specialized companies that subsequently sub-contract the tests to a private laboratory. Other institutions. The Group also provides services to other institutions, including government agencies and other independent clinical laboratories that lack the breadth of testing capabilities of the Group. 6.4.7 Invoicing and payment procedures Billing for laboratory services is a complex process that sometimes involves many payers. According to the billing arrangement and the applicable law of the country in which the Group operates, the payer may be either a third party responsible for providing health insurance coverage to patients (such as a national public health insurance system, a private medical insurance company or an employer), a patient, a practitioner or any other party (such as a hospital, another laboratory or an employer) that referred the patient to the Group, or several of these parties. Generally speaking, the Group bills for clinical testing services on a fee-for-service basis, except in Spain where it has entered into agreements with some private insurance companies on the basis of individual policyholders’ expenditure. Although the Group has no knowledge of material problems with respect to receiving its fees, the Group is exposed to the risk of non-payment by patients or other customers (see section 4.5.1. “Credit or counterparty risk” of this document de base). 94 See section 6.2. “Presentation of sectors in which the Group is active” of this document de base for a description of the billing and payment arrangements in every market in which the Group operates. 6.4.8 Group’s suppliers The main equipment and material required by the Group’s operations are testing equipment and reagents. The Group regularly assesses its equipment (analytical systems, robotics, pre- and post-analytical devices) and has entered into national or pan-European pricing agreements with certain laboratory suppliers chosen after calls for tenders. These agreements set purchase prices for reagents according to an adjustable pricing grid as well as for equipment and maintenance required to ensure satisfactory operation of laboratories. These pricing agreements are entered into for an average term of five years according to the kind of activity but parties are entitled to renegotiate the agreed prices if reimbursement levels set in the relevant county are lowered unless an automatic adjustment in prices was previously provided for in the contract. This provision enables the Group to benefit from the latest technological and medical breakthroughs and strike the right balance between total cost, innovation, flexibility and risk management, as quality remains one of its key priorities. For instance, the quality department steps in at the very beginning of the tendering process by drafting specifications for every category of operation which suppliers must comply with. In 2014, the Group’s three largest suppliers were Siemens, Biomérieux and Roche. They accounted for less than 40% of the Group’s total purchases of reagents and consumables. Several other suppliers can supply the Group with the equipment required to perform its tests. Accordingly, the Group is of the opinion that it does not depend on any one supplier and therefore losing one of its current suppliers would probably not have a material adverse effect on its business. The Group increasingly enters into leasing contracts based on a “fee-for-service” model for its laboratory devices. These contracts provide that suppliers of reagents provide the Group with laboratory devices, and in exchange the Group undertakes to buy reagents exclusively from them. The price paid by the Group to these suppliers accordingly also includes the reagents themselves as well as the rental and maintenance of the equipment. The Group has also implemented since 2009 inventory management policies for its network of laboratories, which are aimed at lowering the level of its inventories by asking suppliers to remain the owners of certain products held in the Group’s inventories and by making supply deliveries flexible according to the needs of its laboratories. The Group regularly launches initiatives aimed at reducing costs for all expenditure items. In particular, in order to lower the cost of raw materials and maintain its margins against a backdrop of price cuts imposed on clinical testing in many countries, the Group launched in 2009 a pan-European program called SPORT. It has resulted in a reduction in consumed purchases by cutting the number of suppliers and by negotiating better business terms and conditions thanks to the use of framework contracts, in particular for purchases of reagents used in clinical testing. For example, four European calls for tenders covering up to five countries were successfully concluded during 2014 in the following fields: consumables, microbiology, electrophoresis (technique for separation and analysis of macromolecules) and immunology-hematology. These initiatives should lead to substantial savings in the relevant product categories. 6.4.9 Information Technology Systems The Group uses IT systems in virtually all aspects of its business, in particular clinical testing, test reporting, billing, customer service, logistics and the management of medical data. The Group’s ability to ensure its operations depends on the continued and uninterrupted performance of its IT systems. The Group has standardized some of its systems and is rolling out standard laboratory information and invoicing systems in all its operations, including those of recently acquired companies. Nonetheless, the Group sometimes still uses non-standardized IT systems for billing and laboratory work as well as centralized information systems for some of the companies it has recently acquired. However, the Group forecasts that rolling out standardized practices and systems throughout its network will take several years; in the meantime, the Group’s operations might be negatively affected because of cases of incompatibility between the various IT systems used within it. The breakdowns in the Group’s IT systems that would result from systems being converted could indeed disrupt its operations and result in customers or business opportunities being lost (see section 4.2. “Risks related to the Group’s technology and intellectual property rights” of this document de base). 95 Faced with growing demand for the electronic delivery of laboratory data and because it is committed to improving its patients’ experience, the Group intends to develop its platforms by adding new capabilities and services. In order to do so, it is automating the workflow in patient service centers as well as data processing in laboratories, while working on integrating patient health records and developing online access to information on services and test results for its customers. In the last few years, the Group has invested heavily in its IT systems to develop the following functionalities: the Group has selected Axional to set up its enterprise resource planning system (ERP). The implementation of the asset management and procurement management modules of the system began in 2012 and the process will continue in 2015. Other system modules will be rolled out once the Group has finished ascertaining its functional needs; an application for the publication of clinical results, owned by the Group, which will enhance the Group’s relationships with patients, healthcare professionals, nurses and hospitals. The Group has launched the project and it should be fully deployed in June 2015 in France. The Group plans to further improve this application by also targeting healthcare professionals and mobile users. Since it was set up in 2007, more than 10 million patients’ case files have been processed and subsequently archived; A shared LIS in Spain, the Group launched the convergence process in 2012 and it should be completed in the first half of 2015; a database for all the subsidiaries, covering the operational, human resources and procurement aspects in an initial phase. Rolled out in 2013 and 2014 in France, the system’s deployment will be completed in late 2015; and a mobile application called “iLab” that enables healthcare professionals and the partners of the Group’s laboratories (nurses, homes for dependent seniors, etc.) to look up catalogues of tests and instructions on how to take samples, and to remain informed of any possible changes. This will result in a better level of services and compliance. The Group has been working on the enhancement of this application and on adapting it to different countries, and plans to deliver a version for patients in 2015. In 2014, the Group launched a project to standardize the laboratory information systems for all network members by 2020. This project, known as “EuroLIS”, aims to replace all the information systems currently used in the network Laboratories to gain significantly in flexibility and productivity. EuroLIS will initially be implemented in 2015 in a pilot Laboratory in France and then rolled out to all the French Laboratories. In a second stage, it will be rolled out Europe-wide. It is estimated that EuroLIS will cost about €10 million to complete successfully. 6.4.10 Sales forces, assistance in performing tests and establishing diagnostics The Group operates in a highly competitive market, i.e. Western Europe, in which referring parties can send tests to the laboratory of their choice. To stand out from its competitors, the Group mainly relies on the quality of its diagnostic services, innovations in its services, the wide range of services it offers, the fact that its laboratories are easily accessible in the various countries in which it operates and, when the hospital laboratory market has been outsourced, on the prices it offers. The Group’s laboratory doctors play an important role in terms of sales and marketing thanks to the relationships they have forged with customers. They are backed by a sales force in charge of identifying potential customers with whom laboratory doctors can subsequently get into touch with when local regulations allow them to do so. In Spain, it is up to management to identify key prospective customers because reimbursement agreements must be negotiated with private insurance companies. The Group’s laboratory doctors are subsequently entrusted with the task of establishing a relationship with said healthcare professionals in Spain. In addition, the Group has developed new applications aimed in particular at healthcare professionals. We have already mentioned the cases of the LabMedica pilot program, a diagnostic support tool, and the mobile application iLab that enables healthcare professionals to look up catalogues of tests and instructions on how to 96 take samples, while remaining informed of any related changes (see section 6.4.9. “Information Technology Systems” of this document de base). 6.5 REGULATION In all the countries in which the Group operates, the medical diagnostic market (including clinical laboratory tests) is subjected to stringent regulation and is supervised by various regulatory bodies. This regulation and this supervision strongly influence the way in which the Group operates. With respect to clinical laboratories, these regulations primarily cover operating standards, professional qualifications laboratory staff must hold, restrictions on equity interests in companies operating laboratories and their corporate governance —such restrictions are noticeably stringent in France— as well as prices, and the levels at which medical tests are reimbursed. By way of illustration, in some countries, regulation on owning and operating laboratories require each laboratory or small group of laboratories to be held through a separate subsidiary. In some countries, such as France, the regulation also governs the legal form of the entities via which laboratories can be held. The Group’s operations are also subject to numerous other legal and regulatory provisions, in particular with respect to handling and storing certain chemical products and reagents, the disposal of infectious healthcare waste (waste from activities with risks of infection), the handling and storing of personal data (mainly the patients’ medical information) and the prevention of fraud to social security systems. 6.5.1 France Description of the regulation applicable in France In France, the functioning (the establishing and operation) of clinical laboratories was historically governed by an administrative authorization. These authorizations were issued by the competent administrative authorities, after review of an application that described in detail the premises, the equipment, the performed tests, the operating procedures, the professional qualifications of the laboratory personnel (including laboratory doctors), the governance of the laboratory as well as its corporate form. The regulation set minimal standards to be met in each of these areas. Any change in the above had to be notified to the competent administrative authorities. A January 13, 2010 decree (ordonnance n°2010-49 relative à la biologie médicale), ratified and amended by the Law of May 30, 2013, replaced this administrative authorization procedure by an accreditation procedure. Accreditation under ISO 15189 (international quality standard for clinical laboratories) is delivered by COFRAC. However, a transitional regime was introduced. Under this regime, existing administrative authorizations will remain in force until the clinical laboratories that hold them are accredited, but until no later than November 1, 2020, when these authorizations will be rescinded. New authorizations can no longer be issued apart from a very small number of cases, and under certain conditions, in the context of the restructuring of existing laboratories or site openings. At the same time, accreditation is gradually made compulsory, in the following manner: as of November 1, 2016, clinical laboratories will no longer be allowed to operate without an accreditation covering 50% of the clinical tests they perform; as of November 1, 2018, clinical laboratories will no longer be allowed to operate without an accreditation covering 70% of the clinical tests they perform; as of November 1, 2020, clinical laboratories will no longer be allowed to operate without an accreditation covering 100% of the clinical tests they perform. Some of the provisions relating to the conditions under which COFRAC will deliver accreditations have yet to be specified in the enforcement decrees. COFRAC may suspend or withdraw a clinical laboratory’s accreditation for all or part of the laboratory’s business, if it fails to comply with the standards and regulatory requirements. A ministerial decree dated October 17, 2012 as amended by a decree dated October 21, 2013 provides that the application to begin the accreditation process must be addressed to COFRAC by any non-accredited laboratory by no later than May 31, 2013; failing that, the non-accredited laboratory’s application must be regularized by no later than October 31, 97 2013. All the Group’s laboratories in France have been notified by COFRAC that it had accepted their applications to enter the accreditation process within the deadlines set out in the mentioned decrees. In addition, the February 23, 2015 decree stated that, in order to comply on November 1, 2016 with the accreditation conditions defined by the regulation, each clinical laboratory submits to COFRAC on April 30, 2015 at the latest: either an initial request for accreditation allowing to cover at least 50% of the clinical tests that it makes, this percentage including at least a test of each of the clinical tests’ categories made by the laboratory, or, for laboratories having a partial accreditation, an accreditation extension request allowing to cover at least the percentage of tests determined according to the modalities set out in the previous case. There were two possible options for entering the accreditation process: route A, consisting in a partial COFRAC accreditation (option chosen by 48% of French clinical laboratories) and route B, consisting in a temporary “Bio Quality” recognition, due to disappear by 2015-2016 (option chosen by 52% of French clinical laboratories). All of the Group’s SELs (except one) have chosen route A as it is a longer lasting solution under the transitional provisions. Laboratories that opted for route B must apply to COFRAC for accreditation by no later than April 30, 2015. Fewer than 20% of laboratories that opted for route B had filed an accreditation application on November 1, 2014. The mandatory timeline stipulated by the Law of May 30, 2013 has had the effect of accelerating consolidation in the French clinical laboratory market, as the laboratories experiencing difficulties in their accreditation process were forced to merge rapidly with accredited structures. Based on discussions with the various market operators, the Group stands out clearly as the network with the highest number of accredited laboratories. As the competent administrative authorities in France, the ARS are responsible for ensuring the clinical laboratories comply with existing sanitary and safety regulations through on-site inspections. In addition, some tests or categories of tests are controlled by specialized agencies as part of an annual quality control program. The ARS may impose administrative sanctions on SELs, as well as, in certain instances, on laboratory doctors, that infringe certain provisions of the applicable regulation, in particular health, safety and quality requirements. These sanctions range from fines to the temporary or permanent closure of the laboratory in the case of particularly serious or repeated violations. A clinical laboratory can be located on one or several sites (such sites in turn can be deemed to be Laboratories as defined by this document de base). There is no limit under French law to the number of sites that a clinical laboratory may operate, but certain legal provisions may restrict the opening of new sites. Accordingly, a clinical laboratory that has not been granted an accreditation covering 100% of the clinical tests it performs may only open a new facility if it does not exceed the same total number of sites open to the public. Moreover, sites of a clinical laboratory cannot be located in more than three adjacent regional health territories, barring an exemption granted by the head of the competent ARS under conditions set out by a decree issued by the government, reviewed for legality by the Conseil d’Etat and included in the regional health organization plan. Lastly, the ARS can, or must, reject any opening of new sites in specific cases defined by the Law of May 30, 2013. For instance, the ARS’ managing directors may oppose the opening of a clinical laboratory or of a site of a clinical laboratory, if it would result, in the relevant regional health territory, in an increase in the supply of clinical tests to a level 25% higher than the population’s needs as defined by the regional health organization plan. They may also oppose, on grounds related to the risk of affecting the continuity of clinical testing supply, an acquisition or restructuring affecting a clinical laboratory, or a site of a clinical laboratory, when said operation would result, in the relevant health territory, in the number of tests performed by the laboratory and resulting from this acquisition or restructuring exceeding the threshold of 25% of all clinical tests carried out. Finally, the acquisition of shares of companies operating a clinical laboratory is not authorized when such an acquisition would enable the acquirer to control, directly or indirectly, in the same health territory, more than 33% of all clinical tests performed. A person or entity is deemed to control more than 33% of all clinical tests carried out in a health territory if they own, directly or indirectly, the majority of the share capital of several companies operating a clinical laboratory and the combined business of those companies represents more than 33% of all clinical tests in that health territory. French law also limits the number of tests that can be outsourced by a clinical laboratory to another clinical laboratory every year, to be analyzed and interpreted, to 15% of the total number of tests carried out by the outsourcing laboratory. Sub-contracting contracts must be registered with the ARS and professional Orders. 98 Clinical laboratories, however, are free to share the tests to be carried out between their various sites as they wish. They can even concentrate all tests on a single production site. Laboratory doctors (doctors or pharmacists), laboratory technicians and nurses who collect samples taken from patients must meet minimum professional qualifications. In France, every clinical laboratory must be supervised by at least one laboratory doctor (called the responsible laboratory doctor) who acts as the legal representative of the laboratory company which operates the Laboratory. This laboratory doctor is responsible for the laboratory’s operations, including the processing of tests outsourced to other laboratories. Each site of a laboratory must also be supervised during opening hours by a laboratory doctor who can be identified at all times. Laboratory doctors working in laboratories are subject to the same rules of professional conduct as doctors and pharmacists, depending on the professional Order they are members of. Laboratory doctors must be registered with the relevant professional association, the Ordre des pharmaciens for qualified pharmacists and the Ordre des médecins for qualified medical doctors. Companies operating a clinical laboratory must also be registered with either one or both Ordres, based on the professional affiliation of the laboratory doctors practicing within such laboratories. The professional Ordres (the “Ordres”) are self-regulating bodies with administrative and disciplinary powers over practicing doctors and pharmacists and over companies that have registered with them. They also represent the collective interests of pharmacists (For the Ordre des pharmaciens) and medical doctors (for the Ordre des médecins) (including in both cases the interests of laboratory doctors), before French public authorities. These Ordres may be called upon to issue opinions on certain issues involving their profession, including when bills and regulations are being drafted. They also monitor compliance by practicing professionals and professional companies with applicable laws and regulations, as well as with ethical rules. The principle of independence, defined in article R. 4235-18 of the French Public Health Code, is one of the professional conduct rules enforced by the Ordre des pharmaciens. Under this principle, a pharmacist must not be subject to any financial, commercial, technical or moral constraint, if such constraint could impair his or her professional independence. As for medical doctors, article R. 4127-5 of the French Public Health Code provides that a medical doctor cannot, in any manner whatsoever, compromise his or her professional independence. To guarantee this independence, when the biologist-in-charge thinks that the decisions taken by a physical or legal person operating a clinical laboratory could endanger patients’ health, public health or the operating rules of the laboratory provided for in the Code of public health, the biologist-in-charge informs the general director of the competent ARS which takes the appropriate measures. The Ordre des pharmaciens and the Ordre des médecins maintain, each as far as it is concerned, a national register of practicing professionals (Tableau de l’Ordre), on which every practicing pharmacist or doctor, as well as every professional corporation, must be registered, thereby regulating access to the profession. New companies operating clinical laboratories must apply for registration in the relevant national register as a prerequisite to obtaining prefectoral agreement. An Ordre may withhold (or suspend) registration if it notices that the applicant has breached the relevant professional conduct rules. Clinical laboratories are subject to ongoing regulatory supervision by the Ordres and must therefore submit to the relevant Ordre or Ordres for review any proposed change in their share capital or in the articles of association (Statuts) of the companies that operate them, any cooperation contract entered into with other clinical laboratories and, more generally, any agreement relating to their operations or governing the relations between their shareholders. After reviewing this information, the Ordre may inform the ARS of any breaches of the regulation. The ARS are not bound by the findings of the Ordres in this respect. Each professional Ordre, using its disciplinary powers, may impose disciplinary sanctions on professional corporations and laboratory doctors (doctors or pharmacists). The Ordre may especially suspend, temporarily or permanently, practicing laboratory doctors who have breached professional conduct rules. Certain illegal activities, including the illegal practice of clinical biology and the misleading use of the title of laboratory doctor, carry criminal penalties that range from the prohibition to practice clinical biology or to operate a clinical laboratory, to imprisonment for natural persons. Furthermore, clinical laboratories may not advertise their services, directly or indirectly, to the general public. However, scientific information given to medical doctors and pharmacists, the public announcement of the 99 existence and location of a clinical laboratory published at the time of its opening or the opening of its sites, and references to the accreditation of the laboratory, are excluded from this prohibition. Most importantly, the French regulation imposes restrictions on the ownership and the corporate governance structure of companies operating a clinical laboratory. These restrictions reflect the traditionally held view in France that laboratories should be operated by small, privately run professional practices. All the Group’s clinical laboratories in France are operated through SELAS (société d’exercice libéral par actions simplifiée), which are a specific category of SELs. This kind of company is governed, in particular, by the following principles: an SEL must have been registered on the national register of the Ordre des médecins or of the Ordre des pharmaciens and must have been approved by the “Préfet” of the area (who usually delegates this task to the ARS); a laboratory doctor can hold the position of the responsible laboratory doctor of only one SEL and can therefore run only one SEL; an SEL can only be run by a laboratory doctor who works for and is a shareholder of that SEL; more than half of the share capital and voting rights of an SEL of laboratory doctors must be held, directly or indirectly (through specific companies), by the laboratory doctors working for the SEL. Exceptionally, SELs of laboratory doctors that had a different capital ownership structure when the Law of May 30, 2013 was promulgated, benefit from a grandfathering exemption that allows them to keep their existing structure, so that laboratory doctors who do not practice within these SELs, as well as other companies operating laboratories, can continue to hold a majority stake in their share capital (but not in their voting rights). Nonetheless, the law gives the laboratory doctors practicing within the SELs who qualify for this exemption a priority right should the shares of the SEL be put up for sale; natural persons or legal entities that are neither laboratory doctors, nor laboratory companies, cannot directly hold more than 25% of the share capital of a SELAS (this limit was confirmed by a decision of the Court of Justice of the European Union on December 16, 2010 (case C-89/09), discussed below); finally, to prevent any conflict of interests, Article L. 6223-5 of the French Public Health Code forbids the following persons, on the basis of their activities or their relations with certain activities in the medical or paramedical sector (the “prohibited investors”), from making any direct or indirect investment in the share capital of a company operating a French clinical laboratory: i. every physical or legal person qualifying as a health profession other than laboratory doctors, activity providers, distributors or manufacturers of In Vitro Medical Diagnostic devices, social or medico social private law health institutes, insurance or capitalization companies, benefit, retirement or mandatory or elective social security institutions; ii. every physical or legal persons holding 10% or more of the share capital of a company providing, distributing or manufacturing medical devices or In Vitro Medical Diagnostic devices, insurance or capitalization companies, benefit, retirement or mandatory or elective social security institutions; and iii. every physical or legal persons holding 10% or more of the share capital of a health professionals company that is authorized to take samples under the conditions mentioned at Article L. 6211-13 and that do not meet the conditions of chapter II title I livre II “Clinical testing” of part 6 of the legislative part of the French Public Health Code. It should also be noted that Law no. 90-1258 of December 31, 1990 on SELs enables public authorities to issue decrees, reviewed for legality by the Conseil d’Etat, preventing a SEL (or an entity operating a clinical laboratory) from holding a majority interest in another SEL or restricting the number of SELs in which a person or a legal entity (practicing as a professional or a nonprofessional) can hold a stake directly or indirectly. Nonetheless, as regards the first of these restrictions, it is probable that the government cannot impose such a restriction with respect to SELs of laboratory doctors insofar as the Law of May 30, 2013 has already made it compulsory for laboratory doctors practicing in an SEL to hold a majority stake in its share capital, while setting 100 out a derogatory regime. As regards the second restriction, the Court of Justice of the European Union has already ruled that limiting to two the number of SELs in which a laboratory doctor or an entity operating a clinical laboratory can be a shareholder, violated Article 43 on the freedom of establishment of the Treaty Establishing the European Community. Certain aspects of this legal regime were examined by the Court of Justice of the European Union. In March 2009, the European Commission launched a procedure against France, challenging two provisions of French law. First of all, the European Commission argued that the 25% limit set on the interests which can be held by non-professional third parties in the share capital was an unfounded restriction of the freedom of establishment provided for in the Treaty Establishing the European Community. Second, the European Commission criticized as overly restrictive the rule under which certain legal or natural persons may not own shares in more than two SELs. In its decision dated December 16, 2010, the Court of Justice of the European Union found in favor of France on the first count, holding that this limit was reasonable in view of the State’s legitimate public health and safety concerns. The Court noted the threat to such independence that might arise from financial pressures placed on laboratory doctors by third-party investors. It argued that a Member State could validly draw the conclusion that the professional independence of laboratory doctors would not be adequately ensured in structures where such professionals would hold only a minority interest in the share capital, regardless of whether they were granted majority voting rights. The Court found against France on the second count, however, holding that the ownership restriction placed by existing regulations on qualified professionals was inadequate and disproportionate with respect to the public health objectives sought to be achieved. Acknowledging the decision of the Court of Justice of the European Union, France repealed the regulatory provision in question in decree no. 2013-117 of February 5, 2013. With regard to pricing and reimbursement, clinical laboratories are bound by the prices set by the CNAM. These prices are revised regularly following negotiations between the Ministry of Social Affairs, Health and Women’s Rights, the CNAM, the Ordre des pharmaciens and the Ordre des médecins. However, an agreement was signed on October 10, 2013 by the main French biologists’ trade unions and UNCAM. The purpose of the agreement is to give clinical laboratories visibility on their financial prospects over a three-year period, whilst also exerting control over healthcare spending. This led to a three-year agreement setting annual growth in clinical laboratory spending at 0.25% for the period 2014-2016. This target will be reached by modest reductions in prices to be spread over the period, and control of prescriptions, in order to offset the natural growth in volumes. Trade unions meet with the Health Insurance Funds (Caisses d’Assurance Maladie) every six months, in order to assess the impact of changes in prices and determine what future changes may be necessary to meet the annual growth target. In late January 2015, CNAM held discussions with professional unions to reiterate its commitment to the three-year agreement and to propose a rate revision commensurate with volume growth projected for 2014 and expected for 2015. The Company estimates that the proposed rate revision will have a gross adverse effect on revenue of around 1.50%, while the 2014 revision had a gross adverse effect of 2.51% and a net adverse effect of around 1.20% (reductions are estimates based on annual test volumes). According to CNAM’s projections, volume growth in 2015 will make up for the decline and result in the 0.25% target for annual clinical expenditure growth set by the agreement being attained. Definitive 2014 figures will be available in late June 2015, as will information about the trend in early 2015. On that basis, a decision will be made regarding a revision to the nomenclature in September 2015, in order to get as close as possible to the 0.25% target regarding growth in annual clinical expenditure. Lastly, it should be noted that the SEL operating anatomical pathology laboratories, which the Group could shortly acquire, are medical doctors’ SELs, and therefore subject to substantially similar rules to those applicable to the SELs operating clinical laboratories. In particular, the restrictions on ownership and corporate governance structure are relatively close. In this respect, a noteworthy point is that, in order to prevent any conflict of interest, any equity interest, whether direct or indirect, in the SELs operating anatomical pathology laboratories is also prohibited for various categories of natural persons and legal entities, because of their business or because of their ties with certain operations in the medical or paramedical sector. This ban includes laboratory doctors and companies operating clinical laboratories. To comply with this regulation, the Group will set up a corporate structure that will enable it to hold SELs that operate anatomical pathology laboratories through some of its subsidiaries that are not affected by this ban. 101 Impact of regulations applicable on the Group’s corporate structure The Company holds its French subsidiaries operating laboratories (in the form of SELAS) directly and indirectly (through an Italian laboratory company) and its foreign subsidiaries indirectly (through several national holding companies and other laboratory companies). In France, in particular, ownership of the share capital and voting rights of the SELs operating clinical laboratories and their corporate governance structure are highly regulated. In particular, the majority of voting rights of the SELs must be held by laboratory doctors practicing within these SELs. In order to comply with this regulation, the Group has put in place a corporate structure under which it holds directly or indirectly, through Istituto il Baluardo S.p.A., one of its Italian subsidiaries, about 99.9% of the share capital of the SELs benefiting from the grandfathering arrangements under the Law of May 30, 2013, while some of the laboratory doctors practicing in said SELs hold the remaining shares. Nonetheless, the articles of association (statuts) of all the Group’s SELs grant the majority of voting rights in all shareholders’ general meetings to laboratory doctors who are shareholders in the SELs in which they practice. The Group will no longer be able to use this approach (which benefits, at the date of this document de base, to the majority of the SELs of the Group) for most of its acquisitions in the wake of the Law of May 30, 2013, which has made it mandatory for more than 50% of the share capital (in addition to 50% of voting rights) of an SEL of laboratory doctors to be held by the laboratory doctors practicing in the SEL. An alternative structure, based on issuing preferred shares within the Group, has therefore been set up to make acquisitions in compliance with the regulation, while enabling the Group to hold virtually all the economic rights in the acquired SELs and exercise control over them. Therefore, in all cases, the Group holds virtually all the economic rights in the SELs and controls them under corporate governance, contractual and organizational structure, in accordance with French regulation and, accordingly, fully consolidates these SELs in its financial statements. Impact of the regulation on the corporate governance of the SELs The laboratory doctors from whom the Group acquires SELs (whether said SEL is or is not covered by the grandfathering exception) or a clinical laboratory, who decide to stay in the Group, continue to run it on a dayto-day basis but are contractually bound to comply with the Group’s policies and standards in terms of reporting, and in particular with respect to financial and accounting information, financing and centralized cash management, budgeting and, insofar as compatible with the French regulatory framework, management of the SEL. In order to improve the coordination of the operations in its clinical laboratories and federate its network, the Group has set up a resource sharing structure, in the form of a French GIE (Groupement d’intérêt économique), Labco Gestion, which encompasses all of the Group’s existing French laboratory companies and some of its foreign companies, including General Lab S.A., Laboratoire d’Analyses Médicales Romain Païs (a Belgian laboratory company) and Istituto il Baluardo S.p.A. (one of the Italian laboratory companies). The GIE provides administrative support for the clinical laboratories of Group member Companies, in particular for purchasing, quality management, legal affairs, information technology, scientific communication and human resources. The GIE is managed by the Company as its sole board member, appointed by a qualified (three-quarters majority) vote of the GIE’s members. The GIE’s activities are financed by its members through contributions of an amount set every year for each member by the Company as sole board member based on, inter alia, the member’s financial capacity, number of employees and utilization of the GIE’s services. The Company as sole board member appoints the other executive officers of the GIE, including regional managers within France, and country managers outside of France, who act as agents of the GIE with respect to its members in their geographical area. Furthermore, as part of the Group’s development, the manner in which the corporate governance arrangements of its SELs could be changed has been considered in order to adapt them to the development of the network’s laboratories (increase in the size of entities and in the number of laboratory doctors working in them, generation renewal, adaptation to the new regulatory framework, etc.). As a result, in strict compliance with French regulation governing clinical laboratories, the Group set up in 2014 a new corporate governance, contractual and organizational structure for its French SELs subsidiaries, giving it control over them. As of the date of this document de base, these new corporate governance arrangements had not yet been implemented in all the 102 Group’s SELs. As of the date of this document de base, a significant percentage of the Group’s SELs, representing approximately 63% of the French contribution to the Group’s revenue for the financial year ended December 31, 2014, had adopted these new corporate governance arrangements. The expected calendar for the deployment of this new corporate governance framework should allow the implementation of these new provisions in practically the entirety of the Group’s SELs before the end of 2015. This structure is based on a set of standard agreements (the “Operating Rules”), including: articles of association (statuts), (which set out in particular that, barring an opposite decision voted by a qualified majority of shareholders, all the distributable income must be allocated to dividend payments for every financial year); a shareholders’ agreement (governing all the corporate governance arrangements specific to the SEL); a private practice agreement (which is individualized to define each laboratory doctor’s specific contractual commitments); the internal rules (règlement intérieur) (governing the daily organization of laboratory doctors within the SELs); and a charter of management board members (governing the manner in which the SEL’s management board operates). These Operating Rules give the Group the power to control the SELs’ strategic and financial operations, qualified as relevant, while strictly complying with the regulation requiring the laboratory doctors which are shareholders of the SELs in which they work, i.e. the API, to own the majority of the voting rights (50.01%) of the SEL. Furthermore, shareholders’ agreements with the APIs define the commitments they accept, including obligations to sell their shares in the SEL, firm and definitive acceptance of the constraints related to the Group’s financing and the commitment to participate fully to the Group’s development and restructuring. Thus, shareholders’ agreements organize in advance the restructurings that would be put in place (in particular, issuance or conversion of existing shares to preferred shares (actions de préférence), change of the voting majority rules in the general assembly) in case of a change of the regulation applicable to SELs regarding the holding of share capital or voting rights. In day-to-day management, the Group exercises exclusive control1 over the SELs through Strategic Committees set up pursuant to the shareholders’ agreements. These Strategic Committees take strategic and financial decisions by a simple majority vote and are composed equally of the APIs who are members of the SELs’ management boards (which are generally composed of three members) and of the representatives of the Group (in equivalent number). Given the contractual commitments made by the API parties to the agreements, including the commitment of loyal adherence to the Group’s legal organization, which includes membership of GIE Labco Gestion and utilization of its central support services (accounting, finance and legal services, purchasing, IT, human resources, scientific information and quality), decisions proposed by the Group are intended to be adopted by consensus, with a favorable vote from the API who are members of the Strategic Committee. We note that the shareholders’ agreements expressly provide that the commitments made by the API shall in no way affect their professional independence and that the Group expressly undertakes not to intervene in the regulated clinical laboratory activities exercised under the sole responsibility of the API. The Group therefore does not, and will never, intervene in the SELs’ purely clinical practice, which is the sole responsibility of the laboratory doctors. 1 The control exercised by the Group’s French entities rely on the corporate governance mechanisms and on contractual agreements, qualified by the Group as de facto control (see Note 3 to the audited consolidated financial statements for the financial year ended December 31, 2014 in Section 20.1.1 “IFRS consolidated financial statements for the financial years ended December 2012, 2013 and 2014” of this document de base). 103 In case of a deadlock, which should be analyzed on a theoretical level for the sole purpose of justifying the Group’s exclusive control cited above and therefore full consolidation under IFRS, the Group will have the following mechanisms to exercise control: The Group will always have the right to remove ad nutum an API from the Strategy Committee of an SEL and appoint one of the other laboratory doctors in the SEL as a replacement (a mediumsized SEL typically has some ten laboratory doctors), who would be favorable to the decision to be adopted pursuant to the Operating Rules. If this mechanism is insufficient, for example in the event of a block opposition from all the laboratory doctors in the SEL, the Group will always have the right to appoint in a SEL a Group laboratory doctor from outside this SEL. This laboratory doctor would then be appointed API and then member of the Strategic Committee of the SEL under the mechanism described in i. above. Lastly, in an extreme case of deadlock, the Group will always have the right to have the strategic and financial decisions reviewed and adopted by the general meeting of SEL shareholders. In accordance with the above i. and ii., the Group would begin by appointing a Group laboratory doctor into the SEL, then would transfer him or her a few shares to enable that person to exercise some of the votes allocated to the API, which, combined with the Group’s voting rights (exercised by the parent SEL(s)) would together give them a majority within the SEL and, therefore, exclusive control over it. This would be possible as, within each category of shareholders (on the one hand the API, collectively holding 50.01% of the voting rights and, on the other, the Group holding 49.99% of the voting rights), the split of voting rights is proportional to the capital held by each shareholder. The Group’s laboratory doctor appointed in accordance with the above i., ii. and iii., qualified as an “aligned” biologist for the purposes of IFRS 10 (concept of agent versus principal), can be considered as taking decisions compliant with those of the Group as he himself would be a member of the Group’s management and aligned to the Group’s interests given his involvement in the Group. The mechanism for transferring shares to the biologist appointed by the Group could be a loan of shares, but other mechanisms could also be considered (e.g. simple sale of shares). It should be noted that, at the date of this document de base, the Group has never yet had to make use of a mechanism for ending a deadlock situation, such as a loan of shares, and that it is neither necessary nor useful to set out the terms contractually insofar as its implementation relies on the use of a mechanism under ordinary law, which has, in any event, already been upheld by previous case law for use within an société d’exercice libéral. However, should the Group consider making use of such a mechanism, the Group is convinced that there would be no infringement of the principle of professional independence by the laboratory doctor receiving the share transfer, as he or she would become a signatory to the relevant shareholders’ agreement guaranteeing total independence in the exercise of his or her medical activity, which is not an element indicative of control. The incoming laboratory doctor would only have to take a position on subjects already explicitly set out in the corporate governance documentation summarized above. Again, in accordance with the regulation, the documentation underlying the legal and organizational structure is systematically sent to the supervisory authorities (professional Ordres and competent ARS) when adopted by a SEL. The supervisory authorities ensure that the operation of the clinical laboratories complies with the regulations and the professional Ordres ensure that they comply with the applicable ethical principles. To date, none of the Operating Rules as summarized above have been contested in any way by the supervisory authorities. Lastly, it should be noted in this respect that certain other legal organizational methods adopted in the past by the Group’s SELs had been criticized and sanctioned by the Governing Board of the Ordre des pharmaciens. These sanctions were contested by the Group, which filed a complaint with the European Commission in 2007. It is mainly on this ground that the European Commission found that the Ordre des pharmaciens had sought to prevent the development of laboratory groups on the French market, in violation of European Union rules on collusion and restrictive commercial practices, and ordered it to pay a very substantial fine in December 2010. 104 This ruling has just been confirmed by a decision of the Court of Justice of the European Union on December 10, 2014 (see section 20.3.1. ““Contentious proceedings – Ordre des Pharmaciens and Ordre des Médecins” of this document de base). On March 5, 2015, the Conseil Central de la Section G, which is the body of the Ordre des pharmaciens competent for clinical testing, closed, during an administrative session, several disciplinary cases without taking any action. These case closings without any action taken result from a withdrawal of the complaints filed by its president against the Group’s medical doctors and SELs. Even though these withdrawals are not motivated, they are probably the result of the decision of the Ordre des Pharmaciens to not appeal its conviction by the European General Court. This specific legal and organizational structure, currently used by the Group, as well as the arrangements described above, entails certain risks (see section 4.1. “Risks related to the business sectors and markets in which the Group operates” of this document de base: further regulatory changes in France, or challenges initiated by the competent administrative authorities or Ordres might call into question the Group’s organizational and legal structure). 6.5.2 United Kingdom In the United Kingdom, there is no specific regulation related to the ownership of a company operating clinical laboratories. However, the authorization to operate may be suspended if the CEO of a company providing services to the NHS fails the so-called “integrity test”. The Care Quality Commission will be able to have such decisions applied more firmly if CEOs fail this integrity test since a measure is to be implemented in April 2015. In order to run a clinical laboratory in the United Kingdom, one needs to obtain several authorizations and accreditations. First of all, the company must register with the Care Quality Commission under the Health & Social Care Act 2008 as a service provider; it must also register the address of every one of its laboratories. If the company is already registered, it has to apply for the registration of any additional laboratory. Every laboratory must also have a sworn-in manager who supervises operations, quality, safety and compliance rules. The other key requirement is CPA accreditation, delivered by UKAS. All British laboratories need to obtain this accreditation. Lastly, according to the type of operation, certain specific authorizations can be required, for example an authorization from the Medicines and Healthcare Regulatory Agency (MHRA) is necessary for blood transfusions. There are no specific regulations covering laboratory staff or defining a minimum number of qualified employees. Biomedical scientists must be registered with the Health and Care Professions Council (HCPC). With respect to accreditation by UKAS, the ratio between qualified laboratory technicians and laboratory technicians being trained is a crucial criterion. The adoption of the Health & Social Care Act 2012 started a real overhaul of the organization of the NHS and the way in which budgets are allocated. This new organization favors and facilitates agreements between the NHS and the private sector. TUPE refers to the “Transfer of Undertakings (Protection of Employment) Employment) regulations 2006” as amended by the “Collective redundancies and Transfer of Undertakings (Protection of Employment) (Amendment) regulations 2014”. They apply to organisations of all sizes and protect employees’ rights when the organization or service they work for transfers to a new employer. A relevant business transfer triggering TUPE occurs when a business or part of a business moves to a new owner or merges with another business to make a brand new employer. However, most of the Group’s UK business does not result from mergers or business transfer but from the sole decision of the Group’s clients to outsource their clinical testing activities. In this case, the following conditions must exist immediately before the transfer for the TUPE regulations to apply: There must be an organized grouping of employees The employees should be assigned to the group The activities should not become overly fragmented The activities should remain fundamentally the same. 105 Employees from the newly-acquired business, service or contract will transfer automatically to the incoming employer. Where an employee transfers under the TUPE regulations, the rights and obligations, powers and liabilities also transfer with them to the incoming employer. Employees who transfer from the outgoing employer to the incoming employer are not regarded as dismissed under TUPE, so a transfer does not trigger an entitlement to redundancy pay or pay in lieu of notice unless there is an actual dismissal. Following a transfer, employers often find they have employees with different terms and conditions working alongside each other and wish to change/harmonies terms and conditions. However, TUPE protects against change/harmonization for an indefinite period if the sole or principal reason for the change is the transfer. Any such changes will be void, even if mutually agreed and favorable to an employee, because the rights provided by the TUPE regulations cannot be signed away. This limits the scope for immediate harmonization of terms and conditions. 6.5.3 Belgium Generally speaking, Belgian legislation does not impose restrictions on the ownership of companies operating clinical biology laboratories (the equivalent of clinical laboratories). However, prescribing doctors are expressly banned from holding a stake in companies operating clinical laboratories or acting as managers or agents of these companies. The legal form is the main regulatory constraint weighing on the opening and operation of clinical laboratory companies. The manager of a clinical laboratory must be a natural person, a private limited liability company (société privée à responsabilité limitée (“SPRL”)), a société en nom collectif, a société coopérative or a nonprofit legal entity. The duration of the royal decree listing the legal forms that clinical laboratory companies are required to adopt in order to be eligible for reimbursement by the Belgian public health insurance company was initially due to expire on December 31, 2009. This duration of the royal decree had been extended retroactively until December 31, 2012 by a Decree dated January 27, 2010. To date, no new extension has been enacted. The provisions of Belgian company law applicable to Belgian legal entities operating clinical laboratories impose certain restrictions on the transferability of the shares of such legal entities. For example, in an SPRL, unless more stringent provisions of the articles of association (statuts), half of the shareholders holding shares representing 75% of the share capital of the company (minus the number of shares being transferred) must consent to the transfer of shares under consideration. There are no requirements as to the legal form of an entity purchasing shares of an SPRL. Belgian law does not provide for any specific rules with respect to the voting rights attached to the shares of a laboratory company. However, such a company must comply with general rules, in particular: (i.) the laboratory company must not have a corporate or statutory purpose other than the operation of clinical laboratories; (ii.) its Articles of Association (statuts) must include a provision to the effect that the company is required to strive for a standard of quality that avoids any act entailing complementary expenses that are not justified by the compulsory healthcare insurer, the patient or the party or parties that insure(s) the payment of these services; (iii.) the laboratory company is required to ensure via a written agreement with the persons performing tests on its behalf, that said people are free to carry out these services as they wish and will have access to all necessary means in order to guarantee the quality of the services rendered. The company operating clinical laboratories must provide the Ministry of Social Affairs, Health and Women’s Rights with an annual list of its members or partners and must maintain accounting records, drawn up in accordance with the accounting standards set by royal decree. Prices and reimbursement levels in the clinical field are set after consultation between the INAMI that organizes, manages and controls compulsory health insurance in Belgium and professionals. Said prices and reimbursement levels are set out in a nomenclature, and depend on budget constraints. As a result, should a risk 106 or significant overshooting of budget objectives be recorded, “claw-back” mechanisms kick in. The INAMI nomenclature is regularly updated. 6.5.4 Switzerland Swiss legislation does not set out any specific constraint with respect to the ownership of companies operating clinical laboratories. The only obligations relate to the personnel working in the laboratory. The laboratory manager and technical managers must hold a federal diploma in medical studies or pharmacy delivered by the FAMH (Foederatio Analyticorum Medicinalium Helveticorum) after four years of specialization. Moreover, half of the employees must hold a diploma qualifying them in medical testing or as laboratory assistants. The laboratory manager can be in charge of no more than three laboratories and must have at least two years’ experience as a technical manager. In order to manage a laboratory, the company must provide local authorities with a complete list of the tests that will be offered as well as the names and diplomas of all the managers: the laboratory manager, the technical managers and the quality managers. Authorizations to operate a laboratory are delivered for five years for microbiology, serology and genetics laboratories. For other disciplines, the authorization must be renewed only if there is a change in managers. Controls and inspections are carried out by cantonal authorities. For microbiology, serology and genetics laboratories, cantons transfer responsibility to federal authorities and inspections are carried out by SwissMedic. With regard to quality control, Qualab (the Swiss Commission for Quality Assurance in Medical Laboratories) was founded by associations of laboratories, doctors, hospitals and insurance companies in order to define and guarantee quality criteria for clinical testing laboratories. The quality of laboratories is assessed with respect to two aspects: (i) internal quality for every series of tests or twice a day for very frequent tests and (ii) external quality, at least four tests per year per laboratory, four bodies (e.g. CSCQ, MQ, etc.) send samples to assess the accuracy of results. Accreditation to the main international standards (e.g. ISO: 17025 or ISO: 15189) is not compulsory in Switzerland. However, most major Groups voluntarily decided to apply and are now accredited. These accreditations make it easier, inter alia, to obtain an authorization to operate. 6.5.5 Spain In Spain, the rules covering the authorization and operation of healthcare centers, establishments and services — including clinical laboratories — are defined at a national level by the Law on General Health (Ley 14/1986, de 25 de abril, General de Sanidad) and by royal Decree 1277/2003. This national legislation sets out the general principles that govern the operation of laboratories and the minimum requirements to be met to obtain administrative authorizations, but each autonomous community (comunidad autonoma) is in charge of implementing this overall model and accordingly defines the manner in which it is adapted. By consequence, the specific requirements to be met to obtain an administrative authorization and operate a clinical laboratory can vary by region. Generally speaking: (i.) prior administrative authorization is required to establish, modify, enlarge, relocate or close clinical laboratories; (ii.) to obtain the relevant authorizations, certain requirements related to the organizational structure, business, facilities, infrastructure and personnel of the laboratory must be met; (iii.) there are no ownership restrictions regarding clinical laboratories, but certain staffing requirements apply. The laboratory must be operated under the responsibility of a qualified laboratory specialist (especialista en analisis clinicos). In addition, certain other categories of laboratory personnel must hold minimum professional qualifications. Laboratory doctors must hold a relevant university degree in fields such as biology, pharmacy, chemistry or medicine, and they must have completed further specialization to qualify as laboratory specialists (especialista 107 en analisis clinicos). In addition, laboratory doctors must be registered with the relevant regional Ordre in order to be permitted to practice. Advertising and all kinds of promotion are governed by ethical rules. Each Ordre is generally speaking in charge of defending the interests of its members, and those of the profession as a whole, and for protecting the rights of consumers of services provided by said profession. The Ordre des médecins and the Ordre des pharmaciens are given by law the right to participate in the drafting of laws and regulations pertaining to their respective professions. In addition, the Ordre des médecins and the Ordre des pharmaciens must approve the rules of professional conduct applicable to their profession, which regulate relations among professionals, interactions with patients and advertising and promotion by professionals of their services to the general public. These Ordres may take disciplinary actions to sanction breaches of the relevant professional conduct. These sanctions range from warnings to temporary or permanent removal from the relevant professional registry. Unfair competitive practices or a criminal conviction are also grounds for removal from the Ordres’ professional registries. Disciplinary sanctions are not exclusive of other proceedings, in particular by governmental institutions, if the incriminating behavior also constitutes a violation of governmental laws or regulations. Clinical laboratories must also comply with other specific rules such as health and safety, bio-waste disposal and data protection. Prices for clinical laboratory services in Spain are not regulated by law. In the private health insurance sector, prices for laboratory tests are set by an agreement between (both public and private) insurers, complementary health insurance providers, hospitals and clinical laboratories. Private laboratories receive a fixed fee based on either the number of patients in their geographical region (model based on the number of inhabitants), or on the number of tests performed (model based on the number of tests). Public hospitals are required to launch public tender offers to select services providers, and the resulting outsourcing contract must set out the terms of the contractual relationship between the two parties, including the prices for testing services performed. 6.5.6 Portugal The clinical laboratory services market sector in Portugal is supervised by the ERS, or national health authority, as well as by the regional health authorities. The ERS is a public body administratively and financially independent from the Portuguese government, in charge of enforcing fair competition rules in the healthcare market, monitoring the quality of healthcare services and the protection of end-users rights. The ERS also ensures that the right to equitable and universal access to public healthcare is complied with. The regional health authorities are in charge of delivering authorizations to private healthcare service providers and applying the regulations in force. Private clinical laboratories are currently primarily regulated by the Law on clinical laboratories (Portaria n° 166/2014 de 21 de Agosto) (as amended). It sets out the standards required to open and operate clinical laboratories. Decree-law no. 279/2009 dated as of October 6, 2009 established a legal regime for the operation of healthcare units. The Decree reinstates an authorization obligation and defines new criteria for the granting of authorizations to clinical laboratories. This new set of regulations became effective for clinical laboratories by ministerial decree n/287/2012 of September 20, 2012. The ministerial application decree published on August 21, 2014, which was the result of discussions between the National Association of Clinical Laboratories (Associação Nacional dos Laboratorios Clinicos), other entities and the government, brought little change compared with previous regulations on opening and operating a laboratory. Laboratories in the process of obtaining accreditation will have a period of two years to comply (Portaria n° 166/2014 de 21 de Agosto). Clinical laboratories must be managed by either a medical doctor registered with the Portuguese Ordre des médecins (Ordem dos Médicos), or a pharmacist registered with the Portuguese Ordre des pharmaciens (Ordem dos Farmaceuticos). In both cases, they must be specialized in clinical pathology or in clinical analysis. Clinical pathology laboratories may be managed only by a medical doctor who is specialized in clinical pathology and registered with the Portuguese Ordre des médecins. The law requires that this doctor or professional pharmacist (Director Técnico) be personally and verifiably available to oversee the operation of the laboratory. 108 The Portuguese Ordre des médecins and Ordre des pharmaciens are professional associations that are in charge of the regulation of their respective professions. They have the power to control and oversee access and exercise of these professions. In addition, each Order is in charge of delivering the title of specialist in clinical pathology or clinical testing, necessary for the practice of the profession of laboratory doctor. Portuguese law does not set any restrictions on ownership of laboratory companies, except that employees of the national healthcare system (SNS) are prohibited from holding more than 10% of the share capital of these companies, or serving on the board of a company that provides services to the SNS (source: Decreto Lei 97/98 de 18 de Abril). Health care services are mainly provided by the SNS intermediary and private laboratories have to enter into reimbursement agreements with this intermediary. Such agreements are not concluded with new market entrants, forcing international groups such as the Company to proceed by acquisitions to penetrate the Portuguese market. For patients covered by the public insurance system, prices and reimbursement levels are set at the national level by the SNS, with some specific prices applicable to certain categories of employees, such as civil servants. For privately insured patients, prices and reimbursement levels are established as a result of negotiations between insurance companies and laboratories As of the date of this document de base, the Portuguese government is analyzing a possible restructuring of the National Healthcare System. This could lead to reforms of the healthcare sector, in particular with respect to the outsourcing of services to national health system patients. 6.5.7 Italy The Italian healthcare system is decentralized. Decisions in the public sector are enacted on both a national and regional basis level (competenza concorrente). National laws and regulations provide a general framework for healthcare policy and regional healthcare budgets. They also set indicative prices for diagnostic services tests. Each region must implement the national legal framework. Each region is responsible for its own annual healthcare budget and for allocating funds to regional health authorities. In turn, regional health authorities allocate funds to both public healthcare facilities and the private facilities meeting the requirements described below. To operate in the public healthcare system (Servizio Sanitario Nazionale/Servizio Sanitario Regionale) and receive reimbursements from public authorities, both public and private healthcare facilities must: (i) obtain accreditation from the competent authority for each facility (which, depending on the regional legislation, may be either the region or the regional health authority), and (ii) enter into agreements with the regional health authorities for the number and type of services that each facility can provide. Maintaining the agreements with the public authorities is likely to become more difficult in the future since it could be requested that the health structure carry out a minimum number of tests per year. In certain regions, such as Lombardy, there are few accreditations and new agreements are in limited number for the time being. As a result, new providers cannot enter into agreements with regional healthcare authorities and new facilities cannot be opened for the time being (for instance, Sampling Centers can be relocated only and new ones cannot be opened). Non-accredited laboratories can be freely opened. In Liguria, it is no longer possible to obtain accreditations or enter into agreements for new laboratories but new Sampling Centers can be opened. In Campania, accreditations are subject to a temporary regime and a final one is expected to be defined within the next two years. So far, new facilities cannot be opened under agreements with regional healthcare authorities but the regulation is still under review by the local authorities. In principle, their objective should be to facilitate consolidation and higher quality levels. Italian laws and regulations provide specifically for data protection requirements in connection with genetic testing, including requirements for security measures (e.g. certified e-mails and coding), detailed information about the scope and purposes of the genetic tests, and genetic counseling. In Lombardy, Campania and Liguria, where the Group operates clinical laboratories, public and private healthcare facilities, including clinical laboratories, are generally subject to the following requirements: (i) managers and certain other staff members must hold specific professional qualifications (e.g. the chief medical 109 officer (direttore sanitario) must be a qualified doctor and a member of the relevant professional association of doctors); (ii) technical, structural and operational conditions must be met; and (iii) legal entities or natural persons operating the facilities must receive authorization from the relevant regional health authority. In Lombardy, private clinical laboratory operators can self-certify that all requirements are met by filing a declaration of commencement of activity (Segnalazione di Inizio Attivita) with the competent regional health authority before a laboratory begins operations. The regional health authority subsequently verifies the accuracy of the information provided in the declaration of commencement of activity. In addition, as a further requirement, clinical laboratories that intend to operate in the public healthcare system must obtain accreditation by enrolling in the register of the accredited facilities and entering into agreements with the regional health authorities. Neither Lombardy, nor Campania or Liguria restrict or limit the ownership of laboratories. However, the Lombardy Region suspended the implementation of new agreements with local health authorities for the extension of ambulatory care services (including clinical laboratory tests and sample collection activities) that can be provided under the coverage of the public healthcare system (Servizio Sanitario Nazionale). The national healthcare system sets prices and reimbursement levels for patients. Prices applicable to both public and private healthcare facilities operating within the public healthcare system (Servizio Sanitario Nazionale) are set at the regional level. Accordingly, different prices are applied by the various regions. Regional health authorities, however, are required to apply a discount to reimbursements due to private clinical laboratories. The discount rate stands at 18% in Lombardy and 8% in Liguria (9% for clinical imaging) and is to be calculated on the basis of prices in force. There is no discount in Campania, after new prices were set in 2013 (source: Legge finanziaria – articolo 1, comma 796, lettera O and Healthcare Ministry Decree no. 95 of July 6, 2012 and subsequent October 18, 2012). Regional health authorities are in charge of reimbursing healthcare facilities and are bound by the framework implemented by their region. Moreover, with respect to patients covered by private insurance, prices are set by an agreement between private insurance companies and laboratories. Health services advertising has been liberalized with regard to the operations of sole practitioners, professional associations of doctors and medical service providers established in the form of companies (including clinical laboratories). 6.6 DATA ON WHICH ANY STATEMENT BY THE COMPANY WITH RESPECT TO ITS COMPETITIVE POSITION IS BASED See section “General Comments” of this document de base. 110 CHAPTER 7 ORGANIZATION STRUCTURE The simplified organizational chart below shows the Group’s position as at December 31, 2014, it should be noted that the percentages listed correspond to the percentage of share capital held. The Group includes Labco, the Group’s ultimate parent company and its consolidated subsidiaries. The Company and the main companies in each country in which the Group operates are described below. None of the companies belonging to the Group were listed companies at the date of this document de base. France Labco is a société anonyme governed by French Law with share capital of €70,679,705 headquartered at 60-62 rue d’Hauteville, 75010 Paris, France and registered with the registre du commerce et des sociétés de Parisunder number 448 650 085. Labco SA is primarily active in establishing shareholdings in clinical testing and anatomical and cytopathology testing companies or laboratories, and more generally in any company whose corporate purchase is to contribute, directly or indirectly, to the establishment of medical diagnoses. Labco Midi is a société d’exercice libérale par actions simplifiée governed by French Law with share capital of €50,000 headquartered at 115 rue de la Haye 34080, Montpellier, France and registered with the Montpellier Trade and Companies Register under number 450 376 603. The Company holds directly or indirectly 99.98% of the share capital of Labco Midi, the principal activities of which are the operation of clinical testing laboratories and the acquisition of shareholdings in other clinical testing laboratories. Groupe Biologic is a société d’exercice libérale par actions simplifiée governed by French Law with share capital of €40,000 headquartered at rue Louis Pasteur, 71600 Paray-le-Monial, France and registered with the Mâcon Trade and Companies Register under number 492 916 150. The Company holds directly or indirectly 99.98% of the share capital of Groupe Biologic, the principal activities of which are the operation of clinical testing laboratories and the acquisition of shareholdings in other clinical testing laboratories. Laboratoire de biologie médicale Delaporte is a société d’exercice libérale par actions simplifiée governed by French Law with share capital of €66,012 headquartered at rue N3 Centre Commercial Carrefour, 77410, Claye Souilly, France and registered with the Meaux Trade and Companies Register under number 390 753 895. The 111 Company holds directly or indirectly 99.99% of the share capital of Laboratoire de biologie médicale Delaporte, the principal activities of which are the operation of clinical testing laboratories and the acquisition of shareholdings in other clinical testing laboratories. Biologistes associés regroupant des laboratoires d’analyses (“BARLA”) is a société d’exercice libérale par actions simplifiée governed by French Law with share capital of €46,086, headquartered at 6 rue Barla, Nice, France and registered with the Nice Trade and Companies Register under number 782 596 670. The Company holds directly or indirectly 98.36% of the share capital of BARLA, the principal activities of which are the operation of clinical testing laboratories and the acquisition of shareholdings in other clinical testing laboratories. United Kingdom Integrated Pathology Partnerships Limited is a private limited company governed by English law with share capital of £100, headquartered at One Southampton Row, London WC1B 5HA, United Kingdom and registered under number 07195844. The Company directly owns 90% of iPP’s shares as well as a call option to purchase the 3% held by Sodexo and a call option to purchase the 7% granted to iPP’s two main executives through the UK Employee Shareholders Scheme (see section 21.1.6 – “Information on the share capital of the Company or of its subsidiaries that is the subject of an option or a conditional or unconditional agreement providing for it to be made subject to an option and details of such options (including the identity of the persons to whom they relate)” of this document de base)2. Labco Diagnostic UK Limited is a private limited company governed by English law with share capital of £2, headquartered at Nunn Brook Road Huthwaite, Sutton-in-Ashfield, Nottingham NG17 2HU, United Kingdom and registered under number 7840843. The Company holds directly or indirectly the entire share capital and all of the voting rights of Labco Diagnostic UK Limited, the principal activity of which is the management and operation of a dialysis laboratory. iPP Facilities Limited is a private limited company governed by English law with share capital of £2, headquartered at 4 Devonshire Street, London W1W 5DT, United Kingdom and registered under number 8867499. The Company holds directly or indirectly the entire share capital and all of the voting rights of iPP Facilities Limited, the principal activity of which is the management and operation of a laboratory. iPP Analytics Limited is a private limited company governed by English law with share capital of £2, headquartered at 3rd Floor, One Southampton Row, London WC1B 5HA, United Kingdom and registered under number 8948045. The Company holds directly or indirectly the entire share capital and all of the voting rights of iPP Analytics Limited, the principal activity of which is the management of laboratory staff. Belgium Labco Finance is a société privée à responsabilité limitée governed by Belgian law with share capital of €10,018,600, headquartered at avenue Louise 350, Ixelles 1050, Belgium and registered under number 0826013990. The Company holds directly or indirectly the entire share capital and all of the voting rights of Labco Finance, the principal business activity of which is the provision of financial services exclusively for related or associate companies. Laboratoire d’analyses médicales Roman Païs S.P.R.L. is a société privée à responsabilité limitée governed by Belgian law with share capital of €21,690.68, headquartered at 11 rue Seutin, 1400 Nivelles, Belgium and registered under number 0440299628. The Company holds directly or indirectly the entire share capital and all of the voting rights of Roman Païs S.P.R.L., the principal activity of which is the operation of a dialysis laboratory. 2 The economic rights attached to the interest held by the two executives vest progressively. As a result, and given the call options held by Labco, these interests are considered, from a financial reporting point of view, as falling within IFRS 2 “Share-based payments” and are therefore not considered as granting minority financial rights. In addition, Labco has a call option to purchase Sodexo’s 3% interest at a fixed price, which does not confer minority financial rights. As a result, the Company’s interest in its subsidiary iPP is deemed to be 100% in the financial statements for the financial year ended December 31, 2014, whereas from a company-law point of view it is considered to be 90%. 112 Switzerland TEST Tailored Efficient Swiss Testing S.A. is a société anonyme governed by Swiss law with share capital of CHF500,000, headquartered at 36 rue de Lausanne, Geneva 1201, Switzerland and registered under IDE-UID business identification number CHE-432 556 812. The Company owns directly or indirectly 48% (and has a call option to purchase at a pre-agreed price, the remaining 52%) of the share capital and voting rights of TEST Tailored Efficient Swiss Testing SA, which provides a fully electronic solution to the needs of independent healthcare professionals enabling them to view the catalogue of tests, select the tests to be performed, enter patient details, view results and order additional tests. Spain Labco Diagnostics España, S.A. is a joint-stock corporation governed by Spanish law with share capital of €16,942,815, headquartered at 28 calle Londres, Barcelona, Spain and registered with the Barcelona Trade Register (“Registro Mercantil”) in Volume 40711, Page 40, Section 8, Sheet B-353263, Entry 13 under tax identification number (“código de identificación fiscal”) CIF A64605538. The Company holds directly or indirectly the entire share capital and all of the voting rights of Labco Diagnostics España S.A., the principal business activity of which is the establishment of shareholdings in clinical testing companies or laboratories in particular in the Iberian peninsula. Labco Spain, S.L. is a limited liability company governed by Spanish law with share capital of €300,000, headquartered at 8 calle Valgrande, Edificio Thanworth II, Madrid, Spain and registered with the Madrid Trade Register (“Registro Mercantil”) in Volume 29196, Page 34, Section 8, Sheet M-525606, Entry 4 under tax identification number (“código de identificación fiscal”) CIF B86367703. The Company holds directly or indirectly the entire share capital and all of the voting rights of Labco Spain, S.L., the principal business activity of which is the establishment of shareholdings in clinical testing companies or laboratories in particular in the Iberian peninsula. Labco Madrid, S.A. is a joint-stock company governed by Spanish law with share capital of €65,000, headquartered at 8 calle Valgrande, Madrid, Spain and registered with the Madrid Trade Register (“Registro Mercantil”) under number M-6.288 folio 133 and following of Volume 315 of Section 8a, and under tax identification number (“código de identificación fiscal”) CIF A79462420. The Company holds directly or indirectly the entire share capital and all of the voting rights of Labco Madrid, S.A., the principal business activity of which is the management and operation of clinical testing companies or laboratories in the Iberian peninsula. General Lab, S.A. is a single-shareholder company governed by Spanish law with share capital of €1,880,000, headquartered at 28 calle Londres, Barcelona, Spain and registered with the Barcelona Trade Register (“Registro Mercantil”) in Volume 42992, Page 34, Section 8, Sheet B-21984, Entry 59 under tax identification number (“código de identificación fiscal”) CIF A59845875. The Company holds directly or indirectly the entire share capital and all of the voting rights of General Lab, S.A., the principal business activity of which is the management and operation of clinical testing companies or laboratories in the Iberian peninsula. Portugal Questão em Aberto S.A. is a sociedade anónima (joint-stock corporation) governed by Portuguese law with share capital of €26,626,600, headquartered at Rua Rodrigues Sampaio, no. 30 C, 3, 1169-067, Freguesia Santo António, concelho de Lisboa, Lisbon, Portugal and registered under identification number (NIPC) 508 592 305. The Company holds directly or indirectly the entire share capital and all of the voting rights of Questão em Aberto S.A., the principal business activity of which is the establishment of shareholdings in clinical testing companies or laboratories. Italy Istituto Il Baluardo S.p.A. is a societá per azioni con socio unico (private company limited by shares) governed by Italian law with share capital of €120,000, headquartered at 4 via del Molo, Genoa, Italy and registered under tax number (“codice fiscale”) 00887530103. The Company holds directly or indirectly the entire share capital and all of the voting rights of Istituto Il Baluardo SpA, the principal business activity of which is to provide clinical testing services or establish shareholdings in clinical laboratory testing laboratories. 113 Labco Italia S.R.L. is a societá a responsabilitá limitata (limited liability company) governed by Italian law with share capital of €15,297,000, headquartered at 61 via Giovanni Pacini, Milan, Italy and registered under tax number (“codice fiscale”) 06254340968. The Company holds directly or indirectly the entire share capital and all of the voting rights in Labco Italia S.R.L., the principal business activities of which are the operation of clinical testing laboratories and the establishment of shareholdings in other clinical testing laboratories. SDN S.P.A. is a societá a responsabilitá limitata (limited liability company) governed by Italian law with share capital of €1,248,000, headquartered at Napoli (NA) via Francesco Crispi 8 CAP 80121, Italy and registered under tax number (“codice fiscale”) 01288650631. The Company directly or indirectly owns the entire share capital and all of the voting rights of SDN S.P.A., the principal business activities of which are the prevention, diagnosis, treatment and rehabilitation of humans and animals, including the preparation and management of tools necessary for that purpose, as well as taking shareholdings in companies with these activities. The Group’s business activities are described in Chapter 6 “Overview of Group businesses” of this document de base. The functions exercised by the Company’s executives in its subsidiaries are described in section 14.1 – ”Members of the administrative, management and supervisory bodies and general management” of this document de base. 114 CHAPTER 8 REAL ESTATE PROPERTIES, PLANTS AND EQUIPMENT 8.1 SIGNIFICANT EXISTING OR PLANNED PROPERTY, PLANT AND EQUIPMENT As at December 31, 2014, the Group owned property, plant and equipment with a gross value of approximately €261.3 million, including €50.8 million in the form of contracts eligible to be accounted for as leases under IFRS. The property, plant and equipment owned by the Company is described in Note 15 to the financial statements for the financial years ended December 31, 2012, 2013 and 2014 described in section 20.1.1 – “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” in this document de base, prepared in accordance with IFRS for the financial years ended December 31, 2012, 2013 and 2014. At the date of this document de base, the non-current assets planned by the Group reflect the current investments and those envisaged as described in section 5.2.2 – “Current investments” and section 5.2.3 – “Future investments” in this document de base. 8.1.1 Real estate properties The Group’s facilities consist primarily of Collection Centers, routine testing laboratories, emergency services hospital laboratories, technical platforms and specialty platforms. The Group’s policy is to lease rather than own its facilities, preferably through long-term leases, except where concentrated activity justifies the acquisition of the buildings in which these activities are conducted. The Group rents its head office in Paris. The Group believes that its facilities are generally adequate for its present needs and that suitable additional or replacement space would be available if required. Barcelona project On October 2, 2014, the Group acquired a building in Barcelona to bring together the activities of five production plants, a storage facility and administrative offices in a single location. This building is ideally located as it is close to the Barcelona ring road (“Ronda de Dalt”) and lies within easy reach of the airport (13 km) and the city center (10 km). The various departments will occupy three floors and a basement providing 5,747 m² in total floor space. This space is due to be increased in the second half of 2016 to 8,957 m². The total cost of this project is estimated at €15.5 million, including the reorganization costs (relating to removal expenses, double rent, redundancy payments, etc.), of which €12.0 million expenditure was incurred in 2014 in acquiring the building and completing initial refurbishment works. Prior to this acquisition, the Group occupied approximately 7,000 m² in space in the Barcelona region at an annual cost of approximately €0.8 million. Bringing operations together at a single location will optimize efficiency and reduce overhead costs, generating economies of scale. Basildon project The Basildon real estate project results from the signature of an outsourcing contract with Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust. The Group has undertaken to build a new technical platform to perform all of the non-urgent tests at the two hospitals. A site has been chosen to host the new laboratory facility in north Basildon, close to the A127 road. The proximity of this major road means that the journey time to Southend hospital will be less than 25 minutes, and the location also offers easy and rapid access to London as well as to the region’s other public hospitals. iPP Facilities Ltd, the subsidiary responsible for managing the Group’s assets in the United Kingdom, completed this acquisition on September 29, 2014. In advance of this purchase, the Group prepared, along with its partner Ashley House Ltd., the refurbishing and conversion of the premises, which at the date of this document de base are currently used as office and warehouse space. The Group is also working with its 115 principal suppliers to equip this new technical platform with all the requisite technology. The laboratory facility is expected to open in the first half of 2016. The total cost of the project is £5.4 million, excluding taxes, consisting of £1.0 million used to acquire the building at the end of September 2014 and £4.4 million to refurbish and convert the premises. This project will give the Group better control and greater flexibility in the management of its real estate portfolio in the United Kingdom. In addition, given the length of the contract entered into with Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust, this project is financially more attractive than a conventional lease. Property in Belgium As part of the acquisition of a laboratory in Belgium in July 2014, the Group acquired a building with floor space of approximately 650 m² via a Belgian real-estate holding company created in 2014 called General Immo, for €1.7 million. A sample-taking laboratory is located on the ground floor of the building, which also contains offices. The building is located on one of Brussels’ main thoroughfares. 8.1.2 Other property, plant and equipment The property, plant and equipment owned by the Group consists mainly of technical equipment and installations and in particular automated devices and tools used to perform clinical testing, office and IT facilities, fixtures and fittings for the premises and vehicles. 8.2 ENVIRONMENT AND SUSTAINABLE DEVELOPMENT The Group’s operations are subject to licensing, authorization and regulation under EU, national and local laws and regulations relating to the protection of the environment and human health and occupational health and safety, including those governing the handling, transportation and disposal of medical samples and biological, infectious and hazardous waste and the clean-up of contaminated sites. All of the Group’s laboratories are subject to strict requirements for the disposal of laboratory samples at authorized facilities, and the Group generally uses external service providers for the disposal of such samples. In addition, the Group has to meet extensive requirements relating to workplace safety for employees in clinical laboratories who could be exposed to various biological risks such as blood-borne pathogens (including HIV and the Hepatitis B virus). These requirements include work practice controls, protective clothing and equipment, training, medical follow-ups, vaccinations and other measures designed to minimize exposure to, and the transmission of, blood-borne pathogens. Although the Group is not aware of any current material non-compliance with or any failure to comply with any specific obligation under environmental, health and safety laws and regulations in connection with its operations, failure to comply with such laws and regulations in the future could result in civil and criminal fines and penalties, remediation costs, enforcement actions, the suspension or termination of its licenses and authorizations to operate or third party claims (see section 4.3 “Risks related to the Group and its commercial activities” of this document de base). Following the listing of the Company’s shares on Euronext Paris, the Company plans to implement the legal and regulatory rules applicable to public companies regarding the report on information on how the Company takes into consideration the social and environmental consequences of its activity as well as its social undertakings in favor of sustainable development and in favor of the fight against discrimination and the promotion of diversity. 116 CHAPTER 9 OPERATING AND FINANCIAL REVIEW OF THE GROUP Investors are invited to read the following information concerning the Group’s results of operations together with the Group’s consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014, as well as the notes to these financial statements, as included in section 20.1.1. “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” of this document de base. The Group’s consolidated financial statements have been prepared in accordance with IFRS as adopted by the European Union and in force for the relevant financial years. The consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014 have been audited by the Company’s statutory auditors. The reports by the Company’s statutory auditors are presented in section 20.1.2. “Statutory auditors’ report on the accounts established in accordance with IFRS principles for the financial years ended December 31, 2012, 2013 and 2014” in this document de base. Unless stated otherwise, the information presented in Chapter 9 “Operating and financial review of the Group” is shown on a historical basis without including the results of the SDN group, which was acquired effective July 30, 2014 (for a description of the acquisition of the SDN group, see section 6.4.3.2 “Overview of the Southern Europe market – Italy” of this document de base). The SDN group has been consolidated since July 30, 2014 and therefore contributed to the Group’s results for 5 months of the financial year ended December 31, 2014. The Group has also prepared pro forma financial information for the 2013 and 2014 financial years as if the SDN group had been acquired effective January 1, 2013. Those pro forma financial data are presented in section 20.1.3. “Pro forma financial information for the financial years ended December 31, 2013 and December 31, 2014” of this document de base. The pro forma financial information is presented solely for illustrative purposes and does not reflect the results that would have been produced had the SDN group acquisition actually been completed on January 1, 2013. This information has also been subject to a limited review by the Company’s statutory auditors, which is presented in section 20.1.4. “Statutory Auditors’ reports on the pro forma financial information for the financial years ended December 31, 2013 and December 31, 2014” of this document de base. 9.1 OVERVIEW 9.1.1 Introduction The Group is one of the leading groups in the European clinical laboratory services market, with the broadest geographical reach. It performs over 150 million tests p.a. at its 165 laboratories. The Group provides its services to more than 20 million patients p.a. and at the date of this document de base, it had almost 6,000 employees and medical staff in the countries in which it operates. The Group’s business activities are organized into two geographical operating segments: (i) Northern Europe encompassing France, the United Kingdom, Belgium, Switzerland, and, until 2013, Germany, and (ii) Southern Europe, encompassing Spain and Portugal, both managed by the same management team, and Italy. In line with IFRS 8, segment reporting is prepared based on the internal management data used to allocate resources to the segments and for the analysis of their performance by the Chief Executive Officer and the executive committee. The segments’ performance is measured based on Revenue and EBITDA in a manner consistent with the income statement published in the consolidated financial statements. The Group’s financial items (including financial income and expenses) and income tax expense are managed centrally by the Group and are not presented in the operating segment figures. Where shared resources, provided in most cases by the Group’s holding companies, are used, this is taken into account in the segment results by reallocating the costs to the segments in proportion to each segment’s Revenue. The Group is the market leader in Spain and Portugal (the Group ranked number one in these markets based on 2014 revenue). In France and Italy, it is one of the two leading players in its business sector. In Italy, it has a strong presence in medical imaging and poly-ambulatory medicine following the acquisition of the SDN group in July 2014 (based on pro forma 2013 and 2014 revenues). The Group also has significant foothold in Belgium, where it ranks third (based on 2014 revenue). In addition, the Group has been present since 2010 in the United Kingdom where it delivers laboratory outsourcing services to hospitals and other healthcare providers. In December 2013, the Group pulled out of the German market to focus on national markets in which it has 117 achieved or may shortly achieve a leadership position, significant market share or a distinctive positioning. The Group also set up a business in Switzerland in 2013 via Test S.A. In addition, the Group provides clinical laboratory testing services to customers in Latin America, the Middle East, Eastern Europe and North Africa, with analyses for those countries performed by the Labco NOÛS specialty laboratory located in Barcelona, and the related revenue is included in the Iberian Peninsula cash-generating unit. In the financial year ended December 31, 2013, Revenue from the Northern Europe segment totaled €357.9 million (representing 65% of the Group total) and EBITDA came to €75.5 million (71% of the Group total), representing an EBITDA margin of 21.1%. Concurrently, Revenue from the Southern Europe segment totaled €189.4 million (35% of the Group total) and its EBITDA amounted to €30.8 million (29% of the Group total), representing an EBITDA margin of 16.2%. In the financial year ended December 31, 2014, Revenue from the Northern Europe segment totaled €401.3 million (representing 65% of the Group total) and EBITDA came to €79.2 million (70% of the Group total), representing an EBITDA margin of 19.7%. Concurrently, Revenue from the Southern Europe segment totaled €214.3 million (35% of the Group total) and its EBITDA amounted to €34.0 million (30% of the Group total), representing an EBITDA margin of 15.9%. The differences in the EBITDA margin between the Southern Europe and Northern Europe segments reflect: (i) differences in profitability of the clinical testing activities between countries where the Group is present given the business models adopted and prices implemented by the public health authorities or private-sector organizations (see the description of the various business models in section 6.2 – “Presentation of sectors in which the Group is active” of this document de base), (ii) differences in profitability between the medical diagnostics activities performed by the Group (routine testing, specialty testing, anatomical pathology testing, medically assisted reproduction and medical imaging); and (iii) the geographical positioning and the size of the Group’s laboratories in the various national markets. The superior profitability of the Northern Europe segment compared with that of the Southern Europe segment in the financial years ended December 31, 2012, 2013 and 2014 reflects the size of France’s contribution to the Northern Europe segment during this period. In addition, the profitability of the Group’s operations in Belgium progressed significantly between 2012 and 2014 with the take-off in nutritional testing. Since the profitability of the Group’s outsourced clinical testing activities in the United Kingdom is below the Group average, the expansion of iPP’s activities in the United Kingdom is having an unfavorable impact on the Northern Europe segment’s margin. In addition, as outlined above, the principal countries in the Southern Europe segment experienced a major economic and financial crisis over the period, which affected the operations of this Group segment. However, with the July 2014 acquisition of the SDN group, which is a leading player in integrated diagnostics with higher profitability than the rest of the Group, profitability should improve in the Southern Europe segment. The Group has a catalogue of over 5000 routine and specialty tests used by health professionals to diagnose their patients and to care for and treat their conditions. Aside from the patients who go to the Group’s clinical testing laboratories, the Group’s customers are also doctors, hospitals, insurance companies and employers in respect of their obligations under medical Law. The Group performs clinical analysis, generally using automated testing equipment or devices. It delivers the results to prescribing doctors and patients and offers assistance with the interpretation of these results through its laboratory doctors. In certain countries, some of the Group’s laboratories are also responsible for the sampling, and the delivery of the samples to its testing facilities. Between 2012 and 2014, the Group recognized significant growth in its Revenue, as a result of solid organic growth as well as profitable, accretive growth through acquisitions, mainly through the purchase of small and medium-sized laboratories that are merged with the Group’s existing organization or strategic acquisitions of new regional platforms. In 2014, the Group’s Revenue totaled €615.6 million (pro forma Revenue of €649.6 million considering the acquisition of the SDN group took place on January 1, 2013) and its EBITDA stood at €113.3 million (pro forma EBITDA of €131.2 million). For further information about the Group’s key figures for the period between 2012 and 2014, see Chapter 3 “Selected financial information” of this document de base. 118 9.1.2 Principal factors influencing the Group’s results from operating activities Certain key factors and certain past events and operations have affected and may continue to affect the Group’s business and results of operations presented below: 9.1.2.1 General economic conditions and legal framework 9.1.2.1.1 Economic environment The Group is exposed to the general economic conditions in the markets in which it operates. Although the market for clinical testing services is not generally regarded as very sensitive to macroeconomic cycles, the Group believes that a downturn in general economic conditions affects demand for its services and thus has a negative impact on its results of operations. This impact derives from the fact that governments and third-party payers (see section 9.1.3.1 – “Revenue” of this document de base for a detailed explanation of third-party payers) are seeking to reduce healthcare expenditure, and existing and potential customers may face financial difficulties. In addition, where patients, directly or indirectly such as through private health insurance premiums, are responsible for all or part of the costs of laboratory tests, individual decisions to reduce out-of-pocket healthcare expenditures may result in weaker demand for the Group’s services. The economic recession and the volatility generated by the economic and financial crisis that began in 2007 have increased the risk associated with conducting the Group’s business in certain countries where it has significant operations, such as the risk of customers defaulting on their payments. Economic difficulties have also given rise to weaker levels of activity and higher unemployment and have led governments, private insurers and other organizations to reduce their healthcare spending, which may curb the Group’s Revenue or margins. Moreover, competition and pricing pressures tend to increase during such periods, which may reduce the Group’s ability to win additional outsourcing contracts and may oblige it to agree to the renegotiation of contracts in force on less favorable terms (see section 4.1 “Risks related to the business sector and markets in which the Group operates” of this document de base: persistent economic weakness may have a detrimental effect on the Group’s business). Yet tough economic conditions may have a positive impact on the outsourcing of clinical laboratory testing insofar as the roll-out of cost-cutting or deficit reduction plans may prompt companies or public-sector institutions to outsource clinical testing services more rapidly and to a greater extent. Likewise, price cuts in Spain have, for example, been offset by volume growth owing to an increase in the number of patients relying on private healthcare plans. The Group believes this trend reflects the erosion of trust in the public health insurance system and a poorer quality of service provided by the public healthcare systems amid fiscal austerity. 9.1.2.1.2 Specific impact of volume and price effects Like most businesses providing healthcare services, the Group’s performance and in particular organic growth in the Group’s business are influenced by the combined effects of trends in volumes and the average price per file, which is in turn affected by pricing trends and the nature of the clinical testing performed (the “Mix Effect”). Volume growth flows from macroeconomic trends and developments in society with the aging of the population, the increase in chronic conditions and shift towards preventative and personalized medicine, for which diagnosis is crucial. In many countries where the Group operates, services are delivered to patients under healthcare programs funded at least to some extent by public organizations, which set prices or a method of determining prices covering all or some of the clinical laboratory testing services provided by the Group. These prices vary significantly from one country to another. Prices may be changed at any time, and recent prices’ revisions have generally resulted in prices’ reductions. Amid the tough economic environment prevailing in Europe, governments and public-sector payers took additional measures to reduce their overall health spending over the period between 2011 and 2014, including the clinical laboratory testing services provided by the Group. Governments typically control healthcare expenditure by cutting prices or reimbursement levels, seeking to reduce the number of tests prescribed by doctors, and limiting the testing services covered by their health, protection and social security programs. These measures and especially those capping or reducing the prices that the Group is allowed to charge for its services or totally excluding some of the Group’s services from the scope of reimbursed health services, have a negative impact on clinical testing volumes and the prices that can be charged for these tests, leading to a 119 negative impact on the Group’s revenue. In addition, the date from which prices reductions and other measures intended to cut health spending may also affect comparisons between the Group’s results and the size of these reductions in a given period. Since 2011, many European governments and in particular those in France, Spain, Portugal, Germany, Italy and the United Kingdom have introduced or announced their intention of introducing austerity measures with a view to reducing public spending, including health expenditure. In France, for example, the government announced its intention of securing at least €110 million in mandatory annual savings on health expenditure from French clinical laboratory testing services in 2012. However, an agreement was signed on October 10, 2013 by the main French biologists’ trade unions and UNCAM. The purpose of the agreement is to give clinical laboratories visibility on their financial prospects over a three-year period, whilst also exerting control over healthcare spending. This led to a three-year agreement setting annual growth in clinical laboratory spending at 0.25% for the period 2014-2016. This target will be reached by modest reductions in prices to be spread over the period, and control of prescriptions, in order to offset the natural growth in volumes. Trade unions meet with the Health Insurance Funds (Caisses d’Assurance Maladie) every six months, in order to assess the impact of changes in prices and determine what future changes may be necessary to meet the annual growth target. In Portugal, the “Memorandum of Understanding on Specific Economic Policy Conditionality” between the European Commission, the International Monetary Fund and the European Central Bank in 2011 was enacted in response to the economic bailout, which resulted in financial aid of €78 billion on the basis of a three-year political program until mid-2014. The Group believes that this program, which included the aim of reducing national spending on healthcare by 10% in each of 2011 and 2012, has resulted in price reductions in excess of 10% for certain tests in Portugal since October 2011. In addition, two public health insurance funds aligned their prices in Portugal during 2012, which has led, in the Group’s opinion, to price cuts applicable to certain tests there since August 2012. In Italy, where prices are set on an indicative basis at the national level and subject to adjustments at the regional level, prices in Liguria were lowered in October 2012, and a fresh reduction of 20% to 30% for molecular imaging services was agreed in fall 2013. Campania also adopted lower prices in March 2013. The Group also has to contend with efforts by non-governmental third-party payers-mainly private health insurers-to reduce utilization and reimbursement of clinical laboratory testing services. In certain markets, the Group receives payment for its services from private health insurers that have gained significant bargaining power by reimbursing healthcare services only if such services are provided by pre-selected providers. These private health insurers negotiate fee structures with healthcare providers, including clinical laboratories, and certain private health insurers have insisted on discounted fee structures as a condition for pre-selection in the past and may insist on further discounted fee structures in the future. If the Group is not pre-selected by private insurers, or is required to accept unfavorable terms to secure such pre-selection, its results of operations may be adversely affected. For example, four private insurance customers in Spain accounted for a significant portion of the Group’s Revenue in Spain in 2014. A major Spanish private health insurer, for which the Group is a preselected provider, introduced significant prices cuts in the first half of 2012. Another major Spanish private health insurer also decided to introduce significant prices reductions effective January 2013. Pressure from private health insurers in other areas of the healthcare sector may also affect the Group indirectly. Private health insurers have exerted pricing pressure on private hospitals which, in turn, have exerted pricing pressure on the Group. 9.1.2.1.3 Influence of the legislative and regulatory environment in which the Group operates The Group is subject to significant regulation and control by various regulatory organizations and has to adapt to frequent legislative and regulatory changes at both national and European level. These regulations mainly pertain to operating standards, professional qualifications of laboratory personnel, ownership and corporate governance constraints on companies operating clinical laboratories, and pricing and reimbursement levels of clinical laboratory tests. The changes made to the law and the regulations have had in the past, and may continue to have in the future, a significant impact on the Group’s results of operations. In particular, compliance by laboratories with operating standards and the professional qualifications of laboratory personnel may drive up the Group’s payroll-related, administrative, legal and operating costs. 120 Until the publication on January 13, 2010 of the ordinance (ordonnance n°2010-49 relative à la biologie médicale), the scope for consolidation in the French market was severely restricted in the French market by limitations placed on the number of clinical laboratories that could be operated by a single laboratory company, while restrictions on outsourcing volumes gave rise to operational inefficiencies. Starting on the date of entry in force of the ordinance, these restrictions have been eased, making it possible to (i) restructure the portfolio of clinical laboratories in France, (ii) set up technical platforms that have enabled and will continue to enable the Group to unlock economies of scale by increasing the volume of tests performed and by maximizing returns on testing equipment and personnel, and (iii) bring back in house certain specialty tests that were previously outsourced. As a result of the easing of all these restrictions, the Group’s results of operations have improved. As outlined in section 6.5.1. “Regulation – France” of this document de base, the Law of May 30, 2013 amended the ownership rules concerning clinical testing laboratory companies in France. The Law of May 30, 2013 may limit the Group’s ability to sell or transfer shares in SELs that it holds at the date of this document de base or that it may acquire in the future, and render more complex any restructuring it might consider for its subsidiaries. The Law of May 30, 2013 extended the French regional health authorities’ oversight of compliance with the concentration rules in a given geographical region and clarified the fact that a natural person or a legal entity could not hold a share exceeding 33% of the testing market in this area, either directly or indirectly, through majority ownership of the capital of several clinical laboratory testing companies. Furthermore, the Law of May 30, 2013 instituted minimum accreditation standards that clinical testing laboratories must satisfy between 2016 and 2020 and the implementation of which will be costly, time and resources-consuming for the Group (see section 6.5.1. “Regulation – France”) of this document de base. It now requires 50% of the clinical testing performed by each laboratory to be accredited from November 1, 2016, 70% from November 1, 2018 and 100% from November 1, 2020 compared with 100% from November 1, 2016 under the previous legislation. Expenses related to the management of quality and accreditations excluding payrollrelated costs came to €1.1 million in the financial year ended December 31, 2014, compared with €0.74 million in the financial year ended December 31, 2013. In addition, since the clinical sector is VAT exempt, clinical testing laboratories have to pay VAT, thereby recognizing operating costs inclusive of VAT. VAT rates, the amount of taxes on sales and other similar taxes may be increased, especially in the current economic and political climate, with certain European governments seeking to raise more revenue from direct and indirect taxation. For example, certain VAT rates were hiked in France on January 1, 2014, with the standard rate being raised from 19.6% to 20.0%, which gave rise to additional purchasing costs for French clinical testing laboratories, barring renegotiations with suppliers to agree improved terms and conditions, fully or partially offsetting the impact of the 0.4 point hike in the VAT rate (see section 4.4.5. “Risks related to VAT and French payroll tax” of this document de base). As stated in section 6.4.3.2. “Overview of the Southern Europe market – Spain” of this document de base), raw materials and products used in clinical laboratory testing services in Spain will no longer qualify for the reduced rate of VAT. In addition, deferred taxes have been capitalized to reflect the future tax savings arising from differences between the carrying amount of assets and liabilities and their tax base, and deferred tax losses from the Group’s subsidiaries. Future crystallization of these assets depends on the tax rules, the outcome of the possible tax audits and the future results of the relevant subsidiaries. At December 31, 2014, the value of the deferred taxes recognized as assets totaled €10.9 million, of which €4.8 million derived from tax loss carryforwards. It is possible that the value of these assets declines following changes in the tax rules. 9.1.2.2 Expansion in the Group’s network of clinical laboratories through acquisitions During the period covered by the financial statements included in this document de base, acquisitions accounted for a significant portion of the Group’s overall expansion strategy. As a result, acquisitions and sales have been, and probably will in the future be, a key factor to take into consideration when analyzing the Group’s operating performance. Since 2009, the Group has chiefly made acquisitions of small clinical laboratories, which may be closed down or converted into Sampling Centers to unlock significant synergies in the short term. The Group has also dedicated more substantial resources to integrating its recent acquisitions. 121 During the financial years ended December 31, 2011, 2012, 2013 and 2014, the Group completed respectively thirty-five, sixteen, eleven and sixteen acquisitions of groups of companies, companies or small- or mediumsized clinical laboratory businesses, chiefly in France, for a total consideration net of cash acquired (excluding earn-outs) of €97.2 million, €45.2 million, €20.4 million and €130.2 million respectively, with the SDN group representing €107.6 million. Earn-out payments linked to the Group’s acquisitions may turn out to be significant. At December 31, 2014, the estimated fair value of liabilities linked to earn-out payments (fixed or subject to performance conditions) stood at €11.5 million. Between 2012 and 2013, the Group did not make any large acquisitions and, at the same time, scaled down the number of such acquisitions it made compared with 2011 for various reasons. Firstly, the Group suspended its acquisition program in Italy, Spain and Portugal until the economy picked up again in late 2013 so that it could instead focus on organic growth and on restructuring its business portfolio. The Group decided to pull out of the German market in the summer of 2013, since it was unable to identify any growth opportunities to achieve critical mass there rapidly. Lastly, the economic, regulatory and tax-related uncertainties in France prompted hesitancy among the major players, sparking a very sharp slowdown in mergers & acquisitions activity in clinical diagnostics. Against this backdrop, the number of opportunities for the Group and that of completed transactions decreased significantly. Even so, on October 25, 2013, the Group acquired almost all of Sodexo’s holdings in the iPP joint venture, set up jointly to expand outsourcing of the clinical diagnostics business for the NHS in the United Kingdom. iPP started up its operations on June 1, 2012 under the contract with Taunton and Somerset NHS Foundation Trust and the Yeovil District Hospital NHS Foundation Trust. Since iPP is now controlled by the Group, it has been fully consolidated since October 25, 2013, while the net profit of the iPP joint venture was previously recognized in the share of profit of associates. In the financial year ended December 31, 2014, the Group completed 16 acquisitions of small- or medium-sized groups of companies, companies or clinical laboratory businesses, chiefly in France for a total consideration net of cash acquired (excluding earn-outs) of €130.2 million. In addition, the Group completed a major acquisition in Italy on July 30, 2014 – SDN Spa and its four subsidiaries – making it a market leader in this market and bolstering its activities in integrated diagnostics, especially in molecular imaging, through the acquisition of the SDN group, which is active in Naples and Campania. The SDN group posted pro forma Revenue of €48.0 million in the financial year ended December 31, 2014, with margins ahead of the Group average. As part of its overall strategy, the Group regularly evaluates its business portfolio and from time to time may sell non-core activities and those regarded as less profitable. During the summer of 2013, the Group completed a strategic review of its operations in Germany and decided to pull out of the German market for the time being to focus on national markets in which it has achieved or may shortly achieve a leadership position, significant market share or a distinctive positioning. The Group thus sold its business activities in Germany to Sonic Healthcare on December 2, 2013 and, in compliance with IFRS 5, the cash-generating unit sold was restated under discontinued operations in the 2012 and 2013 periods in the Group’s consolidated financial statements for the financial year ended December 31, 2013. Acquisitions and disposals affect the Group’s results of operations in different ways. Firstly, its results during periods in which an acquisition takes place may be affected by the inclusion of results from the businesses acquired in the consolidated results from the date on which control is gained, which is generally when ownership of the shares changes hands. Similarly, disposals affect group results, since the divested entity is no longer consolidated from the date control is lost. The acquisitions made in 2012, 2013 and 2014 contributed approximately to 2.3%, 2.1% and 4.5% respectively of the Revenue shown in the audited consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014. Subsequently, the results of the periods are positively impacted by the synergies plus the immediate effect of the inclusion of results from the businesses acquired in the consolidated results. For example, acquisitions can harness economies of scale in procurement through the pooling of purchasing volumes with suppliers to secure rebates in return for larger volumes. The Group acquires clinical laboratories with limited property, plant & equipment assets, for a consideration that exceeds in many cases their net assets, which leads to significant amounts of goodwill being recognized on 122 the Group’s statement of financial position (€702.4 million at December 31, 2014). The Group evaluates the recoverability and measures the potential impairment of goodwill annually or at interim closing dates if an impairment indicator is identified and may recognize charges in case of impairment. Following the annual goodwill impairment review, the Group recognized a €36 million impairment loss allocated to the cashgenerating unit in Germany at December 31, 2012. No impairment loss was recognized in 2013 or 2014. For further information about the analysis of goodwill impairment and corresponding sensitivity analyses, see Note 13 “Goodwill” to the audited consolidated financial statements for the financial year ended December 31, 2014. The Group intends to continue its expansion drive to bolster its geographical coverage and bulk up its network of clinical laboratories. This development is expected to consist of selective acquisitions of clinical laboratories in each of the markets in which the Group is present. The Group also intends to pursue further development in specialty testing, such as genetic testing and other clinical diagnostics, including anatomical pathology and radiological testing for integrated diagnosis, by making selective acquisitions or forging commercial partnerships with biotechnology companies. In addition, the Group could consider selling laboratories in some markets or regions through dynamic management of its asset portfolio, in order to focus on national markets or regions in which it has achieved or may shortly achieve a leadership position, significant market share or a distinctive positioning. 9.1.2.3 Organic growth The Group’s results of operations are also affected by organic growth in the Group’s various businesses, varying from one type of business and one geographical market to another. Organic growth between one given financial period and an earlier comparative financial period is determined by the Group by calculating the growth in Revenue excluding the consolidation perimeter effects arising from acquisitions and disposals completed in either of the financial periods under comparison. In particular, when the Group analyzes organic growth in Revenue between one accounting period (“period n”) and the prior comparative accounting period (“period n1”), the impact of consolidation perimeter effects are determined as follows: Organic growth for acquisitions that took place during financial period n-1 is calculated by comparing Revenue in period n with pro forma Revenue in period n-1 adjusted for the effects of acquisitions, i.e. by adding back Revenue from the acquisitions made during the period prior to the date on which the relevant operations were added to the scope of consolidation. The full-year contribution from the acquisitions made during financial period n-1 consists of the estimate of Revenue recognized by the newly acquired companies prior to the date on which they were added to the perimeter of consolidation; Organic growth for acquisitions that took place during period n is calculated by subtracting Revenue generated by the businesses acquired between the date on which they joined the perimeter of consolidation and the end of period n; The percentage of organic growth is then calculated as the ratio of Revenue in period n restated for the acquisitions completed during period n/ pro forma Revenue in period n-1 adjusted for the impact of the acquisitions. In addition to organic growth, the Group analyzes its results by considering the estimated consequences for Revenue of differences in the number of working days between two periods. Over the period 2012-2014, given the challenging economic conditions prevailing in Europe and additional measures taken to reduce healthcare spending by various governments (as outlined in the paragraph concerning the specific effects of volumes and prices, see section 9.1.2.1.2. “Specific impact of volume and price effects” of this document de base), volume growth was offset by pricing pressures, paving the way for organic growth of 1.8% between 2012 and 2013 and 3.4% between 2013 and 2014. 9.1.2.4 Cost structure and operating performance Synergies and cost reductions have a crucial bearing on the Group’s profitability because they help to offset some of the downward pressure on prices brought to bear by governments and third-party payers, which may negatively impact the Group’s Revenue. 123 Since 2011, the Group has consolidated small clinical laboratories into larger entities and pooled testing volumes at more efficient technical platforms in all the countries in which it operates. Various mergers took place in 2011 (15 entities merged with another in France, 7 in the Iberian Peninsula and 1 in Germany), in 2012 (14 entities merged with another in France, 4 in the Iberian Peninsula and 1 in Italy), in 2013 (17 entities merged with another in France, 7 in the Iberian Peninsula and 1 in Germany), and in 2014 (5 entities merged with another in France, 8 in the Iberian Peninsula). Mergers pave the way for significant economies of scale. The Group regularly introduces measures to reduce costs across all expense categories. In particular, to reduce raw materials costs and protect its margins against the backdrop of prices reductions imposed on the clinical laboratory testing market in a number of countries, the Group has implemented the pan-European SPORT project since 2009, which has allowed a reduction in procurement costs by cutting the number of suppliers it works with and by securing better commercial terms through the use of framework agreements, especially for purchases of the chemical reagents used in clinical laboratory tests. For example, in late 2012, European and national calls for tenders were held to choose two preferred suppliers of reagents and clinical laboratory equipment in each testing category (biochemistry, bacteriology, etc.), which has gradually enabled the Group to benefit from more favorable commercial terms, thereby generating significant savings. The “Safe” project initiated in June 2010 enabled the Group to reduce its operational staff costs through a hiring freeze and laboratory headcount reductions. The Group also implemented the “Deep Dive” program during 2012 and 2013, which involved a full, detailed review of its operations in France in order to restructure the French network by identifying the efficiency gains within its grasp by speeding up the pooling process and conducting a roots-and-branch overhaul, which in particular gave rise to a workforce optimization plan that led to the reduction of 67 FTE positions over 2 years. Lastly, since the fourth quarter of 2011, the Group has implemented major restructuring plans in Spain and Portugal. The headcount in Spain and Portugal was reduced through cutbacks in support functions and the consolidation of laboratories. For example, the Group pooled most of its facilities in Portugal in the cities of Lisbon, Faro and Porto. During 2013, additional restructuring was implemented in Spain in the context of contractual renegotiations with a major customer leading to a major reorganization of the operations of the laboratories handling the contract in line with customer service requirements and, to a lesser extent, a reorganization of the headquarters functions. In late 2014, a restructuring plan was implemented as part of the move to amalgamate all sites in the Barcelona region in Spain within the new building purchased in October 2014. The new site and the related overhaul of logistics should enable the Group to manage volume growth. In Italy, the activities of the CAM laboratory were reorganized with the consolidation of the various sites at a unique facility via Elvezia in Monza, which was optimized and endowed with cutting-edge equipment in late 2012. Even so, these initiatives may lead in certain cases to restructuring costs, impairment losses, redundancy costs and litigation costs. 9.1.2.5 Seasonality 9.1.2.5.1 Seasonality of Revenue and results of operations The Group’s Revenue and results of operations are exposed to seasonal fluctuations owing to the impact of vacation periods, particularly in the summer, on the activities of certain laboratories and the impact of challenging weather conditions, if any, during the winter period. The effect of this seasonal impact varies from one country in which the Group is present to another. 9.1.2.5.2 Seasonality of changes in the working capital requirement The working capital requirements are also subject to seasonal fluctuations owing to the impact of vacation periods on the aforementioned activities and the seasonal effects of payments by the Group’s principal customers, in particular public-sector or public-private organizations, such as INAMI in Belgium and the ASL regional health insurance funds in Italy, which tend to settle all the amounts they owe for the year at December 31. 124 As such, more cash is required to cover the working capital requirement over the first 9 months of the accounting year, while in the 4th quarter the Group enjoys the benefit of the cash generated by the working capital. In addition, the seasonal effect on cash used as or generated by working capital usually tends to increase from one year to the next owing to (organic or acquisition-led) growth in consolidated annual Revenue. 9.1.3 Description of the key income statement items 9.1.3.1 Revenue The Group generates Revenue from a wide range of clinical or diagnostic services which are paid for by private insurers, hospitals, patients, pharmacies and national health insurance funds. These services include clinical laboratory testing, consisting of both routine and specialty testing, anatomopathology diagnostics, histologic and cytologic testing, as well as imaging services including medical imaging and molecular imaging (mainly in Italy). It also consists of other revenue, which mainly comprises interest received on trade receivables and revenue generated by activities not directly related to clinical testing and medical imaging services. Revenue from clinical laboratory testing and medical imaging services is stated at the fair value of the consideration received or receivable net of returns, trade rebates and volume discounts. Revenue from services is recognized when the service is rendered. Revenue is based on the net amount invoiced or “invoiceable”, where this may be estimated reliably. Where it seems probable that a discount will be granted and that its amount can be estimated reliably, the discount is accounted for as a reduction in total revenue when the sale is recognized. The process of estimating the ultimate collection rate of the receivables generated by the clinical laboratory testing business requires the use of significant assumptions and judgments. Services that are reimbursed by third-party payers, including social security systems, are recognized under revenue net of allowances to provisions for the difference between the invoiced amount and the estimated amount of the reimbursement receivable from these third-party payers. Adjustments to these provisions based on actual reimbursements by third-party payers are accounted for upon their payment as an adjustment to net total revenue. Public-sector third-party payers Payments made by public-sector agencies for clinical laboratory testing services are based on pricing scales drawn up by the public authorities. Collection times for these receivables usually depend on full and accurate information being furnished in accordance with the various reporting deadlines. Collection times vary from one country to another. Private insurers Reimbursements by private insurers are based on negotiated fee-for-service schedules and on capitated payment rates. Substantially all of the accounts receivable due from private insurers represent amounts billed under negotiated fee-for-service arrangements. The Group uses a standard approach to establish allowances for doubtful accounts for such receivables, which considers the aging of the receivables, historical collection experience and other factors. Client payers Client payers include doctors, hospitals, employers and other commercial laboratories. The credit risk and ability to pay are more of a consideration for these payers than private insurers and government payers. The Group uses a standard approach to establish allowances for doubtful accounts for such receivables which considers the aging of the receivables as well as specific account reviews, historical collection experience and other factors. Patients (individuals) Patients are charged based on the established patient fee schedules, subject to any limitations on fees negotiated with the mutuals or doctors on behalf of their patients. The collection of receivables due from patients is subject 125 to credit risk and ability of the patients to pay. The Group uses a standard approach to establish allowances for doubtful accounts for such receivables, which considers the aging of the receivables, historical collection experience and other factors. Countries and regions in which the Group operates experience different demand trends owing chiefly to their public health management models and local economic conditions. For a detailed classification of the Group’s customers, see section 6.4.6. “Group’s customers” of this document de base. 9.1.3.2 Cost of sales The Group’s cost of sales consists primarily of variable costs given the high proportion of raw materials costs (chemical reagents) and outsourced tests, and to a lesser extent, transport and logistics costs. The main components of this cost of sales are as follows: Chemical reagents used to perform the clinical laboratory tests purchased from suppliers in the health diagnostics industry (Beckmann, Abbott, Roche, Siemens); Supplies and consumables such as tubes, needles, special conditioning used to collect samples or to condition the samples so that they can be processed on automated equipment or diagnostics devices; Analyses outsourced to other clinical testing laboratories and in particular certain specialty tests that the Group is unable or not authorized to perform; Pre-testing sample collection, where this is not carried out by the Group; Transport and collection costs related to the waste generated by the clinical testing business. The rebates granted by certain suppliers, chiefly suppliers of reagents and consumables and specialty testing laboratories, are accounted for as reductions in the cost of purchasing raw materials, supplies and outsourced tests. 9.1.3.3 Payroll-related expenses Payroll-related expenses consist of fixed and variable wages and salaries, temporary staffing costs, social security contributions and other salary-based taxes (such as the taxe sur les salaires in France), pension contributions or estimated service costs for provisions for post-employment benefit schemes in France recognized in accordance with IFRS IAS 19 and any other expenses payable to employees, such as mandatory employee profit-sharing in France, or related to employees, such as travel expenses. They also consist of the fixed and variable remuneration paid to laboratory doctors under various legal forms, either compensation paid as salary or fees or, mainly for French laboratory doctors, the priority dividends based on current-year profits for the variable portion. As explained in Note 3.1.1 to the consolidated financial statements for the financial year ended December 31, 2014 included in section 20.1.1. “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” of this document de base, the priority dividends due to be paid to certain laboratory doctors in the following year are recognized as employee benefit expense and a liability in the current year. 9.1.3.4 Share-based payments Expenses related to share-based payments represent expenses to be recognized under IFRS 2 and correspond to the estimated theoretical costs of the benefit that employees may gain from the equity instruments (“BSA” warrants or free shares) granted to them as employees performing services to the Group. These expenses also include, eventually, social security expenses to be paid for such granted shares. 126 9.1.3.5 Other operating expenses Other operating expenses mainly include: Rent and service charges related to premises, equipment and vehicles; Maintenance and repair expenses and in particular for testing equipment and systems, as well as insurance costs; Taxes other than on income and in particular land tax and business tax. In France, this category also covers the CET, but not the CVAE; these two levies were both introduced in France on January 1, 2010. The business tax payable by French subsidiaries prior to January 1, 2010 was replaced by the CET and CVAE levies. CET is recognized under “Taxes other than on income”, while CVAE is accounted for under “Income tax”; Expenses and professional fees, such as lawyers’, audit and consultants’ fees; Sales, marketing and quality expenses. Since the clinical laboratory testing industry is highly regulated and particularly so in France with the obligation to secure accreditation based on strict standards, the Group incurs quality-related expenses; Administrative expenses and allowances for doubtful accounts or inventories, when proven and definitive. 9.1.3.6 Transaction costs for usual small size acquisitions Under IFRS 3 revised, transaction costs related to acquired entities have been recorded since 2008 in the consolidated statement of income together with those related to abandoned deals. Given the non-operational, irregular and non-recurring nature of these costs, they are shown on a separate line of the consolidated statement of income, and depending on the amount of costs incurred per acquisition project, they are qualified as transaction costs for usual small size acquisitions recorded as other operating expenses, or they are qualified as transaction costs for significant and unusual transactions recorded as non-recurring operating expenses in the line perimeter effect. 9.1.3.7 EBITDA EBITDA is defined as operating income before non-recurring items restated for depreciation and amortization, impairment losses and additions to provisions net of reversals. Under IFRS, EBITDA does not have to be shown on a separate line of the statement of income. EBITDA does not necessarily represent a useful measure of the Group’s financial condition, liquidity or profitability and should not be considered as an alternative to the results determined in compliance with IFRS for the period, the cash flows determined in compliance with IFRS or any other measure provided for in compliance with IFRS. EBITDA may be used to compare the Group’s performance using consistent criteria over the various periods concerned insofar as it eliminates the impact of items that are not directly related to operating performance even though it includes certain non-operating and unusual items such as share-based payments and transaction costs for usual small size acquisitions, which have been shown on separate lines. The Group believes that EBITDA is a useful measure in this document de base insofar as it provides the same information as that used by the Group’s management to assess the Group’s performance. Even so, EBITDA has a number of limitations as an analytical tool and should not be considered in isolation or instead of an analysis of the Group’s results from operating activities. Since other market participants may not calculate EBITDA in the same manner, the EBITDA shown by the Group may not be comparable with the figures provided by other companies under the same heading. 9.1.3.8 Depreciation, impairment losses and amortization, provisions and reversals Depreciation and amortization reflects the normal wear and tear of property, plant and equipment and intangible assets, including the amortization of intangible assets recognized upon consolidation in respect of fair value 127 adjustments to assets and liabilities within twelve months of an acquisition. Current operating provisions reflect allowances to and reversals from provisions for disputes with employees, customers or third parties and restructuring expenses incurred in the normal course of the Group’s business. This also includes allowances for doubtful accounts and inventories to cover the risks of non-collection of receivables or impairment of inventories. Where the risk of impairment is proven, the provisions are reversed, and a definitive impairment loss is recognized under other operating expenses. 9.1.3.9 Non-recurring income and expenses Non-recurring income and expenses consist of income and expenses that are not considered to be generated or incurred in the recurring operating activities of the Group. This line item primarily reflects impairment losses on goodwill and other non-operating non-current assets, significant non-recurring restructuring costs, provisions for major litigation, expenses incurred through restructuring of the Group’s debt, the transaction costs related to significant and unusual acquisitions involving consolidated subsidiaries, whether or not the planned transaction is abandoned or goes ahead, changes in the fair value of earn-out payments after the one-year evaluation period, and capital gains and losses on the disposal of non-current assets or of investments in consolidated companies. 9.1.3.10 Net finance costs Net finance costs is the sum of financial expense and income and primarily includes (i) interest on outstanding amounts of existing debt and especially the amounts outstanding on the issue of High-Yield Bonds, the revolving credit facility and bank loans and factoring programs, (ii) interests payable on finance leases, (iii) gains and losses caused by fluctuations in exchange rates, (iv) the impact of fair value adjustments on financial instruments held to cover interest rate risks, (v) the interest cost of post-employment benefit obligations, and (vi) income from cash equivalents. 9.1.3.11 Income tax expenses Income tax expenses consist of (i) tax paid on income in all the countries in which the Group operates, including the IRAP in Italy and the CVAE in France, and (ii) any changes in the net deferred taxes accounted for on the Group’s statement of financial position. 9.1.3.12 Share of profit of associates The share of profit of associates represents the share in the profit after tax of associates attributable to the Group. 9.1.3.13 Net profit from discontinued operations Net profit from discontinued operations represents the net profit generated by operations classified as discontinued pursuant to IFRS 5 and thus shown on a separate line of the income statement. A discontinued operation under IFRS 5 is a cash-generating unit (i.e. a component of an entity with activities and cash flows that can be distinguished for operational and financial reporting purposes) representing either a separate major line of business or a geographical area of operations in respect of which a single coordinated plan to dispose has been drawn up. The net profit of the German entities in the relevant periods has thus been reclassified under this heading for the financial years 2012 and 2013. 9.1.4 Main accounting principles For a description of the Group’s significant accounting principles and material accounting estimates, see Note 3 to the Group’s consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014 included in section 20.1.1. “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” of this document de base. 128 9.2 ANALYSIS OF THE RESULTS OF OPERATIONS FOR THE FINANCIAL YEARS ENDED DECEMBER 31, 2014 AND DECEMBER 31, 2013 9.2.1 Overview Preamble To recap, the business in Germany was sold to the Sonic Healthcare group on December 2, 2013 (effective November 30, 2013 for business purposes). As a result, the German cash-generating unit was classified in compliance with IFRS 5 under discontinued operations in the consolidated statement of income, statement of financial position and statement of cash flows with effect from September 30, 2013. (See section 20.1.1. “IFRS consolidated financial statements for the periods ended December 31, 2012, 2013 and 2014 – Note 33 to the consolidated financial statements for the period ended December 31, 2013”.) During 2014, Revenue grew by 12.5% to €615.6 million. EBITDA grew by 6.6% to €113.2 million, but the EBITDA margin contracted by 1 point. The key factors behind these trends were the impact of acquisitions, synergies and efficiency gains, offset by the sharp rise in share-based payments (introduction of the UK Employee Shareholders Scheme in 2014 benefiting the two leading UK managers, and the Group’s performance-shares plan), pricing pressures in France, in the Iberian Peninsula and in Italy and by the impact of the start-up of outsourcing activities in the United Kingdom, as these posted a negative EBITDA. The following table shows trends in the Group’s results of operations in the financial years ended December 31, 2014 and December 31, 2013. Financial years ended December 31 CONSOLIDATED STATEMENT OF INCOME (in millions of euros except for %) 2014 % 2013 % Variation % 611.3 99.3% 544.2 99.4% 67.1 12.3% 4.4 0.7% 3.1 0.6% 1.2 38.7% 615.6 100% 547.3 100.0% 68.3 12.5% Cost of sales (140.1) -22.7% (122.8) -22.4% (17.3) 14.1% Payroll related expenses (256.8) -41.7% (232.5) -42.5% (24.3) 10.5% (2.0) -0.3% (0.3) -0.1% (1.6) 484.0% (102.1) -16.6% (84.5) -15.4% (17.6) 20.8% (1.5) -0.2% (1.0) -0.2% (0.5) 51.5% 113.2 18.4% 106.3 19.4% 7.0 6.6% Revenue Other income Total Revenue (Total proceeds of ordinary activities) Share based payments Other operating expenses Transaction costs for usual small size acquisitions EBITDA EBITDA margin (EBITDA/Revenue) Depreciation, amortization, impairment losses, provisions and reversals Results from operating activities before non-recurring items Non-recurring income and expenses Results from operating activities after non-recurring items Net finance costs Income tax expenses Share of profit of associates Net profit from continuing operations Net profit from discontinued operations Net profit of the period Profit attributable to non-controlling interests Profit attributable to owners of the company 18.4% 19.4% (24.2) -3.9% (19.0) -3.5% (5.2) 27.3% 89.1 14.5% 87.3 15.9% 1.8 2.1% (20.8%) 68.2 -3.4% 11.1% 0.3 87.5 0.0% 16.0% (21.1) (19.3) n.a. -22.1% (64.5) (18.7) 0.4 -10.5% -3.0% 0.1% (59.1) (21.1) (1.3) -10.8% -3.9% -0.2% (5.4) 2.4 1.7 9.1% -11.5% n.a. (14.6) -2.4% 6.0 1.1% (20.6) n.a. (14.6) 6.8 (6.8) n.a. 12.8 (27.4) n.a. 0.4 0.1% 0.1 0.0% 0.3 351.3% (15.0) -2.4% 12.7 2.3% (27.7) n.a. 129 9.2.2 Revenue The Group’s Revenue increased by €68.3 million, or 12.5%, to €615.6 million in the financial year ended December 31, 2014 from €547.3 million in the financial year ended December 31, 2013. This increase was driven mainly by the full-year contribution of the acquisitions completed in 2013, especially the impact of the full consolidation of iPP effective October 25, 2013 and the impact of acquisitions completed in 2014, particularly the acquisition of the SDN group in Italy on July 30, 2014, and organic volume growth, the start of the outsourcing activites of the agreement with Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust and the favorable Mix Effect recorded in most countries in which the Group is present. The increase was partially counterbalanced by a challenging economic environment in the Iberian Peninsula and by price reductions in most countries in which the Group is present and especially in France and Italy. The acquisitions completed in 2014, principally in Italy, France, Spain and Belgium, contributed €27.5 million to Revenue in the financial year ended December 31, 2014. The following table shows a breakdown of Revenue by country in value and as a percentage of the Group’s total Revenue as recorded in the years ended December 31, 2014 and December 31, 2013, as well as an analysis of the increase in Revenue between organic growth (at comparable perimeter) and growth including changes in the perimeter of consolidation (at current perimeter) for each segment and for the Group as a whole. Financial year ended December 31 Revenue (in millions of euros except for %) Northern Europe France 2014 % 401.3 65% 357.9 % Unadjusted Perimeter Constant Perimeter 65% 12.1% 3.3% 0.1% 56% 325.3 59% 5.2% Belgium 30.0 5% 27.2 5% 10.5% 6.9% United Kingdom 27.0 4% 4.4 1% 509.7% 52.7% Switzerland 2.0 0% 0.9 0% 113.3% 113.3% 214.3 35% 189.4 35% 13.1% 3.7% 158.7 26% 151.2 28% 5.0% 2.9% 55.6 9% 38.2 7% 45.4% 7.0% 100% 547.3 100% 12.5% 3.4% Southern Europe Iberian Peninsula Italy 342.3 2013 % Variation 615.6 Total 9.2.2.1 Northern Europe The Group’s Revenue in the Northern Europe segment increased by €43.4 million, or 12.1% on a reported basis, to €401.3 million in the financial year ended December 31, 2014 against €357.9 million in the financial year ended December 31, 2013. The increase in revenue was driven predominantly by the full consolidation for the first time of iPP effective October 25, 2013 and to the full-year contribution from businesses acquired, especially in France during 2013 and to a lesser extent during 2014. Revenue growth also reflects organic growth, which reached 3.3% during the period. Revenue in France grew by €17.0 million, or 5.2%, to €342.3 million in the financial year ended December 31, 2014 from €325.3 million in the financial year ended December 31, 2013, when the Group’s top line was held back by the impact of poor weather conditions in northern France during the first quarter of 2013. This rise was chiefly attributable to the full-year contribution of the acquisitions completed in 2013, organic volume growth and the favorable Mix Effect and, to a lesser extent, the pro rata temporis effect of the acquisitions made in 2014, offset partially by the annual reduction in the prices applied by the French Health Authority. The Group made 5 acquisitions during the financial year ended December 31, 2014, and the acquisition-driven Revenue increase came to €9.8 million in 2014. The full-year contribution made by businesses acquired in 2013 totaled €6.9 million. Organic growth ran at 0.1%, reflecting the impact of the negative annual adjustment of the prices applied by the French Health Authority of around 2.3% in April 2013 and 2.3% in April 2014, offset by volume growth and the favorable Mix Effect. Revenue in Belgium increased by €2.8 million, or 10.5%, to €30.0 million in the financial year ended December 31, 2014 from €27.2 million in the financial year ended December 31, 2013, owing chiefly to expansion in nutritional testing and, to a lesser extent, in routine testing. The Group made one acquisition during the financial 130 year ended December 31, 2014, and the acquisition-driven Revenue increase came to €1.0 million. As a result, organic growth was 6.9%. Revenue in the United Kingdom rose by €22.6 million to €27.0 million in the financial year ended December 31, 2014 from €4.4 million in the financial year ended December 31, 2013. Following the buyout of Sodexo’s holdings in iPP on October 25, 2013, the Group has held full control of iPP. Accordingly, iPP has been fully consolidated since that date and contributed Revenue of €16.9 million in 2014. Labco UK, which manages the subcontracting contract for the Fresenius group’s clinical diagnostic testing business, posted a top-line increase owing mainly to higher volumes. In addition, iPP Analytics and iPP Facilities were set up in 2014 and on October 1, 2014 started activities related to the laboratory services outsourcing contract with Basildon and Thurrock University Hospital NHS Foundation Trust and Southend University Hospital NHS Foundation Trust. Those two entities contributed €8.0 million to Revenue in 2014. Revenue in Switzerland rose by €1.1 million to €2.0 million in the financial year ended December 31, 2014 from €0.9 million in the financial year ended December 31, 2013. Revenue in Switzerland reflects the clinical laboratory testing business generated by Test SA, a 48%-held subsidiary of the Group founded in early 2013 by laboratory doctors, but fully consolidated by the Group as a result of the analysis of the control it exercises. 9.2.2.2 Southern Europe The Southern Europe segment’s Revenue improved by €24.9 million, or 13.1%, to €214.3 million in the financial year ended December 31, 2014 from €189.4 million in the financial year ended December 31, 2013. The increase in Revenue mainly reflects the €14.1 million impact of the SDN group in Italy, which was acquired on July 30, 2014, the full-year contribution of €1.9 million from businesses acquired in 2013 and the impact of €2.6 million from acquisitions completed in the Iberian Peninsula and Italy in 2014. Organic growth ran at 3.7% during this period, reflecting the strong expansion in specialty testing (particularly non-invasive genetic testing for Down’s syndrome) in Spain and Italy and a firm top-line performance in Italy. Revenue in the Iberian Peninsula increased by €7.5 million, or 5.0%, to €158.7 million in the financial year ended December 31, 2014 from €151.2 million in the financial year ended December 31, 2013. The Group made three acquisitions in the Iberian Peninsula during the financial year ended December 31, 2014, and the acquisition-driven Revenue increase came to €2.0 million. The full-year contribution made by businesses acquired in 2013 totaled €1.9 million. In July 2014, the Group sold Sabater Pharma in Spain, which before its disposal contributed €0.9 million to Group Revenue in the financial year 2014 as opposed to €1.7 million in the financial year 2013. Organic growth ran at 3.0%, reflecting the volume growth in Spain and Portugal in routine testing and the strong expansion in specialty testing (particularly non-invasive genetic testing for Down’s syndrome, which posted a significant increase in sales during 2013) and an upbeat performance in Latin America on the back of a favorable currency effect, offset partially by the full-year impact of the price reductions in Spain introduced in early 2013 on certain contracts with hospital laboratories in Spain and the unfavorable Mix Effects affecting the day surgery business in Portugal. Revenue in Italy increased by €17.4 million, or 45.4%, to €55.6 million in the financial year ended December 31, 2014 from €38.2 million in the financial year ended December 31, 2013. The Group carried out the strategic acquisition of the SDN group, the Naples-based leader in integrated diagnostics, on July 30, 2014, and the Visconteo laboratory, resulting in an acquisition-driven Revenue increase of €14.7 million. As a result, organic growth was 7.0%, reflecting volume growth and particularly the launch of non-invasive Down’s syndrome genetic tests, partly offset by the impact of price reductions resulting from budget cuts by ASLs in some regions. 9.2.3 Cost of sales The cost of sales increased by €17.3 million, or 14.1%, to €140.1 million in the financial year ended December 31, 2014 from €122.8 million in the financial year ended December 31, 2013. The cost of sales stated as a percentage of Tevenue came to 22.7% in 2014, compared with 22.4% in 2013. The rise in the cost of sales derived mainly from the full-year contribution of the acquisitions made in 2013, chiefly in France, the impact of the full consolidation of iPP, and the impact of acquisitions in 2014, particularly the SDN group, along with the higher cost of sales in the Iberian Peninsula and in Italy as a result of the development of non-invasive genetic testing for Down’s syndrome and the hike of the VAT rate in Italy (from 21% to 22%) effective October 2013. The increase in the cost of sales as a percentage of Revenue was mainly 131 attributable to the impact of the expansion of specialty testing in Iberia and Italy since the Down’s syndrome genetic testing is outsourced, giving rise to a thinner gross margin but with no material impact on EBITDA margin. This rise was also attributable to the unfavorable impact on Revenue of the price reductions in France and Italy, offset partially by the positive full-year impact of the renegotiations of the commercial terms secured on the Group’s reagent purchases in early 2013. 9.2.4 Payroll related expenses Payroll related expenses increased by €24.3 million, or 10.5%, to €256.8 million in the financial year ended December 31, 2014 from €232.5 million in the financial year ended December 31, 2013. Payroll related expenses stated as a percentage of Revenue came to 41.7% in 2014, compared with 42.5% in 2013. This increase in payroll related expenses was chiefly attributable to the full-year contribution made by the businesses acquired in 2013 and 2014, and in particular the impact of the full consolidation of iPP, which incurred non-recurring restructuring costs in line with the previsions of the first few years of the business plan for the NHS outsourcing contracts. The decline in the payroll related expenses to Revenue ratio was achieved through efficiency gains, which paved the way for reductions in the operational workforce, and through the impact of the restructuring and efficiency plans implemented in Spain, Portugal and France. Another positive factor was the impact of the employment competitiveness tax credit (CICE) in France. In its second year in force, the rate increased from 4% to 6%. This contraction also reflected the productivity gains unlocked in Italy and Belgium. 9.2.5 Share-based payments Expenses linked to share-based payments (BSA warrants and free shares) significantly increased by €1.6 million from €0.3 million in the financial year 2013 to €2.0 million in the financial year 2014. The IFRS 2 expense reflected, only in 2013, the non-cash expense calculated in respect of the 2010 BSA plan in respect of the last year of the vesting period for the estimated rights as adjusted following the departure of certain beneficiaries. The expense recognized in 2014 reflects a profit-based incentive plan set up in April 2014 for the two main managers of the Group’s UK activities and qualifying under IFRS as a cash-settled share-based payments plan with a pro rata temporis vesting period for rights of 5 years. It also includes the IFRS 2 charge on a prorata temporis basis and employer expenses to be paid for French beneficiaries of the Group bonus performance share plan introduced in mid-November 2014. That plan, which features a performance condition and a two-year vesting period, relates to around 1% of the Company’s share capital and qualifies under IFRS as an equitysettled share-based payment plan. 9.2.6 Other operating expenses Other operating expenses increased by €17.6 million, or 20.8%, to €102.1 million in the financial year ended December 31, 2014 from €84.5 million in the financial year ended December 31, 2013. This increase was attributable principally to the full-period contribution made by the businesses acquired in 2013, especially with the full consolidation of iPP and the impact of the acquisitions made in 2014, particularly the acquisition of the SDN group. To a lesser extent, the increase was also caused by the rent increase in Italy with the opening of new sites, the start of activity under the new Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust contract – resulting in one-off start-up costs of €0.7 million – and the higher level of losses on unrecoverable receivables from €2.3 million in 2013 to €4.1 million in 2014. The increase in losses on unrecoverable receivables was due to the write-off of €2.9 million of unrecoverable receivables in France, offset by the release of €1.5 million of impairment provisions. The one-off losses on recoverable receivables in France on December 31, 2014 arise mainly from an in-depth analysis carried out at the end of the financial year 2014 using SELs’ operational management software, which identified €1.7 million of receivables that were older than the prescribed time limit, along with €0.6 million of unsubstantiated differences between amounts invoiced and amounts received on clinical contracts in 2 SELs. The rise in other operating expenses was partially offset by the restructuring-led reduction in equipment leasing costs in the Iberian Peninsula. Other operating expenses stated as a percentage of Revenue came to 16.6% in 2014, compared with 15.4% in 2013. The key factors contributing to this increase were the impact of the full consolidation for the first time of 132 iPP and the acquisition of the SDN group, as these entities have a higher ratio of operating expenses to Revenue than the Group in its other countries and the impact of non-recurring impairments of trade receivables in France. 9.2.7 Transactions costs for usual small size acquisitions Transaction costs for usual small size acquisitions increased by €0.5 million from €1.0 million in 2013 to €1.5 million in 2014 because the number of completed or abandoned acquisition plans was higher in 2014 than the number of acquisitions completed in 2013. 9.2.8 EBITDA EBITDA increased by €7.0 million, or 6.6%, to €113.2 million in the financial year ended December 31, 2014 from €106.3 million in the financial year ended December 31, 2013. This increase flowed mainly from the impact of the SDN acquisition on July 30, 2014 and the full-year contribution made by the businesses acquired in 2013 largely in France, offset partially by the unfavorable impact of prices reductions in France, by pricing pressure in the Iberian Peninsula and by the adverse effect of clinical testing outsourcing by certain NHS trusts in the United Kingdom, which dragged down Group EBITDA by €1.3 million. iPP, which operates the Taunton and Somerset outsourcing contract, has been fully consolidated since October 25, 2013, and iPP Analytics and iPP Facilities, set up in 2014, started activity under the new Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust contract on October 1, 2014. The outsourced provision of laboratory services for certain NHS trusts generates losses in the first few years of the contract while the outsourced operations are restructured and resources are mobilized, before the expected efficiency is achieved. Consolidated EBITDA stated as a percentage of Revenue (EBITDA margin) declined from 19.4% in 2013 to 18.4% in 2014 (see the segmental analysis below). Adjusted for the effects of UK subsidiaries managing laboratory services outsourcing contracts for certain NHS trusts, the EBITDA margin was 19.4% in 2014 as opposed to 19.6% in 2013. The following table shows EBITDA by segment over the indicated periods and also stated as a percentage of each segment’s Revenue. Financial year ended December 31 EBITDA (in millions of euros except for %) 2014 % 2013 % % Variation Northern Europe As a % of consolidated Revenue 79.2 19.7% 70% 75.5 21.1% 71% 5.0% Southern Europe As a % of consolidated Revenue 34.0 15.9% 30.0% 30.8 16.2% 29% 10.6% Total As a % of consolidated Revenue 113.2 18.4% 100% 106.3 19.4% 100% 6.6 9.2.8.1 Northern Europe The Northern Europe segment’s EBITDA increased by €3.7 million, or 5.0%, to €79.2 million in the financial year ended December 31, 2014 from €75.5 million in the financial year ended December 31, 2013. This increase flowed mainly from the full-period contribution made by the businesses acquired in 2013 largely in France and the impact of the acquisitions made during 2014, offset partially by the unfavorable impact of price reductions in France and by the increase in share-based payment expenses, due to the profit-sharing plan granted to the two main managers in the United Kingdom and the impact of a Group bonus performance share plan adopted in November 2014. The provision of outsourced clinical testing for certain NHS trusts in the UK dragged down Group EBITDA by €(1.3) million. The outsourced laboratory services for certain NHS trusts generates losses in the first few years of the contracts while the outsourced operations are restructured and resources are mobilized, before the expected efficiency is achieved. iPP Analytics and iPP Facilities, set up in 2014, started activity under the new Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust contract on October 1, 2014 and dragged down Group EBITDA by €(0.6) million excluding share-based payment expenses, while iPP, which has operated the Taunton and Somerset outsourcing contract since June 1, 2012, made a positive EBITDA contribution of €0.3 million excluding share-based payment expenses after two years of losses. As a percentage of Revenue, EBITDA in this segment fell from 21.1% in 2013 to 19.7% in 2014. Adjusted for the effects of UK subsidiaries managing laboratory services 133 outsourcing contracts for certain NHS trusts, the EBITDA margin was 21.4% in 2014 as opposed to 21.3% in 2013. The relative stability of EBITDA margin was mainly attributable to the stable ratio in France, with the impact of the prices reductions in France and non-recurring expenses recognized in other operating expenses relating to write-offs of receivables at December 31, 2014, offset by the cost efficiency measures implemented in France (concentration of laboratories through mergers or creation of technical platforms, the “Deep Dive” plan optimizing the structure of the workforce, commercial renegotiations with certain suppliers) and an improvement in the EBITDA margin in Belgium on the back of economies of scale flowing from top-line growth. 9.2.8.2 Southern Europe The Southern Europe segment’s EBITDA rose by €3.2 million, or 10.6%, to €34.1 million in the financial year ended December 31, 2014 from €30.8 million in the financial year ended December 31, 2013. This sharp increase was mainly due to the strategic acquisition of the SDN group on July 30, 2014. Adjusted for the impact of consolidating the SDN group, EBITDA in the Southern Europe segment fell €0.5 million due to the decline in the Iberian Peninsula. The impact of pricing pressure in the Iberian Peninsula and in particular price reductions in the Spanish hospital and insurance business and the impact of prices reductions for certain tests in Portugal was not fully offset by the impact of the restructuring in the Iberian Peninsula and the expansion of the specialty clinical laboratory testing business, especially certain genetic tests. The strong performance of Italian activities made up for the increase in rent relating to new collection centers and the increase in VAT. The EBITDA of Southern Europe stated as a percentage of Revenue went down from 16.2% to 15.9% reflecting the impact of the weaker performance by operations in the Iberian Peninsula, partly offset by the positive impact of the SDN acquisition, where margins are higher than in the Group’s other activities. 9.2.9 Depreciation, impairment losses and amortization, provisions and reversals Depreciation, amortization, impairment losses, provisions and reversals increased by €5.2 million, or 27.3%, to €24.2 million in the financial year ended December 31, 2014 from €19.0 million in the financial year ended December 31, 2013. This rise was chiefly attributable to higher depreciation and amortization expense given the impact of acquisitions, particularly that of the SDN group, including amortization of intangible assets recognized on the allocation of the purchase price of certain acquisitions made in the second half of 2013 with business activities focused on outsourcing or subcontracting contracts (iPP and an acquisition in Spain to acquire a subcontracting contract for a clinic). The increase also flowed from the commissioning in late 2013 of the major IT developments completed by the Company. 9.2.10 Non-recurring income and expenses Non-recurring income and expenses showed a net non-recurring expense of €20.8 million in the financial year ended December 31, 2014 compared with net non-recurring income of €0.3 million in the financial year ended December 31, 2013. Non-recurring expenses in 2014 mainly consisted of €8.8 million of non-recurring costs and provisions recognized in relation to the purchase of, or undertaking to purchase, priority dividend rights from certain French laboratory doctors for 4 French SELs, €5.3 million of costs incurred for strategic projects corresponding mainly to advisors’, lawyers’ and financial auditors’ fees in relation to the planned IPO, €1.5 million of redundancy costs and restructuring provisions relating to the restructuring in Spain, €1.7 million of nonrecurring costs relating to an out-of-court settlement with CPAMs (after a French laboratory issued incorrect invoices for several years because of a mistake inputting prices into its information system, it voluntarily informed the CPAMs in its region of the mistake, and an out-of-court settlement was signed on December 2014 in order to repay the incorrectly invoiced sums), and €0.6 million of non-recurring costs relating to a material dispute with an IT service provider after the court found against Group’s Roman Pais laboratory in Belgium on appeal, after 10 years of legal proceedings, and €0.7 million of non-recurring provisions to cover the risk associated with the Dillenburg dispute, which is covered by the seller’s guarantee (garantie de passif) included in the contract to sell the German activities to Sonic Healthcare dated December 2, 2013. They also include perimeter effects corresponding to transaction costs for major planned acquisitions in an amount of €2.0 million, mainly related to the strategic acquisition of the SDN group in Italy, which was completed on July 30, 2014, and the €0.5 million gain on disposals of fixed assets, mainly comprising the gain on the Sabater Pharma disposal. 134 Net non-recurring expense in the financial year ended December 31, 2013 primarily reflected €1.8 million in costs incurred for strategic projects, costs of departures and provisions for restructuring totaling €0.7 million covering the outstanding measures in the 2011 restructuring plan implemented in the Iberian Peninsula with the reversal of the corresponding provisions, €0.8 million in severance payments deriving from the measures in the “Deep Dive” efficiency plan implemented in France during 2013, €0.8 million in provisions for restructuring set aside in the first quarter of 2013 for the new restructuring plan implemented in connection with contractual renegotiations with a major customer and use of €0.5 million of the provision during the last nine months of 2013. These items were partially offset by the impact of the step-up acquisition of iPP with €0.8 million of income from the bad will arising on the acquisition and also a €3.4 million non-recurring gain on the disposal of a fixed asset reflecting the Group’s original 51% interest in iPP previously accounted for as an associate and its re-measurement at fair value at the date of the acquisition. 9.2.11 Net finance costs Net finance costs rose from €59.1 million in 2013 to a total expense of €64.5 million in 2014. The net financing costs were affected proportionally by additional financial expenses arising from the issue in February 2013 of a tap in a principal amount of €100 million bearing interest at the annual rate of 8.5% and due to mature in 2018 (see section 10.4.3. “High Yield Bonds” of this document de base). In 2014, financing costs also included nonrecurring costs arising from amendments that substantially altered the terms of the January 2011 Revolving Credit Facility (RCF), finalized in December 2014 (see section 10.4.4. “RCF” of this document de base). The costs of setting up the previous RCF that were capitalized but not yet amortized were recognized as financial expenses in a non-recurring amount of €3.1 million, while expenses incurred when setting up the amended contract were capitalized and will be amortized over the renegotiated maturity term. 9.2.12 Income tax expenses Income tax expenses fell by €2.4 million to €18.7 million in the financial year ended December 31, 2014 from €21.1 million in the financial year ended December 31, 2013. This decline was chiefly attributable to the positive effect of the tax restructuring measures implemented such as the institution of a single tax group in Portugal and corporate restructuring that led to a reduction in tax expenses, partly offset by the tax expenses attributable to the SDN group for the period following its consolidation within the Group. The Group’s relatively high effective tax rate derived from losses, especially at certain holding companies giving rise to tax loss carryforwards that were not capitalized under deferred tax assets, from non-deductible interests and from the impact of charges not deductible for tax purposes recognized upon consolidation. These non-deductible expenses reflected to a large extent the impact of the restatement of the priority dividends paid out to French laboratory doctors, which are recognized under payroll-related expenses in the consolidated financial statements but are not deductible for tax purposes because they are accounted for as dividends in the individual financial statements. A similar treatment applies to non-recurring expenses related to purchases of these priority dividend rights from certain French laboratory doctors. 9.2.13 Share of profit of associates The share of profit of associates represented a profit of €0.4 million in 2014 as opposed to a loss of €1.3 million in 2013. This improvement was chiefly attributable to the transfer out from the share of profit of associates of the losses posted by iPP, the joint venture with Sodexo in the United Kingdom until October 25, 2013. Prior to this date, iPP’s net loss was included in the share of profit of associates line. Since the Group took full control of iPP on October 25, 2013, it has been fully consolidated. 9.2.14 Net profit from discontinued operations Net profit from discontinued operations came to €6.8 million in the financial year ended December 31, 2013, reflecting the net profit of the German cash-generating unit sold to Sonic Healthcare on December 2, 2013 and accounted for under discontinued operations in compliance with IFRS 5 “Assets and liabilities held for sale and discontinued operations”. 135 9.2.15 Net profit Owing to the factors described above, the Group made a net loss of €14.6 million in 2014, compared to a net profit of €12.8 million in 2013. The 2014 loss was mainly due to non-recurring losses, as set out in section 9.2.10 “Non-recurring income and expenses” of this document de base. 9.3 ANALYSIS OF THE RESULTS OF OPERATIONS FOR THE FINANCIAL YEARS ENDED DECEMBER 31, 2013 AND DECEMBER 31, 2012 9.3.1 Overview Preamble To recap, the business in Germany was sold to the Sonic Healthcare group on December 2, 2013 (effective November 30, 2013 for business purposes). As a result, the German cash-generating unit was classified in compliance with IFRS 5 under discontinued operations in the consolidated statement of income, statement of financial position and statement of cash flows with effect from September 30, 2013. From a methodological standpoint in relation to the presentation required under IFRS 5, discontinued operations are included in the Group’s perimeter of consolidation as far as the comparative figures at December 31, 2012 are concerned, but the comparative figures for the income statement concerning the discontinued operations were restated so as to include these activities on a separate discontinued operations line, rather than as part of each line item (see section 20.1.1 – “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014 – Note 33 to the consolidated financial statements for the financial year ended December 31, 2013” of this document de base). During 2013, Revenue grew by 6.4% to €547.3 million. EBITDA rose by 2.8% to €106.3 million, but the EBITDA margin contracted by 0.7 points. The key factors behind these trends were the impact of acquisitions, synergies and efficiency gains, offset to some extent by pricing pressure in France and the Iberian Peninsula and by the impact of activities starting up in Switzerland and the United Kingdom. The following table shows trends in the Group’s results of operations in the years ended December 31, 2013 and December 31, 2012. Financial year ended December 31 2012 restated in line with IFRS 5 CONSOLIDATED STATEMENT OF INCOME (in millions of euros except for %) 2013 Revenue 544.2 99.4% 511.4 99.4% 32.8 6% 3.1 0.6% 2.9 0.6% 0.3 10% 547.3 100.0% 514.2 100.0% 33.1 6% Cost of sales (122.8) -22.4% (111.3) -21.7% (11.4) 10% Payroll related costs (232.5) -42.5% (219.6) -42.7% (12.8) 5.8% Other income Total Revenue Share based payments % % Difference % (0.3) -0.1% (0.6) -0.1% 0.2 -40% Other operating expenses Transaction costs for usual small size acquisitions EBITDA (84.5) -15.4% (77.7) -15.1% (6.8) 9% (1.0) -0.2% (1.5) -0.3% 0.6 -36% 106.3 19.4% 103.4 20.1% 2.8 3% EBITDA margin (EBITDA/Revenue) Depreciation, amortization, impairment losses, provisions and reversals Results from operating activities before non-recurring items Non-recurring income and expenses Results from operating activities after non-recurring items Net finance costs Income tax expenses Share of profit of associates 19.4% 20.1% (19.0) -3.5% (17.6) -3.4% (1.4) 8% 87.3 15.9% 85.8 16.7% 1.4 2% 0.3 0.0% (6.5) -1.3% 6.8 n/a 87.5 16.0% 79.3 15.4% 8.2 10% (59.1) (21.1) (1.3) -10.8% -3.9% -0.2% (53.8) (18.6) (0.1) -10.5% -3.6% 0.0% (5.3) (2.6) (1.2) 10% 14% n/a 136 Net profit from continuing operations Net profit from discontinued operations Net profit for the period Profit attributable to non-controlling interests Profit attributable to owners of the company 9.3.2 6.0 6.8 12.8 1.1% 6.9 (35.0) (28.1) 1.3% (0.9) 41.8 40.9 n/a n/a n/a 0.1 0.0% 0.4 0.1% (0.3) -74% 12.7 2.3% (28.5) -5.5% 41.2 n/a Revenue The Group’s consolidated Revenue increased by €33.1 million, or 6.4%, to €547.3 million in the financial year ended December 31, 2013 from €514.2 million in the financial year ended December 31, 2012. This rise was chiefly attributable to the full-year contribution of the acquisitions completed in 2012 and, to a lesser extent, the effect of the acquisitions made in 2013, the organic growth in volumes and the favorable Mix Effect in most of the countries in which the Group is present. The increase was partially offset by a challenging economic environment in Iberia and price reductions in most countries where the Group is present and especially in France and Iberia. The acquisitions completed in 2013, primarily in France, contributed €11.7 million to Revenue in the financial year ended December 31, 2013. The following table shows a breakdown of Revenue by country in value and stated as a percentage of total Revenue as recorded in the financial years ended December 31, 2013 and December 31, 2012, respectively, as well as an analysis of the increase in Revenue between organic growth (at comparable perimeter) and growth including changes in the scope of consolidation (at current perimeter) for each segment and for the Group as a whole. Financial year ended December 31 % variation Revenue (in millions of euros except for %) 2013 % 2012 restated in line with IFRS 5 % Unadjusted Northern Europe Constant scope 357.9 65% 328.9 64% 8.8% 1.8% France 325.3 59% 301.8 59% 7.8% -0.5% Belgium 27.2 5% 25.9 5% 4.8% 4.8% United Kingdom 4.4 1% 1.3 0% 253.8% 62.6% Switzerland 0.9 0% 0.0 0% n/a n/a 189.4 35% 185.3 36% 2.2% 1.9% Iberian Peninsula 151.2 28% 149.4 29% 1.2% 0.4% Italy 38.2 7% 35.8 7% 6.7% 8.0% 547.3 100% 514.2 100% 6.4% 1.8% Southern Europe Total 9.3.2.1 Northern Europe The Northern Europe segment’s Revenue progressed by €28.9 million, or 8.8% on a reported basis, to €357.9 million in the financial year ended December 31, 2013 from €328.9 million in the financial year ended December 31, 2012. Revenue growth flowed predominantly from acquisitions, especially in France during 2012 and to a lesser extent in 2013. Revenue growth also reflected organic growth, which reached 1.8% during the period. Revenue in France increased by €23.6 million, or 7.8%, to €325.4 million in the financial year ended December 31, 2013 from €301.8 million in the financial year ended December 31, 2012. This rise was chiefly attributable to the full-year contribution of the acquisitions completed in 2012, the organic growth in volumes and the favorable Mix Effect. To a lesser extent, it was due to the prorata contribution from acquisitions carried out in 2013 and the positive effect of the change in accounting treatment of outsourced specialty testing services following regulatory changes in France in February 2012. That change in the accounting treatment means that the income and expenses generated by outsourced specialty testing services are recognized respectively under Revenue and cost of sales. Those effects were offset by the annual reduction in prices applied by the French Health Authority (Autorité Française de Santé) and the lower number of business days in 2013. The Group made eight acquisitions during the financial year ended December 31, 2013, and the acquisition-driven Revenue increase came to €6.8 million in 2013. The full-year contribution made by businesses acquired in 2012 totaled 137 €18.4 million. The organic contraction came to 0.5%, reflecting the impact of the annual adjustment to the prices applied by the French Health Authority (Autorité Française de Santé) of around 2.3% in April 2013, offset by volume growth and the favorable Mix Effect. Revenue in Belgium increased by €1.3 million, or 4.8%, to €27.2 million in the financial year ended December 31, 2013 from €25.9 million in the financial year ended December 31, 2012, owing chiefly to expansion in nutritional testing and, to a lesser extent, in routine testing. Revenue in the United Kingdom rose by €3.2 million to €4.4 million in the financial year ended December 31, 2013 from €1.3 million in the financial year ended December 31, 2012. Following the buyout of Sodexo’s interest in iPP on October 25, 2013, the Group has held full control of iPP. Accordingly, iPP has been fully consolidated since that date and contributed Revenue of €2.8 million (iPP recognized Revenue of €16.0 million over the full year). Labco UK, which manages the subcontracting contract for the Fresenius group’s clinical diagnostic testing business, posted a top-line increase owing mainly to higher volumes. Revenue in Switzerland came to €0.9 million, reflecting the clinical laboratory testing business generated by Test SA, a 48%-held subsidiary of the Group founded in early 2013 by laboratory doctors, but fully consolidated by the Group as a result of the analysis of the control it exercises. 9.3.2.2 Southern Europe The Southern Europe segment’s Revenue improved by €4.2 million, or 2.2%, to €189.4 million in the financial year ended December 31, 2013 from €185.3 million in the financial year ended December 31, 2012. Revenue growth mainly reflects organic growth, which reached 1.9% during the period. Revenue in the Iberian Peninsula increased by €1.8 million, or 1.2%, to €151.2 million in the financial year ended December 31, 2013 from €149.4 million in the financial year ended December 31, 2012. The Group made two acquisitions in the Iberian Peninsula during the financial year ended December 31, 2013, and the businesses acquired in 2013 generated a Revenue increase of €1.1 million, while no acquisitions took place in 2012. Organic growth ran at 0.4%, reflecting the volume growth in Spain and Portugal in routine clinical testing and the strong expansion in specialty testing, partially offset by the impact of the price reductions in Spain and prices reductions in Portugal. This growth was chiefly fuelled by business growth in Spain with the increase in volumes and in particular the strong expansion in specialty testing (especially non-invasive genetic testing for Down’s syndrome), partially offset by price reductions in the Spanish hospital and insurance business, as well as prices pressure and the unfavorable currency effect impacting the Group’s activities in Brazil. Business trends in Portugal remained stable with higher volumes for hospital laboratories offset by the unfavorable impact of a reduction in the price of certain tests from August 2012 and a contraction in the day surgery business. Revenue in Italy grew by €2.4 million, or 6.7%, to €38.3 million in the financial year ended December 31, 2013 from €35.8 million in the financial year ended December 31, 2012, mainly on the back of the volume growth generated by the ramp-up in the Monza medical center, combined with the opening of new Sampling Centers. In addition, the Group sold the Centro Diagnostico Missori entity in June 2012. 9.3.3 Cost of sales The Group’s cost of sales increased by €11.4 million, or 10.3%, to €122.8 million in the financial year ended December 31, 2013 from €111.3 million in the financial year ended December 31, 2012. The cost of sales stated as a percentage of Revenue came to 22.4% in 2013, compared with 21.7% in 2012. The rise in the cost of sales derived mainly from the full-year contribution of the acquisitions made in 2012, chiefly in France, from the impact of the acquisitions made in 2013 and in particular the full consolidation of iPP, and the higher cost of sales in the Iberian Peninsula as a result of the development of specialty testing. The increase in the cost of sales as a percentage of Revenue was mainly attributable to the impact of the expansion of the specialty testing business in the Iberian Peninsula. Genetic testing for Down’s syndrome is outsourced, and so it gives rise to a thinner gross margin, but has no material impact on the EBITDA margin. This rise was also attributable to the unfavorable impact on Revenue of the price reductions in France and the Iberian Peninsula, offset partially by the positive full-year impact of the renegotiations of the commercial terms secured on the Group’s reagent purchases. 138 9.3.4 Payroll related expenses Payroll related expenses increased by €12.8 million, or 5.8%, to €232.5 million in the financial year ended December 31, 2013 from €219.6 million in the financial year ended December 31, 2012. Payroll related expenses stated as a percentage of Revenue came to 42.5% in 2013, compared with 42.7% in 2012. This rise was chiefly attributable to the full-year contribution made by the businesses acquired in 2012, mainly in France, to the increase in mandatory employee profit-sharing and incentive plans (including the related social security charges) in France as a result of the mergers completed, to the impact of the higher payroll charges introduced by the French government and the impact of the businesses acquired in 2013, including the switch to full consolidation of iPP. The increase was partially offset by efficiency gains, which paved the way for reductions in the operational workforce, the impact of the restructuring and efficiency plans implemented in Spain, Portugal and France and the impact of the French CICE (competitiveness tax credit) during its first year in force in France. The decline in the ratio of payroll-related expenses to Revenue was attributable to efficiency gains, especially in Italy and Belgium. 9.3.5 Share based payments Expenses linked to share based payments (BSA warrants and free shares) decreased by €0.2 million from €0.6 million in 2012 to €0.3 million in 2013. The IFRS 2 expense in 2013 solely reflects the non-cash expense calculated annually in respect of the 2010 BSA plan adjusted following the departure of certain beneficiaries, while the 2012 figures include the pro rata temporis non-cash expense in respect of the 2011 free share plan for the first quarter of 2012. The 2011 free share plan was cancelled in the second quarter of 2012, leading to the residual expense under IFRS 2 of €1.6 million initially intended to be spread over the vesting period of the rights to be recognized immediately in the consolidated income statement as a non-recurring expense. 9.3.6 Other operating expenses Other operating expenses increased by €6.8 million, or 8.7%, to €84.5 million in the financial year ended December 31, 2013 from €77.7 million in the financial year ended December 31, 2012, owing chiefly to the full-year contribution made by businesses acquired in 2012 (mainly in France), the impact of acquisitions in 2013, in particular with the full consolidation of iPP and rise in real estate rental costs in France, Italy and Belgium with the opening of new sites and the contractual increase in the rental index, offset partially by a reduction in equipment leasing costs and cleaning expenses in the Iberian Peninsula as a result of the restructuring. Other operating expenses stated as a percentage of Revenue came to 15.4% in 2013, compared with 15.1% in 2012. The key factors contributing to this increase were the impact of the full consolidation for the first time of iPP, as it has a higher ratio of operating expenses to Revenue than the Group has in its other countries. 9.3.7 Transaction costs for usual small size acquisitions Transaction costs for usual small size acquisitions decreased by €0.6 million from €1.5 million in 2012 to €1.0 million in 2013 because the Group made fewer and smaller acquisitions in 2013 than in 2012. 9.3.8 EBITDA EBITDA increased by €2.8 million, or 2.8%, to €106.3 million in the financial year ended December 31, 2013 from €103.4 million in the financial year ended December 31, 2012. This increase flowed mainly from the fullyear contribution made by the businesses acquired in 2012 largely in France, offset partially by the unfavorable impact of prices reductions in France and pricing pressure in the Iberian Peninsula, and by the first year of consolidation of iPP and TEST SA, which generated negative EBITDA in their start-up phases. Consolidated EBITDA stated as a percentage of Revenue (EBITDA margin) declined from 20.1% to 19.4% (see the segmental analysis below). The following table shows EBITDA by segment over the indicated periods and also stated as a percentage of each segment’s Revenue. 139 Financial year ended December 31 EBITDA (in millions of euros except for %) Northern Europe As a % of consolidated Revenue Southern Europe As a % of consolidated Revenue Total As a % of consolidated Revenue 9.3.8.1 2013 75.5 % 71% 21.1% 30.8 71.7 % % change 69% 5.2% 31% -2.9% 100% 2.8% 21.8% 29% 16.2% 106.3 2012 restated in line with IFRS 5 31.7 17.1% 100% 19.4% 103.4 20.1% Northern Europe The Northern Europe segment’s EBITDA increased by €3.8 million, or 5.2%, to €75.5 million in the financial year ended December 31, 2013 from €71.7 million in the financial year ended December 31, 2012. This increase flowed mainly from the full-year contribution made by the businesses acquired in 2012 largely in France, offset partially by the unfavorable impact of prices reductions in France. The iPP subsidiary, which handles outsourced clinical laboratory testing for certain NHS trusts and is set to incur losses in the first few years of the contracts while the outsourced operations are restructured to achieve the expected efficiency, contributed negative EBITDA of €0.25 million to the Group’s results. Likewise, the clinical laboratory testing business established in Switzerland during the first half of 2013 gave rise to negative EBITDA during the start-up phase. The EBITDA of Northern Europe stated as a percentage of Revenue edged down from 21.8% to 21.1%. Restated for the impact of the Swiss laboratory Test SA and the iPP subsidiary, the EBITDA margin of Northern Europe came to 21.5% in 2013, down 0.3 points. This decline was chiefly attributable to the impact of the price reductions in France, which were not fully offset by commercial renegotiations with certain suppliers and immediate effects of the cost efficiency measures taken in France (concentration of laboratories through mergers or creations of technical platforms, Deep Dive plan optimizing the structure of the workforce). 9.3.8.2 Southern Europe The Southern Europe segment’s EBITDA declined by €0.9 million, or 2.9%, to €30.8 million in the financial year ended December 31, 2013 from €31.7 million in the financial year ended December 31, 2012. This decline was chiefly attributable to the impact of pricing pressure in the Iberian Peninsula and in particular price reductions in the Spanish hospital and insurance business and the impact of price reductions for certain tests in Portugal, offset partially by the impact of the restructuring in the Iberian Peninsula and the expansion of the specialty clinical laboratory testing business, especially certain genetic tests, and the firm performance of the businesses in Italy. The EBITDA of Southern Europe stated as a percentage of Revenue went down from 17.1% to 16.2% reflecting the impact of the weaker performance by operations in the Iberian Peninsula. 9.3.9 Depreciation, amortization, impairment losses, provisions and reversals Depreciation, amortization, impairment losses, provisions and reversals increased by €1.4 million, or 8.0%, to €19.0 million in the financial year ended December 31, 2013 from €17.6 million in the financial year ended December 31, 2012. This increase was chiefly attributable to the higher depreciation and amortization expense given the impact of acquisitions and the specific effect in 2012 of a reversal of a provision covering the dispute with a French laboratory doctor, while the amount paid out (€0.3 million) was recognized as a non-recurring expense. 9.3.10 Non-recurring income and expenses Non-recurring income and expenses showed net recurring income totaling €0.3 million in the financial year ended December 31, 2013 compared with €6.5 million in net expense in the financial year ended December 31, 2012. 140 Net non-recurring expense in the financial year ended December 31, 2013 primarily reflected €1.8 million in costs incurred for strategic projects, costs of departures and provisions for restructuring totaling €0.7 million covering the outstanding measures in the 2011 restructuring plan implemented in the Iberian Peninsula with the reversal of the corresponding provisions, €0.8 million in severance payments deriving from the measures in the “Deep Dive” efficiency plan implemented in France during 2013, €0.8 million in provisions for restructuring set aside in the first quarter of 2013 for the new restructuring plan implemented in connection with contractual renegotiations with a major customer and use of €0.5 million of the provision during the last nine months of 2013. These items were partially offset by the impact of the step-up acquisition of iPP with a €0.8 million income from the bad will arising on the acquisition and also a €3.4 million non-recurring gain on the disposal of a fixed asset reflecting the Group’s original 51% interest in iPP previously accounted for as an associate and its re-measurement at fair value at the date of the acquisition. Non-recurring expenses in the financial year ended December 31, 2012 primarily comprised €2.8 million in costs incurred on strategic projects, €3.2 million in costs arising from employee departures and provisions for restructuring costs under the 2011 restructuring plans in Spain and Portugal and the 2012 plans in France (especially Deep Dive), with €1.9 million of the provisions used, €1.8 million in net non-recurring income owing to the settlement receivable in connection with the early termination of a clinical services contract in France net of estimated restructuring costs, €1.5 million of IFRS 2 charges reflecting the cancellation of the 2011 free share plan, and €2.7 million arising from perimeter effects in respect of changes in the fair value of earn-out payments and in particular €2.3 million in earn-out payments anticipated upon the acquisition of the CIC group (since renamed Labco NOÛS), which were recognized in expenses. 9.3.11 Net finance costs Net finance costs moved up from €53.8 million in 2012 to a total expense of €59.1 million in 2013. Net financing costs were affected proportionally by additional financial expense arising from the issue in February 2013 of a tap in a principal amount of €100 million bearing interest at the annual rate of 8.5% and due to mature in 2018 (see section 10.4.3. “High Yield Bonds” of this document de base). 9.3.12 Income tax expenses Income tax expenses rose by €2.6 million to €21.1 million in the financial year ended December 31, 2013 from €18.6 million in the financial year ended December 31, 2012. This increase was chiefly attributable to the specific impact in 2012 deriving from the capitalization of €4.2 million in deferred taxes representing a portion of the Group’s tax loss carryforwards following a review of the prospects of these tax loss carryforwards reversing over the subsequent 5-year period given changes in the Group’s projections as a result of the tax restructuring carried out coupled with the impact of the new tax legislation enacted in certain countries during 2012. Despite the impact of certain corporate restructuring measures that have led to a reduction in tax expense, the Group capitalized in 2013 a smaller amount of €0.3 million in additional deferred tax assets on tax loss carryforwards. Furthermore, it incurred the full-year impact of €0.7 million arising from the dividend tax introduced in France during the summer of 2012. The Group’s relatively high effective tax rate derived from losses, especially at certain holding companies giving rise to tax loss carryforwards that were not capitalized under deferred tax assets, from non-deductible interests and from the impact of charges not deductible for tax purposes recognized upon consolidation. These non-deductible expenses reflected to a large extent the impact of the restatement of the priority dividends paid out to French laboratory doctors, which are recognized under payroll related expenses in the consolidated financial statements but are not deductible for tax purposes because they are accounted for as dividends in the individual financial statements. 9.3.13 Share of profit of associates The share of profit of associates declined by €1.2 million to a loss of €1.3 million in 2013 from a loss of €0.1 million in 2012. This trend was chiefly attributable to the loss posted by iPP, the joint venture with Sodexo in the United Kingdom until October 25, 2013. Prior to this date, iPP’s net loss was included in the share of profit of associates line. Since the Group took full control of iPP on October 25, 2013, it has been fully consolidated. In 2012, the loss recorded by iPP, which has been in a start-up phase since June 2012 incurring restructuring costs in its first few years in activity, was offset by the positive earnings of three clinical testing laboratories in France (Brigout, Degraef Pouliquen, and Sèvre et Loire Biologies) in which the Group had a minority holding and were thus accounted for under associates. In late 2012, the Group acquired a number of shares that gave it control of these three laboratories and so they were fully consolidated in 2013. 141 9.3.14 Net profit from continuing operations Owing to the factors described above, the Group made a net profit from continuing operations of €6.0 million in 2013, compared to a net profit of €6.9 million in 2012. 9.3.15 Net profit from discontinued operations Net profit from discontinued operations reflected the net profit of the German cash-generating unit sold to Sonic Healthcare on December 2, 2013 and accounted for under discontinued operations in compliance with IFRS 5 “assets and liabilities held for sale and discontinued operations”. Net profit over the first 11 months of 2013 came to €7.6 million, which was partially offset by the consolidated capital loss of €0.8 million on disposal recorded in the financial year ended December 31, 2013, compared with a loss of €35.0 million in the financial year ended December 31, 2012, including an impairment loss of €36.0 million on the German cash-generating unit’s goodwill. 142 CHAPTER 10 CAPITAL RESOURCES 10.1 GENERAL PRESENTATION The Group’s main financing needs relate to acquisitions, investments, the working capital requirement, the repayment of borrowings and the payment of related interests. As part of its business activities, the Group’s main financing sources are as follows: • Available cash. The Group retains cash and cash equivalents to cover its ordinary financing needs. The minimum amount of cash and cash equivalents is €15 million, due to constraints arising under the covenants applying to the Group under the Amended RCF. The Group’s cash position is denominated almost entirely in euros, except for cash held by Group subsidiaries located in the UK, in Latin America and in Switzerland. Cash and cash equivalents amounted to €56.6 million for the financial year ended December 31, 2012, €167.8 million for the financial year ended December 31, 2013 and €74.1 million at December 31, 2014. The increase in cash and cash equivalents in 2013 arose mainly from the disposal of the German business for a net amount of €73 million, and from the residual proceeds, after repaying funds drawn on the RCF, of the February 2013 issue of High-Yield Bonds, fungible with previously issued bonds, in a principal amount of €100 million. • Net cash flows from operating activities. The Group’s main source of liquidity consists of cash flows from its operating activities. The Group’s ability to generate cash from operating activities going forward depends on its future operating performance which, in turn, depends on economic, financial, competition, market, regulatory and other factors, most of which are not under the Group’s control. Net cash flows from operating activities totaled €90.2 million for the financial year ended 2012, €93.0 million for the financial year ended 2013 and €85.7 million for the financial year ended 2014 (see cash flow statement included in the audited Group consolidated financial statements in section 20.1.1. “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” below); and • Debt. The Group refinanced its debt in 2011 by (i) issuing bonds with a principal amount of €500 million and maturing in 2018 (the High-Yield Bonds described in section 10.4.3. “High Yield Bonds” of this document de base) and (ii) arranging a revolving credit facility, which had an initial amount of €125 million, subsequently increased to €135 million, and an amount of €128.25 million following the amendment signed in December 2014. In early 2013, the Group carried out a tap, an additional issue of bonds fungible with the existing HighYield Bonds in a principal amount of €100 million. The proceeds were used to repay amounts drawn on the RCF (for a description of those transactions, please see Note 5.2 to the Group’s audited consolidated financial statements for the period ended December 31, 2013 included in section 20.1.1 – “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” of this document de base). The revolving credit facility arranged in 2011 was restructured in December 2014 as part of the signature of a new RCF contract in an amount of €128.5 million (the Amended RCF as described in section 10.4.4. “RCF” of this document de base). At December 31, 2014, debt consisted mainly of the High-Yield Bonds in a principal amount of €600 million, drawings on the Amended RCF – which had a principal amount of €75 million – along with finance leases and some residual bank loans (see Note 23 to the Group’s consolidated financial statements included in section 20.1.1. “IFRS financial statements for the financial years ended December 31, 2012, 2013 and 2014” of this document de base and the description of the High-Yield Bonds, of the RCF and of the Amended RCF in section 10.4.3. “High-Yield Bonds” and section 10.4.4. “RCF” of this document de base.) On February 11, 2015, the Group carried out an issue of bonds fungible with previously issued bonds in a principal amount of €100 million, in order to repay €100 million of drawings on the Amended RCF, which amounted to €108 million at January 31, 2015. As described in section 13.1.2 “Group forecasts for the financial year ending December 31, 2015” of this document de base and based on updated treasury forecasts, the Group’s management believes that the Group will be able to cover its liquidity requirements in the twelve months following the date of the registration of this document de base, and to cover interest payments and debt repayments over the same period. 143 10.2 PRESENTATION AND ANALYSIS OF THE MAIN WAYS IN WHICH THE GROUP USES CASH 10.2.1 Acquisitions Acquisitions consist of purchases of groups of companies, companies and/or businesses for consideration, net of cash acquired and the payment, in certain cases, of fixed or conditional earn-outs, which can be sub-divided as follows: bolt-on acquisitions made in regions where the existence of the Group’s technical platforms allows industrial synergies to be harnessed rapidly; acquisitions that extend territorial coverage, mainly the acquisition of platforms in new territories from which the Group can apply its strategy of consolidating smaller operators; acquisitions of medical expertise designed to give the Group additional scientific and technological capacities. For the financial years ended December 31, 2012, 2013 and 2014, the Group carried out respectively sixteen, eleven and sixteen acquisitions of groups of companies, companies or the business assets of small and mediumsized laboratories for a total purchase price, net of cash acquired (and excluding earn-out payments) of €45.2 million in 2012, €20.4 million in 2013 and €130.2 million in 2014, including €107.6 million for the strategic acquisition of the SDN group on July 30, 2014. In addition, the Group made fixed or performance-related earnout payments in respect of the aforementioned acquisitions or those carried out previously. The Group’s liability for earn-out payments, estimated at fair value, amounted to €11.5 million at December 31, 2014. 10.2.2 Investments The Group’s investments fall into the following categories: Purchases of laboratory materials and devices; Purchases of information systems: hardware, software, licenses and IT developments; and Purchases of fixtures and fittings in rented premises and, in some rare cases, real-estate transactions. The Group’s investment expenditure net of disposal effects totaled €15.4 million in 2012, €18.7 million in 2013 and €35.5 million in 2014 (see section 5.2 – “Investments” of this document de base, which explains past, current and future investment expenditure). 10.2.3 Payment of interests and repayment of debts A large proportion of the Group’s cash flow goes on servicing (paying interests) and repaying debts. The Group paid interests and financial expenses of €51.1 million in 2012, €52.0 million in 2013 and €57.9 million in 2014. It also paid (after offsetting transactions involving the revolving credit facility (RCF)) €10.6 million to repay borrowings in 2012, €9.2 million in 2013 and €4.8 million in 2014. It paid €6.7 million to repay finance-lease debt in 2012, €6.0 million in 2013 and €6.9 million in 2014. 10.2.4 Financing the working capital requirement The working capital requirement mainly consists of the value of trade receivables and other operational receivables, plus inventories and minus trade payables and other operating payables. Structurally, the Group’s working capital requirement reflects its business models in each geographical zone in which it operates, and is structurally negative in France and the UK. 144 10.3 THE COMPANY’S CONSOLIDATED CASH FLOWS 10.3.1 A business model that generates large amounts of cash The Group’s business model is characterized by its ability to generate large amounts of cash, due in particular to: high operating margins; low capital intensity, with investments relating mainly to fitting out rented premises and buying hightech testing equipment. Suppliers of chemical reagents for clinical tests currently use a model in which they provide machines or rent out equipment under comprehensive contracts covering the leasing of equipment, the supply of chemical reagents and maintenance services; a structurally negative working capital requirement in certain countries in which the Group operates, due in particular to very rapid collection of receivables. The working capital requirement is structurally negative in France and the UK for the following reasons: receivables are collected quickly from public-sector payers. For example, Health Insurance Bodies (Caisses d’Assurance Maladie) in France pay within 15 days of reimbursement requests being sent; very low inventory levels, partly because certain reagent suppliers bill on the basis of reported billable tests, which means that suppliers retain ownership of their inventories and have incurred the calibration work costs; the usual payment terms in force in the European Union for purchases of goods, consumables and services. In addition, the EBITDA/cash flow conversion rate is very high, and was over 80% in the periods under review. The EBITDA/cash flow conversion ratio is calculated with – a numerator of: o cash flow from (used in) operating activities restated for discontinued activities; o plus flows from (used in) non-recurring charges; o minus net cash flows from acquisitions and disposals of tangible and intangible assets; o plus income tax paid; and – EBITDA as denominator. This ratio was 93% in 2012, 91% in 2013 and 83% in 2014. For the financial year ended December 31, 2014, the restated ratio for exceptional property, plant and equipment acquisitions related to real estate projects of €15.3 million, amounted to 96%. 10.3.2 Group cash flows in the financial years ended December 31, 2013 and 2014 The following table summarizes the Group’s cash flows for the years ended December 31, 2013 and 2014 as published. It therefore includes, for the financial year ended December 31, 2013, data relating to the German business that was sold in late 2013, prompting the presentation pursuant to IFRS 5 of cash flows relating to the divested German entities in specific line items relating to discontinued activities. 145 Financial year ended December 31 Cash flow 2014 (in millions of euros except for %) 2013 Cash flows from operating activities 85.7 Cash flows from investing activities (163.1) 27.7 Cash flows from financing activities (16.0) (9.3) Net increase/(decrease) in cash and cash equivalents (93.5) 111.4 10.3.3 93.0 Group cash flows from operating activities in the financial years ended December 31, 2013 and 2014 The table below shows the Group’s cash flows from operating activities in the financial years ended December 31, 2013 and December 31, 2014. Financial year ended December 31 Cash flow 2014 (in millions of euros except for %) EBITDA 2013 113.2 Change in working capital requirement 106.3 14.3 7.3 Income tax paid (29.3) (24.5) Net cash used in non-recurring expenditure (14.3) (5.6) 1.7 1.4 85.7 93.0 Other cash flows Cash flows from operating activities in relation to discontinued operations 8.0 Net cash flows from operating activities Total cash flows from operating activities fell €7.3 million or 7.9% to €85.7 million in 2014, as opposed to €93.0 million in 2013, including €8.0 million of cash flows from discontinued operations. The decline is the result of cash flows from discontinued operations, which amounted to €8.0 million in 2013 and consisted of non-recurring income arising from the settlement of the litigation with former shareholders of the Dillenburg laboratory. Adjusted for discontinued operations, net cash flows from operating activities increased, mainly because of a €7.0 million rise in EBITDA and a positive contribution of €7.0 million from the change in the working capital requirement deriving mainly from the specific 2014 effect of the seasonality of the SDN given the acquisition date on July 30, 2014 and, to a lesser extent, the impact of non-recurring elements described in Section 9.2.10 “Non-recurring income and expenses” of this document de base, partly offset by a €8.7 million increase in cash used in non-recurring expenses, relating in particular to the purchase of priority dividend rights from certain French laboratory doctors, and a €4.8 million increase in income tax paid due to tax paid by the SDN group, which was acquired on July 30, 2014 but whose annual tax was paid in the second half of 2014. 10.3.4 Group cash flows from investing activities for the financial years ended December 31, 2013 and 2014 The table below shows the Group’s cash flows from investing activities in the financial years ended December 31, 2013 and December 31, 2014. Financial year ended December 31 Cash flow 2014 (in millions of euros except for %) Purchases and disposals of property, plant and equipment and intangible assets Purchases of investments net of cash acquired and changes in debt relating to acquisition Net decrease (increase) in other assets 2013 (35.5) (125.2) (2.4) Cash flows from investing activities in relation to discontinued operations Cash flows from (used in) investing activities (20.2) 73.1 (6.5) (163.1) 146 (18.7) 27.7 Total cash flows from investing activities went from a €27.7 million inflow in 2013, including €6.5 million relating to discontinued operations, to a €163.1 million outflow in 2014. This change was mainly due to the strategic acquisition of the SDN group on July 30, 2014, whereas in 203, the Group had sold its German activities for an amount, net of sale fees, of €73 million. Cash used in acquisitions of tangible and intangible assets, net of disposals, increased from €18.7 million in 2013 to €35.5 million in 2014, due in particular to realestate investments in Barcelona, in Brussels relating to the acquisition of a laboratory and in the United Kingdom relating to the new platform for the new Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust contract, along with, to a lesser extent, Group investments in information systems. 10.3.5 Group cash flows from financing activities in the financial years ended December 31, 2013 and 2014 The table below shows the Group’s cash flows from financing activities in the financial years ended December 31, 2013 and December 31, 2014. Financial year ended December 31 Cash flow 2014 (in millions of euros except for %) 2013 Proceeds from share capital increase (19.3) 0.0 Net cash from (used in) net financial profit (loss) (57.9) (52.0) New borrowings and other financial liabilities 429.8 192.4 Repayment of borrowings and other financial liabilities (361.8) (142.2) Repayment of finance lease liabilities (6.9) (6.0) Dividends paid to minority interests in fully consolidated companies (0.0) (0.1) Cash flows from (used in) financing activities of discontinued operations Cash flows from (used in) financing activities (1.5) (16.0) (9.3) N.B.: cash flows from new borrowings and the repayment of borrowings include cash flows arising from amounts drawn and repaid on the revolving credit facility, either for very short periods for cash management purposes, or for longer periods. Cash flows used in financing activities totaled €16.0 million of outflow in 2014 because the Group used €57.9 million of net cash on financial expenses, €6.9 million on repaying finance-lease debt and a net €4.2 million on repaying other debts. In addition, the Group distributed share premiums in an amount of €21.9 million to shareholders in the first half of 2014, of which it paid €21.5 million on December 31, 2014, and carried out a €2.2 million capital increase reserved for laboratory doctors who had sold their laboratories to the Group (see section 21.1.7 – “Changes in the share capital over the past three financial years”). The Group also drew a net €75 million on its revolving credit facility, particularly in order to finance the acquisition of the SDN group, and incurred €2.8 million of issuance costs when renegotiating the Amended RCF on December 18, 2014. Cash flows used in financing activities totaled €9.3 million of outflow for the financial year 2013 because the Group used €1.5 million of net cash on the financing of discontinued operations, €52.0 million on financial expenses and €6.0 million on repaying finance-lease debt. In 2013, the Group issued a tap, i.e. additional bonds in a principal amount of €100 million, and at that time repaid the revolving credit facility in a net amount of €41.0 million and other debts in an amount of €8.8 million. 10.3.6 Group cash flows in the financial years ended December 31, 2012 and 2013 The business in Germany was sold to Sonic Healthcare on December 2, 2013, with economic effect from November 30, 2013 (see section 9.3.1 – “Analysis of the results of operations for the financial years ended December 31, 2013 and December 31, 2012 – Overview” of this document de base). As a result, in compliance with IFRS 5, the German cash-generating unit was treated as a discontinued operation from September 30, 2013 in the consolidated statement of income, statement of financial position and statement of cash flows. From a methodological point of view, in accordance with the presentation required under IFRS 5, discontinued operations are included in the Group’s scope of consolidation in the comparative figures for the six months ended December 31, 2012, but the comparative cash-flow statement figures relating to discontinued operations 147 have been adjusted to include those operations in specific “discontinued operations” line items, instead of within each accounting caption. The following table summarizes the Group’s cash flows for the financial years ended December 31, 2012 and 2013. Financial year ended December 31 Cash flow 2012 restated in line with IFRS 5 2013 (in millions of euros except for %) Cash flows from (used in) operating activities 93.0 90.2 Cash flows from (used in) investing activities 27.7 (60.0) (9.3) (41.8) 111.4 (11.6) Cash flows from (used in) financing activities Net increase/(decrease) in cash and cash equivalents 10.3.7 Cash flows from operating activities The table below shows the Group’s cash flows from operating activities in the financial years ended December 31, 2012 and December 31, 2013. Financial year ended December 31 Cash flow 2012 restated in line with IFRS 5 2013 (in millions of euros except for %) EBITDA 106.3 Change in working capital requirement Income tax paid Net cash from (used) in non-recurring expenditure 103.4 7.3 5.0 (24.5) (19.6) (5.6) (6.7) Other cash flows 1.4 1.6 Cash flows from (used in) operating activities of discontinued operations 8.0 6.5 93.0 90.2 Net cash flows from operating activities Total cash flows from operating activities increased €2.8 million or 3.1% to €93.0 million in 2013, including €8.0 million of cash flows from discontinued operations, as opposed to €90.2 million in 2012, including €6.5 million of cash flows from discontinued operations. The increase mainly resulted from a €2.8 million rise in EBITDA from continuing operations, a positive contribution of €2.4 million from a reduction in the working capital requirement, a €1.1 million reduction in cash used in non-recurring expenses and a €1.5 million increase in cash flow generated by discontinued operations in Germany, partly offset by a €4.8 million increase in income tax paid. The higher amount of tax paid related mainly to France, because of catchup in down payments made in 2013 on the basis of 2012 tax, and the balancing tax payment for 2012, whereas in 2012, down payments were low since they were based on the 2011 tax, which was sharply reduced by non-recurring expenses arising from the 2011 refinancing. 10.3.8 Cash flows from investing activities The table below shows the Group’s cash flows from (used in) investing activities in the financial years ended December 31, 2012 and December 31, 2013. 148 Financial year ended December 31 Cash flow 2012 restated in line with IFRS 5 2013 (in millions of euros except for %) Purchases and disposals of property, plant and equipment and intangible assets (18.7) (14.0) Purchases of investments, net of cash acquired and changes in debt related to acquisitions (20.2) (44.5) Net Decrease (increase) in other assets 73.1 (0.1) Cash flows from (used in) investing activities of discontinued operations (6.5) (1.3) Cash flows from (used in) investing activities 27.7 (60.0) Total cash flows from (used in) investing activities went from a €60.0 million outflow in 2012 to a €27.7 million inflow in 2013. The improvement resulted mainly from the €73.0 million of net proceeds received in 2013, net of costs to sell, from the disposal of German entities. In addition, cash used to acquire investments, net of cash acquired and changes in debt related to acquisitions, fell from €44.5 million in 2012 to €20.2 million in 2013, since acquisitions were less numerous and of a smaller size in 2013 than in 2012. Cash used to acquire tangible and intangible assets rose from €14.0 million in 2012 to €18.7 million in 2013. 10.3.9 Cash flows from financing activities The table below shows the Group’s cash flows from (used in) financing activities in the financial years ended December 31, 2012 and December 31, 2013. Financial year ended December 31 Cash flow 2012 restated in line with IFRS 5 2013 (in millions of euros except for %) Proceeds from share capital increase Net cash from (used in) net financial profit (loss) New borrowings and other financial liabilities 0.0 27.4 (52.0) (50.6) 192.4 616.4 (142.2) (627.1) Repayment of finance lease liabilities (6.0) (6.0) Dividends paid to minority interests in fully consolidated companies (0.1) (0.1) Cash flows from (used in) financing activities of discontinued operations (1.5) (1.7) Cash flows from (used in) financing activities (9.3) (60.0) Repayment of borrowings and other financial liabilities N.B.: cash flows from new borrowings and the repayment of borrowings include cash flows arising from amounts drawn and repaid on the revolving credit facility, either for very short periods for cash management purposes, or for longer periods. Cash flows used in financing activities totaled €9.3 million in 2013 because the Group used €1.5 million of net cash on the financing of discontinued operations, €52.0 million on financial expenses and €6.0 million on repaying finance-lease debt. In 2013, the Group issued a tap, i.e. additional bonds in a principal amount of €100 million, and as a result repaid the revolving credit facility in a net amount of €41.0 million and other debts totaling €8.8 million. In 2012, cash flows used in financing activities totaled €41.8 million because the Group used €1.7 million of net cash on the financing of discontinued operations, €50.6 million on financial expenses and €6.0 million on repaying finance-lease debt. The Group also repaid the revolving credit facility in a net amount of €7.0 million and other debts totaling €3.9 million. In addition, the Group carried out several capital increases in 2012 totaling €27.4 million (net of related costs). 10.4 EQUITY AND FINANCIAL DEBT 10.4.1 Group equity The Group’s equity attributable to equity holders of the parent was €143.1 million at December 31, 2014, €177.9 million at December 31, 2013 and €165.3 million at December 31, 2012. Changes in equity during that 149 period were mainly the result of (i) changes in the Group’s net profit, as described in section 9.2.15 – “Net profit” and section 9.3.15.“Net profit from discontinued operations” of this document de base, as well as (ii) share capital transactions during the concerned period, mainly capital increases, including the March 28, 2012 capital increase with preferential subscription rights maintained in a nominal amount of €24.7 million and (iii) the distribution of share premiums to Company shareholders on March 17, 2014 in an amount of €21.9 million. 10.4.2 Financial liabilities The Group’s financial liabilities amounted to €580.6 million at December 31, 2012, €649.7 million at December 31, 2013 and €724.5 million at December 31, 2014. The increase in gross debt during that period was mainly due to financing requirements arising from acquisitions. The table below breaks down the Group’s gross debt on the dates indicated: Financial year ended December 31 Cash flow 2014 2013 2012 594.8 593.1 491.0 RCF Syndicated loans at effective interest rate 72.3 (4.5) 35.1 Secured bank loans at effective interest rate 13.6 13.9 15.9 Accrued interests on the Senior Secured Bonds 23.5 23.5 19.5 Finance Lease liabilities 19.4 22.4 16.7 Other financial loans 0.9 1.2 2.4 724.5 649.7 580.6 (in millions of euros except for %) 8.5% Senior Secured Bonds at effective interest rate 1 2 Total financial liabilities 1 Net of capitalized issuance costs in accordance with IAS 39 2 Idem (*) On February 11, 2015, the Company issued additional 8,5% Notes due 2018 for an aggregate nominal of €100 million. Consequently to this issuance of €100 million, the total aggregate amount of 8,5% Notes due 2018 is €700 million. For a breakdown of non-derivative financial liabilities at December 31, 2014 by contractual maturity date, please see section 4.5.4 “Liquidity risk” of this document de base. 10.4.3 High Yield Bonds The Company issued bonds in a principal amount of €500 million on January 24, 2011, and two taps, i.e. additional bonds fungible with those bonds on February 13, 2013 and February 11, 2015, which took the total principal amount of the High-Yield Bonds to €700 million (together the High-Yield Bonds) (see section 10.1 – “ General Presentation” of this document de base). The High-Yield Bonds bear interest at an annual rate of 8.5% and are due for redemption on January 15, 2018. Interest on the High-Yield Bonds is payable every six months, on January 15 and July 15 of each year. The Group used the proceeds from the High-Yield Bonds to redeem part of its existing debt (mezzanine debt and syndicated loans). It also used the proceeds from the tap to repay all drawings on the Amended RCF (€67 million). The High Yield Bonds are listed for trading on the Global Exchange Market of the Irish Stock Exchange (organized multilateral trading facility within the meaning of European Parliament and Council Directive 2004/39/EC of April 21, 2004 as amended). From January 15, 2014 and subject to a notice period of at least 30 days but not more than 60 days before the proposed redemption date, the Company may redeem some or all of the High Yield Bonds early, in which case each High Yield Bond may be redeemed for an amount equal to the sum of (a) its principal amount plus an early redemption premium equal to (i) 6.375% of that principal amount if the redemption takes place on or after January 15, 2014 and before January 15, 2015, (ii) 4.250% of that principal amount if the redemption takes place on or after January 15, 2015 and before January 15, 2016 and (iii) 2.125% of that principal amount if the redemption takes place on or after January 15, 2016 and before January 15, 2017, and (b) any accrued interests that are due, along with any additional amount due until the redemption date. No redemption premium shall be due in respect of voluntary early redemptions from January 15, 2017 onwards. The Company intends to redeem 150 part of the High-Yield Bonds early by using some or all of the net proceeds from the capital increase taking place as part of the Company’s IPO. If tax regulations change and impose new withholding taxes or other deductions on amounts due with respect to the High Yield Bonds or on guarantees, the Company may repurchase, subject to a notice period of at least 30 days but not more than 60 days before the redemption date, all (and not merely part) of the High Yield Bonds at par plus any accrued interest that is due, along with any additional amount due until the repurchase date. If the Company undergoes a “change of control”, defined as (i) the sale of all or substantially all of the Group’s assets, (ii) the adoption of a plan to wind up or liquidate the Company, (iii) a transaction after which a third party owns over 50% of the Company’s voting rights or (iv) a situation where persons making up a majority of the Company’s Board of directors for a period of two consecutive years cease to be members of that board, the Company shall be required to offer to repurchase the High-Yield Bonds at 101% of their nominal value (plus any accrued interest that is due and any additional amount due until the repurchase date), subject to a notice period of at least 30 days but not more than 60 days before the repurchase date. As an exception, the Company shall not be required to offer to repurchase the High Yield Bonds in the event of a “change of control” if (i) an identical proposal is made by a third party instead of the Company, in accordance with the Indenture stipulations applicable to the Company in the event of a “change of control” and buys all of the High-Yield Bonds tendered by holders of the High-Yield Bonds as a result of that offer or if (ii) a notification of redemption has been duly submitted in accordance with the Indenture provisions applicable to the aforementioned voluntary early redemptions. The Indenture provides for accelerated maturity situations, including payment default, violation of certain obligations in the Indenture, cross-default with other debts, certain bankruptcy and insolvency events, and court orders to pay sums of money. The Indenture specifies commitments towards holders of High-Yield Bonds, partly intended to limit the ability of the Company and certain Group Companies to: take out additional debt; pay dividends in excess of certain limits or carry out any other distribution; after the Company’s IPO, and subject to certain restrictions, the Company will be authorized to pay dividends up to an annual amount equal to 6% of the funds received by the Company through the placement forming part of the IPO; carry out certain payments or investments, including investments above an overall authorized amount of €20 million; provide collateral or guarantees; sell assets (except assets whose Fair Market Value is less than €5 million); in particular, it is stipulated that the proceeds from the relevant sales or disposals, subject to limited exceptions, be used to redeem the High-Yield Bonds; carry out transactions with affiliated companies; and merge or combine with other entities. Those limitations are subject to various conditions and limited exceptions. In addition, the Indenture required the Company to provide certain accounting information to holders of HighYield Bonds, including the Group’s quarterly and annual financial statements. To comply with the principle of equivalent disclosure, the Group intends to coordinate the provision of such information with the financial disclosures made to the market when the Company’s shares are listed on Euronext Paris. The High-Yield Bonds are guaranteed by the Company and by some of its subsidiaries, including Labco Midi, Bio-Alpes, Groupe Biologic, Biopar (formerly Bioval), Biologistes Associés Regroupant des Laboratoires d’Analyses, Laboratoire de Biologie Médicale Delaporte, Biofrance, Biopaj, Laboratoire Bioliance, Bioalliance, 151 Laboratoire d’Analyses de Biologie Médicale Christine Pépin – Philippe Leluan – Patricia Sannier – Didier Guillo, Norden, Novabio Diagnostics, Normabio, Labco Finance, Laboratoire d’Analyses Médicales Roman Païs, Oxabio, Centre Biologique, Institut de Biologie Clinique, Labco Italia S.r.l., C.A.M. Centro Analisi Monza S.p.A., Istituto il Baluardo S.p.A., Laboratório Médico Dr. David Santos Pinto e Dr. Fernando Teixeira, SA, Flaviano Gusmao S.A., General Lab Portugal S.A., Gnóstica – Laboratório de Análises Clínicas S.A., General Lab S.A.U., Labco Madrid, Labco Diagnostics España S.A., Mazarin, Isolab, Sylab, Dr. Macedo Dias Laboratório de Anatomia Patológica, SA, Labco Germany (formerly Labco Deutschland Gmbh), Axilab, BioRhône, SDN S.p.A. and Ellipsys (together the “Guarantors”). Those guarantees are subject to various limitations based on rules related to the protection of the corporate interest, rules relating to financial assistance and any other equivalent rule applicable to the companies concerned. In addition, under the Indenture, holders of the High-Yield Bonds benefit from the following first-ranking pledges granted by the Company and its subsidiaries on the securities of holding companies and certain operating entities. Those pledges relate mainly to capital securities and existing or future receivables. The list of companies whose shares are pledged is provided in section 10.4.4.5 – “Security interests and guarantees” of this document de base. The High-Yield Bonds are governed by the law of New York State. On the date of this document de base, the Company had a financial rating of B2 from Moody’s, B+ from Standard & Poor’s and B+ from Fitch, while the High-Yield Bonds had financial ratings of B3, B+ and BBrespectively. 10.4.4 RCF The Company and certain members of the Group entered into a Revolving Credit Facility Agreement, drafted in English and governed by English law, on January 21, 2011 with parties including Crédit Suisse International, Deutsche Bank AG, London Branch, UBS Limited and Natixis as Mandated Lead Arrangers, Natixis as Agent and Issuing Bank, Deutsche Bank AG, London Branch as Security Agent and Credit Suisse International, Deutsche Bank A.G., London Branch, Natixis and UBS Limited as Original Lenders (hereinafter the “RCF”). The RCF was amended for the first time on April 12, 2012. Under an Amendment and Restatement Agreement dated December 18, 2014, the RCF underwent certain other amendments. The RCF thus modified is hereinafter referred to as the “Amended RCF”. Some provisions of the Amended RCF differ according to whether or not the Company’s shares are listed following a Qualifying Offering, i.e. an offering in which the ratio of Total Net Debt on the day following the offering to EBITDA calculated on a proforma basis over the last 12 months (LTM EBITDA) on the last day of the month preceding the offer, is lower than 3.75:1, subject to compliance with the general undertaking to maintain a minimum liquidity balance of at least €15 million. The following description is a description of the Amended RCF provisions applicable once the Company’s shares are listed. The application of these provisions is subject to customary conditions, including the listing of the Company’s shares for trading on Euronext Paris and the settlement-delivery of the initial public offering transaction being duly notified to the lenders that are parties to the Amended RCF, and compliance with the aforementioned ratio conditions. 10.4.4.1 Amount, usage, term Senior Credit Facility The Amended RCF is a revolving credit facility in a maximum principal amount of €128.25 million (the “Senior Credit Facility”), which can be used in the form of multi-currency advances for terms of 1, 2, 3 or 6 months (or any other term agreed with the Agent) or in the form of offerings of letters of credit. 152 The Senior Credit Facility’s maturity date is December 15, 2017, one month before the maturity date of the High-Yield Bonds. However, if the High-Yield Bonds are refinanced or repurchased, the Senior Credit Facility’s maturity date will be postponed until the sooner of (i) February 15, 2019 and (ii) the date falling one month before the final maturity date of the instrument refinancing the High-Yield Bonds, and if the final maturity date of the High-Yield Bonds is postponed, the maturity date of the Senior Credit Facility will be postponed until the sooner of (i) February 15, 2019 and (ii) the date falling one month before the final maturity date of the High-Yield Bonds. The Senior Credit Facility may be used to (i) finance items such as Capital Expenditure, the purchase price, costs and expenses related to Permitted Acquisitions and investments in Permitted Joint Ventures, and costs and expenses related to any business restructuring in relation thereto, (ii) refinance the debts of entities acquired through Permitted Acquisitions and (iii) finance the Group’s working capital requirement and general requirements. The Senior Credit Facility may be used by several of the company’s French and foreign subsidiaries, with the joint and several guarantee of the Company and certain of the French and foreign subsidiaries that are guarantors (see paragraph 10.4.4.5 below). Additional Credit Facility The Amended RCF also provides that the Company may request an additional unconfirmed revolving credit facility (the “Additional Credit Facility”), in which lenders under the Amended RCF may choose to take part, provided that the Intercreditor Agreement is amended as required to authorize the provision of that Additional Credit Facility. The maturity date of the Additional Credit Facility may not be before the final maturity date of the Senior Credit Facility. The Additional Credit Facility will be available to finance or refinance Permitted Acquisitions or investments in Permitted Joint Ventures. Ancillary Facilities The Company and a lender under the Amended RCF may agree that the lender provides to one or more borrowers some or all of its commitment with respect to the Amended RCF in the form of an Ancillary Facility, which may only take the form of a letter of credit. The terms and conditions of the Ancillary Facility shall be agreed between the Company and the lender concerned, it being understood that the maturity date may not be before the Senior Credit Facility’s maturity date. Any amount provided by a lender through such an Ancillary Facility shall reduce commensurately the commitment of the lender concerned under the Amended RCF. 10.4.4.2 Interests, commitment fee Advances granted under the Senior Credit Facility or which may be granted under the Additional Credit Facility bear interest at the Libor rate or, for advances in euros, the Euribor rate applicable to the selected term, plus a margin that depends on the Leverage Ratio, defined as the ratio of Total Net Debt to Adjusted EBITDA, as follows: Leverage Ratio Less than or equal to 2.00x Less than or equal to 2.50x but more than 2.00x Less than or equal to 3.00x but more than 2.50x Less than or equal to 3.25x but more than 3.00x Less than or equal to 3.50x but more than 3.25x Less than or equal to 3.75x but more than 3.50x Less than or equal to 4.00x but more than 3.75x Over 4.00x Margin (per year) 1.50% 1.75% 2.00% 2.25% 2.50% 2.75% 3.00% 3.25% The Company is also required to pay a fee equal to 35% of the applicable margin, based on the unused available portion of the Senior Credit Facility. In the event that advances are made under the Additional Credit Facility, the Company will be required to pay a fee, the amount of which will be determined in a separate letter. 153 Any unpaid amount under the Amended RCF will bear interest at a rate equal to the rate at which that amount would have borne interest if, during the period during which it was unpaid, it had constituted an advance under the Senior Credit Facility, plus 1 percentage point. The Company is also required to pay agent-to-agent and security agent fees, the amounts of which are determined in separate letters. The Company or the borrowers are required to pay various fees related to the usage of the Senior Credit Facility in the form of letters of credit. 10.4.4.3 Repayment of the principal Advances made under the credit facility are due for repayment at the end of the interest period selected by the borrower and further drawings may be made until one month before the final expiry date (see section 10.4.4.1 – “Amount, usage, term” of this document de base). 10.4.4.4 Early repayment The Amended RCF stipulates a certain number of circumstances that would trigger early repayment of advances under the Senior Credit Facility and, if applicable, a reduction or termination of the Senior Credit Facility. Full mandatory early repayment - The Senior Credit Facility will be terminated and all advances provided by the lenders will be repayable early in the event of: a Change of Control defined as one or more third parties acting in concert (other than 3i, the Company’s shareholders on the date of the Amended RCF or related parties) directly or indirectly owning more than 30% of the Company’s capital or voting rights; or a disposal of all or substantially all of the Group’s assets. Partial mandatory early repayment In the event of asset disposals or the receipt of insurance compensation, the Company is required, subject to certain exceptions and situations regarding the reuse of proceeds, to use proceeds from the disposal or compensation to make an early repayment of the Senior Credit Facility. Provisions applicable to the early redemption of the High-Yield Bonds In the event that the High-Yield Bonds are redeemed partially in a proportion of 50% or more, the Company is required to repay advances under the Senior Credit Facility and reduce the Senior Credit Facility to the extent of the percentage of High-Yield Bonds redeemed in excess of 50%. Termination or early repayment at the option of the Company The Company may terminate early some or all of the Senior Credit Facility, subject to a minimum amount of €5 million, and any Group Company may repay early some or all drawings on the Senior Credit Facility, subject to a minimum amount of €5 million. 10.4.4.5 Security interests and guarantees Under the Amended RCF, the obligations of the Obligors with respect to the Finance Documents are jointly and severally guaranteed by the Company and some of its French and foreign subsidiaries as guarantors. The list of Guarantors is provided above in section 10.4.3 – “High Yield Bonds” of this document de base. The Company undertakes that the combined EBITDA of all guarantors shall at all times represent 70% of the Restricted Group’s EBITDA. The obligations of guarantors may be limited by applicable laws in their place of incorporation. 154 Under the Amended RCF, the Senior Credit Facility must be guaranteed by the following real security interests: (i) pledges of shares of any company acquired where that company’s EBITDA is equal to or over €3,000,000 and (ii) pledges of the Company’s receivables resulting from any intragroup loan where the receivables exceed €5 million. However, until the High-Yield Bonds are refinanced, pledges of shares and receivables made in accordance with previously applicable thresholds (€750,000 and €1 million respectively) shall remain in place. At the moment, real security interests guaranteeing the Senior Credit Facility are: - Pledges of shares or equity securities – the companies whose shares are pledged are the following: Labco Midi, Groupe Biologic (anciennement Laboratoire d’analyses médicales Jorion), Laboratoire de Biologie Médicale Delaporte, Biopar (anciennement Bioval), Biologistes Associés Regroupant des Laboratoires d’Analyses, Labco Finance SPRL, Labco Germany GmbH (anciennement Labco Deutschland GmbH), Labco Italia Srl, Labco Diagnostics Espana SA (anciennement Labco Diagnostics Espana SL), Laboratoire d’Analyses Médicales Carron, Bioalliance (anciennement Eslab), Bio-Rhône (anciennement Laboratoire de l’Avenue), Institut de Biologie Clinique (anciennement Laboratoire Schaffner), Biofrance, Isolab, Laboratoire de Biologie Medicale Aubert (anciennement Laboratoire d’analyse de biologie médicale Aubert H), Oxabio (anciennement Laboratoire Gaudaert – Dauchy – Leclercq – Capelle – Bourlart), Norden, Novabio Diagnostics (anciennement SELAS Tixier – Pierfitte – Avot), Biopaj, SDN S.p.A., Istituto Il Baluardo S.p.A., Labco Lombardia Srl, C.A.M. - Centro Analisi Monza S.p.A., Laboratorio Médico Dr. David Santos Pinto E Dr. Fernando Teixeira, S.A., Flaviano Gusmao S.A., Gnostica – Laboratorio de Analises Clinicas, S.A., General Lab Portugal S.A., General Lab S.A.U (anciennement General Lab SA), Labco Diagnostics Sweden AB 3, Ellipsys SCA et Laboratoire d’Analyses Médicales Roman Païs SC SPRL - Pledges of receivables – the companies that have pledged amounts receivable by them under an intragroup loan or intragroup loans to which they are party are the following: la Société, Labco Midi, Groupe Biologic (anciennement Laboratoire de Biologie Médicale Jorion), Laboratoire de Biologie Médicale Delaporte, Biologistes Associés Regroupant des Laboratoires d’Analyses, Biopar (anciennement Bioval), Labco Finance SPRL, Labco Germany GmbH (anciennement Labco Deutschland GmbH) et Labco Diagnostics Espana SA (anciennement Labco Diagnostics Espana S.L). In addition, - any asset pledged to guarantee the High-Yield Bonds must also be pledged to guarantee the Senior Credit Facility in accordance with the principles set out in appendix 16 (Agreed Security Principles); and - any Material Company, mentioned in the annex to the Amended RCF, or whose EBITDA equals 5% or more of the Group’s EBITDA or a company that directly owns shares in such a company, shall be an Additional Guarantor. Assuming that the High-Yield Bonds are refinanced, lenders under the Amended RCF will benefit from pledges guaranteeing the debt taken out to refinance the High-Yield Bonds and at least the following security interests: 1) Guarantee from: Bioalliance (anciennement Eslab), Bio-Alpes, Biofrance, Biologistes Associés Regroupant des Laboratoires d’Analyse, Biopaj, Biopar (anciennement Bioval), Bio-Rhône (anciennement Laboratoire de l’Avenue), Centre Biologique, Groupe Biologic (anciennement Laboratoire d’analyses médicales Jorion), Institut de Biologie Clinique (anciennement Laboratoire Schaffner), Isolab, Labco Midi S.A., Laboratoire Bioliance, Laboratoire d’Analyses de Biologie Médicale Christine Pepin – Philippe Leluan – Patricia Sannier – Didier Guillo, Laboratoire de Biologie Médicale Delaporte, Mazarin, Norden, Normabio, Novabio Diagnostics (anciennement SELAS Tixier – Pierfitte – Avot), Oxabio (anciennement Laboratoire Gaudaert – Dauchy – Leclercq – Capelle – Bourlart), Sylab, C.A.M. Centro Analisi Monza S.p.A., Istituto Il Baluardo S.r.l., Labco Italia S.R.L., SDN S.p.A., Labco Germany GmbH (anciennement Labco Deutschland GmbH), Labco Finance Sprl, Laboratoire d’Analyses Médicales Roman Païs SC SPRL, Dr. Macedo Dias – Laboratorio de Anatomia patologica, S.A., Flaviano Gusmao S.A., General Lab Portugal S.A., Gnostica – Laboratorio de Analises Clinicas S.A., Laboratorio Medico Dr. David Santos Pinto e Dr. Fernando Texeira S.A., 3 Being dissolved 155 General Lab S.A.U. (anciennement General Lab SA), Labco Diagnostics Espana S.A. (anciennement Labco Diagnostics Espana SL), Labco Madrid S.A., Axilab et Ellipsys SCA; 2) Pledges or shares from the following companies: Biologistes Associés Regroupant des Laboratoires d’Analyses, Biofrance, Biopaj, Biopar (formerly Bioval), C.A.M. – Centro Analisi Monza Spa, Laboratoire de Biologie Médicale Delaporte, General Lab SA, General Lab Portugal S.A., Groupe Biologic, Institut de Biologie Clinique, Isolab, Istituto Il Baluardo Srl, Labco Diagnostics Espana SL, Labco Finance, Labco Italia, Labco Midi, Laboratoire Bioliance, Novabio Diagnostics, Oxabio, Laboratoire d’Analyses Médicales Roman Païs and SDN S.p.A.; 3) Pledges of shares in any company acquired by a guarantor or a borrower as part of a Permitted Acquisition and whose EBITDA is at least €3 million; 4) Pledges of shares in any subsidiary of any Additional Borrower or Additional Guarantor whose EBITDA is at least €3 million; and 5) Pledges of any intragroup receivable of a principal amount of over €5 million. Under clause 14 (Application of Proceeds) of the Intercreditor Agreement (see section 10.4.5 – “Intercreditor Agreement” of this document de base), if security interests are enforced, the lenders under the Amended RCF shall be paid out of proceeds from enforcing those security interests ahead of holders of High-Yield Bonds. 10.4.4.6 Undertakings - Restrictive clauses - Financial covenants The Amended RCF provides undertakings to disclose information, in particular accounting and financial information (provision of quarterly, half-year and annual financial statements, it being stipulated that the obligation to provide disclosure about the Group’s financial position, assets or activities is subject to compliance with applicable legislation and regulations, rules applicable to regulated markets and all confidentiality obligations), or information provided to the Lenders in order to meet their obligations in respect of moneylaundering legislation, as well as certain other positive undertakings relating to patents and intellectual property, maintaining authorizations, tax, maintaining insurance policies and constituting and maintaining security interests. The Amended RCF contains certain negative undertakings, which forbid the Group or limit its ability to: - take out additional debt, subject to certain exceptions relating in particular to loans between members of the Group, all debts in respect of the High-Yield Bonds, the financing of Permitted Acquisitions to the extent permitted by the Amended RCF, and provided in particular that such additional debt does not rank higher than the Senior Credit Facility; - grant security interests; - carry out acquisitions other than Permitted Acquisitions or form joint ventures other than Permitted Joint Ventures; in particular, permission to carry out an acquisition is subject to compliance with an Incurrence Ratio; - pay dividends or buy back shares except where (i) the Leverage Ratio calculated on a pro forma basis (taking into account such distribution) is not higher than 4.25:1 and (ii) the general undertaking to maintain a minimum level of liquidity (the Minimum Cash Balance Covenant), i.e. €15 million is complied with; to the extent that such condition differs from that stipulated in the terms of the HighYield Bonds, which allow dividends to be distributed up to an annual limit of 6% of the funds received from the IPO plus the amount of specific distribution reserves authorized under the terms of the HighYield Bonds in a combined total amount of at least €50 million. Of the condition stipulated in the Amended RCF and that stipulated in the terms of the High-Yield Bonds, the more restrictive condition shall apply until the High-Yield Bonds have been refinanced. Those undertakings limit the Group’s ability to distribute dividends, but they should not be capable of preventing it from pursuing its dividend distribution policy. The Group’s initial aim is to distribute dividends with respect to 2016, with a planned payout rate of around 20% of the Group’s net profit (see section 12.2 – “Medium-term outlook” of this document de base); and 156 - sell assets and carry out mergers. In addition, the Company’s undertakings with respect to the Amended RCF include compliance with negative undertakings included in the terms of the High-Yield Bonds, which are duplicated, mutatis mutandis, in appendix 15 (Restrictive Covenants) of the Amended RCF. The Amended RCF Lenders have undertaken to examine, when the High-Yield Bonds are refinanced, the amendments to be made to undertakings under the Amended RCF, particularly in order to reflect the terms of the instrument refinancing the High-Yield Bonds. Such amendments must be approved by a majority of lenders (Majority Lenders), i.e. by lenders whose commitments make up at least 66 2/3% of the lenders’ total commitments. The Amended RCF requires compliance with covenants and financial ratios: (i.) a Super Senior Gross Leverage Ratio (the ratio of Senior Total Gross Debt to adjusted EBITDA) which must not exceed 1.75:1 until December 31, 2017 and 1.50:1 from January 1, 2018; and (ii.) an undertaking to maintain a Minimum Cash Balance of at least €15 million. The Amended RCF also contains certain declarations made by the Company on its own behalf or on behalf of the Group and/or other Group members that are usual in this kind of credit agreements. 10.4.4.7 Accelerated maturity situations The Amended RCF stipulates accelerated maturity situations that are usual in this kind of credit arrangements, allowing the Majority Lenders to require acceleration of all current drawings and or of any “cash cover” in relation to a Letter of Credit, and the termination of the Senior Credit Facility. Those situations include: 4 - a failure to pay an amount due in respect of the Senior Credit Facility; - a failure to comply with a covenant or financial ratio; - a failure to comply with another obligation stipulated by the Amended RCF; - an inaccurate declaration; - the acceleration of another borrowing, a payment default or a situation where a creditor of such a debt requires acceleration of that debt (subject to a unit threshold of €3,000,000 and a combined threshold of €10,000,000); - the cessation of payment or the commencement of insolvency proceedings for certain Group companies; - the discontinuation of activities of certain Group companies; - the failure to comply with provisions of the Intercreditor Agreement; - the occurrence of a litigation that could have an adverse outcome for the Group resulting in a Material Adverse Effect on the Group;4 - the occurrence of an event or circumstance that has or is reasonably likely to have a Material Adverse Effect; Defined as a material adverse effect on (i) the Group’s business or assets as a whole, (ii) the ability of the Obligors to honor their obligations, or (iii) subject to exceptions provided for by law and enforceability formalities, the validity or rank of security interests that must be granted in accordance with the Amended RCF. 157 - a material reservation expressed in the statutory auditors’ report on the Company’s annual consolidated financial statements. 10.4.4.8 Applicable law – Competent courts The Amended RCF is governed by English law, although the undertakings provided for in appendix 15 (Restrictive Covenants) and relating to certain undertakings made by the Company and the Group are governed by the law of New York State. The English courts have jurisdiction over any dispute. 10.4.5 Intercreditor Agreement When the initial RCF was arranged and when the High-Yield Bonds were issued, the Company and each of its subsidiaries that was a borrower or guarantor with respect to the Senior Credit Facility or the High-Yield Bonds (hereinafter the “Debtors”) entered into an Intercreditor Agreement with parties including the Lenders that were parties to the initial RCF, the Agent, the Security Agent and the Trustee acting on behalf of holders of the HighYield Bonds on January 24, 2011 and on which counterparties to the Hedging Agreements may rely in order to benefit from the security interests. The Intercreditor Agreement governs aspects including: - the relative ranking of the various categories of the Debtors’ creditors (including with respect to their guarantees); - the order of priority for the payment of creditors in the event of a payment default with respect to the Senior Credit Facility or the High-Yield Bonds and specifically assuming that security interests are enforced; - the relative ranking of security interests granted by the Debtors; - the payments authorized in respect of certain of the Debtors’ liabilities; - the respective rights of creditors benefiting from security interests to initiate enforcement proceedings and the terms for enforcing such security interests; - obligations for a creditor to return any amount unduly received from a Debtor; - the terms under which guarantees and security interests may be released to allow, in certain specific cases: (i) a disposal of pledged assets or (ii) the refinancing in full or in part of the Senior Credit Facility or the High-Yield Bonds or (iii) the arrangement of additional debt stated under the Intercreditor Agreement as similar to the Senior Credit Facility or High-Yield Bonds. In the event of a conflict between the provisions of the Intercreditor Agreement and the provisions of the Amended RCF or High-Yield Bonds, those of the Intercreditor Agreement shall prevail. 10.4.5.1 Ranking and Priority Under the Intercreditor Agreement, Debtors’ liabilities with respect to the Senior Credit Facility (“Revolving Creditor Liabilities”), Debtors’ liabilities with respect to all Hedging Agreements (“Hedging Liabilities”) and Debtors’ liabilities with respect to the High-Yield Bonds shall rank pari passu and without preference between them as regards priority of payment, except where real security interests are enforced (as described below). The Intercreditor Agreement also provides that certain intragroup Liabilities and the liabilities of Debtors to the Company’s shareholders (“Subordinated Liabilities”) are subordinated to the Senior Credit Facility, the Hedging Liabilities and the Liabilities with respect to the High-Yield Bonds. The Intercreditor Agreement also provides that the security interests granted by the Debtors with respect to the Senior Credit Facility, the Hedging Agreements and High-Yield Bonds guarantee the corresponding liabilities in 158 the following order and with the following priority (provided that the security interests are stipulated as guaranteeing the liabilities stated below): 1) Firstly, the payment of fees, costs and expenses owed to the Agent, the Trustee and the Security Agent pari passu and without any preference between them; 2) Secondly, the payment of (i) Revolving Creditor Liabilities and (ii) 20% of the Priority Hedging Liabilities, pari passu and without any preference between them; and 3) Thirdly, the payment of (i) Liabilities with respect to the High-Yield Bonds and (ii) Non-Priority Hedging Liabilities. The proceeds from enforcing security interests shall be divided in accordance with the foregoing through the application of article 14.1 of the Intercreditor Agreement. 10.4.5.2 Parallel debt The Intercreditor Agreement provides: - that each Debtor under the Indenture and the High-Yield Bonds shall assume with respect to the Security Agent, as Secured Notes Parallel Debt Creditor, a payment obligation parallel to that assumed by the Debtor concerned with respect to the holders of High-Yield Bonds and which shall exist alongside, without adding to, the said payment obligation (the “Secured Notes Parallel Debt”); and - that each Debtor under the Amended RCF and the Senior Credit Facility shall assume with respect to the Security Agent a payment obligation parallel to that assumed by the Debtor concerned with respect to the Lenders and which shall exist alongside, without adding to the said payment obligation (the “Senior Credit Facility Parallel Debt”). For the purposes of applying the Intercreditor Agreement, the Secured Notes Parallel Debt and the Senior Credit Facility Parallel Debt constitute payment obligations treated in the same way as the principal payment obligations in relation to the High-Yield Bonds or Senior Credit Facility, respectively, whose regime and rights apply to them as set out by the Intercreditor Agreement, and which benefit from the same security interests. 10.4.5.3 Permitted Payments The Intercreditor Agreement permits, among other things: - payments of amounts due with respect to the Amended RCF at any time in accordance with the RCF; - payments of amounts due with respect to the High-Yield Bonds in accordance with the provisions of the Indenture; - payments of the following amounts due with respect to the Hedging Liabilities: (i.) any payment provided for by the relevant hedging agreement coming to its contractual term; (ii.) any payment under clauses regarding the reimbursement of expenses, tax and regulatory costs and certain other technical provisions of hedging agreements; (iii.) any payment resulting from an out-of-court or contractual early termination of the relevant hedging agreement; (iv.) any payment resulting from an early termination following a default relating to the relevant hedging agreement and where no default event exists in respect of the Amended RCF; and (v.) any payment taking place with the prior agreement of the “Majority Senior Creditors”, defined as creditors representing, at a given date, over 50% of all commitments in respect of the Senior Credit 159 Facility, pf amounts remaining due under any Hedging Agreement that has, at the relevant date, already been terminated and of the High-Yield Bonds; - payments of amounts due with respect to intragroup borrowings, provided that at the relevant payment date no acceleration of the Senior Credit Facility and/or of the High-Yield Bonds has been announced, except where the Majority Senior Creditors have authorized the payment in question; and - payments of sums due in respect of the Subordinated Liabilities where (i) at the date of the payment in question, the payment is authorized as a Permitted Distribution under the Amended RCF or is not contrary to the provisions of the Indenture or (ii) the Majority Senior Creditors have authorized the payment in question. 10.4.5.4 Enforcement of security interests The Intercreditor Agreement governs the procedure for enforcing security interests, the implementation of which requires (i) a decision to do so by the majority of the Senior Credit Facility Lenders (representing more than two thirds of the commitments in respect of the Senior Credit Facility), i.e. a majority of holders representing over 50% of the principal amount of the High-Yield Bonds and (ii) the reception by the Security Agent of enforcement instructions from the representatives of the creditors (the Trustee of the High-Yield Bonds or the Agent, as applicable). If the representatives of the creditors send contradictory instructions to enforce Security Interests, a disclosure and consultation process is provided for (except in an insolvency situation or if one of the two creditors groups believes in good faith that the use of the consultation process would seriously harm its ability to enforce the Security Interests). If, after the end of that consultation, the Trustee and Agent do not give the same instructions, the Trustee’s instructions shall prevail. However, if the Lenders have not been fully repaid within six months or if no measure to enforce the security interests has been taken within three months of the corresponding instruction being received, the instructions of the Majority of the Lenders shall prevail. The Intercreditor Agreement provides that the payment of any balance due to the creditors benefiting from security interests, where those security interests are enforced, can be delayed, although not after the date on which the creditors have received all sums deemed to be due to them under the Amended RCF, the Indenture or the hedging agreements, as applicable, and under the Intercreditor Agreement. 10.4.5.5 Release of guarantees and security interests The Intercreditor Agreement allows the Security Agent, subject to certain conditions, to release security interests in order (i) to allow the sale of a pledged asset to the extent that such sale is authorized by the Amended RCF and the High-Yield Bonds or provided for by the Intercreditor Agreement or (ii) to allow equally ranked security interests to be pledged in relation to the Senior Credit Facility or the High-Yield Bonds and any new debt described in section 10.4.5.6 below. 10.4.5.6 Additional debt The Intercreditor Agreement provides that the Senior Credit Facility may be increased or refinanced by a new revolving credit facility and that new high-yield bonds may be issued additionally, or as part of a refinancing of the High-Yield Bonds, in all cases subject to compliance with conditions set out in the Amended RCF and Indenture (particularly regarding financial ratios). Such additional debt shall be treated in the same way as the Senior Credit Facility or, as the case may be, the High-Yield Bonds and will be covered by the same security interests as the Senior Credit Facility or, as the case may be, the High-Yield Bonds. Existing creditors in respect to the Senior Credit Facility and High-Yield Bonds and the creditors of the new financing arrangements thus permitted shall be free to determine between themselves the ranking of their receivables. 10.4.5.7 Amendments Amendments to the Intercreditor Agreement require, subject to specific exceptions, the consent of the Company, the Agent, the Security Agent and the Trustee of the High-Yield Bonds. 160 For those purposes, the Agent generally acts on the instructions of the majority of Lenders or, for certain usual matters, on the unanimous instructions of the Lenders, and the Trustee of the High-Yield Bonds acts on the instructions of holders representing over 50% of the principal amount of the High-Yield Bonds. Notable exceptions include: (i) minor and purely administrative or technical amendments (such as the correction of material errors) that only require the consent of the Company and the Security Agent; (ii) amendments of certain clauses that affect the rights and obligations of the Lenders, which require the consent of parties mentioned in the first paragraph above and that of all Lenders; and (iii) any amendment of a clause specifically affecting the rights and obligations of a party, which also requires the consent of the party in question. 10.4.5.8 Applicable law The Intercreditor Agreement is governed by English law. 10.4.6 Other financial debts The Group has also entered into finance-lease agreements under which debt amounted to €16.7 million at December 31, 2012, €22.4 million at December 31, 2013 and €19.4 million at December 31, 2014. For a detailed explanation of these agreements, please see Note 3.7 and Note 15 to the audited consolidated financial statements for the financial year ended December 31, 2013 included in section 20.1.1 – “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” of this document de base. The Group has also taken out some residual bank loans, the amounts of which were €15.9 million at December 31, 2012, €13.9 million at December 31, 2013 and €13.6 million at December 31, 2014. For a detailed explanation of this debt, please see Note 23 to the audited consolidated financial statements for the financial year ended December 31, 2013 included in section 20.1.1 – “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” of this document de base. 10.5 OFF-BALANCE SHEET COMMITMENTS The Group’s off-balance sheet commitments consist mainly of guarantees given by the Group as part of its investing and financing activities, including guarantees given as part of the operation of the group cash management agreement, or in relation to the High-Yield Bonds and the Amended RCF. The Company’s obligations under the High-Yield Bonds and the obligations of the Company’s direct subsidiaries that borrow money under the Amended RCF have been guaranteed through real security interests, including pledges of securities and receivables resulting from intragroup loans, given by certain companies of the Group referred to as Guarantors (see Note 30 to the audited consolidated financial statements for the period ended December 31, 2014 as included in section 20.1.1. “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” of this document de base). The Group has also given material off-balance sheet commitments relating to its business activity. As part of the outsourcing contract obtained by its subsidiary iPP from NHS hospital trusts in the UK, the Company gave guarantees to the trusts under which it guarantees the performance of iPP’s operating obligations as defined in the outsourcing contracts, for a period covering that of the contract plus two years (see Note 30 to the audited consolidated financial statements for the period ended December 31, 2014 as included in this document de base). The Company’s board of directors approved, on March 5, 2015 the payment, as part of the Company’s IPO, of bonuses. These payments, for a variable amount comprised between €0 and an estimated maximum of €4.5 (net of social security and employer contributions that are not deductible), could be paid to 29 executives and managers of the Group, including a variable amount comprised between €0 and an estimated maximum of €945,000 that would be awarded to Mr. Philippe Charrier, the Company’s Chief Executive Officer. The exact amount of these bonuses and their combined amount will depend on the Company’s share price set for the IPO. The remuneration of the Chief Executive Officer after the initial public offering of the Company will be set by the then-incumbent board of managers. 161 A performance share plan was implemented in the UK in April 2014, benefiting iPP’s two main managers through a “UK Employee Shareholders Scheme” Under that plan, 7% of iPP’s shares were awarded to those managers. The economic rights to those shares are subject to a 5-year vesting period (20% per year). Those managers have been granted a put option that can be exercised when all the economic rights to the shares have vested. The Company has a call option that can be exercised one year after the end of the vesting period and at any time in the event of a change in control of the Company or of iPP. The valuation of the shares is based on a price formula specified in the contract relating to that option. The estimated charge corresponding to the share award plan is subject to regular measurement in the Company’s financial statements. For an analysis of the off-balance sheet commitments made with respect to the 10x Warrants and the Mezzanine Warrants and their financial impact, see section 19.1.3 “Settlement agreements” of this document de base. With the exception of the material commitments set out above, the Group is not party to any off-balance sheet commitment that has or could reasonably have a material impact, currently or in the future, on its financial position, operating results, cash position, investment expenditure or financial resources. 10.6 RESTRICTION ON THE USE OF CAPITAL THAT HAS MATERIALLY INFLUENCED OR COULD MATERIALLY INFLUENCE, DIRECTLY OR INDIRECTLY, THE COMPANY’S BUSINESS ACTIVITY The terms of the High-Yield Bonds and the Amended RCF described in section 10.2 – “Presentation and analysis of the main ways in which the Group uses cash” of this document de base contain certain restrictive provisions for the Company and for the Group, which have limited and could continue to limit the Group’s ability to carry out certain transactions or, more generally, make unconstrained use of its liquidity or make financial undertakings not authorized by those terms or those provisions. The Group has at all times complied with the financial undertakings and the specific ratios provided for by the High-Yield Bonds and by the RCF, including leverage ratios of 5.5 in 2011, 5.75 in 2012 and 2013 and 5.50 in 2014. The leverage ratio is calculated as follows: Financing Net Debt/Financing EBITDA. This leverage ratio amounted to 4.68 for the financial year 2012, 4.58 for the financial year 2013 and 4.87 for the financial year 2014. The Company’s statutory auditors delivered statements regarding this ratio, set under the RCF and the Amended RCF, for the financial years 2012, 2013 and 2014, as required under the RCF and the Amended RCF. 10.7 EXPECTED SOURCES OF FINANCING FOR FUTURE INVESTMENTS As in the financial years ended December 31, 2012, 2013 and 2014, the Group expects its financing requirements in 2015 to relate mainly to the financing of acquisitions (see section 20.1.1. “IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014” of this document de base), investments expenditure (see section 5.2.2. “Current investments” of this document de base), its working capital requirements (see section 10.2.4. “Financing the working capital requirement” of this document de base) and the repayment of borrowings and payment of related interests. To finance its future investments, the Group will use its traditional financing sources, i.e. available cash, net cash flows from operating activities and debt (including drawings on the RCF), as well as accessing the capital markets. In particular, on February 11, 2015, the Group carried out an issuance of bonds fungible with previously issued bonds in a principal amount of €100 million. The funds obtained were used in particular to repay €100 million drawings on the Amended RCF, with the effect that €120.25 million is available under the Amended RCF at the date of this document de base, subject to compliance with covenants and financial undertakings and other standard undertakings (see section 10.4. “Equity and financial debt” of this document de base). 162 CHAPTER 11 RESEARCH & DEVELOPMENT, PATENTS AND LICENSES The Company places considerable emphasis on using equipment at the cutting edge of technology in every laboratory it runs. It does not have any specific research and development centers or employee categories devoted to research because it relies in this regard on the results of the research and development research obtained by its equipment and test suppliers. However, since the acquisition of the SDN group in July 2014, the Group now runs a research and development activity through the non-profit scientific foundation in which SDN actively participates (see section 6.4.3.2 – “Overview of Southern European market-Italy “ of this document de base). The Group operates under several different business names, trademarks and service brands. Although the Group has consolidated a large portion of its business under the “Labco” name, it sometimes operates under the business name of the laboratory acquired to gain access to the latter’s customer base and reap the benefit of the prestige associated with the trademark or business name of the laboratory concerned. Ten brands, including the Labco (device) trademark, the Labco NOÛS Advanced Special Diagnostics (device) trademark and the SDN (device) trademark were the object of a community registration and are therefore protected in each of the 28 countries of the European Union, including in France (see section 4.2 “Risks related to the Group’s technology and intellectual property rights” of this document de base). The Labco (device) trademark was also registered in Switzerland and other trademarks have been registered in Spain, Portugal and Italy. Other than the “Labco” trademark, the Group does not believe that any of its other business names, service brands and trademarks are essential for its business activities. The Group has also registered several domain names. The Group actively protects its intellectual property and in particular by registering its trademarks and business names. 163 CHAPTER 12 OUTLOOK FOR 2016-2017 The Group intends to continue pursuing its strategy of expansion across all the markets in which it operates and reap the benefit of the opportunities created by the structural trend towards outsourcing that the Group has observed in both its Northern Europe and Southern Europe business segments. In particular, at a time when laboratory services are increasingly being outsourced to the private sector, the Group aims to strengthen its position as a leader in the UK market by 2017 by setting up new technical platforms and entering into new outsourcing partnerships over the period. In 2016-2017, the Group aims to grow Revenue at an average annual rate of approximately 1.5% excluding the Group’s business in the United Kingdom, which should generate annual revenue of approximately €75 million by 2017. The average annual growth of Revenue, including the activities of the Group in the UK should be, for this period, approximately 3.5%. Factoring in the acquisitions envisaged over the period and the contribution of the Group’s UK business, the Group aims to grow Revenue at an average annual rate of approximately 10%. In addition, taking into account acquisitions envisaged over the period, the Group is aiming for an average annual EBITDA growth of approximately 10% in 2016-2017, excluding the impact of the Group’s UK business. Given the costs involved in setting up major outsourcing partnerships in the United Kingdom, the Group believes that the EBITDA corresponding to its UK business should amount to approximately €4 million by the financial year ending December 31, 2017 and an amount of approximately €10 million to Group EBITDA by the financial year ending December 31, 2018. The Group also believes that, from 2017, its recurring investment expenditure – excluding acquisitions and net of disposals – on property, plant and equipment and intangible assets will fall as a percentage of Revenue relative to 2015 and 2016 to approximately 2.5%. After strengthening its financial position through a partial repayment of the High Yield Bonds by using a large portion of the net proceeds of the capital increase planned as part of the IPO and the refinancing of the remainder of the High Yield Bonds the Group expects to state an amount in taxes of approximately 35% of the income before taxes in 2017. This reduction in the level of tax excludes any potential use in future financial years (i) of tax loss carryforwards which totaled approximately €100 million at the date of this document de base and (ii) any deferred non-deductible interest, which totaled approximately €50 million at the date of this document de base. The Group estimates that the use of tax loss carryforwards and deferred non-deductible interest will allow them to state an amount in taxes lower than 35% of the income before taxes in the medium term. The Group also intends, subject to approval from the Company’s shareholders in its annual general meeting, to adopt a dividend payout rate of around 20% of Group net profit, with the first dividend payment taking place in 2017 with respect to the financial year ending December 31, 2016. The Group does not envisage paying a dividend in respect of the financial year ending December 31, 2015. The Group intends to devote approximately €200 million to its policy of acquisitions in 2016 and 2017, excluding transformational acquisitions. The acquisitions would mostly be selective deals in the markets in which the Group currently operates. As part of its strategy, the Group aims to maintain a year-end Financing Net Debt to Financing EBITDA leverage ratio not exceeding 3.25x in 2016 and 2017. 164 CHAPTER 13 PROFIT FORECASTS OR ESTIMATES 13.1 FORECASTS 13.1.1 Assumptions The Group has based its forecasts on the annual consolidated financial statements for the financial year ended December 31, 2014. Those forecasts are based on the following assumptions: (a) the full-year impact of the acquisitions made during financial year 2014; (b) the acquisitions made by the Group during 2015 being included in the scope of the consolidation of acquisitions from the date on which those acquisitions were completed; (c) exchange rates being stable at a rate of €1.35 to the pound; (d) the regulatory and tax frameworks being unchanged from those in force as at December 31, 2014; (e) as regards the Group’s business in France, new prices for clinical services in accordance with the threeyear agreement agreed in October 2013 between the main French laboratory doctor unions and UNCAM; as well as the impact in 2015 of the effective start in the fourth quarter of 2014 of iPP’s supply agreement for laboratory testing services in the United Kingdom with Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust; 13.1.2 Group forecasts for the financial year ending December 31, 2015 Based on the assumptions stated above, the Group forecasts Revenue in the region of €710 million for the financial year ending December 31, 2015, representing an increase of approximately 15% from the Revenue of €615 million recorded by the Group in the financial year ended December 31, 2014. This growth is expected to include: an increase in Revenue in the region of €40 million owing to the full-year impact of acquisitions made by the Group in 2014; organic growth of approximately 1.5% excluding the contribution of the Group’s UK business, and a revenue in France, not taking into account the acquisitions made by the Group during the financial year 2015, stable relative to 2014; an increase in Revenue in the order of €20 million owing to the contribution of acquisitions made by the Group in 2015 (only including acquisitions mentioned in section 5.2.1.1 – “Acquisitions and disposals of groups of companies, companies and/or businesses” and section 5.2.2.1 – “Acquisitions of groups of companies, companies and/or businesses” of this document de base); a near-doubling in the Group’s UK revenue, which should be approximately €50 million, chiefly attributable to the effective start of the agreement concluded in May 2014 by the Group with Basildon and Thurrock University Hospitals NHS Foundation Trust and Southend University Hospital NHS Foundation Trust. After taking into account the additional full-year impact of only those acquisitions mentioned in section 5.2.1.1 – “Acquisitions and disposals of groups of companies, companies and/or businesses” and section 5.2.2.1 – “Acquisitions of groups of companies, companies and/or businesses” of this document de base, the Group’s Revenue should be close to €730 million. 165 Before taking into account any negative contribution from the United Kingdom, which is forecast to post negative EBITDA of around €7 million, the Group believes that: its EBITDA in the financial year ending December 31, 2015 should exceed €135 million; After taking into account the additional full-year impact of only those acquisitions mentioned in section 5.2.1.1 – “Acquisitions and disposals of groups of companies, companies and/or businesses” and section 5.2.2.1 – “Acquisitions of groups of companies, companies and/or businesses” of this document de base, the EBITDA should be approximately €140 million. This EBITDA (excluding the United Kingdom) for the 2015 financial year will include: transaction costs of approximately €1.5 million relating to the acquisitions mentioned in sections 5.2.1.1 - “Acquisitions and disposals of groups of companies, companies and/or businesses” and 5.2.2.1 - “Acquisitions of groups of companies, companies and/or businesses” of this document de base; a charge, not affecting cash, of an amount of approximately €2.1 million relating to the allocation of performance shares at the end of 2014, as described in section 15.1 – “Compensation and benefits of any nature allocated to directors and corporate officers and members of administrative, management and supervisory bodies during the financial years ending December 31, 2013 and December 31, 2014” of this document de base. However, this EBITDA does not include the Group’s share of equity accounted companies, which amounts to approximately €0.5 million. The Group expects normal investment expenditure of around €17 million and non-recurring investment expenditure of around €17 million in relation to the start of the new technical platform in Barcelona, the new service contract in Essex, United Kingdom, and information systems development projects. The Group intends to devote a budget of approximately €75 million to acquisitions in the financial year ending December 31, 2015, it should be noted that at the date of this document de base, the Group has already made 3 acquisitions for a total amount of approximately €47 million and has also issued letters of intent regarding the acquisition of laboratories for a total of approximately €71 million. During the 2015 financial year, the Group estimates that it will pay taxes amounting to approximately €30 million, impacting cash. In connection with the Company’s initial public offering, the Group is contemplating a capital increase of a gross amount of approximately €320 million and is expecting fees relating to the initial public offering to be approximately €15 million. The Group expects to achieve a Financing Net Debt to Financing EBITDA ratio of around 3.25x between December 1 and December 31, 2015 after taking into account the allocation of a significant portion of the net procceds of the capital increase to the partial repayment of the financial indebtedness and all of the costs relating to refinancing the remainder of the High Yield Bonds. From the date of the initial public offering of the Company and in light of market conditions, the Group envisages allocating a significant portion of the net proceeds of the capital increase to the early repayment of a portion of the High Yield Bonds issued against the payment of a redemption premium expected to be equal to 4.25% of their par value. In addition, the Group is currently in discussions with banking institutions to determine the best financial instrument in order to refinance the High Yield Bonds still in circulation. The Group estimates that the effects of repaying indebtedness on the one hand and the planned refinancing on the other, will allow the Group to significantly reduce the average cost of its’ financial indebtedness for the 2015 financial year for the two following reasons: the Group intends to refinance its existing 8.5% per annum High Yield Bonds due in 2018 through a long-term financing with an annual interest rate inferior to 4% (in view of current market conditions); 166 13.2 the margin paid on the Amended RCF is determined according to a scale based on the Financing Net Debt to Financing EBITDA ratio. Taking into account the lowering of this ratio to 3.25 for December 1 to December 31, 2015 due to the intended capital increase, the margin paid on the Amended RCF will be reduced from 375pts to 225pts. STATUTORY AUDITORS’ REPORT ON THE PROFITS FORECASTS Aplitec Aplitec 4-14, rue Ferrus 4-14, rue Ferrus 75014 Paris Deloitte & Associés 67, rue de Luxembourg 59777 Euralille 75014 Paris LABCO Société Anonyme 60 - 62, rue d’Hauteville 75010 Paris Statutory Auditors' report on the profit forecasts for the period of January 1, 2015 to December 31, 2015 To the General Manager In our capacity as Statutory Auditors of your company and in accordance with Commission Regulation (EC) no809/2004, we hereby report to you on the consolidated EBITDA forecasts of Labco set out in section 13 of the registration document (document de base), registered by the French financial markets authority (Autorité des marchés financiers – the “AMF”) on April 7, 2015. It is your responsibility to compile the consolidated EBITDA forecasts, together with the material assumptions upon which they are based, in accordance with the requirements of Commission Regulation (EC) n°809/2004 and ESMA’s recommendations on profit forecasts. It is our responsibility to express an opinion, based on our work, in accordance with Annex I, item 13.2 of Commission Regulation (EC) n°809/2004, as to the proper compilation of these forecasts. 167 We performed the work that we deemed necessary according to the professional guidance issued by the French Institute of Statutory Auditors (Compagnie nationale des commissaires aux comptes – CNCC) for this type of engagements. Our work included an assessment of the procedures undertaken by management to compile the forecasts as well as the implementation of procedures to ensure that the accounting policies used are consistent with the policies applied by Labco for the preparation of the consolidated financial statements of December 31, 2014. Our work also included gathering information and explanations that we deemed necessary in order to obtain reasonable assurance that the forecasts have been properly compiled on the basis stated. Since forecasts, by nature, are uncertain and may differ significantly from actual results, we do not express an opinion as to whether the actual results reported will correspond to those shown in the forecasts. In our opinion: a) the consolidated EBITDA forecasts have been properly compiled on the basis stated; and b) that basis of accounting used for the forecasts is consistent with the accounting policies of Labco. This report has been issued solely for the purposes of filing the registration document with the AMF and, if applicable, the admission to trading on a regulated market, and/or a public offer, of shares of Labco in France and in other EU member states in which a prospectus, including the registration document and a securities note (note d’opération), approved by the AMF is notified; and cannot be used for any other purpose. Lille and Paris, April 7, 2015 The Statutory Auditors Deloitte & Associés Aplitec Gérard BADIN Pierre LAOT 168 CHAPTER 14 ADMINISTRATIVE, MANAGEMENT AND SUPERVISORY BODIES AND GENERAL MANAGEMENT 14.1 MEMBERS OF THE ADMINISTRATIVE, MANAGEMENT AND SUPERVISORY BODIES AND GENERAL MANAGEMENT Unless otherwise stated, references to the Articles of Association and to the Internal Regulations in this Chapter 14 “Administrative, management and supervisory bodies and general management” and in Chapter 16 “The functioning of the Company’s administrative and management bodies” of this document de base refer either (i) to the Company’s Articles of Association adopted by the General Meeting of shareholders of the Company on October 2, 2014, applicable with effect from the date of settlement/delivery of the Company’s shares issued or sold in connection with its initial public offering on the Euronext Paris regulated market; or (ii) to the Internal Regulations of the board of directors as approved by the Company’s board of directors before completion of the Company’s initial public offering, approval of which must be reiterated by the newlyconstituted board of directors which will meet immediately after the completion of the Company’s initial public offering. The Company is a société anonyme with a board of directors governed by current laws and regulations and by its Articles of Association. On the date of settlement/delivery of the Company’s shares issued or sold in connection with its initial public offering on the Euronext Paris regulated market, the Company’s management will be entrusted to a board of directors comprising 9 members, 7 of whom will be independent. The General Meeting held on October 2, 2014 elected Philippe Charrier as a member of the Company’s board of directors with effect from the date of settlement/delivery of the Company’s shares issued or sold in connection with its initial public offering on the Euronext Paris regulated market. He is expected to be appointed as Chairman of the Company’s board of directors at its first meeting to be held after the settlement/delivery of the Company’s shares issued or sold in connection with its initial public offering on the Euronext Paris regulated market. Philippe Charrier will also keep his position as Chief Executive Officer of the Company. The main provisions of the Articles of Association and of the Internal Regulations relating to the board of directors, its committees and the general management of the Company, and in particular concerning their powers and the way in which they function, are described in Chapter 16 “The functioning of the Company’s administrative and management bodies” of this document de base. 14.1.1 The board of directors 14.1.1.1 Composition of the board of directors The following table sets out the anticipated composition of the board of directors following the settlement/delivery of the Company’s shares issued or sold in connection with its initial public offering on the Euronext Paris regulated market. 169 Name and forename(s), or company name Philippe Charrier Age Nationality Date of first appointment Expiry of term of office 60 French January 12, 2012 General Meeting held to approve the financial statements for the year ending December 31, 2016 Main position within the Company CEO Directorships and other offices held outside the Company (within or outside the Group) in the last five years Directorships and other offices held as of the date of registration of this document de base (within the Group): – – – – Director, Istituto il Baluardo SPA Director, Baluardo Servizi Sanitari SPA Director, Labco Diagnostics UK Director, Integrated Pathology Partnership Limited Directorships and offices held as of the date of registration of this document de base (outside the Group): – – – – – – Chairman, Clubhouse France Director, Lafarge Director, Rallye Chairman of the board of directors, Alphident (personal holding company) Chairman of the board of directors, Dental Emco Director and Chairman, UNAFAM Nationale (association) Directorships and offices held in the last five years but no longer held as of the date of this document de base (within the Group): – N/A Directorships and offices held in the last five years but no longer held as of the date of this document de base (outside the Group): – Daniel Bour 58 French January 12, 2012 General Meeting held to approve the financial statements for the year ending December 31, 2015 Independent Director N/A Directorships and other offices held as of the date of registration of this document de base (within the Group): – N/A Directorships and offices held as of the date of registration of this document de base (outside the Group): – – – Chairman and CEO, Générale du Solaire Chairman, Randopar Chairman, Professional Union Enerplan Directorships and offices held in the last five years but no longer held as of the date of this document de base (within the Group): – Member of the Strategy Committee, Labco SAS (until it became an SA) Directorships and offices held in the last five years but no longer held as of the date of this document de base (outside the Group): – 170 N/A Name and forename(s), or company name Erin Elizabeth Gainer Age Nationality Date of first appointment Expiry of term of office 41 American Irish October 2, 2014 on nonretroactive condition precedent General Meeting held to approve the financial statements for the year ending December 31, 2017 Main position within the Company Independent Director Directorships and other offices held outside the Company (within or outside the Group) in the last five years Directorships and other offices held as of the date of registration of this document de base (within the Group): – N/A Directorships and offices held as of the date of registration of this document de base (outside the Group): – – Chairman, Laboratoire HRA Pharma Chairman of the Supervisory Board, Celogos Directorships and offices held in the last five years but no longer held as of the date of this document de base (within the Group): – N/A Directorships and offices held in the last five years but no longer held as of the date of this document de base (outside the Group): – Jean-Yves Guedj 59 French October 2, 2014 on nonretroactive condition precedent General Meeting held to approve the financial statements for the year ending December 31, 2015 Independent Director N/A Directorships and other offices held as of the date of registration of this document de base (within the Group): – N/A Directorships and offices held as of the date of registration of this document de base (outside the Group): – – Doctor specializing in intensive care anesthesia, chairman of the operating theatre at the Clinique du Trocadéro in Paris and individual contractor at the American Hospital of Paris (Neuilly) Chairman and CEO, Clinique Mozart (clinic currently inactive) Directorships and offices held in the last five years but no longer held as of the date of this document de base (within the Group): – N/A Directorships and offices held in the last five years but no longer held as of the date of this document de base (outside the Group): – Anne France LaclideDrouin 46 French October 2, 2014 on nonretroactive condition precedent General Meeting held to approve the financial statements for the year ending December 31, 2017 Independent Director Directorships and other offices held as of the date of registration of this document de base (within the Group): – N/A Directorships and offices held as of the date of registration of this document de base (outside the Group): – – 171 N/A Member of the Management Board, Oberthur Technologies Holding Chief Financial Officer, Oberthur Technologies Group Name and forename(s), or company name Age Nationality Date of first appointment Expiry of term of office Main position within the Company Directorships and other offices held outside the Company (within or outside the Group) in the last five years – Member of the Executive Committee, Oberthur Technologies Directorships and offices held in the last five years but no longer held as of the date of this document de base (within the Group): – N/A Directorships and offices held in the last five years but no longer held as of the date of this document de base (outside the Group): – – – – Heather Lawrence 65 British October 2, 2014 on nonretroactive condition precedent General Meeting held to approve the financial statements for the year ending December 31, 2017 Independent Director Chief Financial Officer, Elis group Member of the Executive Committee, Elis CFO, GrandVision BV group CEO, GrandVision BV’s Multibrands holding company Directorships and other offices held as of the date of registration of this document de base (within the Group): – N/A Directorships and offices held as of the date of registration of this document de base (outside the Group): – – – – – – – Director, NMC Health PLC Member of the Audit Committee, NMC Health PLC Member of the Clinical Governance Committee, NMC Health PLC Director, Monitor Healthcare Regulator Member of the Remuneration Committee, Monitor Healthcare Regulator Chairman of the Nominations Committee, Monitor Healthcare Regulator Director in charge of the “Trust Development Authority Productivity Program”, Heather Lawrence Consulting Directorships and offices held in the last five years but no longer held as of the date of this document de base (within the Group): – N/A Directorships and offices held in the last five years but no longer held as of the date of this document de base (outside the Group): – Marie-Laure Pochon 56 French October 2, 2014 on nonretroactive condition precedent General Meeting held to approve the financial statements for the year ending December 31, 2017 Independent Director Directorships and other offices held as of the date of registration of this document de base (within the Group): – N/A Directorships and offices held as of the date of registration of this document de base (outside the Group): – – – 172 CEO, Chelsea and Westminster Hospital NHS Foundation Trust CEO, Financière Acteon SAS Chairman, SOPRO SA CEO, Apicéa group Name and forename(s), or company name Age Nationality Date of first appointment Expiry of term of office Main position within the Company Directorships and other offices held outside the Company (within or outside the Group) in the last five years Directorships and offices held in the last five years but no longer held as of the date of this document de base (within the Group): – N/A Directorships and offices held in the last five years but no longer held as of the date of this document de base (outside the Group): – – – – – – – – – Denis Ribon 45 French January 12, 2012 General Meeting held to approve the financial statements for the year ending December 31, 2015 Independent Director Director, Mauna Kea Technology Chairman Europe, GN Store Nord Member of the Management Committee, GN Resound Chairman, GN Hearing SAS Director of Commercial Operations, H. Lundbeck A/S Member of the Executive Committee, H. Lundbeck A/S Member of the Management Committee, H. Lundbeck A/S Regional Director, H. Lundbeck A/S Chairman and CEO, Lundbeck France Directorships and other offices held as of the date of registration of this document de base (within the Group): – Director, iPP Directorships and offices held as of the date of registration of this document de base (outside the Group): – – – – Chairman of the board of directors, ArchiMed Chairman of the board of directors, MediStream Chairman of the Remuneration Committee, MediStream Chairman of Ardechio SAS (family holding) Directorships and offices held in the last five years but no longer held as of the date of this document de base (within the Group): – – Member of the Strategic Committee, Labco SAS (until it became an SA) Observer at iPP Directorships and offices held in the last five years but no longer held as of the date of this document de base (outside the Group): – – – – Eric Souêtre 59 French January 12, 2012 General Meeting held to approve the financial statements for the year ending December 31, 2015 173 Director Director, Quintiles: member of the audit committee, remuneration committee and governance committee Director, Loxam Director, WFCI Director, Carso Current directorships Directorships and other offices held as of the date of registration of this document de base (within the Group): – N/A Name and forename(s), or company name Age Nationality Date of first appointment Expiry of term of office Main position within the Company Directorships and other offices held outside the Company (within or outside the Group) in the last five years Directorships and offices held as of the date of registration of this document de base (outside the Group): – – – – – – – – – – – – – – Director, Careventures Asia Director, Careventures Europe Director, Labasia Director, Tizi SPR Director, Caret SPRL Director, SARK Ltd. Director, Sark II Ltd. Director, ARZ SPRL Director, Dentak Director, Genepoc Director, Acta SA Director, Eussa SA Member of the Strategy Committee, Cathay Capital Partner Member of the Strategy Committee, Erys Group Directorships and offices held in the last five years but no longer held as of the date of this document de base (within the Group): – – Member of the Strategy Committee, Labco SAS (until it became an SA) Chairman, Labco SAS Directorships and offices held in the last five years but no longer held as of the date of this document de base (outside the Group): – N/A For the purposes of their corporate offices, the address of the members of the board of directors is that of the Company’s registered office. 14.1.1.2 Biographies of the members of the board of directors Philippe Charrier, 60, has been the Company’s Chief Executive Officer since January 2012. From 1978 to 2006, he held various positions at Procter & Gamble, where among other things he acted as management controller in France and in the United States (at the head office in Cincinnati), chief financial officer and marketing director. In 1996, he became chief executive officer in Morocco in charge of Beauty Products in the Africa and Middle East regions, before being appointed as chairman and chief executive officer of Procter & Gamble France. He then joined Oenobiol, the leading European company in nutritional, health and beauty supplements, as chief executive officer, a position that he retained after the acquisition of the company by Sanofi-Aventis in 2009. In January 2011, he was appointed Chairman of the Company and member of the Strategy Committee (equivalent to the board of directors when the Company was a société par actions simplifiée – SAS). Philippe Charrier is also Chairman of the board of directors of Alphident and Dental Emco, as well as being a director of Lafarge and of Rallye (the conglomerate controlling Casino, Franprix, Leaderprice and Monoprix, among others). He has been the Chairman of the charity Clubhouse France since 2010 and director and Chairman of UNAFAM Nationale since 2012. He is a graduate of Ecole des Hautes Etudes Commerciales (HEC Business School) and is qualified as a chartered accountant (DECF Diploma). 174 Daniel Bour, 58, has been an independent director of the Company since 2008. He is currently chairman of Générale de Solaire, a company that develops, builds and operates solar power plants. He is also chairman of Randopar. He began his career as an engineer in the rural, water and forests department of the Ministry of Agriculture before joining Compagnie Générale des Eaux in 1987. In 1988, he was appointed as the executive officer in charge of development at Générale de Santé and he became chief executive officer of Générale de Santé International in 1990 and then of Générale de Santé in 1996. He was elected chairman and chief executive officer of Générale de Santé from 1997 to 2003 and then chairman of the Management Board until 2007. Daniel Bour is a graduate of the Ecole Nationale du Génie Rural et des Eaux et des Forêts and of the Institut National Agronomique of Paris and has a degree in Economics from the Université de Paris Sorbonne. Erin Elizabeth Gainer, 41, began her career as a research associate at the Institute for Genomic Research in Rockville, US, before becoming a science teacher with the US Peace Corps in Zaka, Zimbabwe. In 2000, after roles as an epidemiologist in Baltimore and a risk analyst in the United States, she joined HRA Pharma in Paris, a laboratory specializing in treatments in the field of women’s health and endocrinology, as project manager. She was appointed executive director of research and development of HRA Pharma and became Chairman in 2009. She has also been chairman of the Supervisory Board of Celogos since 2006. Erin Gainer holds a bachelor’s degree in Biology and English from Rice University in Houston, United States (1995), a PhD in Epidemiology from the University of Paris XI and an executive MBA from INSEAD. Jean-Yves Guedj, 59, is a specialist in intensive care anesthesia, chairman of the operating theatre of the Trocadéro clinic in Paris. Until 1986, he held various positions with the Paris SAMU (emergency medical service), he was responsible for the emergency room at the American Hospital of Paris (Neuilly) where he is still an independent contractor. From 1986 to 1996 he was, in parallel, medical director at Johnson & Johnson (Critical Care department). A consultant and medical expert with the Mayo Clinic Rochester and Professor Muchada’s INSERM unit, he has taken part in extensive research work on anesthesia and nutrition, as well as the development and marketing of leading anesthesiology products such as Arrow catheters in 1991, the Close Loop System in 1993 and the Oxyshuttle in 1996. During his career, Jean-Yves Guedj has also been a founder and director of many private health establishments in France and abroad. He holds a degree in medicine, specializing in intensive care anesthesia, is a former intern at the Hôpitaux de Paris, former endocrinology clinic manager at the Bichat teaching hospital and former assistant director of the Hôpitaux privés de la Croix-Rouge. Anne-France Laclide, 46, has been chief financial officer and a member of the executive committee of the Oberthur Technologies Group since October 2013. She has been also a member of the management board of Oberthur Technologies Holding since 2013. She began her career with PricewaterhouseCoopers and subsequently held various positions in the finance departments of international groups. In 2001, she became CFO of Guilbert and retained her position at the time of the group’s partial acquisition by Staples. She then joined AS Watson and then GrandVision BV, world number two and European leader in the eyewear market, as group CFO and CEO of the Multibrands Holding company. In 2012, she became CFO and a member of the executive committee of the Elis group, leaving in October 2013 after managing and organizing the company’s debt refinancing. Anne France Laclide is a graduate of the Institut Commercial de Nancy (ICN), holds a degree in business studies and finance from the university of Mannheim, and is a qualified accountant (DESCF diploma). 175 Heather Lawrence, (Officer of the Most Excellent Order of the British Empire (OBE)), 65, began her career in the healthcare sector after training as a nurse and teacher. She then held various management positions in mental health hospitals in the United Kingdom, before being appointed CEO of London teaching hospital, Chelsea and Westminster Hospital NHS Foundation Trust, in 2000. Since July 2012, she has been a consultant working on coordinating and promoting various London hospital and health services. Heather Lawrence is a director, member of the audit committee and member of the clinical governance committee of NMC Health PLC, a top-rate healthcare services provider in the United Arab Emirates. She is also a director, member of the remuneration committee and chair of the nominations committee of Monitor Healthcare Regulator. In 1974, she obtained a degree in nursing from the University of London and she also has a teaching certificate from the University of Westminster. Marie-Laure Pochon, 56, has been CEO of the Acteon Group, world leader in small appliances and consumables for dentistry, since October 2014. She has a broad knowledge of the pharmaceuticals industry where she has held a number of positions of increasing importance: beginning in the marketing function, she was promoted to head of a business unit at the age of 30, became chairman of the French subsidiary of an international group at the age of 39, and chairman of the Europe region at the age of 49. She has worked in large US groups, including MSD for twelve years and Pfizer for four years, as well as listed European groups, including Schwarz Pharma for eight years and Lundbeck for six years. In 2012, she broadened her experience to non-pharmaceutical companies, but still in the healthcare industry: GN Store Nord as Chairman, Europe and then the Apicéa Group, French leader in the digitization of relations between hospitals, city practitioners and patients. Marie-Laure Pochon was a director of Lundbeck and Mauna Kea. For six years, she also sat on the board of Leem and co-founded an association of pharmaceuticals companies (CRIP). She has an engineering degree from ESPCI Paris Tech (Physics and Chemistry engineering college) and holds an MBA from the Institut Supérieur des Affaires (HEC). Denis Ribon, 45, has been a director of the Company since 2008. Having worked for management consulting firm A.T. Kearney, he became global head of healthcare at the private equity company 3i and co-managed 3i France until April 2014. During his thirteen years at 3i, he invested nearly €1 billion in some 20 companies in all areas of the healthcare sector, whether in medical equipment, pharmaceuticals, public health or personal care products. Since April 2014, Denis Ribon has managed the company ArchiMed, which he created and which manages funds invested in SMEs in the healthcare sector in Europe. Denis Ribon is also a director of MediStream (Switzerland) and of iPP. He was a member of the board of directors of several listed and unlisted companies in the healthcare sector, including Quintiles, Cair, Vétoquinol, Bioprofile, Neftys Pharma and Carso. He has an MBA from the Ecole des Hautes Etudes Commerciales (HEC Business School), as well as a doctorate in veterinary science from the Ecole Vétérinaire in Lyon. Eric Souêtre, 59, is co-founder of the Company and currently a director. He began his career in medical research as clinical head at the Universities of Nice and then Paris and at the National Institute of Health (NIH) in Washington DC, USA, between 1985 and 1989. In 1990, he founded and directed the international health economics research and consultancy company Benefit (as Chief Executive Officer), which was the European leader when it was acquired by the Quintiles Group (USA) in 1995, of which he was appointed Vice-President Regulatory Affairs and director. In 2003, as Chairman and CEO of the Company, he created Labco with a group of French biologists. From 2003 to 2010, he steered the Group to becoming European leader in diagnostics and was appointed director in 2010. 176 He is also Executive Chairman of Careventures (Luxembourg), a private equity fund specializing in the European healthcare sector, and of Careventures (Belgium), a private equity fund specializing in the healthcare sector in emerging countries. Eric Souêtre is qualified as a medical doctor (M.D.), has a certificate in psychiatry from the University of Nice (1983), a doctorate in neuroscience from the University of Marseilles (Ph.D., 1985) and an MBA from the Ecole des Hautes Etudes Commerciales (HEC Business School) (1990). Eric Souêtre is also a healthcare economist, expert adviser to the European Commission and author of many international publications. He is a director of and adviser to many healthcare companies in Europe, Asia and North America. 14.1.1.3 Declarations relating to the members of the board of directors As far as the Company is aware, there are no family connections between the members of the Company’s board of directors identified above. During the last 5 years, none of the members of the board of directors identified above: 14.1.2 has been found guilty of fraud, been indicted, or been the subject of any official public sanction imposed against him by statutory or regulatory authorities; has been involved in a bankruptcy, sequestration of assets or liquidation as a director or corporate officer; has been prevented by a court from acting as a member of an administrative, management or supervisory body or from being involved in the management or the conduct of the affairs of an issuer. General Management On the date of this document de base, Philippe Charrier occupies the positions of Chief Executive Officer and director of the Company. As indicated in section 14.1 “Members of the administrative, management and supervisory bodies and general management” of this document de base, he is expected to become Chairman of the Company’s board of directors. 14.1.3 Executive Committee The Group Executive Committee comprises the key members of Group Senior Management, including: 14.2 Philippe Charrier, Chief Executive Officer of the Company since January 2012; Etienne Couëlle, CEO France, director of 3i seconded to Labco in 2009; Albert Sumarroca, CEO Spain and Portugal; Luis Miguel da Palma Vieira, head of business development Europe and CEO Italy; Dr. Stuart Quinn, CEO United Kingdom since January 2014. Formerly an investor in 3i, he helped the Company to set up its joint venture iPP in the United Kingdom; Santiago Valor, Chief Medical Officer; Vincent Marcel, Chief Financial Officer since 1 February 2012; Ginette Leclerc, Legal Counsel since 8 September 2014; Philippe Cailly, Head of Group information systems. CONFLICTS OF INTEREST IN THE ADMINISTRATIVE BODIES AND IN GENERAL MANAGEMENT On the date of this document de base, and as far as the Company is aware, there are no actual or potential conflicts of interest concerning the Company between the duties of the persons referred to in section 14.1 ”Members of the administrative, management and supervisory bodies and general management” of this document de base and their private interests and other duties. 177 CHAPTER 15 COMPENSATION AND BENEFITS 15.1 COMPENSATION AND BENEFITS OF ANY NATURE ALLOCATED TO DIRECTORS AND CORPORATE OFFICERS AND MEMBERS OF ADMINISTRATIVE, MANAGEMENT AND SUPERVISORY BODIES DURING THE FINANCIAL YEARS ENDING DECEMBER 31, 2013 AND DECEMBER 31, 2014 The compensation and benefits granted to the Chairman of the board of directors, the Company’s Chief Executive Officer and the non-executive directors (namely the other members of the board of directors) comprising the board of directors on the date of settlement/delivery of the Company’s shares issued or sold in connection with its initial public offering on the Euronext Paris regulated market (in respect of the functions that they exercised in the Company during the financial years ending December 31 2013 and 2014), are set out below. At the date of this document de base, Andreas Gaddum is Chairman of the board of directors and Philippe Charrier is Chief Executive Officer. Philippe Charrier is expected to be appointed Chairman of the board at the first board meeting held after settlement/delivery of Company’s shares issued or sold in connection with its initial public offering on the Euronext Paris regulated market. At the shareholders’ meeting of October 2, 2014, Philippe Charrier was elected director effective subject to the non-retroactive condition precedent settlement/delivery of the Company’s shares issued or sold in connection with the initial public offering on the Euronext Paris regulated market. 15.1.1 Summary of the compensation of the Chairman of the board and of the Chief Executive Officer in respect of the financial years 2013 and 2014 The following table summarizes the compensation, options and shares allocated to Messrs. Andreas Gaddum and Philippe Charrier during the financial years ending December 31, 2013 and 2014. Table 1 – Summary of the compensation, options and shares allocated to the Chairman of the board and to the Chief Executive Officer (AMF nomenclature) Financial year ending December 31, 2013 Financial year ending December 31, 2014 (In euros) Andreas Gaddum, Chairman of the board Compensation due in respect of the financial year 191,6451 125,000 - - - - - - 763,040 584,780 Value of multi-year term variable compensation allocated during the financial year Value of the options allocated during the financial year - - - - Value of the performance shares allocated during the financial year - - 954,685 709,780 Value of multi-year variable compensation allocated during the financial year Value of the options allocated during the financial year Value of the performance shares allocated during the financial year Philippe Charrier, Chief Executive Officer Compensation due in respect of the financial year Total 1 Including the service contract through the company Acand and the fixed-term employment contract from October 1, 2014 to June 30, 2015 15.1.2 Compensation and benefits of any nature allocated to the Chairman of the board and to the Chief Executive Officer The following table contains a breakdown of the fixed and variable compensation and other benefits granted to Messrs. Andreas Gaddum and Philippe Charrier during the financial years ending December 31, 2013 and 2014. 178 Table 2 – Table summarizing the compensation of the Chairman of the board and of the Chief Executive Officer (AMF nomenclature) 2014 2013 Amount due Amount paid Amount due Amounts paid Fixed compensation 65,6352 65,635 Variable annual compensation 67,500 67,500 0 0 0 0 Multi-year variable compensation 0 0 0 0 Exceptional compensation 0 0 0 0 Directors’ fees 0 0 0 0 Benefits in kind 0 0 0 0 450,000 450,000 450,000 450,000 (In euros) Andreas Gaddum, Chairman of the board1 3 Philippe Charrier, Chief Executive Officer Fixed compensation Variable annual compensation 4 5 308,000 232,000 132,000 200,000 Multi-year variable compensation 0 0 0 0 Exceptional compensation 0 0 0 0 Directors’ fees 0 0 0 0 Benefits in kind 5,040 5,040 2,780 2,780 Total 828,675 752,675 652,280 720,280 6 1 2 3 4 5 6 Andreas Gaddum receives no direct compensation from the Company or any Group company. He does however receive compensation under a service contract through the company Acand (see section 19.1.4 – “Executive Administrator Agreement and other agreements with Andreas Gaddum” of this document de base). Including the service contract through the company Acand and the fixed-term employment contract from October 1, 2014 to June 30, 2015. Idem. Including the hardship allowance granted to Philippe Charrier (covering his travel abroad as well as the number of days spent abroad), as well as a bonus for meeting certain quantitative objectives such as increasing Group Revenue, increasing EBITDA and other qualitative objectives such as the creation of synergies and, if applicable, fulfilling certain important external growth transactions. Includes the amount due in respect of 2013 and a part payment of variable compensation due in respect of 2014. Company car. 15.1.3 Compensation and benefits of any nature allocated to the directors The following table contains details of the amount of directors’ fees and other compensation paid to the Company’s directors by the Company or by any Group Company during the financial years ending December 31, 2013 and December 31, 2014. Table 3 – Table showing directors’ fees and other compensation received by the directors (AMF nomenclature) Directors Amount paid during the financial year 2014 Amount paid during the financial year 2013 0 0 Andreas GADDUM, Chairman of the board Directors’ fees 1 Other compensation 126,010 57,5002 Philippe CHARRIER - - Directors’ fees 0 0 Other compensation 0 0 179 Directors Amount paid during the financial year 2014 Amount paid during the financial year 2013 Daniel BOUR - - Directors’ fees 0 70,000.003 Other compensation 0 0 Directors’ fees N/A N/A Other compensation N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A 0 0 0 0 0 0 0 0 Erin Elizabeth GAINER Heather LAWRENCE Directors’ fees Other compensation Jean-Yves GUEDJ Directors’ fees Other compensation Anne-France LACLIDE Directors’ fees Other compensation Marie-Laure POCHON Directors’ fees Other compensation Denis RIBON Directors’ fees Other compensation Eric SOUÊTRE Directors’ fees Other compensation 1 2 3 Including the service contract through the company Acand and the fixed-term employment contract from October 1, 2014 to June 30, 2015. Including the service contract through the company Acand and the fixed-term employment contract from October 1, 2014 to June 30, 2015. Payment for 2011 and 2012. Voted upon but not approved at the time. 15.1.4 Subscription and share purchase options allocated by the Company or by any Group Company to the Chairman of the board and to the Chief Executive Officer Not applicable. 15.1.5 Subscription and share purchase options exercised by the Chairman of the board and by the Chief Executive Officer Not applicable. 180 15.1.6 Performance shares allocated to the Chairman of the board and to the Chief Executive Officer Shares allocated for free to each corporate officer Shares allocated for free by the shareholders’ general meeting during the financial year to each corporate officer by the issuer and by each group company (nominative list) Philippe Charrier Andreas Gaddum TOTAL 1 2 No. and date of plan Plan no.1 Date: 02/10/2014 Plan no.1 Date: 02/10/2014 Number of shares allocated during financial year Value of the shares according to the method used for the consolidated accounts (in euros) Date of acquisition Date of availability Performance conditions 68,736 434,411.52 November 13, 2016 November 13, 2018 Yes1 20,620 130,318.40 November 13, 2016 November 13, 2018 Yes2 89,356 564,729.89 See section 15.1.11 “History of allocations of free shares – Information on shares allocated for free” of this document de base. Idem 15.1.7 Performance shares that became available to the Chairman of the Board and to the Chief Executive Officer Not applicable. 15.1.8 History of allocations of subscriptions and acquisitions of share purchase options The Company did not allocate any share purchase options or subscriptions. 15.1.9 History of allocations of acquisitions of share purchase warrants As indicated in section 21.1.4 “Potential Capital” of this document de base, since 2005 the Company has issued share purchase warrants that have been acquired by two of the Group’s executives. At the date of this document de base, Andreas Gaddum holds 25,407 2008-1-1 share purchase warrants, which have vested. Daniel Bour holds 14,078 C1-10xT1 share purchase warrants, that he acquired. 181 History of acquisition of share purchase warrants Date of meeting January 31, 2008 July 21, 2008 Date of Strategic Committee of the Company (when in used to be in the form of SAS) March 5, 2008 N/A Total number of shares capable of subscription of which the number capable of subscription or acquisition by: 39,925 8,411 Corporate officers: Daniel Bour 0 14,078 ABSA C1-10xT1 Andreas Gaddum 25, 407 BSA 2008-1-1 0 Start date for exercise of share purchase warrants June 1, 20151 From the “exit” date as per the issuing contract Expiry date December 31, 2017 July 24, 2023 Subscription price of warrants at issuance 0.80 euros 14.206582 euros Procedure for exercise (when the plan includes several tranches) N/A N/A Number of shares subscribed for on the date of this document de base 0 0 Cumulative number of canceled or null share purchase warrants 0 0 Share purchase warrants remaining at the end of financial year 2015 N/A 0 1 2 2 Period of exercise amended by the Extraordinary General Meeting of October 2, 2014, after authorization by the General Meeting of 2008-1-1 Warrant holders, on the non-retroactive condition precedent of settlement/delivery of the Company’s shares issued or sold in connection with the initial public offering of the Company. Subscription price of one ABSA C1. 15.1.10 Share purchase options allocated to the top ten non-corporate officer employees Not applicable. 15.1.11 History of allocations of free shares – Information on shares allocated for free No free shares were allocated during the financial years ending December 31, 2013 and 2012. The free shares allocated in 2011 were cancelled during the financial year 2012. The introduction of a free allocation plan of performance shares was proposed and approved at the General Meeting of shareholders of the Company on October 2, 2014. The beneficiaries of this plan are the members of the Company’s management and the Group’s key management personnel (namely 67 persons). The performance shares allocated on November 13, 2014 in connection with this plan represent 1.0% of the Company’s capital (i.e. 687,361 shares). These shares will vest after a period of two years and the beneficiaries will be required to hold the shares for the minimum period set by the board of directors, i.e. two years from the vesting date. The board of directors have made the vesting of the shares conditional on the fulfillment of certain performance criteria related to recurrent EBITDA growth (defined as EBITDA as per the consolidated accounts as of December 31, 2014, excluding transaction costs, IFRS 2 and 3 expenses, restructuring costs and start up costs in the United Kingdom) for the 2014 financial year in comparison to the 2013 financial year, corresponding to an amount of at least €2.2 million. The condition imposed by the board of directors was fulfilled. 182 History of allocations of free shares – Information on shares allocated for free (AMF nomenclature) Plan no. 1 Date of plan (date of shareholders’ meeting) 10/02/2014 Date of Board meeting 11/13/2014 Total number of shares allocated free of charge, including the number allocated to: Philippe Charrier Andreas Gaddum Date of acquisition of the shares Date of the end of the retention period 1% of share capital, i.e. 687,361 ordinary shares 10% of the plan, i.e. 68,736 shares 3% of the plan, i.e. 20,620 shares 2 years from the award date 2 years from the date of acquisition Number of shares subscribed on the date of this document de base 0 Cumulative number of cancelled or lapsed shares 0 Remaining shares allocated free of charge at the end of the financial year The entire plan awarded on 11/13/2014 15.1.12 Change in compensation of executive corporate officers and directors’ fees paid to members of the Board of directors after the initial public offering In connection with the Company’s initial public offering, the board of directors’ approved, on March 5, 2015, the payment of exceptional compensation. This payment, for a variable amount comprised between €0 and an estimated maximum of €4.5 (net of social security and employer contributions that are not deductible), could be paid to 29 executives and managers of the Group, including a variable amount comprised between €0 and an estimated maximum of €945,000 that would be awarded to Mr. Philippe Charrier, the Company’s Chief Executive Officer. The exact amount of these bonuses and their combined amount will depend on the Company’s share price set for the IPO. The remuneration of the Chief Executive Officer will be decided by the then-incumbent board of directors following the initial public offering. After the initial public offering directors’ fees will be determined in the following manner: the General Meeting will fix an overall budget, the amount of which will be divided between the directors by the board of directors, it being noted that the Compensation Committee of the Company has proposed that the budget for the financial year ending December 31, 2015 be fixed at €250,000, half of which will be allocated on the basis of the attendance conditions. 183 15.1.13 Employment contracts, retirement benefits and payments in the event of termination of the functions of the Chairman of the board and of the Chief Executive Officer (In euros) Employment contracts yes Andreas Gaddum, Chairman of the board of directors Start of term of office: January 12, 2012 no Supplementary pension scheme yes no 1 X X X X Payments or benefits due or likely to be due as a result of the termination or alteration of functions yes no Payments relating to a noncompetition clause yes no X X End of term of office2 Philippe CHARRIER, Chief Executive Officer Start of term of office: January 12, 2012 X X End of term of office3 1 2 3 Fixed-term employment contract from October 1, 2014 to June 30, 2015. Date of the shareholders’ meeting called for 2018 to vote on the financial statements for the financial year ending December 31, 2017. Date of the shareholders’ meeting called for 2018 to vote on the financial statements for the financial year ending December 31, 2017. Employment contract Philippe Charrier has entered into an employment contract with the Company. That contract was suspended at the time of his appointment as Chairman of the Company with effect from January 3, 2011, when the Company was still incorporated as a société par actions simplifiée. That suspension was maintained due to his appointment as Chief Executive Officer of the Company with effect from January 12, 2012 (the Company having then been reincorporated as a société anonyme). During the suspension of his employment contract, Philippe Charrier receives compensation in respect of his office as Chief Executive Officer. (see sections 19.2.1 “Statutory auditor’s special report on regulated agreements for the financial year ended December 31, 2013” and 19.2.2. “Statutory auditor’s special report on regulated agreements for the financial year ended December 31, 2012” of this document de base). Supplementary pension scheme No executive corporate officer has the benefit of a supplementary pension scheme. Payments and benefits due or likely to be due as a result of a change of duties or termination of employment (after the initial public offering) In the context of a transaction affecting the Company’s share capital involving only a partial or total sale of the capital to a new strategic investor, a decision taken by the board of directors to revoke or not to renew the corporate office of Philippe Charrier, within twelve months of the final completion of such a transaction by the Company irrevocably undertakes to pay him a severance payment equal to twelve months’ gross basic fixed monthly compensation (excluding variable compensation) if he cannot resume the performance of his employment contract as Chief Operating Officer on the same financial terms as his current corporate office as Chief Executive Officer (see sections 19.2.1. “Statutory auditor’s special report on regulated agreements for the financial year ended December 31, 2013” and 19.2.2. “Statutory auditor’s special report on regulated agreements for the financial year ended December 31, 2012” of this document de base). In addition, in the context of a transaction affecting the Company’s share capital followed by decisions resulting in the revocation or non-renewal of the corporate office of Philippe Charrier, within twelve months of the final completion of such a transaction the Company shall pay him a severance payment equal to twelve months’ gross basic fixed monthly compensation (excluding variable compensation). 184 Payments due relating to a non-competition clause Philippe Charrier’s corporate officer’s contract also contains a non-compete clause, which, if implemented, would entitle him to compensation for a gross fixed amount of €337,500 payable in 24 monthly installments of equal amounts, subject to compliance with the non-compete obligation (see sections 19.2.1. “Statutory auditor’s special report on regulated agreements for the financial year ended December 31, 2013” and 19.2.2. “Statutory auditor’s special report on regulated agreements for the financial year ended December 31, 2012” of this document de base). The term of this non-compete clause is 24 months as of the termination date (whether as director, directly or through any entity, or as corporate officer, member of the strategy committee or employee). Pursuant to this clause, Philippe Charrier may not: 15.2 be involved directly or indirectly in any way whatsoever (and particularly as director, corporate officer, employee, consultant or freelancer), either on his own account or on account of another person, in any business that could directly or indirectly compete with the Group’s business activities; acquire or subscribe for shares, directly or indirectly through one or more holding companies, in a company that conducts a business that could directly or indirectly compete with the Group’s business activities (with the exception of acquiring minority interests of less than 5% in listed companies for financial or wealth management purposes); approach customers or commercial or other partners of the Group, or encourage them to terminate, cancel or refrain from entering into a relationship (contractual or otherwise) with the Group. AMOUNTS PLACED IN RESERVES BY THE GROUP TO PAY PENSIONS, RETIREMENTS OR OTHER BENEFITS TO SENIOR EXECUTIVES As indicated in section 15.1.13. “Employment contracts, retirement benefits and payments in the event of termination of the functions of the Chairman of the board and of the Chief Executive Officer” of this document de base, no executive corporate officer benefits from a specific pension scheme. Both in respect of his employment contract and his corporate office, Philippe Charrier benefits from the same pension scheme as the company’s employees. The company has therefore not made provision for any specific sum in respect of the payment of pensions, retirements or other similar benefits to the executive corporate officers. 185 CHAPTER 16 FUNCTIONING OF THE COMPANY’S ADMINISTRATIVE AND MANAGEMENT BODIES The functioning of the Company’s board of directors is determined by legal and regulatory provisions, the Company’s Articles of Association and its own internal regulations, the main provisions of which are set out in section 16. “Functioning of the Company’s administrative and management bodies.” The Articles of Association described in this document de base are those that will enter into force subject to the non-retroactive condition precedent of settlement/delivery of the Company’s shares issued or sold in connection with its initial public offering on the Euronext Paris regulated market. The internal regulations of the board of directors described in this document de base are those of the Company as approved by the Company’s board of directors before the completion of the Company’s initial public offering and whose approval shall be reiterated by the newly constituted board of directors immediately after completion of the Company’s initial public offering. The expiry dates of the terms of the members of the board of directors, in its new form, and of the Company’s management are set out in section 14.1. “Members of the administrative, management and supervisory bodies and general management” of this document de base. 16.1 THE FUNCTIONING OF THE COMPANY’S ADMINISTRATIVE AND MANAGEMENT BODIES 16.1.1 Board of directors 16.1.1.1 Composition of the board of directors The number of directors and independent directors (Article 14 of the Articles of Association, Article 2 of the Internal Regulations) The Company is administered by a board of directors composed of between three and 18 members. The upper limit of 18 members can be increased, if necessary, by directors representing employee shareholders, appointed in accordance with Article 14.8 of the Company’s Articles of Association, by directors representing employees, appointed in accordance with Article 14.9 of the Company’s Articles of Association and in the event of a merger, in accordance with Article L. 225-95 of the French Commercial Code. Appointments to the board of directors are made in order to ensure that men and women are equally represented, in accordance with the provisions of Article L. 225-17 of the French Commercial Code. In accordance with the AFEP-MEDEF Code, the Internal Regulations of the board of directors provide that a director is independent if he or she has no relationship of any kind with the Company or with any company or entity controlled directly or indirectly by the Company within the meaning of Article L. 233-3 of the French Commercial Code (a Group Company), or with their management, that could compromise the exercise of his or her freedom of judgment. The Internal Regulations of the board of directors also provide that, every year, the independent status of each of the directors must be discussed by the Nominations and Compensation Committee and examined on a case by case basis by the board of directors having regard to the criteria regarding qualification as an independent director set out below. The independent status of directors is also discussed when a new director is appointed and when the term of office of the directors is renewed. The shareholders are informed of the conclusions of the board of directors’ examination of the directors’ independent status in the report of the Chairman of the board of directors to the Company’s Annual Ordinary General Meeting. The criteria that must be examined by the Nominations and Compensation Committee and by the board of directors, and that must all be satisfied in order to qualify as an independent director, are as follows: not to be an employee or executive director of the Company or an employee or director of its parent company or of a company within its scope of consolidation, and not to have held any such positions in the previous 5 years; not to be a corporate officer of a company in which an employee appointed as such or a corporate officer of the Company (whether currently or in the previous 5 years) holds office as a director or as a member of the supervisory board; 186 not to be a customer, supplier, investment banker or commercial banker that is significant for the Company or for the Group, or of whose business the Company or the Group represents a significant proportion; in the case of directors exercising functions in one or more banks, not to have taken part (i) in the preparation or solicitation of offers of services by any of those banks to the Company or to a Group Company; (ii) in the work of any of those banks in performance of a mandate entrusted to that bank by the Company or by a Group Company; or (iii) in the vote on any resolution concerning a project in which the bank concerned is or could be involved as an adviser; not to have any close family connections with a corporate officer of the Company or of a Group Company; not to have been a statutory auditor of the Company’s accounts in the last 5 years; not to have been a member of the Company’s board of directors for more than 12 years, on the understanding that independent member status will only be lost upon the expiry of a term of office during which the 12-year period is exceeded. In the case of members of the board of directors holding 10% or more of the Company’s share capital or voting rights, or who represent a legal person holding such a shareholding, the board of directors will make a decision on independent status based on a report from the Nominations and Compensation Committee, taking account, in particular, of the composition of the Company’s capital and the existence of potential conflicts of interest. The board of directors can, however, take the view that a director should not be given independent status having regard to his or her particular situation, even though he or she satisfies the criteria set out above. The Company’s General Meeting of shareholders appointed seven independent directors in light of the criteria set out above, subject to the non-retroactive condition precedent of settlement/delivery of the Company’s shares issued or sold in connection with its initial public offering on the Euronext Paris regulated market. Term of office of the directors (Article 16 of the Articles of Association) Subject to the applicable legislative and regulatory provisions in the case of appointments made provisionally by the board of directors, directors are appointed for a period of 4 years. Exceptionally, the General Meeting has, upon the appointment of certain members of the board of directors, provided that their terms of office shall be less than 4 years, in order to arrange for the staggered renewal of the terms of office of the members of the board of directors. The meeting of October 2, 2014 made provision for such staggered renewals in accordance with the information provided in section 14.1.1.1. “Composition of the board of directors.” Directors’ functions will cease at the end of the Ordinary General Meeting convened to approve the accounts for the previous financial year during the year in which their term of office expires. Directors may be re-elected. Age limit (Article 16 of the Articles of Association) The number of directors (whether natural persons or representatives of legal persons) aged over 70 years may not exceed a quarter of the directors in office, rounded up, if necessary, to the nearest whole number. No person aged over 70 years may be appointed as a director if his or her appointment has the effect of increasing the number of directors aged over 70 years to more than a quarter of the directors in office, rounded up, if necessary, to the nearest whole number. If the number of directors aged over 70 years comes to represent more than a quarter of the directors in office, then unless a director aged over 70 years resigns, the oldest director will automatically be deemed to have resigned from office. 187 Number and status of directors representing the Group’s employees (Article 14 of the Articles of Association) Pursuant to Article L. 225-27-1 of the French Commercial Code, the board of directors includes one or two directors representing the Group’s employees, depending on the number of directors. The number of directors representing employees is two if there are more than 12 directors on the date of appointment of the directors representing employees, and one if the number of the directors is twelve or less on the date of appointment of the director representing employees (in each case, not counting directors representing employee shareholders and directors representing employees). The reduction of the number of directors to twelve or less (not counting directors representing employee shareholders and directors representing employees) will not have any effect on the current terms of office of directors representing employees, which will continue until their expiry. However, upon expiry of the terms of office of directors representing employees, and in the event that the number of directors is still twelve or less on the date of appointment of directors representing employees (not counting directors representing employee shareholders and directors representing employees), the number of directors representing employees will be reduced to one. If, at a later date, the number of directors becomes more than twelve (not counting directors representing employee shareholders and directors representing employees), a second director representing employees will be appointed in accordance with the provisions below, within a period of six months from the date of cooptation by the board of directors, or appointment by the Ordinary General Meeting, of the new director. Directors representing employees are elected as per the conditions provided by Article L. 225-28 of the French Commercial Code and in the manner described below. Employee directors are elected by all employees qualifying as electors in a single electoral college. In accordance with Article L. 225-28 of the French Commercial Code, the election will take place in one round using a proportional representation list system based on the highest remainder and without transferable votes. Each list must include a number of candidates that is double the number of seats to be filled, and must strictly observe equality between men and women. No deputies will be elected. The lists of candidates will be presented exclusively by one or more representative union organizations at Group level. Elections will be organized by General Management. The timetable (and in particular the date for declaration of candidates and the date of the ballot) and any terms and conditions of the electoral operations not specified by the legal or regulatory provisions in force or by the Articles of Association (including the choice of voting methods) will be decided by General Management after consultation with the representative union organizations. The timetable will be drawn up in such a way that the result of the elections can be announced no later than 15 days before the end of the terms of office of the outgoing directors. In the case of the first election taking place pursuant to Law no. 2013-504 of 14 June 2013, the timetable will be drawn up in such a way that the results of the elections can be announced, at the latest, before the expiry of the six-month period following the Extraordinary General Meeting that amended the Articles of Association, as referred to in Article L. 225-27-1 III of the French Commercial Code. At the time of each election, General Management will settle the list of the Company’s direct or indirect subsidiaries whose registered office is in France in accordance with Articles L. 225-27-1 and L. 225-28 of the French Commercial Code. Votes will be cast either electronically, on paper or by post, or by a combination of these methods. When votes are cast electronically, this may be done at the workplace or remotely within a period not exceeding 15 days. The design and installation of the electronic voting system can be entrusted to an external service 188 provider. The system must ensure the confidentiality of the data transmitted, and the security of the means of authentication, signature, recording and counting of votes. In the event of a lack of candidates in the college, the relevant seat or seats will remain vacant until the next elections to renew the term of office of the directors representing employees. In the event of a definitive vacancy for a director representing employees, the vacant seat will be filled in accordance with the provisions of Article L. 225-34 of the French Commercial Code, namely by the candidate appearing on the same list immediately after the last candidate elected. Directors representing employees are not taken into account in determining the minimum and maximum number of directors provided by paragraph 14.1 above. The term of office of directors representing employees is five years. In the event of termination of their employment contract, directors representing employees are automatically deemed to have resigned, and will be replaced in the manner set out above. Newly-elected directors representing employees will take office upon the expiry of the term of office of the outgoing directors representing employees. Directors representing employees are not taken into account for the purposes of the provisions set out in paragraph 16.3 below. In the event that the legal conditions relating to the scope of the obligation to appoint one or more directors representing employees are no longer satisfied, the term of office of directors representing employees will cease at the end of the meeting at which the board of directors notes that the obligation no longer applies. The number of shares of the Company owned by members of the board of directors (Article 11 of the Internal Regulations) Every director, other than those representing employee shareholders, must own at least 1,000 shares of the Company in pure registered form. 16.1.1.2 Directors’ duties The Internal Regulations of the board of directors supplement the legal provisions and the provisions of the Articles of Association relating to the rights and duties of the directors, and take account of the recommendations of the AFEP-MEDEF Code. They are also subject to the obligations the terms of which are summarized below. General obligations (Article 6 of the Internal Regulations) Each member of the board of directors must, before accepting office, ensure that he or she is familiar with his or her general and particular obligations. In particular, he or she must be familiar with the legislative and regulatory provisions in force in connection with his or her function, with the Company’s Articles of Association and with the Internal Regulations of the board of directors, all of the provisions of which are binding on him or her. Each member of the board of directors must also ensure that he or she complies with the legislative and regulatory provisions governing the functions of members of the board of directors of a société anonyme, the provisions of the Company’s Articles of Association and the Internal Regulations of the board of directors, and in particular with the rules relating to: - the definition of the powers of the board of directors; the cumulative number of offices held; the grounds for incompatibility and incapacity; agreements concluded directly or indirectly between a member of the board of directors and the Company; and 189 - the holding and use of privileged or confidential information. Obligation of loyalty and management of conflicts of interest (Article 7 of the Internal Regulations) Members of the board of directors must not in any circumstances act in their own interests contrary to those of the Company. Every member of the board of directors is under an obligation to inform the board of directors of any conflict of interest situation, even potential, between him or her (or any natural person with whom he or she has a family connection) and the Company, any of the companies in which the Company has a shareholding or any company with which the Company is contemplating entering into an agreement of any kind whatsoever. The member of the board of directors concerned must refrain from attending and voting in the deliberations of the board of directors in respect of which he or she has a conflict of interest and in the discussions preceding that vote, unless the agreement concerned is a standard agreement entered into on standard terms. Non-compete obligation (Article 8 of the Internal Regulations) Throughout his or her term of office, every member of the board of directors must refrain from exercising any function in a business in competition with the Company or with any Group Company without first obtaining the consent of the Chairman of the board of directors. General information obligation (Article 9 of the Internal Regulations) Every member of the board of directors must, in accordance with the legislative and regulatory provisions in force both in France or at a European level, provide the board of directors with complete information relating to the remuneration and benefits of any kind paid to him or her by the Company or by any Group Company, to his or her corporate offices and functions in any companies and other legal persons, and to any convictions. Confidentiality obligation (Article 10 of the Internal Regulations) In general, all documentation for meetings of the board of directors and all information gathered during meetings of the board of directors or elsewhere in relation to the Group, its business and its prospects, is confidential, without exception, regardless of whether the information gathered was presented as confidential. Apart from the simple obligation of discretion provided by the legislative and regulatory provisions in force, every member of the board of directors must regard himself or herself as subject to an absolute obligation of professional secrecy. Obligations relating to the ownership of financial instruments issued by the Company (Article 11 of the Internal Regulations) In accordance with the legislative and regulatory provisions in force, every member of the board of directors undertakes to comply with the requirements relating to declaratory obligations as regards the AMF. In addition, members of the board of directors and persons connected to them within the meaning of the applicable legislative and regulatory provisions, must not carry out any operation in relation to the Company’s securities during the period of 30 calendar days preceding the date of publication of the consolidated annual and half-yearly results, or during the period of 15 calendar days preceding the date of publication of the quarterly accounts. Obligation of diligence (Article 12 of the Internal Regulations) All members of the board of directors must devote the necessary time and attention to their functions. Except in the event of an insurmountable impediment, every member of the board of directors undertakes to be assiduous in attending, in person, all meetings of the board of directors, all General Meetings of Shareholders and the meetings of any committees created by the board of directors of which he or she is a member, if necessary by means of video-conferencing or telecommunication. 190 Obligation to keep personal records (Article 13 of the Internal Regulations) Members of the board of directors are under an obligation to keep personal records. The board of directors and each of its members can obtain all documents or information that they consider useful or necessary to complete their tasks. Members of the board of directors may make requests for information to the Chairman of the board of directors, who will be responsible for ensuring that they are satisfied. 16.1.1.3 Powers of the board of directors (Article 19 of the Articles of Association, Section II of the Internal Regulations) On a proposal from the Chief Executive Officer, the board of directors shall determine the strategy for the Company’s business and ensure that it is implemented. Subject to the powers expressly attributed to shareholders’ meetings and within the limitations of the corporate purchase, the board of directors will deal with any questions concerning the proper running of the Company and make decisions by way of resolutions on matters of concern to it. It shall carry out such inspections and checks as it sees fit within the limitations of its functions. The Internal Regulations of the board of directors provide that, in addition to the powers provided by law, regulations and the Articles of Association, the following operations and decisions must be expressly approved by the board of directors in advance before being implemented by the Company’s Chief Executive Officer or, if applicable, Deputy Chief Executive Officer, in connection with the Group’s internal organization: decisions relating to a significant establishment in France or abroad, whether directly by the creation of an establishment, business, branch, direct or indirect subsidiary, or indirectly by the acquisition of an investment; decisions to withdraw such establishments in France or abroad; any merger, spin-off, partial asset contribution or similar operation; the conclusion, amendment or termination of any commercial or industrial cooperation agreement, joint venture, consortium or combination with a third party (excluding agreements concluded in the normal course of business) liable to have a significant impact on the Group’s business or to have a significant impact in the context of a future reconfiguration of the Company’s capital (in particular in change of control clauses or other provision(s)); significant transactions liable to affect the Group’s strategy and to alter its financial structure or the scope of its business; the transfer of ownership of patents used in the Company’s key technologies and the grant of any licenses relating to such key technologies other than in the normal course of business; the acquisition or sale of any investment in any company in existence or to be formed, participation in the formation of any company, grouping or organization and subscriptions for any issue of shares or bonds, excluding treasury operations; the creation of security rights over corporate assets. The Chief Executive Officer and any other person duly authorized to implement such transactions will be responsible for assessing the significant nature of the transactions referred to above: 5 each of the following transactions or decisions involving an investment or disinvestment for the Company or for any Group company5 in an amount equal to or exceeding €10,000,000: the acquisition or sale of buildings; This prior approval procedure does not apply, however, to transactions and decisions giving rise to the conclusion of agreements exclusively between entities controlled by the Company and the Company itself. 191 each of the following transactions or decisions involving an investment or disinvestment, a commitment of expenditure or guarantee for the Company or for any Group company in an amount equal to or exceeding €10,000,000: - any exchanges of property, securities or assets, with or without balancing cash payments, excluding treasury operations; in the event of litigation, the signature of any agreements or settlements, and acceptance of any arbitrage and compromises. the conclusion of any loans, borrowings, credits and advances; the acquisition or assignment of any debt by any means; any industrial or commercial project regarded as significant by the Company’s Chief Executive Officer. 16.1.1.4 Resolutions of the board of directors (Article 18 of the Articles of Association, Section IV of the Internal Regulations) Meetings of the board of directors shall take place as often as is required in the interests of the Company and at least once per quarter and shall be convened by its Chairman or, in the event of his death or temporary unavailability, by at least a third of the directors, by any means in writing, ten calendar days before the date of the meeting, this period being capable of reduction in the event of a duly-justified emergency. The board of directors can nevertheless deliberate even in the absence of a notice of meeting, if all its members are present or represented. At least a third of the directors can either request the Chairman to convene a meeting of the board of directors, or can directly convene such a meeting, on a specific agenda, if the board of directors has not met for more than one month. The Chief Executive Officer or, if applicable, a Deputy Chief Executive Officer, can also ask the Chairman to convene a meeting of the board of directors on a specific agenda. In both cases, the Chairman is bound by the requests made to him and must convene a meeting of the board no less than three calendar days after the request, this period being subject to reduction in an emergency. Meetings of the board of directors will preferably take place at the registered office, or in any other place stated in the notice of meeting. Meetings of the board of directors will be chaired by the Chairman of the board of directors. In the event of the Chairman’s absence, the board of directors will appoint the Chairman of the meeting from among the directors. The board of directors may only validly deliberate if at least half of the directors are present. Decisions of the board of directors are taken by a simple majority of the votes of the members present or represented, and each director present may only represent a single director. In the event of a tied vote, only the current Chairman of the board of directors will have a casting vote. If the current Chairman of the board of directors does not attend the meeting of the board, the ad hoc Chairman of the meeting will not have such a casting vote. For the purposes of calculating the quorum and the majority, directors taking part in meetings of the board of directors by means of video-conferencing or telecommunication that comply with the technical characteristics laid down by the legislative and regulatory provisions in force, will also be deemed to be present under the conditions and in the manner provided by the Internal Regulations of the board of directors. 16.1.1.5 Directors’ remuneration (Article 17 of the Articles of Association, Article 23 of the Internal Regulations) The main provisions of the Articles of Association and Internal Regulations governing directors’ remuneration are described in paragraph 15.1. “Compensation and benefits of any nature allocated to directors and corporate officers and members of administrative, managerial and supervisory bodies during the financial years ending December 31, 2013 and December 31, 2014” of this document de base. 192 16.1.2 General Management Pursuant to the provisions of the Articles of Association and Internal Regulations, general management falls under the responsibility of either the Chairman of the board of directors, who, in this case, will have the title of Chairman and Chief Executive Officer, or of another natural person appointed by the board of directors from among its members or elsewhere, who in this case will have the title of Chief Executive Officer. The board of directors shall choose between these two methods of general management in a decision taken by a majority of the directors present or represented. If the board of directors decides to separate the functions of the Chairman of the board of directors and the Chief Executive Officer, it shall appoint a Chief Executive Officer. If the general management of the Company is the responsibility of the Chairman of the board of directors, the provisions relating to the Chief Executive Officer will apply to him. On a proposal from the Chief Executive Officer, the board of directors may appoint one or two natural persons to assist the Chief Executive Officer, with the title of Deputy Chief Executive Officer, from among its members or elsewhere. 16.1.2.1 Chairman of the board of directors (Article 15 of the Articles of Association, Article 14 of the Internal Regulations) The Chairman of the board of directors is appointed for a period that cannot exceed his or her term of office as a director. He or she can be re-elected. He or she can be dismissed by the board of directors at any time. The age limit for exercising the functions of Chairman of the board of directors is 70 years. The Chairman of the board of directors organizes and manages the work of the board of directors, and accordingly reports to the General Meeting. He ensures that the Company’s corporate bodies, including its committees, function correctly and in particular, ensures that the directors are in a position to carry out their tasks, including on the committees. The Chairman of the board of directors is available at all times to the members of the board of directors, who can ask him any question regarding their tasks, and ensures that the members of the board of directors devote the necessary time to matters of interest to the Company and to Group Companies. The main provisions of the Articles of Association and of the Internal Regulations governing the remuneration of the Chairman of the board of directors are described in section 15.1. “Compensation and benefits of any nature allocated to directors and corporate officers and members of administrative, management and supervisory bodies during the financial years ending December 31, 2013 and December 31, 2014” of this document de base. 16.1.2.2 Chief Executive Officer (Articles 21, 22, 24, 25 and 26 of the Articles of Association, Article 5 of the Internal Regulations) The Chief Executive Officer is appointed by the board of directors, who set his term of office, which cannot exceed if applicable his or her term of office as a director, as well as his or her remuneration. The Chief Executive Officer can be dismissed by the board of directors at any time. The age limit to exercise the functions of Chief Executive Officer is 70 years. The Chief Executive Officer is invested with the broadest powers to act in all circumstances on the Company’s behalf. He exercises those powers within the limitations of the corporate purchase, subject to the powers expressly attributed to shareholders’ meetings and to the board of directors by the legislative and regulatory provisions in force, and subject to the prior approvals to be obtained from the board of directors in accordance with the provisions of the Internal Regulations of the board of directors. 193 The board of directors can, in addition, place limitations on the powers of the Chief Executive Officer in the decision relating to his appointment, as well as specific limitations on his powers for a particular transaction, which will, if necessary, be recorded in the minutes of the decision of the board of directors authorizing said transaction. The Chief Executive Officer represents the Company in its relations with third parties. The main provisions of the Articles of Association and in the Internal Regulations governing the remuneration of the Chief Executive Officer are described in section 15.1. “Compensation and benefits of any nature allocated to directors and corporate officers and members of administrative, management and supervisory bodies during the financial years ending December 31, 2013 and December 31, 2014” of this document de base. 16.1.2.3 Deputy Chief Executive Officers (Articles 23 to 26 of the Articles of Association, Article 5 of the Internal Regulations) On a proposal from the Chief Executive Officer, the board of directors can appoint one or two Deputy Chief Executive Officers from among its members or elsewhere. The Deputy Chief Executive Officer can be dismissed by the board of directors at any time, by a proposal from the Chief Executive Officer. The age limit for exercising the functions of Deputy Chief Executive Officer is 70 years. In agreement with the Chief Executive Officer, the board of directors determines the scope and duration of the powers conferred on each of the Deputy Chief Executive Officers. The board of directors can also place specific limitations on their powers for a particular transaction, which will, if necessary, be recorded in the minutes of the decision of the board of directors authorizing said transaction. Deputy Chief Executive Officers will have the same powers as the Chief Executive Officer with regard to third parties. The main provisions of the Articles of Association and in the Internal Regulations governing the remuneration of Deputy Chief Executive Officers are described in section 15.1. “Compensation and benefits of any nature allocated to directors and corporate officers, members of administrative, management and supervisory bodies during the financial years ending December 31, 2013 and December 31, 2014” of this document de base. 16.2 SERVICE CONTRACTS BETWEEN MEMBERS OF ADMINISTRATIVE, MANAGEMENT AND SUPERVISORY BODIES AND THE COMPANY OR ITS SUBSIDIARIES As far as the Company is aware, on the date of registration of this document de base and subject to the following, no service contracts have been concluded between the Company or the Group Companies and any of the members of the board of directors identified in section 14.1.1.1. ”Composition of the board of directors” of this document de base. However, it should be noted that Andreas Gaddum, Chairman of the board of directors on the date of this document de base, has a service contract through the company Acand, over which he has total control, under which he bills advisory fees, on a case by case basis, the amount of which is agreed with the Company in accordance with the terms of the contract. Andreas Gaddum also has a fixed-term employment from October 1, 2014 to June 30, 2015. 16.3 OBSERVERS (ARTICLE 20 OF THE ARTICLES OF ASSOCIATION AND ARTICLES 21.5 TO 21.8 OF THE INTERNAL REGULATIONS) The main provisions of the Articles of Association and of the Internal Regulations governing the status of observers are described respectively in Article 20 of the Articles of Association and Articles 21.5 to 21.8 of the Internal Regulations. Appointment of observers The Ordinary General Meeting can appoint observers to the board of directors, from among the shareholders. 194 The number of observers cannot exceed three. Observers are appointed for a term of three years, on the understanding that the Company’s Ordinary General Meeting can dismiss them at any time. Their functions cease at the end of the Ordinary General Meeting convened to approve the accounts for the previous financial year held in the year in which their term of office expires. Observers can be re-elected. Any observer reaching the age of 70 years is automatically deemed to have resigned. The missions, and, if applicable, the terms of compensation of observers are matters within the remit of the board of directors and are described in the Internal Regulations of the board of directors. Powers and obligations of observers Observers are invited to attend all meetings of the board of directors. They are asked to attend meetings of the Board of directors as observers and can be consulted by the board. The board of directors can allocate specific tasks to the observers. They take part in the deliberations in an advisory capacity. Observers are bound to comply with the confidentiality obligations referred to in Article 10 of the Internal Regulations. 16.4 COMMITTEES Subject to the non-retroactive condition precedent of settlement/delivery of the Company’s shares issued or sold in connection with its initial public offering on the Euronext Paris regulated market, the Company has created an Audit and Risks Committee (which replaces the previous Audit Committee), a Nominations and Compensation Committee and a Strategy and Major Projects Committee. In addition, it may decide to create any other committees of the board of directors responsible for studying questions referred to them by the board of directors or its Chairman for their examination and opinion. The tasks of the committees are to prepare the decisions of the board of directors, make recommendations and issue opinions on matters within their remit. The composition, manner of functioning and remit of these committees are laid down in the Internal Regulations of the board of directors. The members of each committee will be nominated by the board of directors appointed upon the initial public offering of the Company. 16.4.1 The Audit and Risks Committee 16.4.1.1 Composition of the Audit and Risks Committee (Articles 25 and 28 of the Internal Regulations) The Audit and Risks Committee is composed of at least 3 members, including its Chairman. These members are chosen from among the directors, other than the Chairman of the board of directors, who does not exercise management functions within the Company. Two thirds of the members of the Audit and Risks Committee, including its Chairman, must be independent directors (excluding directors representing employee shareholders and directors representing employees), pursuant to the criteria set out in section 14.1.1.1. “The board of directors” of this document de base. The members of the Audit and Risks Committee must be competent in financing and accounting matters. 195 All the members of the Audit and Risks Committee must be provided at the time of their appointment with information on the specific accounting, financial and operational features of the Company. 16.4.1.2 The remit of the Audit and Risks Committee (Articles 25 and 26 of the Internal Regulations) The primary tasks of the Audit and Risks Committee are to examine the accounts and monitor matters relating to the preparation and audit of accounting and financial information. In this respect, it the Audit and Risks Committee will be responsible, in particular: for examining the draft quarterly, half-yearly and annual parent company accounts and consolidated financial statements before their presentation to the board of directors, and in particular: for ensuring the relevance and permanence of the accounting methods adopted in the preparation of the parent company account and consolidated financial statements; for examining any difficulties encountered in the application of accounting methods; and more specifically, for examining major transactions in respect of which a conflict of interest might arise; for examining the financial documents distributed by the Company at the time of settlement of annual, half-yearly and quarterly accounts; for examining the draft accounts prepared for specific transactions such as contributions, mergers or spin-offs, or for the payment of interim dividends; for examining certain transactions proposed by the Chief Executive Officer and submitted to the board of directors, some of which for prior approval, from a financial point of view, such as: capital increases; equity investments; and acquisitions or disposals; for assessing the reliability of the systems and procedures used in the preparation of the accounts and forecasts and the validity of positions taken to process significant transactions; for ensuring the legal auditing of the annual accounts and consolidated accounts by the statutory auditors; for examining the methods and procedures for reporting and restating accounting information derived from foreign Group companies. It is also the task of the Audit and Risks Committee to verify the effectiveness of the Company’s internal control and risk management systems. In this respect, the Audit and Risks Committee is responsible, in particular: for assessing, with the persons responsible for such activities, the Group’s internal control systems; for examining, with the persons responsible for such activities at Group level, and with the support of the internal audit: - targets and intervention and action plans in the area of internal control; - the conclusions of the interventions and actions taken by those responsible within the Group; and - the recommendations made, and the steps taken to follow up on such interventions and actions by those responsible; for examining internal audit methods and results; 196 for verifying that the procedures used by the internal audit contribute to ensuring that the Company’s accounts: - accurately reflect the Company’s true financial situation; and comply with the accounting rules; for examining the relevance of the procedures for analyzing and monitoring risks, while ensuring that a process is put in place to identify, quantify and prevent the main risks arising from the Group’s business; for examining and monitoring the rules and procedures applicable to conflicts of interest; and for examining the draft report of the Chairman of the board of directors on internal control and risk management procedures. It is also the task of the Audit and Risks Committee to verify the effectiveness of the Company’s external control and the independence of the statutory auditors. In this respect, the Audit and Risks Committee is responsible, in particular: for examining with the statutory auditors, every year: - their action plan and conclusions; and their recommendations and the follow-up action taken; for issuing a recommendation regarding the statutory auditors put forward for appointment by the Company’s General Meeting; for assuring itself of the independence of the Company’s statutory auditors; for examining the remuneration of the Company’s statutory auditors, which must not call into question their independence and objectivity. In order to enable the committee to monitor the rules relating to the independence and objectivity of the statutory auditors throughout their term of office, the Audit and Risks Committee must be informed, every year: of the statutory auditors’ statement of independence; of the amount of fees paid to the statutory auditors’ network by companies controlled by the Company and the entity that controls it in respect of services that are not directly connected with the mission of the statutory auditors; and of any services carried out that are directly connected with the mission of the statutory auditors. In addition, the Audit and Risks Committee must examine with the statutory auditors any risks that could affect their independence and the protective measures taken to mitigate such risks. In particular, they must be satisfied that the amount of the fees paid by the Company and the Group, or the proportion the fees represent in the revenue of the firms and networks, are not such as to undermine the independence of the statutory auditors. The mission of the statutory auditors must exclude any other services unconnected with legal auditing as defined in the statutory auditors’ Code of conduct and applicable professional standards. The auditors selected must agree, on their own behalf and on behalf of the network to which they belong, not to engage in any advisory work (whether legal, fiscal, IT, etc.) directly or indirectly for the company that has chosen them or to the companies that it controls. However, with the prior approval of the Audit and Risks Committee, incidental work or work that is directly complementary to the auditing of the accounts may be carried out, such as acquisition or post-acquisition audits, but excluding valuation and advisory work. 197 Finally, the Audit and Risks Committee must ensure, periodically, that its rules and manner of functioning enable it to assist the board of directors to deliberate validly on the matters within its remit. 16.4.1.3 The functioning of the Audit and Risks Committee (Articles 25, 27 and 29 of the Internal Regulations) The Audit and Risks Committee shall meet as often as required and in any event at least twice a year at the request of its Chairman, the majority of its members, the Chairman of the board of directors or a third of the directors. The Audit and Risks Committee can only meet if more than half of its members are present. Its opinions, proposals or recommendations are adopted by a simple majority of the members of the Committee present. In the event of a tied vote, the Chairman of the Committee does not have a casting vote. In order to carry out its mission, the Audit and Risks Committee in general and each of its members in particular can ask to be provided with such information as they consider relevant, useful or necessary for that purpose. The Audit and Risks Committee can ask to interview the statutory auditors or officers of the Company including the members of the Company’s General Management and the Chief Financial Officer. Such interviews may take place, if necessary, without the members of General Management being present. Finally, if it considers it necessary, the Audit and Risks Committee may commission an independent investigation. The Audit and Risks Committee shall report regularly to the board of directors on the performance of its missions and shall inform the board of directors without delay of any difficulty encountered. These reports will either be inserted in the minutes of the meetings of the board of directors concerned, or will be attached to those minutes. 16.4.2 The Nominations and Compensation Committee 16.4.2.1 The composition of the Nominations and Compensation Committee (Articles 25 and 32 of the Internal Regulations) The Nominations and Compensation Committee is composed of at least three members, including its Chairman. The Chairman of the board of directors and, in the event that the functions of Chief Executive Officer are exercised by a director other than the Chairman of the board of directors, the Chief Executive Officer, cannot be members of the Nominations and Compensation Committee. The majority of the members of the Nominations and Compensation Committee, including its Chairman, must be independent directors pursuant to the criteria set out in section 16.1.1.1. ”The board of directors” of this document de base. 16.4.2.2 The remit of the Nominations and Compensation Committee (Articles 25 and 30 of the Internal Regulations) The missions of the Nominations and Compensation Committee in relation to appointments, are: to assist the board of directors in its choice: – of members of the board of directors; – of members of the committees of the board of directors; and – of Chief Executive Officer and, if applicable, Deputy Chief Executive Officer(s); to select potential members of the board of directors that satisfy the independence and equal representation of men and women criteria, and to submit the list to the board of directors; every year, before publication of the Company’s annual report, to examine the situation of each member of the board of directors having regard to the independence criteria, and to submit its opinion 198 to the board of directors for examination by the board of the situation of each of the persons concerned by those criteria; and to prepare the succession: – of members of the Company’s General Management; and – of the Chairman of the board, the Chief Executive Officer and, if applicable, Deputy Chief Executive Officer(s). The missions of the Nominations and Compensation Committee in relation to remuneration are to make recommendations and proposals to the board of directors concerning, for the members of the board of directors who would be the beneficiaries thereof: the allocation of directors’ fees; all other items of remuneration, including the conditions applicable to their term of office; if applicable, any payments made to observers; potential amendments or changes to the pension and welfare scheme; benefits in kind and miscellaneous pecuniary entitlements; and if applicable: – the grant of share subscription or purchase options; and – the allocation of free shares. It will also be the mission of the Nominations and Compensation Committee to make recommendations to the board of directors concerning: the policy of remuneration of management executives, including the criteria used to define the variable part of such remuneration, which must be consistent with the Group’s strategy; and profit-sharing mechanisms of any kind for the staff of the Company and more broadly for Group Companies, including: – employee savings plans; – supplementary pension schemes; – reserved issues of securities giving access to capital; – the grant of share subscription or purchase options; and – the allocation of free shares. In particular, it will be the task of the Nominations and Compensation Committee to make recommendations to the board of directors relating to the performance criteria to be used, if applicable, to determine the variable part of management executives’ remuneration, the grant or exercise of any share subscription or purchase options and any allocation of free shares. These performance criteria must be simple to draw up and explain, satisfactorily reflect the Group’s financial performance and development targets, at least in the medium term, provide transparency for shareholders in the annual report and at General Meetings and match the objectives of the business and the normal practices of the Company with regard to the remuneration of its managers. Every year, before publication of the Company’s annual report, the Nominations and Compensation Committee will examine the situation of each member of the board of directors having regard to the independence criteria, and submit its opinion to the board of directors, for the examination by the board, of the situation of each of the persons concerned by those criteria. Finally, the Internal Regulations of the board of directors specify that the Nominations and Compensation Committee must periodically ensure that its rules and manner of functioning enable it to assist the board of directors to validly deliberate on matters within its remit. 199 16.4.2.3 The functioning of the Nominations and Compensation Committee (Article 25, 31 and 33 of the Internal Regulations) The Nominations and Compensation Committee shall meet as often as required and in any event at least once a year at the request of its Chairman, the majority of its members, the Chairman of the board of directors or a third of the directors. The Nominations and Compensation Committee can only meet if more than half of its members are present. Its opinions, proposals or recommendations are adopted by a simple majority of the members of the Committee present. In the event of a tied vote, the Chairman of the Committee does not have a casting vote. In carrying out its mission, the Nominations and Compensation Committee can propose to the board of directors that any external or internal investigations be carried out, at the Company’s expense, that may assist the deliberations of the board of directors. It can also interview one or more members of the Company’s General Management, and in particular the Chief Executive Officer or, if applicable, the Deputy Chief Executive Officer(s). It shall report on its work at every meeting of the board of directors. 16.4.3 The Strategy and Major Projects Committee 16.4.3.1 The composition of the Strategy and Major Projects Committee (Articles 25 and 36 of the Internal Regulations) The Strategy and Major Projects Committee is composed of at least three members, including its Chairman. The Strategy and Major Projects Committee is composed of the Chairman of the Company’s board of directors, an independent director and one other member of the board of directors chosen for their expertise in relation to medical biology and the medical analysis laboratory market. 16.4.3.2 The remit of the Strategy and Major Projects Committee (Articles 25 and 34 of the Internal Regulations) The Strategy and Major Projects Committee shall consider and express its opinion on the following subjects presented by General Management to the board of directors: The major strategic policy orientations of the Company and the Group; The Group’s development policy; and The major projects or development programmes that it is envisaged shall be carried out by the Company or a Group company in relation to new tests and examinations; The Strategy and Major Projects Committee will investigate and examine: The drafts of strategic and partnership agreements; External growth transactions and those affecting the Group’s structures: – Significant asset acquisition projects; – Significant establishment projects in France or abroad; – Projects for the creation of significant subsidiaries; Projects for the acquisition or disposal of significant share holdings. The Strategy and Major Projects Committee will be responsible for assessing the significant nature of a project presented by General Management, whether, for example, in relation to the strategic acquisition of a new 200 clinical testing laboratory or the launch of a new test, and shall base its decision in particular on the amount of the commitments or revenue associated with the project concerned. In general, the Strategy and Major Projects Committee shall give its opinion on any other strategic matter submitted to it by the board of directors. 16.4.3.3 The functioning of the Strategy and Major Projects Committee (Article 25, 35 and 37 of the Internal Regulations) The Strategy and Major Projects Committee shall meet as often as required and, in any event, at least once a year. A calendar of meetings of the Strategy and Major Projects Committee shall be fixed by the board of directors, without prejudice to the provisions of the Internal Regulations relating to the convening of committee meetings. In any event, the members of the board of directors shall be informed when meetings of the Strategy and Major Projects Committee are convened. 16.5 DECLARATION RELATING TO CORPORATE GOVERNANCE For the sake of transparency and informing the public, the Company intends, with effect from its initial public offering, to comply with the corporate governance principles defined in the recommendations issued by the Association Française des Entreprises Privées (AFEP) and the Mouvement des Entreprises de France (MEDEF), in the AFEP-MEDEF Code. In particular, the Company intends to ensure the presence of independent members on its board of directors (see the table at section 14.1.1 – “The board of directors” of this document de base, to create specialized board committees responsible for making recommendations to it in relation to strategy, the auditing of the accounts and the remuneration of managers, and to make the implementation of a certain number of major decisions capable of having a substantial impact on the business of the Company or of any Group Company, or on their assets and results, subject to prior approval by the board of directors. In this context, the board of directors adopted internal regulations on September 11, 2014, subject to the nonretroactive condition precedent of settlement/delivery of the Company’s shares issued or sold in connection with its initial public offering on the Euronext regulated market, which contains the terms and conditions governing the composition, organization and functioning of the board of directors and of its committees, as well as the rights and obligations of the directors, the main terms of the internal regulations being described in section 16. “Functioning of the Company’s administrative and management bodies.” The internal regulations of the board of directors described in this document de base are the Company’s as approved by the Company’s board of directors before completion of the Company’s initial public offering and whose approval shall be reiterated by the newly constituted board of directors that will meet immediately after completion of the Company’s initial public offering. 16.6 INTERNAL CONTROL AND CORPORATE GOVERNANCE Since the shares of the Company are not admitted to trading on a regulated market on the date of registration of this document de base, the Chairman of the board of directors is not obliged to prepare a report relating to the composition of the board of directors and to the application of the principle of balanced representation of men and women on the board, to the manner in which the work of the board is prepared and organized and to the internal control and risk management procedures implemented by the Company, in accordance with Article L. 225-37 of the French Commercial Code. With effect from the date of settlement/delivery of the shares of the Company issued or sold in connection with its initial public offering on the Euronext regulated market, the Company intends to implement the legal and regulatory provisions applicable to listed companies with regard to the internal control procedures and will do so in accordance with corporate governance principles. In particular, the Chairman of the board of directors will, in accordance with Article L. 225-37 of the French Commercial Code, draw up the report on internal control mentioned above. 201 Nevertheless, the Company already has an internal control environment based on the roles and responsibilities associated with the different functions of its employees and with official internal control provisions at the level of the main Group entities. In addition, at Group level, a process relating to structured mergers and acquisitions requiring the approval of the board of directors for all material projects and a procedure relative to investment expenditure (i.e. capex or tangible asset acquisition) have been implemented. This process and procedure lay out the framework and the tools of functioning of the mergers and acquisitions departments as well as those responsible for each entity by specifying the responsibilities of those involved and the authorized expenditure commitment threshold. In addition, within the SPORT project, starting in 2010, the Group implemented a purchasing policy at European level, in particular relating to the purchase of reagents and medical analysis materials, thus reducing the number of suppliers in order to focus purchases on selected suppliers with which privileged business conditions have been negotiated under a European framework contract. Finally, for sensitive transactions, such as the payment of invoices or payments owed to employees, that require a segregation of duties, functions have been analyzed in order to put in place the necessary segregation of duties or, if necessary, adjusted balancing controls. In particular, in France, a shared services center responsible for accounting processes was set up at the end of 2010 and a shared services center responsible for the payroll was set up at the end of 2013. On the date of registration of this document de base, almost all French laboratories use the two shared services centers and the integration process for new acquisitions provides that that companies newly acquired in France shall rapidly transfer accounting and payroll tasks to the shared services centers. In addition, at Group level, a centralized European treasury team was put in place, enabling the development of centralized treasury management starting in 2010. 202 CHAPTER 17 EMPLOYEES 17.1 PRESENTATION 17.1.1 Number and breakdown of employees General presentation of workforce On the date of this document de base, the Group employed almost 6,000 people. Its workforce is increasing continuously, mainly due to the Group’s external growth policy. During the financial year ended December 31, 2014, staff costs payable by the Group amounted to approximately €232.8 million versus approximately €213.5 million for the financial year ended December 31, 2013, adjusted for staff costs incurred by the Group’s German business, which was sold on December 2, 2013. Staff costs include gross salaries paid to employees and the corresponding employer’s social security contributions, as well as fees paid to self-employed (TNS) professionals. Staff costs (in € million) Wages and salaries Social security charges Total 2014 180.7 52.1 232.8 2013 163.6 49.9 213.5 Breakdown of employees Breakdown of employees by country The table below shows a breakdown of the Group’s employees by country at December 31, 2014: France Spain Portugal Number of employees 1 2,907 1,350 538 United Kingdom 542 Italy Belgium 471 Switzerland South America1 Total 9 26 5,981 138 Presence in Brazil, Peru, Colombia and Mexico Until 2014, human resources management was decentralized to each of the Group’s entities, and in some cases, was outsourced to specialized independent firms (particularly in France). A coordinated, consistent human resources policy, introduced in the third quarter of 2014, is currently being implemented at Group level. This policy aims to harmonize the presentation of the workforce according to whether they are linked to one of the three production phases (pre-analytical, analytical and post-analytical) or to so called “support” services. As at December 31, 2014, the breakdown of the Group’s workforce was as follows: Workforce (percentage) 1 France Spain Portugal United Kingdom Italy Belgium Switzerland South America1 48.6% 22.6% 9.0% 9.1% 7.9% 2.3% 0.2% 0.4% Presence in Brazil, Peru, Colombia and Mexico As at December 31, 2014, 71% of the Group’s workforce was employed in France and Spain. Between 2013 and 2014, the salaried workforce increased by 78% in Italy and by 223% in the United Kingdom, following the acquisition of the SDN group on July 31, 2014 on the one hand and the transfer of NHS employees pursuant to the agreements with Basildon and Thurrock University Hospital NHS Foundation Trust and Southend University Hospital NHS Foundation Trust on the other. Breakdown of employees by country and occupational category The following table shows a breakdown of the Group’s employees by occupational category and by country at December 31, 2014: 203 December 2014 Production Lab doctor Management1 Technician Sampling2 Messenger Secretaries Lab assistant3 Support Central support function Local support function 1 2 3 4 France Spain Portugal United Kingdom Italy Belgium Switzerl and Latin America 84 6 1,137 271 218 840 144 109 9 682 188 13 189 10 52 0 240 0 17 68 134 0 35 246 53 14 32 134 76 4 53 24 13 151 42 0 1 47 1 23 38 2 1 0 3 0 3 0 1 0 0 0 0 0 0 0 1094 106 19 6 1 0 0 0 98 44 8 22 107 26 1 26 Management of technical teams only Includes nurses Includes maintenance personnel Inclut les équipes de la Société pour l’ensemble du Groupe The following table shows a breakdown of the Group’s employees by occupational category at December 31, 2013 (excluding Latin America): December 2013 Production Lab doctor Management1 Technician Sampling2 Messenger Secretaries Lab assistant3 Support Central support function Local support function 1 2 3 4 France Spain Portugal United Kingdom Italy Belgium Switzerland 82 6 1,123 219 188 766 119 106 14 649 169 11 187 10 52 0 239 0 17 68 137 0 10 90 0 0 7 52 39 1 15 17 9 95 23 0 1 45 4 24 34 3 1 0 3 0 2 0 0 1004 98 21 2 1 25 0 97 40 8 7 65 0 1 Management of technical teams only Includes nurses Includes maintenance personnel Inclut les équipes de la Société pour l’ensemble du Groupe Proportion of women in the Group’s workforce The Group’s staff is predominantly female in the European countries: with women representing more than 80% of the Group’s employees at December 31, 2014 (excluding South America). Proportion men/women in 2014 Men Women Number of Group employees 1,119 4,836 Percentage of Group employees 19% 81% Breakdown of employees by age The following table shows a breakdown of the Group’s employees by age band at December 31, 2014 (excluding South America): Age range Less than 20 years 20-29 years 30-39 years 40-49 years 50-59 years 60-65 years Over 65 years Number of Group employees1 34 1,049 1,594 1,417 1,540 258 63 204 Percentage of Group’s workforce 1% 18% 27% 24% 26% 4% 1% 1 Not known for South America Breakdown of employees by type of employment contract The Group prefers to enter to into indefinite employment contracts in its contractual relations with its employees. However, there may be some differences among various countries in how certain contracts are classified. For example, in France and Spain, interns and apprentices are classified as fixed-term contracts while in Portugal and Italy, they are a separate category. The table below shows a breakdown of Group employees by contract type at December 31, 2014 (excluding South America): Full-time Indefinite Fixed-term Number of Group employees 5,322 633 Percentage of Group employees 89% 11% Breakdown of workforce between full-time and part-time The Group complies with the local legislation on working hours in each country where it operates. The working week ranges from 35 hours in France to 40 hours in the United Kingdom and in Portugal. The table below shows a breakdown as at December 31, 2014 of the Group’s employees in Europe for full-time and part-time workers (excluding South America): Number of Group employees 4,362 1,593 Full-time Part-time 17.1.2 Percentage of Group employees 73% 27% Employment and working conditions The Group’s business activities do not expose it to workplace safety issues and most accidents that have arisen were of a benign character. Two factors could potentially pose a risk within the Group: (i) risk of exposure to blood and (ii) car accidents involving messengers. By way of illustration, the number of accidents declared within the Group in France is roughly ten for the financial year 2014. The level of absenteeism, calculated as the number of days absent divided by the total number of days of work, in the Group is generally between 4% and 6% depending on the country, for the financial year 2014. By way of illustration, it was 5.7% in France, 4.1% in Spain, 6.2% in Portugal and 4.6% in the United Kingdom for this period.6 In Spain, a staff reduction plan involving 45 redundancies was presented to the Works Council on December 30, 2014. All possible measures will be taken to mitigate the social impact of this plan, predominantly by seeking to place the employees concerned with local partners of the various Spanish entities. 17.1.3 Training As of the date of this document de base, the Group has not set up a training program common to all Group companies. The local entities establish their own training plans on the basis of regulatory requirements and their own skill needs. Priority actions in 2013 and 2014 were: (i.) France: 6 The elements used in calculating the rate of absenteeism differ for each country. A policy of harmonization is currently being implemented by the Group. 205 CPD: involves a new regulatory continuing professional development system for healthcare professionals, in particular doctors and clinical pharmacists (including both employees and selfemployed professionals). The Labco training center is an approved “CPD organization” and the Group has designed and organized training programs despite the administrative difficulties involved in implementing them; Training related to accreditation: in this area, the Group has an active training policy, which has led to it being the leader at December 31, 2014 in terms of the number of laboratory accreditations in France with a rate of 90% of its SELs accredited ISO 15189, it being specified that among the remaining 10%, on the date of this document de base, 5% of SEL are awaiting COFRAC’s decision and 5% are awaiting a COFRAC audit. (ii.) United Kingdom: Work on skills development, particularly through the implementation of an action plan designed to define all skills and how to develop them further; Training program for technical tools; A training committee that monitors all training activities. 17.1.4 Compensation policy The Group determines its compensation policy on a country by country basis in order to adapt its compensation methods to local practices. In France, the Group’s external growth has led to an increasing number of different compensation practices within the Group. A policy to harmonize compensation practices was introduced in 2012, beginning by insourcing the payroll system. A second stage consists in determining which compensation practices are in keeping with the Group’s interests and with market practices allowing the Group to eliminate a number of local customs or practices. By way of example, the treatment of the long-service bonus was unified in 2014 (there were seven different systems in 2012) and the number of existing bonuses has been reduced from 77 to 16 in 2014. This plan should be implemented before the end of 2015. 17.1.5 Employee representation Elections for the employee representative bodies have been held in each of the Group’s subsidiaries in accordance with the applicable legislation. The rights, obligations and operating methods of these bodies vary from one country to another, depending on local legislation. In France, social dialogue is structured at company level. Each company has, if necessary, a works council and trade union representatives or a single staff representative body depending on the number of employees and the complexity of the company’s structure. The management of each company negotiates agreements with the representative trade unions on subjects such as incentive plans, gender equality and working time reduction and flexibility. Management of each company chairs the bodies and can negotiate company-wide agreements with the company’s trade union representatives. In the United Kingdom, in-depth work has been carried out with social partners to define the rules and organization governing the transfer of staff from the National Health Service (NHS) to the Group’s structures under a public/private partnership. On the date of this document de base, there is no staff representation at Group level. 17.2 EMPLOYEE INCENTIVE AND PROFIT-SHARING PLANS In France, the Company is not required to have a mandatory employee profit-sharing plan (“participation”) as it does not meet the requisite conditions. Other Group companies in France that do meet the requisite conditions have implemented profit-sharing plans based on the statutory calculation method. For the financial year ending December 31, 2014, thirteen Group companies were required to have an employee profit-sharing plan. 206 17.2.1 Employee incentive plan The employee incentive plan (“intéressement”) is an optional mechanism designed to give employees a stake in the company’s results and performance through the payment of immediately available bonuses, calculated on the basis of a formula, in accordance with the provisions of Article L. 3312-1 of the French Labor Code. Incentive plans have been implemented at the level of certain French subsidiaries for a fixed period of three years. Each agreement sets out its own calculation formula. For the financial year ending December 31, 2014, twelve Group companies were required to have an employee incentive plan. 17.2.2 Employee profit-sharing plan French companies with at least 50 employees have a profit-sharing plan. As such plans have been implemented by the relevant French companies within the Group. A special profit-sharing reserve is due when the entity’s taxable income is higher than a 5% return on equity in accordance with the provisions of Articles L. 3322-2 and L. 3324-1 of the French Labor Code. In France, the Company is not required to have an employee profit-sharing plan as it does not meet the requisite conditions. Other Group companies in France that do meet the requisite conditions have implemented profitsharing plans based on the statutory calculation method. In 2014, thirteen Group companies were required to have an employee profit-sharing plan. 17.3 MULTI-COMPANY EMPLOYEE SHARE OWNERSHIP PLANS Companies that have a profit-sharing plan are required by law to set up an employee share ownership plan in accordance with the provisions of Article L. 3332-3 of the French Labor Code. An employee share ownership plan of the Company or the Group is a collective system of saving, enabling the employees of a member company to build up a portfolio of securities with the help of their employer. In particular, sums received under a profit-sharing or incentive plan can be invested in the share ownership plan and employees can also make voluntary contributions. Sums invested in an employee share ownership plan are blocked for a period of five years, except in some specific circumstances provided for by law. A multi-company employee share ownership plan has been set up in France. It enables Group employees to transfer to the plan, all of the sums paid to them immediately. On the date of this document de base, three Group companies out of the seven eligible companies are members of this plan. 17.4 INCENTIVES PAID TO EXECUTIVE CORPORATE OFFICERS AND DIRECTORS’ SHARE DEALINGS The implementation of a free share award plan was proposed and approved at the shareholders’ meeting of October 2, 2014. Senior executives of the Company and key management personnel (67 people) are the beneficiaries of the plan. The performance shares awarded on November 13, 2014 under the plan represent 1.0% of the Company’s share capital (i.e. 687,361 shares) (see section 15.1.11 “History of allocations of free shares – Information on shares allocated for free” of this document de base). 17.5 SELF-EMPLOYED WORKERS 17.5.1 Legal definition of self-employed workers Definition Self-employed workers (“travailleurs non-salariés” or “TNS”) are people who do not have an employment relationship with the company and therefore do not have an employment contract. One of the three elements that characterize an employer/employee relationship (work, compensation and subordination) is missing in the relationship between a self-employed worker and the principal (who is therefore not their employer). In practice, self-employed workers are not legally in a position of subordination with respect to an employer as they do not obey the employer’s power of management and control. As this element is missing, they should not be considered as employees. 207 Diversity of the category TNS are typically categorized by members of independent professions. There are many types of activity covered by this category including in particular pharmacists, lawyers, independent retailers, certain corporate officers, truck drivers, commercial agents. The term “self-employed” therefore covers workers with very different social security and fiscal regimes. This diversity makes it impossible to provide a general overall description and requires analysis on a case by case basis. On the date of this document de base, the self-employed workers in the Group, particularly in France, are all doctors or pharmacists. 17.5.2 Social law applicable to self-employed workers A person is considered to be self-employed within the meaning of social security laws when that person has exclusive control over his or her working conditions or by the contract concluded with the principal 7. This presumption can be rebutted by proof of an employment relationship. Social protection Self-employed workers benefit from specific social protection regimes, which are different from those of employees or agricultural workers. These regimes provide the same kind of protection afforded to employees (sickness/maternity, pension, disability) but the rules are different. Depending on the protection afforded and the category of self-employed workers, protection may or may not be available, may be mandatory (on a principal or supplementary basis) or optional, “institutional” or private, collective or individual. The position is further complicated by the fact that self-employed workers may sometimes have the option of being covered by the employee regime or in some cases may be automatically covered by the employee regime by law. By way of illustration, all self-employed workers are obliged to pay into a national pension system, which differs according to the type of activity (professions other than lawyers, lawyers, industrialists and retailers, tradesmen, non-salaried agricultural workers) and a different regime applies to each type of activity (doctor or pharmacist) depending on the retirement fund. Special regimes may apply to certain professions, such as the national pension scheme for lawyers. Conversely, all categories benefit from family allowances (in exchange for contributions common to all selfemployed workers, but different from the contributions made by employees). Unemployment insurance is optional for all self-employed workers. National health and pension funds Each category, and in certain cases, each profession, has a special fund responsible for collecting contributions and paying the corresponding benefits (e.g. Caisse d’Assurance vieillesse des pharmaciens or the Caisse nationale des barreaux français, etc.). These are not the same as the funds for employees and are often subject to different regulations. For each type of protection and each profession or category of self-employed worker, there are specific bases and rates for contributions and benefits. 17.5.3 Self-employed workers and the independent practice agreement The Group’s self-employed workers may work within an independent practice agreement (“convention d’exercice libéral”). This Agreement sets out the terms on which the person will work with the Group and stipulates the rights and obligations of each party. Most independent practice agreements refer to the relevant company’s internal regulations. When the self-employed person is a partner in the company, the agreement refers to the relevant company’s Articles of Association. 7 Article L. 311-11 of the French Social Security Code and Article L. 8221-6-I of the French Labor Code relating to natural persons registered on the company or trade registry, commercial agents, truck drivers, school bus drivers, etc. 208 The independent practice agreements sometimes set out the amount of fixed and variable fees paid, notice period for terminating the agreement and arrangement for cover when the person is absent. 17.5.4 Self-employed workers in the Group’s staff The concept of self-employed worker exists in all the countries where the Group operates, under various names. The legal provisions governing this category of worker vary according to the country. In France, most of the Group’s self-employed workers belong to the medical professions (doctors and clinical pharmacists). In other countries, they may also include so called “support” staff for laboratories, as well as messengers. The table below only includes TNS in the medical profession, who consist mainly of lab doctors except for in Italy where the specificity of the services offered by the Group (such as radiology, outpatient surgery and work medical services) requires a large number of independent doctors. In the United Kingdom, lab doctors are employed by the NHS. December 2014 Number of medical professional TNS France Spain Portugal United Kingdom Italy Belgium Switzerland 290 48 8 0 437 10 1 209 CHAPTER 18 PRINCIPAL SHAREHOLDERS 18.1 IDENTIFICATION OF SHAREHOLDERS 18.1.1 Ownership of share capital and voting rights At the date of this document de base, the share capital is made up of shares, all of which carry the same dividend and voting rights, falling into five categories according to the identity of the shareholders that own them. Given the application of new articles of association, subject to the non-retroactive condition precedent of settlement/delivery of the Company’s shares issued or sold as part of the IPO on the regulated market of Euronext Paris, only one category of ordinary shares will exist from the time of settlement/delivery of those shares. Shareholder 3i GC Holding Lab 1 and 3i GC Holding Lab 2 Eric Souêtre1 CM-CIC Investissement Vikings Limited Labco Invest FCPR IXEN Investissement Other1 Total 1 2 Number of shares % of share capital % of voting rights 12,170,434 17.22% 17.22% 6,311,350 4,685,897 4,405,133 3,396,349 2,271,727 37,438,815 70,679,705 8.93% 6.63% 6.23% 4.81% 3.21% 52.97% 100.00% 8.93% 6.63% 6.23% 4.81% 3.21% 52.97% 100.00% Through the companies ACTA and EUSSA. All shareholders owning less than 3% of the share capital, a large proportion of whom are laboratory doctors, Group employees and members of their families. The equity interests set out above are calculated on the basis of shares held directly and indirectly via entities controlled by the shareholders concerned. 18.1.2 Changes in ownership of the share capital and voting rights in the last three financial years At the end of the 2014, 2013 and 2012 financial years, ownership of the Company’s share capital and voting rights broke down as follows: Shareholder As of 12/31/2014 Number of shares 3i GC Holding Lab 1 and 3i GC Holding Lab 2 Eric Souêtre1 CM-CIC Investissement Vikings Limited Labco Invest FCPR IXEN Investissement Other2 Total 1 2 % of share capital As of 12/31/2013 % of voting rights Number of shares % of share capital As of 12/31/2012 % of voting rights Number of shares % of share capital % of voting rights 12,170,434 5,698,479 17.71% 8.29% 17.71% 8.29% 12,170,434 4,998,479 17.78% 7.30% 17.78% 7.30% 12,170,434 4,998,479 17.78% 7.30% 17.78% 7.30% 4,685,897 4,405,133 3,396,349 6.82% 6.41% 4.94% 6.82% 6.41% 4.94% 4,385,897 4,405,133 3,396,349 6.41% 6.43% 4.96% 6.41% 6.43% 4.96% 4,385,897 4,405,133 3,396,349 6.41% 6.43% 4.96% 6.41% 6.43% 4.96% 2,271,727 36,108,168 68,736,187 3.30% 52.53% 100.0% 3.30% 52.53% 100.0% 2,271,727 36,831,303 68,459,322 3.32% 53.80% 100.0% 3.32% 53.80% 100.0% 2,271,727 36,831,303 68,459,322 3.32% 53.80% 100.0% 3.32% 53.80% 100.0% Through the companies ACTA and EUSSA. All shareholders owning less than 3% of the share capital, a large proportion of whom are laboratory doctors, Group employees and members of their families. The equity interests set out above are calculated on the basis of directly held shares and indirectly held shares via entities controlled by the shareholders concerned. 210 18.2 VOTING RIGHTS Article 31.1 of the Company’s articles of association due to come into force from the settlement/delivery of the Company’s shares issued or sold as part of the IPO on the regulated market of Euronext Paris provides that each shareholder shall have as many votes as the number of shares that the shareholder owns or represents. However, Article 31.3 provides for a mechanism to limit voting rights held by shareholders in a shareholders’ general meeting. No shareholder will be able to exercise, either directly or through a proxy, more than 39% of the voting rights attached to the Company’s shares (see section 21.2.3.7 – “Articles of Association – Limitations on voting rights (Article 31 of the Articles of Association)” of this document de base. Article 31.2 explicitly rules out the introduction of a system of double voting rights. 18.3 SHAREHOLDERS PACT, OWNERSHIP UNDERTAKINGS AND PARTIES ACTING IN CONCERT To the Company’s knowledge, at the date of this document de base, there are several shareholders pacts to which the various categories of shareholders and Warrant holders are party. All of these pacts shall become null and void and will cease to be effective from the time of settlement/delivery of the Company’s shares issued or sold as part of the IPO on the regulated market of Euronext Paris. The financial shareholders that must hold more than 0.5% of the share capital of the Company following the initial public offering have agreed, in principle, on entering into an agreement under which they will, following the initial public offering of the Company, proceed, in an organized manner, in sale transactions of the Company’s shares that may take place on the market for a period ending on the earliest of (i) the first anniversary of settlement/delivery of the initial public offering and (ii) the 180th day after the expiry of the undertaking to retain ownership of the shares under a guarantee that will be entered into by the guarantor entities. This agreement will be described in the note d’opération submitted to the Authorité des marchés financiers. 18.4 CONTROL OF THE COMPANY At the date of this document de base, the Company is not controlled by any shareholder within the meaning of Article L. 233-3 of the French Commercial Code. 18.5 AGREEMENTS THAT MAY CAUSE A CHANGE IN CONTROL OF THE COMPANY To the Company’s knowledge, there is no agreement as of the date of this document de base whose implementation might, at a later date, lead to a change in the Company’s control or to the acquisition of control over the Company. 211 CHAPTER 19 RELATED PARTY TRANSACTIONS This chapter describes agreements between the Company and its subsidiaries and between the Company or its subsidiaries and related companies at the date of this document de base. 19.1 INTRAGROUP AGREEMENTS OR AGREEMENTS WITH RELATED PARTIES Substantial transactions entered into by, or continuing between the Company and related parties since January 1, 2012, were as follows: 19.1.1 Guarantees The Company has granted certain guarantees to its subsidiaries or to their benefit: (i.) a first demand guarantee granted in 2013 to Deutsche Bank AG, London Branch and covering sums owed by Labco Diagnostic UK Limited in relation to the operation of the BACS (bankers automated clearing services) system arranged for its benefit; (ii.) a first demand guarantee granted for a maximum amount of €13 million to Deutsche Bank AG, Deutsche Bank Sociedad Anónima Espanola, Deutsche Bank S.p.A. and Deutsche Bank (Portugal) S.A. and covering sums owed with respect to the cross-border cash-pooling agreement set up by Labco Finance: a. by affiliates of the Company in 2013; and b. by Labco Diagnostic UK Limited in 2012; (iii.) comfort letters whereby the Company has undertaken to assist the following subsidiaries so they are able to repay their debts when payments fall due: a. the iPP subsidiary, for the 2013 financial year for a period of 12 months from the date on which the comfort letter is signed; b. the Belgian subsidiary Labco Finance for a period of 12 months following the signature of the comfort letter so that it is able to pay its debt to its creditors; c. the Belgian subsidiary Labco Belgium, for a period of 12 months following the signature of the comfort letter so that it is able to pay its debt to its creditors; d. the Labco Diagnostics España subsidiary to the Company to assist its business for a period of at least 12 months following the signature of the comfort letter; e. the Labco Germany subsidiary, until December 31, 2015 (in particular to allow for the certification of the accounts of Labco Germany); (iv.) letters of intent under which the Company has undertaken: a. to Société Générale to provide Bialiance with the means of repaying its loan which amounts to around €1 million; b. to USMEN s.r.l., the Company confirming that SDN S.p.a. will not be sold to a third party of the Labco Group in the 24 months following its acquisition; (v.) guarantees to Sonic Healthcare in the context of the sale of the German entities, related to the eventual triggering of the guarantee provisions (clauses de garantie de passif). These guarantees will expire on June 2015, except mainly those of fiscal nature which will expire at the end of the limitation period. 212 (vi.) a joint and several guarantee, since 2011, to SAS Bioliance with respect to the liability of the general partners (associés commandités) of SEL Chauvet-Douet-Lissajoux; (vii.) the Indenture relating to the High-Yield Bonds, which contains an undertaking to compensate the banks, including if that compensation arises from an inaccurate representation made by one of the guarantor subsidiaries; (viii.)Parent company guarantees, under which the Company has undertaken to guarantee the proper performance of all obligations of iPP, iPP Facilities and iPP Analytics to: a. Southwest Pathology Services LLP and SPS Facilities LLP under the contracts entitled “supply chain agreement” and “management deed” with the subsidiaries iPP and iPP Facilities; b. Taunton and Somerset NHS Foundation Trust and Yeovil District Hospital NHS Foundation Trust under the contracts entitled “members’ agreement in relation to Southwest Pathology Services LLP” with these same subsidiaries iPP and iPP Facilities; and c. Southend University Hospital NHS Foundation and Basildon and Thurrock University Hospital NHS Foundation under the contract entitled “Supply Chain Agreements” entered into between subsidiaries iPP Analytics and iPP Facilities and Pathology First and Facilities First. Those parent company guarantees are valid for two years after the dates on which the agreements lapse. 19.1.2 Financing agreements The Company has entered into the following financing agreements to which certain of its subsidiaries are party: (i.) the Indenture under which the Company issued High-Yield Bonds in a principal amount of €500 million on January 14, 2011, bonds fungible with those bonds in a principal amount of €100 million on February 13, 2013 and further bonds fungible with those bonds in a principal amount of €100 million on February 11, 2015 and to which certain subsidiaries are party, as guarantors; (ii.) covenant agreements relating to the High-Yield Bonds under which certain consolidated French subsidiaries have made irrevocable undertakings to the Company to assume and perform certain commitments; (iii.) the Amended RCF in an initial amount of €128,250,000, entered into in December 2014 and involving parties including the Company as a guarantor, certain subsidiaries as original borrowers or guarantors, and certain financial institutions; and (iv.) the Intercreditor Agreement entered into in January 2011 by parties including the holders of the HighYield Bonds and the parties to the RCF agreement. 19.1.3 Settlement agreements Given the difficulties in interpreting the terms and conditions regarding the exercisability of the 10x Warrants between the 10x Warrant holders and certain shareholders of the Company, who are SEL managers as well, the Company, at the request of those SEL managers and the 10x Warrant holders and in order to avoid potential litigation with certain important shareholders, entered into an agreement on January 21, 2015, after approval thereof by the board of directors in its meeting on the same day in accordance with articles L. 225-38 and following of the French Commercial Code, in order to resolve the situation (see section 21.1.4.2 – ““Investor” warrants” of this document de base). Concomitantly with the signature of the 10x Warrant Agreement (see section 21.1.4.2 – ““Investor” warrants” of this document de base) and given the purpose of the Mezzanine C Warrants, which is to protect holders against dilution resulting from the exercise of the 10x Warrants, the Company and the holders of the Mezzanine C Warrants entered into an agreement, on the same date and after 213 approval by the board of directors in its meeting on the same day in accordance with articles L. 225-38 and following of the French Commercial Code, in order to address the consequences of the Company’s possible purchase of the 10x Warrants (see section 21.1.4.3 – ““Mezzanine” Warrants” of this document de base). 19.1.4 Executive Administrator Agreement and other agreements with Andreas Gaddum All of the fees invoiced to the Company under the Executive Administrator Agreement with Acand (a company controlled by Andreas Gaddum, Chairman of the board of directors at the date of this document de base), whether for Mr. Gaddum’s compensation for his role as chairman of the board of directors, or for specific services, totaled €425,495 between 2012 and 2014, €232,360 of which were for specific services. The Executive Administrator Agreement will not continue to have effect following the initial public offering of the Company. 19.1.5 Authorization of the reorganization of the legal structure In 2012, the Company’s board of directors authorized a restructuring of the legal structure (involving intragroup acquisitions and transformations) involving entities such as Roman Païs, Labco Diagnostics España, Institut de Biologie Clinique (IBC) and Oxabio, some of which are managed by members of the board of directors. 19.1.6 Agreements with the company Immobilière de Laboratoires Certain Group SELs have entered into real-estate leases with the company Immobilière de Laboratoires. That company is a société civile immobilière managed by Philippe Dauchy, a director and shareholder of the Company at the time of this document. Its main purpose is to build or renovate commercial or industrial premises with a view to renting them out to certain Group SELs. The lease agreements feature the usual commercial terms and conditions, although the leases have longer terms than usual commercial leases and, in accordance with articles L. 145-18, L. 145-21 and L. 145-24 of the French Commercial Code, the leases have a fixed term, i.e. the lessee may not terminate the lease at the end of each 3year period. At the date of this document, 12 leases have been entered into by Société Immobilière de Laboratoires and certain Group SELs. The annual rent paid to the Group by SELs for the 12 existing leases entered into with Société Immobilière de Laboratoires and in force on December 31, 2014 amounts to €1.1 million. Leases concerning premises housing laboratories or technical platforms used by the Group’s SELs and arranged for by Société Immobilière de Laboratoires, usually provide Group SELs with premises that meet their technical expectations and are located in the same geographical areas as the Group’s clinical laboratories. 214 19.2 STATUTORY AUDITORS’ SPECIAL REPORTS ON REGULATED AGREEMENTS 19.2.1 Statutory auditors’ special report on regulated agreements for the financial year ended December 31, 2012 Pierre-Henri Scacchi et Associés 185, avenue Charles de Gaulle 92200 Neuilly-sur-Seine Deloitte & Associés 67, Rue de Luxembourg 59777 Euralille LABCO French public limited company (“Société Anonyme”) 60-62, rue d’Hauteville 75010 Paris Statutory Auditors’ special report on regulated agreements Shareholders’ general meeting approving the financial statements for the financial year ended December 31, 2012 This is a free translation into English of the Statutory Auditors’ special report on regulated agreements with third parties that is issued in the French language and is provided solely for the convenience of English speaking readers. This report on regulated agreements should be read in conjunction with, and construed in accordance with, French law and professional auditing standards applicable in France. It should be understood that the agreements reported on are only those provided by the French Commercial Code and that the report does not apply to those related party transactions described in IAS 24 or other equivalent accounting standards. To the Shareholders, In our capacity as Statutory Auditors of your Company, we hereby report to you on regulated agreements. The terms of our engagement require us to communicate to you, based on information provided to us, the principal terms and conditions of those agreements brought to our attention or which we may have discovered during the course of our audit, without expressing an opinion on their usefulness and appropriateness or identifying such other agreements, if any. It is your responsibility, pursuant to article R.225-31 of the French Commercial Code (Code de Commerce), to assess the interest involved in respect of the conclusion of these agreements for the purpose of approving them. Our role is also to provide you with the information stipulated in article R.225 -31 of the French Commercial Code relating to the implementation during the past year of agreements previously approved by the Shareholders’ Meeting, if any. We conducted the procedures we deemed necessary in accordance with the professional guidelines of the French National Institute of Statutory Auditors (Compagnie Nationale des Commissaires aux Comptes) relating to this engagement. These procedures consisted in agreeing the information provided to us with the relevant source documents. 215 AGREEMENTS SUBMITTED TO SHAREHOLDERS FOR APPROVAL Agreements authorized during the financial year under review In accordance with Article L. 225-40 of the French Commercial Code, we have been informed of the following agreements that have obtained prior approval from your Board of directors. Executive Administrator Agreement with “Acand” Persons concerned: Acand GmbH, Chief Executive of the Company (when it used to be in the form of an SAS); Andreas Gaddum, legal representative of Acand, Chairman of the Company’s Strategy Committee (when it used to be in the form of an SAS) then Chairman of the Company’s Board of directors (in the form of an SA). Nature and purpose: Authorization granted by the Board of directors on April 5, 2012 (twelfth resolution) to amend the agreement with Acand and set the amount of fees that may be invoiced by that company at €2,500 per day of work, including VAT. Details: As of December 31, 2012, the fees invoiced to the Company under that agreement amounted to €102,976 including VAT. Agreement appointing Philippe Charrier as corporate officer of the Company (Chief Executive Officer) Person concerned: Philippe Charrier, member of the Strategy Committee and Chairman of the Company (when it used to be in the form of an SAS) then member of the Board of directors and CEO of the Company (when it used to be in the form of an SA). Nature and purpose: Authorization granted by the Board of directors on May 14, 2012 (ninth resolution) relating to the agreement appointing Philippe Charrier as a corporate officer. Details: Agreement providing for certain obligations with respect to the office of CEO (exclusivity, non-compete, nonsolicitation etc.). Authorization of two Parent Company Guarantees for the benefit of Integrated Pathology Partnerships Limited (“iPP”) Persons concerned: Luis Vieira, member of the Strategy Committee and deputy CEO of the Company (when it used to be in the form of an SAS) and member of iPP’s Board of directors; Denis Ribon, member of the Company’s Strategy Committee (when it used to be in the form of an SAS) and member of the Company’s Board of directors (in the form of an SA), and non-voting member of iPP’s Board of directors. 216 Nature and purpose: Authorization granted by the Board of directors on January 25, 2012 (second resolution) to provide a guarantee for iPP. The agreement was unanimously authorized, with no explicit reference to the directors who did not take part in the vote. Details: Labco undertook to guarantee the proper and timely performance of all obligations, duties and undertakings of its joint-venture subsidiary “iPP” to: - Southwest Pathology Services LLP that arise from the supply chain agreement and management deed; Taunton and Somerset NHS Foundation Trust and Yeovil District Hospital NHS Foundation Trust that arise from the members’ agreement in relation to Southwest Pathology Services LLP. Authorization of a comfort letter in favor of iPP Persons concerned: Luis Vieira, member of the Strategy Committee and deputy CEO of the Company (when it used to be in the form of an SAS) and member of iPP’s Board of directors; Denis Ribon, member of the Company’s Strategy Committee (when it used to be in the form of an SAS) then member of the Company’s Board of directors (in the form of an SA), and non-voting member of iPP’s Board of directors. Nature and purpose: Authorization granted by the Board of directors on September 6, 2012 (thirteenth resolution) to provide a comfort letter in favor of iPP. The agreement was unanimously authorized, with no explicit mention of the directors who did not take part in the vote. Details: For 2012, Labco undertook to assist its joint-venture subsidiary iPP in honoring its debts when they fell due, but only to the extent that other funds were not available to iPP. Authorization of a first demand guarantee in favor of Labco Diagnostics UK Person concerned: Philippe Charrier, member of the Strategy Committee and Chairman of the Company (when it used to be in the form of an SAS), member of the Board of directors and CEO of the Company (in the form of an SA) and member of Labco Diagnostics UK’s Board of directors. Nature and purpose: Authorization granted by the Board of directors on July 5, 2012 (fifth resolution) to provide a first demand guarantee in favor of “Labco Diagnostics UK”. The agreement was unanimously authorized, with no explicit reference to the directors who did not take part in the vote. 217 Details: For financial year 2012, Labco undertook with respect to its subsidiary Labco Diagnostics UK Limited to provide a first demand guarantee covering sums owed by that subsidiary to Deutsche Bank AG, Deutsche Bank Sociedad Anónima Espanola, Deutsche Bank S.p.A. and Deutsche Bank (Portugal) S.A. in respect of the cashpooling system supervised by Labco Finance. Authorization of a comfort letter in favor of Labco Diagnostics España Person concerned: Albert Sumarroca, member of the Strategy Committee and Deputy CEO of the Company (when it used to be in the form of an SAS), permanent representative of a member of the Company’s Board of directors (in the form of an SA) and permanent representative of a director of Labco Diagnostics España. Nature and purpose: Authorization granted by the Board of directors of the Company on September 6, 2012 (fifteenth resolution) to provide a first demand guarantee in favor of the Labco Diagnostics España. The agreement was unanimously authorized, with no explicit reference to the directors who did not take part in the vote. Details: For 2012, Labco undertook to assist its subsidiary Labco Diagnostics España in honoring its debts when they fell due. Authorization of restructuring transactions involving entities such as Roman Païs, Labco Diagnostics España, Institut de Biologie Clinique (IBC) and Laboratoire Goudaert-Dauchy-Leclercq-CapelleBourlart (GDLCB) (now known as “Oxabio”) Persons concerned: Stéphane Chassaing, member of the Strategy Committee and Deputy CEO of the Company (when it used to be in the form of an SAS) then member of the Company’s Board of directors (in the form of an SA) and manager of Roman Païs; Albert Sumarroca, member of the Strategy Committee and Deputy CEO of the Company (in the form of an SAS) then permanent representative of a member of the Company’s Board of directors (in the form of an SA) and permanent representative of a director of Labco Diagnostics España. Thierry Mathieu, member of the Company’s Board of directors and Chairman of the Institut de Biologie Clinique; Philippe Dauchy, member of the Company’s Board of directors and member of the Board of directors and CEO of “Laboratoire Goudaert-Dauchy-Leclercq-Capelle-Bourlart” (now known as “Oxabio”). Nature and purpose: Authorization granted by the Board of directors on December 6, 2012 (second resolution) to carry out a tax restructuring. The agreement was unanimously authorized, with no explicit reference to the directors who did not take part in the vote. 218 Details: Labco authorized various transactions to restructure its subsidiaries, some of which are managed by members of its Board of directors. Those transactions include: - the authorization for Ellipsys to acquire 39 shares in Roman Païs from Labco Diagnostics España, the authorization for the Company to acquire one share in Ellipsys, the authorization to turn Ellipsys into a société en commandite par actions, to appoint the Company as general partner (commandité) of Ellipsys, and to appoint André Verhoeft as the Company’s permanent representative at Ellipsys, the authorization for IBC and Laboratoire Schemitick to acquire the shares held by Roman Païs in Labco Diagnostics España, the authorization for GDLCB and Isolab to acquire 99% of the shares held by Ellipsys in Labco Diagnostics España. Agreements authorized since the closing date We have been informed of the following agreements that have been authorized since the closing date of the financial year under review which obtained prior approval from your Board of directors. Termination compensation payable to Philippe Charrier Person concerned: Philippe Charrier, member of the Strategy Committee and Chairman of the Company (when it used to be in the form of an SAS) then member of the Board of directors and CEO of the Company (in the form of an SA). Nature and purpose: Authorization granted by the Board of directors on March 13, 2013 (fifteenth resolution) in relation to the termination compensation payable to Philippe Charrier. Details: As part of a transaction involving Labco’s share capital, solely comprising a partial or total sale of share capital to a new strategic investor (Cerba, Unilabs etc.) that would take place and be followed, within twelve (12) months of that transaction, by a decision by the Board of directors to terminate or not renew Philippe Charrier’s term as corporate officer, Labco undertook irrevocably to pay to him, as termination compensation, a sum equal to twelve months of his gross fixed remuneration (excluding variable remuneration) if Mr Charrier could not resume performance of his employment contract as Chief Operating Officer on the same financial terms as his current position as CEO. That compensation would not be payable if the termination or non-renewal was based on an established gross professional misconduct by Mr Charrier in the performance of his corporate office. Similarly, if a transaction involving Labco’s share capital was followed, within twelve (12) months of that transaction being completed, by decisions leading to the termination or non-renewal of Philippe Charrier’s term as corporate officer, Labco would pay termination compensation equal to twelve months of his gross fixed remuneration (excluding variable remuneration). That compensation would not be payable if Mr Charrier expressly agreed to resume his role as Chief Operating Officer on the same financial terms as his current position as CEO. Amendment to the contract appointing Philippe Charrier as corporate officer (CEO) Person concerned: Philippe Charrier, member of the Strategy Committee and Chairman of the Company (when it used to be in the form of an SAS) then member of the Board of directors and CEO of the Company (in the form of an SA). 219 Nature and purpose: Authorization granted by the Board of directors on April 26, 2013 (sixth resolution) relating to the contract with Philippe Charrier appointing him as corporate officer (CEO). Details: The contract appointing Philippe Charrier as corporate officer, dated May 14, 2012, includes a non-compete clause prohibiting Mr Charrier, directly or indirectly, until the end of a 24-month period from the end of all of his functions within the Group, from carrying out any activity, that could directly or indirectly compete with the Group’s activities. On April 26, 2013, the Board of directors authorized the payment to Philippe Charrier, as compensation for the aforementioned non-compete undertakings, from the end of Mr Charrier’s functions within the Company, of a gross amount of €337,500 payable in 24 equal monthly instalments, subject to Mr Charrier complying with his non-compete undertakings. AGREEMENTS ALREADY APPROVED BY SHAREHOLDERS IN SHAREHOLDERS’ MEETINGS Agreements entered into in previous financial years which continued to be performed during the financial year under review In accordance with article R. 225-30 of the French Commercial Code, we were informed that the following agreements, already approved by shareholders in shareholders meetings in previous financial years, continued to be performed in the period under review. Senior Note Issuance Agreement Nature: On January 24, 2011, an agreement to issue €500,000,000 of senior secured notes maturing in 2018 governed by the law of the State of New York and drafted in English (the Indenture) between parties including the Company as Issuer, certain direct and indirect subsidiaries of the Company as guarantors, (iii) Deutsche Trustee Company Limited as trustee, (iv) Deutsche Bank AG, London Branch as paying agent and Deutsche Bank Luxembourg S.A. as transfer agent and registrar. Deutsche Trustee Company Limited (Trustee) was replaced, with the authorization of the Board of directors on May 14, 2012, by Deutsche Bank AG, London Branch (Successor Trustee). Covenant agreements (undertakings relating to the senior notes) Nature: Under these agreements, certain consolidated French subsidiaries made an irrevocable undertaking to the Company to assume and perform certain covenants applicable to them under the Indenture and the Covenant agreements. Revolving Credit Facility Agreement Nature: On January 21, 2011, an agreement relating to a Revolving Credit Facility in an amount of €135,000,000 expiring in 2017, drafted in English and entitled Senior Multicurrency Revolving Facility Agreement between parties including the Company as guarantor, (ii) certain subsidiaries as Original Borrowers, certain of the Company’s direct and indirect subsidiaries as Guarantors, and certain financial institutions in particular Mandated Lead Arrangers, Facility Agent, Security Agent and Original Lenders. 220 Pursuant to an authorization granted by the Board of directors on May 14, 2012, the Company requested and obtained amendments to the RCF agreement in particular, relating to the leverage ratio, the incurrence ratio and the definition of EBITDA. Intercreditor Deed Nature: On January 24, 2011, an agreement between creditors drafted in English and entitled Intercreditor Deed between parties including the parties to the Senior Notes Issuance Agreement, the Senior Notes Subscription Agreement and the Revolving Credit Facility Agreement and all counterparties of the Company with respect to all interestrate hedging agreements (and/or all other debtors with respect to these agreements). Contract appointing Philippe Charrier as corporate officer (Chairman) Nature: Signature of the contract appointing Mr Charrier as corporate officer on January 20, 2011, determining the terms of Mr Charrier’s appointment (remuneration, investment, exclusivity, non-compete etc.) and the terms and conditions regarding the termination of Mr Charrier’s corporate office member of the Strategy Committee and Chairman of the Company when it used to be in the form of an SAS, performance of which continued until January 12, 2012. Employment contract entered into with Philippe Charrier Nature: Mr Charrier’s recruitment was authorized by the Strategy Committee on November 4, 2010. Mr Charrier’s employment contract was suspended when he was appointed Chairman on January 3, 2011 and remains suspended due to his appointment as CEO from January 12, 2012. Neuilly-sur-Seine and Lille, October 22, 2013 The Statutory Auditors Pierre-Henri Scacci et Associés Deloitte & Associés Serge Gruber Gérard Badin 221 19.2.2 Statutory auditors’ special report on regulated agreements for the financial year ended December 31, 2013 Aplitec 4-14 rue Ferrus 75014 Paris Deloitte & Associés 67, Rue de Luxembourg 59777 Euralille LABCO French public limited company (“Société Anonyme”) 60-62, rue d’Hauteville 75010 Paris Statutory Auditors’ special report on regulated agreements Shareholders’ general meeting approving the financial statements for the financial year ended December 31, 2013 This is a free translation into English of the Statutory Auditors’ special report on regulated agreements with third parties that is issued in the French language and is provided solely for the convenience of English speaking readers. This report on regulated agreements should be read in conjunction with, and construed in accordance with, French law and professional auditing standards applicable in France. It should be understood that the agreements reported on are only those provided by the French Commercial Code and that the report does not apply to those related party transactions described in IAS 24 or other equivalent accounting standards. To the Shareholders, In our capacity as Statutory Auditors of your Company, we hereby report to you on regulated agreements. The terms of our engagement require us to communicate to you, based on information provided to us, the principal terms and conditions of those agreements brought to our attention or which we may have discovered during the course of our audit, without expressing an opinion on their usefulness and appropriateness or identifying such other agreements, if any. It is your responsibility, pursuant to article R.225-31 of the French Commercial Code (Code de Commerce), to assess the interest involved in respect of the conclusion of these agreements for the purpose of approving them. Our role is also to provide you with the information stipulated in article R.225 -31 of the French Commercial Code relating to the implementation during the past year of agreements previously approved by the Shareholders’ Meeting, if any. We conducted the procedures we deemed necessary in accordance with the professional guidelines of the French National Institute of Statutory Auditors (Compagnie Nationale des Commissaires aux Comptes) relating to this engagement. These procedures consisted in agreeing the information provided to us with the relevant source documents. 222 AGREEMENTS SUBMITTED TO SHAREHOLDERS FOR APPROVAL Agreements authorized during the financial year under review In accordance with Article L. 225-40 of the French Commercial Code, we have been informed of the following agreements that have obtained prior approval from your Board of directors. Authorization of a settlement agreement Persons concerned: David Robin, member of the Company’s Board of directors and deputy CEO of TCR Capital, the management company of FCPR Labco Invest; Daniel Bour, member of the Company’s Board of directors; Philippe Taranto, member of the Company’s Board of directors and manager of iXEN Investissement; Nature and purpose: Authorization granted by the Board of directors on January 17, 2013 (third resolution). Details: The agreement was intended to put an end to existing litigation and to prevent future litigation between Labco and certain of its shareholders or warrant-holders relating to (i) the exercisability of certain warrants and (ii) the procedure for making amendments to certain warrants. Additional Senior Notes Subscription Agreement Persons concerned: BIOLOGISTES ASSOCIES REGROUPANT DES LABORATOIRES D’ANALYSES, BIOFRANCE, BIOPAJ, BIOVAL SAS, CENTRE BIOLOGIQUE, LABORATOIRE DE BIOLOGIE MEDICALE DELAPORTE, LABORATOIRE BIOLIANCE, BIOALLIANCE (formerly ESLAB), OXABIO (formerly LABORATOIRE GOUDAERT-DAUCHY-CAPELLE-BOURLART (GDLCB)), GROUPE BIOLOGIC (formerly JORION), LA BIOLOGIE MEDICALE (acquired on April 3, 2014), LABCO MIDI, NORDEN, NORMABIO, LABORATOIRE D’ANALYSES MEDICALES PEPIN-LELUAN-SANNIER-GUILLO, INSTITUT DE BIOLOGIE CLINIQUE, LABORATOIRE DE BIOLOGIE MEDICALE SCHEMITICK, SYLAB, NOVABIO DIAGNOSTICS, ISOLAB LABORATOIRE D’ANALYSES MEDICALES ROMAN PAIS, LABCO FINANCE, LABCO DEUTSCHLAND GMBH, AESCULABOR-KARLSRUHE, 223 MVZ DR. SINTERHAUF, DR. MED. LAMMERT LABORATOTIUMSMEDIZIN MEDIZINISHE MIKROBIOLOGIE UND INFEKIONDEPIDEMIOLOGIE GESUNDHEITSFORDERUNGS GMBH, MVZ MEDIZINISHE FACHLABOR DILLENBURG GMBH, MEDIZINISHE VERSORGUNGSZENTRUM LABOR SAAR GMBH, LABCO ITALIA S.R.L., CENTRO ANALISI MONZA S.P.A, ISTITUTO IL BALUARDO S.P.A, CLINICA DE DIAGNOSCTICS DR. FERNANDO TEIXEIRA, S.A, FLAVIANO GUSMAO, LDA, GENERAL LAB PORTUGAL S.A, GNOSTICA – LABORATORIO DE ANALISES CLINICAS, S.A, GENERAL LAB S.A, SANILAB S.A, SABATER ANALISIS S.A, MACEDO DIAS, Gilles Meshaka, member of the Company’s Board of directors and manager of the Bioalliance subsidiary; Thierry Mathieu, member of the Company’s Board of directors and manager of the Institut de Biologie Clinique subsidiary; Xavier Merlen, member of the Company’s Board of directors and manager of the Novabio Diagnostics subsidiary; Philippe Sellem, member of the Company’s Board of directors and manager of the Biopaj subsidiary; Philippe Dauchy, member of the Company’s Board of directors and manager of the Oxabio subsidiary; Stéphane Chassaing, member of the Company’s Board of directors and manager of the Labco Belgium and Laboratoire d’Analyses Medicales Roman Pais subsidiaries; Barsedana Inversions SL, permanent representative of Barsedana Inversions SL, member of the Company’s Board of directors and manager of the General Lab SA, Labco Diagnostics España, SA, Sampletest Spain and Sanilab SA subsidiaries. Nature and purpose: In January 2013, an agreement to issue €100,000,000 of additional senior secured notes maturing in 2018, governed by the law of the State of New York and drafted in English (the Indenture) between parties including the Company as Issuer, certain direct and indirect subsidiaries of the Company as Guarantors, (iii) Deutsche Bank AG, London Branch as Successor Trustee, (iv) Deutsche Bank AG, London Branch as paying agent and Deutsche Bank Luxembourg S.A. as transfer agent and registrar. The additional senior secured notes have identical terms and conditions as those issued on January 24, 2011 and are governed by the Senior Secured Notes Issuance Agreement, without any amendment to that agreement, which applies to both the initial issue of January 24, 2011 (Initial Notes) and the additional issue. The Additional Senior Secured Notes Subscription Agreement contains a compensation undertaking from the Company in favor of the banks, including in the event that the cause of that compensation arises from an inaccurate representation made by one of the guarantor subsidiaries. Consequently, the Company’s execution of the Additional Senior Secured Notes Subscription Agreement constitutes a guarantee as provided for by article L. 225-35(4) of the French Commercial Code. Authorizations granted by the Board of directors on January 17, 2013 (third resolution) and January 31, 2013 (second and third resolutions). Authorization of a first demand guarantee as part of the cash-pooling system managed by the Labco Finance subsidiary. 224 Persons concerned: Philippe Charrier, member of the Company’s Board of directors and CEO of the Company, and member of the Board of directors of Labco Diagnostics UK Limited; Gilles Meshaka, member of the Company’s Board of directors and manager of the Bioalliance subsidiary; Thierry Mathieu, member of the Company’s Board of directors, manager of the Institut de Biologie Clinique subsidiary and director of Labco Belgium; Xavier Merlen, member of the Company’s Board of directors and manager of the Novabio Diagnostics subsidiary; Philippe Sellem, member of the Company’s Board of directors and manager of the Biopaj subsidiary; Philippe Dauchy, member of the Company’s Board of directors and manager of the Oxabio subsidiary; Stéphane Chassaing, member of the Company’s Board of directors and manager of the Labco Belgium and Laboratoire d’Analyses Medicales Roman Pais subsidiaries; Barsedana Inversions SL, permanent representative of Barsedana Inversions SL, member of the Company’s Board of directors and manager of the General Lab SA, Labco Diagnostics España, SA, Sampletest Spain and Sanilab SA subsidiaries. Nature and purpose: Authorization granted by the Board of directors on March 13, 2013 (seventeenth resolution) to provide a first demand guarantee in respect of the cash-pooling system supervised by Labco Finance, for the benefit of Deutsche Bank AG, Deutsche Bank Sociedad Anónima Espanola, Deutsche Bank S.p.A. and Deutsche Bank (Portugal) S.A. Details: Labco undertook to provide a first demand guarantee covering sums due by its affiliates to Deutsche Bank AG, Deutsche Bank Sociedad Anónima Espanola, Deutsche Bank S.p.A. and Deutsche Bank (Portugal) S.A. in respect of the cash-pooling system supervised by Labco Finance. Authorization of a first demand guarantee in favor of Labco Diagnostics UK Limited Persons concerned: Philippe Charrier, member of the Company’s Board of directors and CEO of the Company, and member of the Board of directors of Labco UK; Gilles Meshaka, member of the Company’s Board of directors and manager of the Bioalliance subsidiary; Thierry Mathieu, member of the Company’s Board of directors, manager of the Institut de Biologie Clinique subsidiary and director of Labco Belgium; Xavier Merlen, member of the Company’s Board of directors and manager of the Novabio Diagnostics subsidiary; Philippe Sellem, member of the Company’s Board of directors and manager of the Biopaj subsidiary; Philippe Dauchy, member of the Company’s Board of directors and manager of the Oxabio subsidiary; Stéphane Chassaing, member of the Company’s Board of directors and manager of the Labco Belgium and Laboratoire d’Analyses Medicales Roman Pais subsidiaries; Barsedana Inversions SL, permanent representative of Barsedana Inversions SL, member of the Company’s Board of directors and manager of the General Lab SA, Labco Diagnostics España, SA, Sampletest Spain and Sanilab SA subsidiaries. Nature and purpose: Authorization granted by the Board of directors on March 13, 2013 (eighteenth resolution) to provide a first demand guarantee in favor of the Labco Diagnostics UK Limited subsidiary. 225 The agreement was unanimously authorized, with no explicit mention of the directors who did not take part in the vote. Details: Labco made an undertaking to its Labco UK subsidiary to provide a first demand guarantee for sums due by it to Deutsche Bank AG, London Branch relating to the operation of the BACS system arranged for its benefit. Authorization of a comfort letter in favor of iPP, a 97%-owned subsidiary since October 25, 2013 Persons concerned: Denis Ribon, member of the Company’s Board of directors, and observer of iPP’s Board of directors. Nature and purpose: Authorization granted by the Board of directors on September 9, 2013 (fifth resolution) to provide a comfort letter in favor of iPP. The agreement was unanimously authorized, with no explicit mention of the directors who did not take part in the vote. Details: Labco undertook, for a period of at least 12 months from the date on which its 2013 annual financial statements were approved, to assist its joint-venture subsidiary iPP in honoring its debts when they fell due, but only to the extent that other funds were not available to iPP. Authorization of a comfort letter in favor of Labco Diagnostics España SA Person concerned: Albert Sumarroca, permanent representative of “Barsedana Inversions SL”, member of the Company’s Board of directors and permanent representative of a director of Labco Diagnostics España. Nature and purpose: Authorization granted by the Board of directors on June 25, 2013 (second resolution) to provide a first demand guarantee in favor of the Labco Diagnostics España subsidiary. The agreement was unanimously authorized, with no explicit mention of the directors who did not take part in the vote. Details: Labco undertook, for a period of at least 12 months from the date on which its 2013 annual financial statements were approved, to assist its Labco Diagnostics España subsidiary in honoring its debts when they fell due. Authorization of a comfort letter in favor of Labco Belgium subsidiaries Persons concerned: Stéphane Chassaing, member of the Company’s Board of directors and director of Labco Belgium; Thierry Mathieu, member of the Company’s Board of directors and director of Labco Belgium. 226 Nature and purpose: Authorization granted by the Board of directors on July 11, 2013 (second resolution) and September 9, 2013 (fourth resolution) to provide a comfort letter in favor of the Labco Belgium subsidiaries. The agreement was unanimously authorized, with no explicit mention of the directors who did not take part in the vote. Details: Labco gave an undertaking to its Labco Belgium subsidiary until July 11, 2014 and for a period of 12 months following the signature of the comfort letter, to help it honor its debts to the Portuguese company Questao Em Aberto. AGREEMENTS ALREADY APPROVED BY SHAREHOLDERS IN SHAREHOLDERS’ MEETINGS Agreements entered into in previous financial years which continued to be performed during the financial year under review In accordance with article R. 225-30 of the French Commercial Code, we were informed that the following agreements, already approved by shareholders in shareholders meetings in previous financial years, continued to be performed in the period under review. Senior Note Issuance Agreement Nature: On January 24, 2011, an agreement to issue €500,000,000 of senior secured notes maturing in 2018 governed by the law of the State of New York and drafted in English (the Indenture) between parties including the Company as Issuer, certain direct and indirect subsidiaries of the Company as Guarantors, (iii) Deutsche Trustee Company Limited as trustee, (iv) Deutsche Bank AG, London Branch as paying agent and Deutsche Bank Luxembourg S.A. as transfer agent and registrar. Deutsche Trustee Company Limited (Trustee) was replaced, with the authorization of the Board of directors on May 14, 2012, by Deutsche Bank AG, London Branch (Successor Trustee). Covenant agreements (undertakings relating to the senior notes) Nature: Under these agreements, certain consolidated French subsidiaries made an irrevocable undertaking to the Company to assume and perform certain covenants applicable to them under the Indenture and the Covenant agreements. Revolving Credit Facility Agreement Nature: On January 21, 2011, an agreement relating to a Revolving Credit Facility in an amount of €135,000,000 expiring in 2017, drafted in English and entitled Senior Multicurrency Revolving Facility Agreement, between parties including the Company as guarantor, (ii) certain subsidiaries as Original Borrowers, certain of the Company’s direct and indirect subsidiaries as Guarantors, and certain financial institutions as Mandated Lead Arrangers, Facility Agent, Security Agent and Original Lenders. Pursuant to authorization by the Board of directors on May 14, 2012, the Company requested and obtained amendments to the Revolving Credit Facility agreement, in particular relating to the leverage ratio, the incurrence ratio and the definition of EBITDA. 227 Intercreditor Deed Nature: On January 24, 2011, an agreement between creditors drafted in English and entitled Intercreditor Deed between parties including the parties to the Senior Notes Issuance Agreement, the Senior Notes Subscription Agreement and the Revolving Credit Facility Agreement and all counterparties of the Company with respect to all interestrate hedging agreements (and/or all other debtors with respect to these agreements). Unlimited-term employment contract entered into with Philippe Charrier Nature: Mr Charrier’s recruitment was authorized by the Strategy Committee on November 4, 2010. Mr Charrier’s employment contract was suspended when he was appointed Chairman on January 3, 2011 and remains suspended due to his appointment as CEO from January 12, 2012. Executive Administrator Agreement with Acand Nature and purpose: Authorization granted by the Board of directors on April 5, 2012 (twelfth resolution) to amend the agreement with Acand and set the amount of fees that may be invoiced by that company at €2,500 including VAT, per day worked. Details: At December 31, 2013, the fees invoiced to the Company under this agreement amounted to €136,250 excluding VAT. Authorization of two Parent Company Guarantees for the benefit of Integrated Pathology Partnerships Limited (iPP) Nature and purpose: Authorization granted by the Board of directors on January 25, 2012 (second resolution) to provide a guarantee to iPP, a 97%-owned subsidiary since October 25, 2013. Details: Labco undertook to guarantee the proper and timely performance of all obligations, duties and undertakings by its iPP joint-venture subsidiary in favor of: - Southwest Pathology Services LLP as part of the supply chain agreement and management deed; and Taunton and Somerset NHS Foundation Trust and Yeovil District Hospital NHS Foundation Trust as part of the members’ agreement in relation to Southwest Pathology Services LLP. Authorization of a first demand guarantee in favor of Labco Diagnostics UK Limited Nature and purpose: Agreement by the Board of directors on July 5, 2012 (fifth resolution) to provide a first demand guarantee in favor of its Labco Diagnostics UK subsidiary. The agreement was unanimously authorized, with no explicit mention of the directors who did not take part in the vote. 228 Details: Labco gave an undertaking to its subsidiary Labco Diagnostics UK Limited to provide a first demand guarantee covering sums due by that subsidiary to Deutsche Bank AG, Deutsche Bank Sociedad Anónima Espanola, Deutsche Bank S.p.A. and Deutsche Bank (Portugal) S.A. in respect of the cash-pooling system supervised by Labco Finance Agreements approved with respect to the period under review We have been informed of the performance, during the period under review, of the following agreements already approved by shareholders in the shareholders meeting of November 15, 2013, covered by the statutory auditors’ special report of October 22, 2013 Termination compensation payable to Philippe Charrier Person concerned: Philippe Charrier, member of the Company’s Board of directors and CEO of the Company. Nature: Authorization granted by the Board of directors on March 13, 2013 (fifteenth resolution) relating to the termination compensation payable to Philippe Charrier and approved by shareholders in the ordinary shareholders meeting of November 15, 2013. Details: As part of a transaction involving Labco’s share capital, only in the case of a partial or total sale of the share capital to a new strategic investor (Cerba, Unilabs etc.) taking place and followed by, within the twelve (12) months following completion of that transaction, a decision by the Board of directors to terminate or not to renew Philippe Charrier’s term as corporate officer, Labco irrevocably undertook to pay to him, as termination compensation, a sum equal to twelve months of his gross fixed remuneration (excluding variable remuneration) if Mr Charrier could not resume performance of his employment contract as Chief Operating Officer on the same financial terms as his current position as CEO. This compensation shall not be payable if the termination or non-renewal were due to established gross professional misconduct by Mr Charrier in the performance of his corporate office. Similarly, as part of a transaction involving Labco’s share capital taking place and followed by, within the twelve (12) months following completion of that transaction, decisions leading to the termination or nonrenewal of Philippe Charrier’s term as corporate officer, Labco shall pay termination compensation equal to twelve (12) months of his gross fixed remuneration (excluding variable remuneration). This compensation shall not be payable if Mr Charrier expressly accepts to resume his role as Chief Operating Officer on the same financial terms as his current position as CEO. Amendment to the contract appointing Philippe Charrier as corporate officer (CEO) Person concerned: Philippe Charrier, member of the Company’s Board of directors and CEO of the Company. Nature and purpose: Authorization by the Board of directors on April 26, 2013 (sixth resolution) relating to the contract with Philippe Charrier appointing him as corporate officer (CEO) and approved by shareholders in the ordinary shareholders meeting of November 15, 2013. 229 Details: The contract appointing Philippe Charrier as corporate officer, dated May 14, 2012, includes a non-compete clause prohibiting Mr. Philippe Charrier, directly or indirectly, for a 24-month period from the termination of all of his functions within the Group, from carrying out any activity, directly or indirectly in any way whatsoever, that could compete directly or indirectly with the Group’s business activities. On April 26, 2013, the Board of directors authorized the payment to Philippe Charrier, as compensation for the aforementioned non-compete undertakings, from the termination of Mr Charrier’s functions within the Company, of a gross amount of €337,500 payable in 24 equal monthly instalments, subject to Mr Charrier complying with his non-compete undertakings. Paris and Lille, June 2, 2014 The Statutory Auditors Aplitec Deloitte & Associés Pierre Laot Gérard Badin 230 19.2.3 Statutory auditors’ special report on regulated agreements for the financial year ended December 31, 2014 LABCO French public limited company (“Société Anonyme”) 60-62, rue d’Hauteville 75010 Paris Statutory Auditors’ special report on regulated agreements Shareholders’ general meeting approving the financial statements for the financial year ended December 31, 2014 This is a free translation into English of the Statutory Auditors’ special report on regulated agreements with third parties that is issued in the French language and is provided solely for the convenience of English speaking readers. This report on regulated agreements should be read in conjunction with, and construed in accordance with, French law and professional auditing standards applicable in France. It should be understood that the agreements reported on are only those provided by the French Commercial Code and that the report does not apply to those related party transactions described in IAS 24 or other equivalent accounting standards. To the Shareholders, In our capacity as Statutory Auditors of your Company, we hereby report to you on regulated agreements. The terms of our engagement require us to communicate to you, based on information provided to us, the principal terms and conditions of those agreements brought to our attention or which we may have discovered during the course of our audit, without expressing an opinion on their usefulness and appropriateness or identifying such other agreements, if any. It is your responsibility, pursuant to article R.225-31 of the French Commercial Code (Code de Commerce), to assess the interest involved in respect of the conclusion of these agreements for the purpose of approving them. Our role is also to provide you with the information stipulated in article R.225 -31 of the French Commercial Code relating to the implementation during the past year of agreements previously approved by the Shareholders’ Meeting, if any. We conducted the procedures we deemed necessary in accordance with the professional guidelines of the French National Institute of Statutory Auditors (Compagnie Nationale des Commissaires aux Comptes) relating to this engagement. These procedures consisted in agreeing the information provided to us with the relevant source documents. AGREEMENTS SUBMITTED TO THE APPROVAL OF THE SHAREHOLDERS' MEETING Agreements authorized during the financial year under review In accordance with Article L. 225-40 of the French Commercial Code, we have been informed of the following agreements that have obtained prior approval from your Board of Directors. Authorization of a letter of intent in favor of the subsidiary “Labco Diagnostics España SA” (LDE): Person concerned: Albert Sumarroca, permanent representative of “Barsedana Inversions SL”, member of the board of directors of the Company and, in addition, permanent representative of a director of “Labco Diagnostics España SA”; Nature and purpose: Authorization granted by the Board of directors on June 19 th, 2014 (eighth resolution) to sign a letter of intent towards its subsidiary “Labco Diagnostics España SA”; 231 Details: By this letter, Labco undertook to support its subsidiary “Labco Diagnostics España SA” for a period of 12 months following the signing of this letter. Authorization of a comfort letter in favor of the subsidiaries “Labco Belgium”: Persons concerned: Stéphane Chassaing, member of the Board of directors of the Company and, in addition, board member of “Labco Belgium”; Thierry Mathieu, member of the Board of directors of the Company and, in addition, board member of “Labco Belgium”; Nature and purpose: Authorization granted by the board of directors on June 19, 2014 (ninth resolution) to sign a comfort letter in favor of its subsidiary “Labco Belgium”, Details: By this letter signed on June 26, 2014, Labco undertook to assist its subsidiary “Labco Belgium” in honoring its debts to its creditors, for a period of 12 months following the signing of the comfort letter; Authorization of a comfort letter in favor of “iPP”: Persons concerned: Denis Ribon, member of the board of directors of the Company and, in addition, board member of “iPP”; Nature and purpose: Authorization granted by the board of directors on September 5 th, 2014 (twenty second resolution) to sign a comfort letter in favor of its subsidiary Labco iPP; Details: Labco undertook to assist its subsidiary iPP in honoring its debts when they fell due, for a period of 12 months following the signing of the letter; Authorization to sign the “Amendment and Restatement Agreement”: Persons concerned: Philippe Sellem, member of the board of directors of the Company and, in addition, manager of the subsidiary BIOPAJ; Gilles Meshaka, member of the board of directors of the Company and, in addition, manager of the subsidiary BIOALLIANCE (formerly ESLAB); Philippe Dauchy, member of the board of directors of the Company and, in addition, manager of the subsidiary OXABIO (formerly LABORATOIRE GOUDAERT-DAUCHY-CAPELLE-BOURLART (GDLCB)); Thierry Mathieu, member of the board of directors of the Company and, in addition, manager of the subsidiary INSTITUT DE BIOLOGIE CLINIQUE; Xavier Merlen, member of the board of directors of the Company and, in addition, manager of the subsidiary NOVABIO DIAGNOSTICS; Stéphane Chassaing, member of the board of directors of the Company and, in addition, manager of the subsidiary LABORATOIRE D’ANALYSES MEDICALES ROMAN PAIS; Albert Sumarroca, permanent representative of BARSEDANA INVERSIONS S.L., member of the board of directors of the Company and, in addition, managers of the subsidiaries: - - LABORATORIO MEDICO DR. DAVID SANTOS PINTO E DR. FERNANDO TEIXEIRA, S.A. (formerly LABORATORIO MEDICO DR. DAVID SANTOS PINTO, S.A.). FLAVIANO GUSMÃO, S.A. (formerly FLAVIANO GUSMAO, LDA); 232 - GENERAL LAB PORTUGAL, S.A.; GNOSTICA – LABORATORIO DE ANALISES CLINICAS, S.A.; LABCO DIAGNOSTICS ESPANA S.A.; GENERAL LAB S.A.; LABCO MADRID S.A.; DR. MACEDO DIAS – LABORATORIO DE ANATOMIA PATOLOGICA, S.A. (formerly MACEDO DIAS); Nature and purpose: The board of directors granted to the Company on November 13, 2014 the right to sign an amendment to the credit agreement drafted in English and entitled Senior multicurrency Revolving Facility Agreement Details: Amendment of the credit agreement drafted in English and entitled Senior multicurrency Revolving Facility Agreement between, mainly,(i) the Company as guarantor, (ii) some of its subsidiaries as original borrowers, (iii) some direct and indirect subsidiaries of the Company as guarantors and (iv) some financial institutions acting as, notably, mandated lead arrangers, facility agent, security agent and original lenders. Authorization of an amendment to the agreement between creditors entitled “intercreditor Agreement”: Persons concerned: Philippe Sellem, member of the board of directors of the Company and, in addition, manager of the subsidiary BIOPAJ; Gilles Meshaka, member of the board of directors of the Company and, in addition, manager of the subsidiary BIOALLIANCE (formerly ESLAB); Philippe Dauchy, member of the board of directors of the Company and, in addition, manager of the subsidiary OXABIO (formerly LABORATOIRE GOUDAERT-DAUCHY-CAPELLE-BOURLART (GDLCB)); Thierry Mathieu, member of the board of directors of the Company and, in addition, manager of the subsidiary INSTITUT DE BIOLOGIE CLINIQUE; Xavier Merlen, member of the board of directors of the Company and, in addition, manager of the subsidiary NOVABIO DIAGNOSTICS; Stéphane Chassaing, member of the board of directors of the Company and, in addition, manager of the subsidiary LABORATOIRE D’ANALYSES MEDICALES ROMAN PAIS; Albert Sumarroca, permanent representative of BARSEDANA INVERSIONS S.L., member of the board of directors of the Company and, in addition, manager of the subsidiaries: - LABORATORIO MEDICO DR. DAVID SANTOS PINTO E DR. FERNANDO TEIXEIRA, S.A. (formerly LABORATORIO MEDICO DR. DAVID SANTOS PINTO, S.A.); - FLAVIANO GUSMÃO, S.A. (formerly FLAVIANO GUSMAO, LDA); - GENERAL LAB PORTUGAL, S.A.; - GNOSTICA – LABORATORIO DE ANALISES CLINICAS, S.A.; - LABCO DIAGNOSTICS ESPANA S.A.; - GENERAL LAB S.A.; - LABCO MADRID S.A.; - DR. MACEDO DIAS – LABORATORIO DE ANATOMIA PATOLOGICA, S.A. (formerly MACEDO DIAS); Nature and purpose: The board of directors authorized on November 13, 2014 an amendment to the Intercreditor Agreement entered into between the creditors and drafted in English, and is entered into between, mainly, parties to the Senior Multicurrency Revolving Facility Agreement. 233 Details: Amendment of the Intercreditor Agreement entered into between creditors and drafted in English. Authorization to sign the Deed confirming the French Securities: Persons concerned: Philippe Sellem, member of the board of directors of the Company and, manager of the subsidiary BIOPAJ; Gilles Meshaka, member of the board of directors of the Company and, in addition, manager of the subsidiary BIOALLIANCE (formerly ESLAB); Philippe Dauchy, member of the board of directors of the Company and, in addition, manager of the subsidiary OXABIO (formerly LABORATOIRE GOUDAERT-DAUCHY-CAPELLE-BOURLART (CDLCB)); Thierry Mathieu, member of the Board of directors of the Company and, furthermore, executive director of the subsidiary INSTITUT DE BIOLOGIE CLINIQUE; Xavier Merlen, member of the board of directors of the Company and, in addition, manager of the subsidiary NOVABIO DIAGNOSTICS; Albert Sumarroca, (permanent representative of BARSEDANA INVERSIONS S.L.), member of the board of directors of the Company and, in addition, manager of LABCO DIAGNOSTICS ESPANA S.A.; Nature and purpose: The Board of directors on November 13, 2014 authorized the company to enter into a deed confirming the French securities set up under the credit contract drafted in English and entitled Senior Multicurrency Revolving Facility Agreement referred to in the previous paragraph above; Details: Setting up French securities under the contract drafted in English and entitled Senior Multicurrency Revolving Facility. AGREEMENTS ALREADY APPROVED BY THE SHAREHOLDERS MEETING Agreements entered into in previous financial years a) which continued to be performed during the financial year under review In accordance with article R. 225-30 of the French Commercial Code, we were informed that the following agreements, already approved by shareholders in shareholders’ meetings in previous financial years, continued to be performed in the period under review. Senior Notes Issuance Agreement and additional Senior Notes: Nature and purpose: Senior Secured Notes Issuance Agreement of €500 00 000 was entered into on January 24, 2011, and an additional issuance of €100 000 000 on January 15, 2013. Those issuances will mature in 2018. Those agreements, entitled the Indenture, are governed by the law of the State of New York and drafted in English between parties including (i) the Company as Issuer, (ii) certain direct and indirect subsidiaries of the Company as guarantors, (iii) Deutsche Trustee Company Limited as trustee, (iv) Deutsche Bank AG, London Branch as paying agent and (v) Deutsche Bank Luxembourg S.A., as transfer agent and as registrar. Details: On January 24, 2011, signature of an agreement, the Indenture, to issue €500,000,000 of senior secured notes maturing in 2018 governed by the law of the State of New York and drafted in English between parties including (i) the Company as Issuer, (ii) certain direct and indirect subsidiaries of the Company as guarantors, (iii) Deutsche Trustee Company Limited as trustee, (iv) Deutsche Bank AG, London Branch, as paying agent and (v) Deutsche 234 Bank Luxembourg S.A., as transfer agent and registrar. An additional senior secured notes issuance of €100 000 000 was made on January 15, 2013. The additional senior secured notes have identical terms and conditions to those issued on January 24, 2011 and are governed by the Senior Secured Notes Issuance Agreement, without any amendment, considering that this agreement applies to the issuance of the initial notes of January 24, 2011 and to the additional issuance. Deutsche Trustee Company Limited (“Trustee”) has been replaced, with the authorization of the Board of directors on May 14, 2012, by Deutsche Bank AG, London Branch (“Successor Trustee”). Covenant agreement (relating to the Senior Notes): Nature and purpose: Pursuant to these agreements, some French consolidated subsidiaries irrevocably committed towards your Company, to undertake and perform certain obligations, the covenants, that are applicable to them according to the terms and conditions of the Indenture and of the Covenants agreements. Details: According to these agreements, certain French consolidated subsidiaries irrevocably committed towards your Company to undertake and perform certain obligations, the covenants, that are applicable to them according to the terms and conditions of the Indenture and of the Covenants agreements. Revolving Credit Facility Agreement: Nature and purpose: On January 21, 2011, conclusion of a revolving credit facility agreement to issue €135,000,000 of senior secured notes maturing in 2017 drafted in English and entitled Senior Multicurrency Revolving Facility Agreement between parties including (i) the Company as Issuer, (ii) certain subsidiaries as original borrowers, (iii) certain direct and indirect subsidiaries of the Company as guarantors and, (iv) certain financial institutions acting mainly as Mandated Lead Arrangers, Facility Agent, Security Agent and Original Lenders. Intercreditor Deed: Nature: On January 24, 2011, conclusion of an agreement between creditors entitled Intercreditor Agreement and drafted in English entered into between, notably, parties to the Senior Notes Issuance Agreement, the Senior Notes Subscription Agreement, the Revolving Credit Facility Agreement, as well as any party with the company to a rate hedging agreement (and/or any other debtor under such contracts). Executive Administrator Agreement with “Acand”: Nature and purpose: Agreement with the Company Acand, initially authorized by the strategic Committee on November 4, 2010, and amended by the Board of directors on April 5, 2012 (twelfth resolution) setting the amount of fees that may be invoiced by that company at €2,500 including VAT, per day worked. Details: On December 31, 2014, the fees invoiced to your Company under this agreement amounted to €203,145 excluding taxes. Unlimited-term employment contract entered into with Philippe Charrier Nature: Mr Charrier’s recruitment was authorized by the Strategy Committee on November 4, 2010. Philippe Charrier’s employment contract was suspended 235 when he was appointed Chairman as of January 3, 2011 and remains suspended due to his appointment as Chief Executive Officer as of January 12, 2012. b) which were not performed during the financial year under review Furthermore, we have been informed that the following agreements, already approved by the General Assembly of shareholders during previous financial years, were not performed during the financial year under review. Authorization of two Parent Company Guarantees to the benefit of Integrated Pathology Partnerships Limited “iPP”: Nature and purpose: Authorization granted by the Board of directors on January 25, 2012 (second resolution) to provide a guarantee to iPP, a 97%-owned subsidiary since October 25, 2013. Details: Labco undertook to guarantee the proper and timely performance of all obligations, duties and undertakings by its joint-venture subsidiary iPP in favor : - of “Southwest Pathology Services LLP” as part of the supply chain agreement and management deed; - of “Taunton and Somerset NHS Foundation Trust” and “Yeovil District Hospital NHS Foundation Trust” in connection with “members’ agreement in relation to Southwest Pathology Services LLP. Authorization of a first demand guarantee in favor of the subsidiary “Labco Diagnostics UK Limited” : Nature and purpose: Authorization granted by the Board of directors on July 5, 2012 (fifth resolution) to provide a first demand guarantee in favor of its subsidiary Labco Diagnostics UK. The agreement was unanimously authorized, with no explicit mention of the directors who did not take part in the vote. Details: Labco undertook in favor of its subsidiary “Labco Diagnostics UK Limited” to provide a first demand guarantee covering sums due by that subsidiary to Deutsche Bank AG, Deutsche Bank Anónima Espanola, Deutsche Bank S.p.A. and Deutsche Bank (Portugal) S.A. in connection with the cashpooling supervised by Labco Finance. Authorization of a first demand guarantee in favor of the subsidiary “Labco Diagnostics UK Limited” Nature and purpose: Authorization granted by the Board of directors on March 13, 2013 (eighteenth resolution) to issue a first demand guarantee in favor of the subsidiary “Labco Diagnostics UK Limited”. This agreement was unanimously authorized, with no explicit mention of the directors who did not take part in the vote. Details: Labco undertook in favor of its subsidiary Labco UK to provide a first demand guarantee covering sums due by that subsidiary to Deutsche Bank AG, London branch, in connection with the BACS system set up in its favor. 236 Authorization of a comfort letter in favor of the subsidiary iPP: Nature and purpose: Authorization granted by the Board of directors on September 9, 2013 (fifth resolution) to provide a comfort letter in favor of the subsidiary iPP. This agreement was unanimously authorized, with no explicit mention of the directors who did not take part in the vote. Details: Labco undertook, for a period of at least 12 months from the date on which its 2013 annual financial statements were approved, to assist its jointventure subsidiary iPP in honoring its debts when they fall due, but only to the extent that other funds were not available to iPP. Authorization of a comfort letter in favor of the subsidiary “Labco Diagnostics España SA”: Nature and purpose: Authorization granted by the Board of directors on June 25, 2013 (second resolution) to issue a first demand guarantee in favor of the subsidiary “Labco Diagnostics España”. This agreement was unanimously authorized, with no explicit mention of the directors who did not take part in the vote. Details: Labco undertook, for a period of at least 12 months from the date on which its 2012 annual financial statements were approved, to assist its jointventure subsidiary “Labco Diagnostics España” in honoring its debts when they fall due. Authorization of a comfort letter in favor of the subsidiaries “Labco Belgium” Nature and purpose: Authorization granted by the Board of directors on July 11, 2013 (second resolution) and on September 9, 2013 (fourth resolution) to provide a comfort letter in favor of its subsidiaries “Labco Belgium” and “Labco Finance”. This agreement was unanimously authorized, with no explicit mention of the directors who did not take part in the vote. Details: Labco undertook until July 11, 2014 and then for a period of 12 months following the date of signing the comfort letter, to assist its subsidiary “Labco Belgium” in honoring its debts towards its creditors. Authorization of a first demand guarantee in connection with the cash-pooling managed by “Labco Finance”: Nature and purpose: Authorization granted by the Board of directors on March 13, 2013 (seventeenth resolution) to issue a first demand guarantee in connection with the cash-pooling system supervised by Labco Finance in favor of Deutsche Bank AG, Deutsche Bank Sociedad Anonima Espanola, Deutsche Bank S.p.A and Deutsche Bank (Portugal) S.A. Details: Labco undertook to provide a first demand guarantee for the amounts due by its affiliates in favor of the Deutsche Bank AG, Deutsche Bank Sociedad Anónima Espanola, Deutsche Bank S.p.A. and Deutsche Bank (Portugal) S.A. in connection with the cash-pooling system supervised by Labco Finance. 237 Contract with Philippe Charrier appointing him as corporate officer (Chief Executive Officer) Nature and purpose: Authorization by the Board of directors on April 26, 2013 (sixth resolution) to amend the contract with Philippe Charrier appointing him as chief executive officer (CEO) and approved by shareholders in the ordinary shareholders meeting of November 15, 2013. Details: The contract appointing Philippe Charrier as chief executive officer, includes a non-compete clause prohibiting Mister Philippe Charrier, directly or indirectly, until the expiration of a twenty-four-month (24) period from the termination of all of his functions within the Group, from carrying out any activity, directly or indirectly in any capacity whatsoever, that could compete directly or indirectly with the Group’s business activities. On April 26, 2013, the Board of directors authorized the payment to Philippe Charrier, as compensation for the aforementioned non-compete undertakings, starting from the termination of Mr Charrier’s functions within the Group, of a gross fixed amount of €337,500 payable in 24 equal monthly payments, subject to Mr Charrier complying with his non-compete undertakings. Termination compensation payable to Philippe Charrier Nature: Authorization granted by the Board of directors on March 13, 2013 (fifteenth resolution) relating to the termination compensation payable to Philippe Charrier and approved by shareholders in the ordinary shareholders meeting of November 15, 2013. Details: As part of a transaction involving Labco’s share capital, only in the case of a partial or total sale of the share capital to a new strategic investor (Cerba, Unilabs, …) taking place and followed by, within the twelve (12) months following completion of that transaction, a decision by the Board of directors to terminate or not to renew Philippe Charrier’s term as corporate officer, Labco irrevocably undertook to pay to him, as termination compensation, a sum equal to twelve months of his gross fixed remuneration (excluding variable remuneration) if Mr Charrier could not resume performance of his employment contract as Chief Operating Officer on the same financial terms as his current position as CEO. This compensation shall not be payable if the termination or non-renewal were due to established gross professional misconduct by Mr Charrier in the performance of his corporate office. Similarly, as part of a transaction involving Labco’s share capital taking place and followed by, within the twelve (12) months following completion of that transaction, decisions leading to the termination or non-renewal of Philippe Charrier’s term as corporate officer, Labco shall pay a termination compensation equal to twelve (12) months of his gross fixed remuneration (excluding variable remuneration). This compensation shall not be payable if Mr Charrier expressly accepts to resume his role as Chief Operating Officer on the same financial terms as his current position as CEO. 238 Paris and Lille, April 2, 2015 The Statutory Auditors Aplitec Deloitte & Associés Pierre Laot Gérard Badin 239 CHAPTER 20 FINANCIAL INFORMATION CONCERNING THE COMPANY’S ASSETS AND LIABILITIES, FINANCIAL POSITION AND RESULTS 20.1 IFRS FINANCIAL REPORTING 20.1.1 IFRS consolidated financial statements for the financial years ended December 31, 2012, 2013 and 2014 Financial year ended December 31, 2012 Contents CONSOLIDATED STATEMENT OF INCOME 242 CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME 243 CONSOLIDATED STATEMENT OF FINANCIAL POSITION 244 CONSOLIDATED STATEMENT OF CHANGES IN EQUITY 245 CONSOLIDATED STATEMENT OF CASH FLOWS 246 NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS FOR THE YEAR ENDED 31 DECEMBER 2012 NOTE 1 NOTE 2 NOTE 3 NOTE 4 NOTE 5 NOTE 6 NOTE 7 NOTE 8 NOTE 9 NOTE 10 NOTE 11 NOTE 12 NOTE 13 NOTE 14 NOTE 15 NOTE 16 NOTE 17 NOTE 18 NOTE 19 NOTE 20 NOTE 21 NOTE 22 NOTE 23 NOTE 24 NOTE 25 NOTE 26 NOTE 27 NOTE 28 NOTE 29 NOTE 30 NOTE 31 NOTE 32 NOTE 33 NOTE 34 REPORTING ENTITY 247 BASIS OF PREPARATION 247 SIGNIFICANT ACCOUNTING POLICIES 250 FINANCIAL RISK MANAGEMENT 263 SIGNIFICANT EVENTS 266 ACQUISITIONS OF SUBSIDIARIES 270 GEOGRAPHICAL INFORMATION 272 PAYROLL RELATED EXPENSES 272 OTHER OPERATING EXPENSES 273 NON RECURRING INCOME AND EXPENSES 273 NET FINANCE COSTS 274 INCOME TAX EXPENSES 276 GOODWILL 278 INTANGIBLE ASSETS 281 PROPERTY, PLANT AND EQUIPMENT 282 INVESTMENTS IN ASSOCIATES 284 OTHER NON-CURRENT ASSETS 286 DEFERRED TAX ASSETS AND LIABILITIES 287 INVENTORIES 287 TRADE RECEIVABLES AND OTHER CURRENT ASSETS 288 CASH AND CASH EQUIVALENTS 289 CAPITAL AND RESERVES ATTRIBUTABLE TO OWNERS OF THE PARENT BORROWINGS AND OTHER FINANCIAL LIABILITIES 293 EMPLOYEE BENEFIT LIABILITIES 297 SHARE BASED PAYMENT SCHEMES 298 PROVISIONS 300 LITIGATIONS AND CONTINGENT LIABILITIES 301 TRADE AND OTHER LIABILITIES 304 FINANCIAL INSTRUMENTS 304 CAPITAL COMMITMENTS AND CONTINGENCIES 307 EARNINGS PER SHARE 314 RELATED PARTY TRANSACTIONS 315 GROUP ENTITIES 317 EVENTS AFTER THE REPORTING PERIOD 321 240 289 247 NOTE 35 AUDITOR FEES 322 241 Consolidated statement of income For the year ended 31 December 2012 242 Consolidated statement of comprehensive income For the year ended 31 December 2012 The accompanying notes are an integral part of the financial statements 243 Consolidated statement of financial position As at 31 December 2012 CONSOLIDATED STATEMENT OF FINANCIAL POSITION (€ 000) ASSETS Notes Goodwill Intangible assets Property, Plant and Equipment Investments in associates Other non-current assets Deferred tax assets Note 13 Note 14 Note 15 Note 16 Note 17 Note 18 NON CURRENT ASSETS Inventories Trade Receivables Other current assets Cash and cash equivalents Note 19 Note 20 Note 20 Note 21 CURRENT ASSETS Assets classifed as held for sale 31.12.2012 620 619 12 606 59 853 2 340 8 938 7 614 616 124 10 163 54 528 2 452 7 354 3 453 711 969 694 073 8 938 97 442 13 704 56 595 9 697 98 992 11 557 69 833 176 679 190 079 Note 5.1.2 TOTAL ASSETS EQUITY & LIABILITIES Notes Share Capital Additional paid-in capital Reserves attributable to owners of the parent Currency translation adjustements Net income (Group share) Equity attributable to owners of the parent Non-controlling interests TOTAL EQUITY 31.12.2011 0 0 888 648 884 152 31.12.2012 31.12.2011 68 459 210 995 ( 85 583) ( 169) ( 28 454) 43 337 208 727 40 453 ( 59) ( 128 984) 165 250 163 474 1 168 1 366 166 417 164 839 Provisions - non current Employee benefits liabilities Borrowings and other financial liabilities - non current Other non-current liabilities Deferred tax liabilities Note 26 Note 24 Note 23 Note 28 Note 18 858 8 158 548 675 2 310 2 369 344 7 370 555 555 3 417 2 302 NON-CURRENT LIABILITIES Provisions - current Current financial liabilities Trade Liabilities Other current liabilities Note 26 Note 23 Note 28 Note 28 562 370 5 846 31 917 56 971 65 129 568 988 7 625 33 996 52 688 56 015 159 862 150 324 0 0 888 648 884 152 CURRENT LIABILITIES Liabilties classifed as held for sale Note 5.1.2 TOTAL EQUITY AND LIABILITIES The accompanying notes are an integral part of the financial statements 244 Consolidated statement of changes in equity As at 31 December 2012 Share Capital € 000 Balance at 1 January 2012 43 337 Share premium 208 727 Stock Option Plan reserve 4 037 Fair value reserve Retained Earnings ( 0) ( 91 922) Currency translation Own shares reserve ( 59) ( 646) Noncontrolling interest Total Equity 163 474 1 366 164 840 ( 28 454) 382 ( 28 073) Total comprehensive income for the period Net result of the period ( 28 454) Other comprehensive income Effective portion of changes in fair value of cash flow hedges, net of tax 0 Net change in fair value of cash flow hedges, transferred to profit & loss, net of tax 0 Actuarial gains or losses on pension obligations 198 0 0 0 198 198 Other changes 0 0 Total other comprehensive income 0 0 0 0 198 0 0 199 0 199 Total comprehensive income for the period 0 0 0 0 ( 28 256) 0 0 ( 28 256) 382 ( 27 874) 25 123 2 268 Transactions with owners, recorded directly in equity Contributions by and distributions to owners Capital increase 27 391 Dividends 0 (533) Share-based payment transactions 2 634 2 101 Treasury shares Total contributions by and distributions to owners 27 391 ( 165) ( 165) 2 101 0 25 123 2 268 ( 533) 0 2 634 Other variations Changes in ownership interests in subsidiaries that do not result in a loss of control 0 0 ( 110) Acquisition of non-controlling interest 29 492 0 ( 165) ( 110) 649 29 327 ( 110) 649 ( 414) 0 0 0 0 649 0 0 649 ( 414) 235 Total transactions with owners 25 123 2 268 ( 533) 0 3 283 ( 110) 0 30 031 ( 580) 29 452 Balance at 31 December 2012 68 459 210 995 3 504 ( 0) ( 116 894) ( 169) ( 646) 165 250 1 168 166 417 Fair value reserve Retained Earnings Currency translation reserve ( 2 478) 37 575 Total changes in ownership interests in subsidiaries 235 As at 31 December 2011 Share Capital € 000 Balance at 1 January 2011 43 064 Share premium 204 607 Stock Option Plan reserve 1 926 Own shares ( 646) Noncontrolling interest Total Equity 284 048 1 008 285 056 ( 128 984) 241 ( 128 744) Total comprehensive income for the period Net result of the period ( 128 984) Other comprehensive income Effective portion of changes in fair value of cash flow hedges, net of tax Net change in fair value of cash flow hedges, transferred to profit & loss, net of tax Actuarial gains or losses on pension obligations 2 280 ( 406) Other changes 0 0 2 280 2 280 ( 406) ( 406) 0 0 Total other comprehensive income 0 0 0 2 280 ( 406) 0 0 1 874 0 1 874 Total comprehensive income for the period 0 0 0 2 280 ( 129 390) 0 0 ( 127 110) 241 ( 126 869) 273 4 120 ( 109) ( 109) Transactions with owners, recorded directly in equity Contributions by and distributions to owners Capital increase 4 393 Dividends 0 2 111 Share-based payment transactions 2 111 Treasury shares Total contributions by and distributions to owners Total transactions with owners Balance at 31 December 2011 2 111 0 273 4 120 2 111 Other variations Changes in ownership interests in subsidiaries that do not result in a loss of control Acquisition of non-controlling interest Total changes in ownership interests in subsidiaries 4 393 0 0 0 198 ( 60) ( 59) 0 6 504 0 ( 109) 79 ( 46) 6 395 79 ( 46) 226 0 0 0 0 ( 46) 0 0 ( 46) 226 180 273 4 120 2 111 198 ( 106) ( 59) 0 6 536 117 6 653 43 337 208 727 4 037 ( 0) ( 91 922) ( 59) ( 646) 163 474 1 366 164 840 The accompanying notes are an integral part of these consolidated financial statements. 245 180 Consolidated statement of cash flows For the year ended 31 December 2012 CONSOLIDATED STATEMENT OF CASH FLOW (€ 000) Notes EBITDA Other calculated revenues and expenses Dividends received from associates Cash from (used in) non recurring expenses net Changes in inventories Changes in trade and other receivables from operations Changes in trade and other payables from operations Changes in other receivables and payables Income tax paid CASH FLOWS FROM (USED IN) OPERATING ACTIVITIES (A) Purchases of intangible, property, plant and equipment Proceeds on disposals of intangible, property, plant and equipment Purchases of investments, net of cash acquired and changes in debt related to acquisitions Net decrease (increase) in other assets Changes effet in consolidation scope CASH FLOWS FROM (USED IN ) INVESTING ACTIVITIES (B) Proceeds from share capital increase Cash from (used in) net financial profit (loss) New borrowings and other financial liabilities Repayment of borrowings and other financial liabilities Repayment of finance lease liabilities Dividends paid 2012 2011 110 213 92 212 1 237 2 778 372 302 ( 8 066) ( 11 265) 1 132 ( 278) 679 ( 9 309) 8 412 ( 857) ( 3 832) 7 109 ( 19 979) ( 18 218) 90 167 62 475 ( 15 714) ( 13 498) 344 1 561 ( 44 473) ( 93 315) 216 ( 2 755) ( 349) 1 817 ( 59 976) ( 106 190) 27 391 4 394 ( 51 088) ( 43 201) 616 398 678 771 ( 627 681) ( 603 089) ( 6 699) ( 5 973) ( 97) ( 97) CASH FLOWS FROM (USED IN) FINANCING ACTIVITIES (C) ( 41 776) 30 804 TOTAL CASH FLOWS (A+B+C) ( 11 584) ( 12 911) 67 740 80 676 Cash and cash equivalent at the begining of the period Change effect in foreign exchange rate Cash and cash equivalent at the end of the period Note 21 NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS ( 27) ( 26) 56 129 67 740 ( 11 584) ( 12 911) The accompanying notes are an integral part of these consolidated financial statements. 246 Notes to the consolidated financial statements for the year ended 31 December 2012 Reporting entity Note 1 Labco SAS (the “Company”), converted in Labco SA, société anonyme, on January 12, 2012, is a company domiciled in France. The address of the Company’s registered office is 60 - 62, rue de Hauteville, 75010, Paris, France. The consolidated financial statements of the Company as at and for the year ended 31 December 2012 comprise the Company and its subsidiaries (together referred to as the “Group” and individually as “Group entities”) and the Group’s interest in associates. The Group primarily is involved in clinical diagnostics testing and screening services mainly in France, Spain, Portugal, Italy, Belgium, Germany and the United Kingdom and we also provide clinical laboratory testing services to customers in Latin America, the Middle East and North Africa. Basis of preparation Note 2 2.1. Statement of compliance The consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (IFRSs), as adopted by the European Union (EU) and IFRS as published by IASB effective as at December 31, 2012. As a reminder, the Group’s consolidated financial statements have been prepared for the first time in 2010 in accordance with IFRSs, the opening IFRS balance sheet being prepared as of January 1, 2009. The accounting policies retained are the same as those used in preparing the consolidated financial statements at 31 December 2011, except for The Standards and Interpretations adopted by the European Union applicable as from 1 January 2012, which have no significant effect on the consolidated financial statements of the Group Certain Standards and Interpretations adopted by the European Union not mandatorily applicable as from 1 January 2012 but for which the Group has elected to implement early adoption and which have limited impact on the consolidated financial statements of the Group: o Amendment to IAS 1 – Presentation of Items of Other Comprehensive Income o Amendment to IAS 19 – Employee Benefits The consolidated financial statements were authorised for issue by the Board of directors on April 4, 2013. 2.2. IFRS basis adopted 2.2.1. Standards, amendments and interpretations effective as of January 1, 2012 The Group’s consolidated financial statements comply with the amendments to published standards and interpretations which came into effect on January 1. 2012 and have been adopted by the European Union. The following amendments and interpretations are mandatorily applicable as of January 1. 2012: Amendment to IFRS 7 – Disclosures – Transfers of Financial Assets This amendment has no material impact on the consolidated financial statements. 247 2.2.2. Standards, amendments and interpretations not mandatorily applicable as of January 1, 2012 The Group has elected to early adopt the following amendment whose application is not mandatory as of January 1, 2012: Amendment to IAS 1 –Presentation of Items of Other Comprehensive Income Amendment to IAS 19 – Employee Benefits The impact for the Group of this amendment is limited since the Group already recognizes all actuarial gains and losses in other comprehensive income. 2.2.3. New standards, amendments and interpretations not applicable as of January 1. 2012 A number of new standards, amendments to standards and interpretations are not yet effective for the year ended 31 December 2012, and have not been applied in preparing these consolidated financial statements. IFRS 9 – Financial Instruments: Classification and Measurement Amendment to IFRS 7 – Financial Instruments: Disclosures and Amendment to IAS 32 Financial Instruments: Presentation Amendment to IAS 12 – Deferred Tax: Recovery of Underlying Assets IFRS 10 – Consolidated Financial Statements, IFRS 11 – Joint Arrangements and IFRS 12 – Disclosures of Interests in Other Entities, as well as the resulting revised IAS 27 and IAS 28 IFRS 13 – Fair Value Measurement The Group is currently reviewing these standards, amendments and interpretations to assess their possible effect on its financial information. 2.2.4. Summary of options used on the first time adoption of IFRS As a first time adopter in 2010, the opening IFRS balance sheet has been prepared as of January 1, 2009 (i.e. date of transition to IFRS) using IFRSs as adopted by the European Union effective December 31, 2010. In accordance with IFRS 1, the Group has elected to use the following main exemptions for the preparation of its first IFRS financial statements: business combinations that occurred before the date of transition to IFRS are not retrospectively restated in accordance with IFRS 3 – Business Combinations; the long term employee benefits have been fully recorded; for the share based payment transactions, only the 2008 scheme has been restated according to IFRS 2, and financial instruments held have all been classified as financial assets available for sale at the date of transition, with the exception of liabilities and receivables and trade receivables. 2.3. Basis of measurement The consolidated financial statements have been prepared on the historical cost basis except for the following items in the statement of financial position: 248 derivative financial instruments are measured at fair value certain long term financial assets are measured at fair value 2.4. Functional and presentation currency These consolidated financial statements are presented in euro, which is the Company’s functional currency. All financial information presented in euro has been rounded to the nearest thousand. 2.5. Use of estimates and judgments The preparation of the consolidated financial statements in conformity with IFRSs requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected. Information about critical judgments in applying accounting policies that have the most significant effect on the amounts recognized in the consolidated financial statements is included in the following notes: Note 3.1.1 – Subsidiaries and consolidation method Note 3.2.2 – Derivative financial instruments, including hedge accounting Note 3.7 – Leased assets Note 3.9.2 – Non-financial assets Information about assumptions and estimation uncertainties that have a significant risk of resulting in a material adjustment within the next financial year are included in the following notes: Note 3.6.1 and Note 6 – Goodwill and acquisition of subsidiaries 249 Significant accounting policies Note 3 The accounting policies set out below have been applied consistently to all periods presented in these consolidated financial statements, unless otherwise indicated. The accounting policies have been applied consistently by Group entities. 3.1. Basis of consolidation 3.1.1. Subsidiaries and consolidation method Subsidiaries are entities controlled by the Group. Control is the power to govern the financial and operating policies of an entity so as to obtain economic benefits from its activities. In assessing control, the Group takes into consideration potential voting rights that currently are exercisable. Regulations governing the ownership and certification of laboratories in certain jurisdictions require us to hold each clinical laboratory or a limited number of the clinical laboratories through a separate subsidiary. Certain countries also regulate the corporate form through which laboratories may be held, such as “SELs” (société d’exercice liberal) in France or “MVZs” (Medizinisches Versorgungszentrum) in Germany. In France, we are subject to regulatory constraints on the ownership of share capital and voting rights of SELs operating clinical laboratories by persons other than laboratory doctors and laboratory companies. To comply with such constrain, we have established a specific corporate structure pursuant to which and subject to a few exceptions, we directly and indirectly hold shares representing approximately up to 99.9% of the share capital of our SELs and the laboratory doctors operating such SELs hold the remainder. However, the articles of association of all of our SELs grant to the laboratory doctors operating them 50.01% of the voting rights at all the shareholders’ general meetings. Although we cannot have the majority of the voting rights in our SELs, we have put in place mechanisms that grant us substantially all of the economic rights over such SELs and allow us to control, within the French regulatory framework, and fully consolidate our French network: As we acquire SELs, we change their articles of association to implement the capital structure described above but also to adopt specific provisions, in particular with respect to governance. The financial statements of subsidiaries are included in the consolidated financial statements from the date that control commences until the date that control ceases. The acquisition date is the date on which control is transferred to the acquirer. Judgment is applied in determining the acquisition date and determining whether control is transferred from one party to another. The accounting policies of subsidiaries have been changed when necessary to align them with the policies adopted by the Group. Non-controlling interests (“minority interests”) represent the part of net income or loss, and of net equity not held by the Group. They are presented in the consolidated Income Statement, the Consolidated Statement of Comprehensive Income and in equity in the Consolidated Statement of Financial Position, separately from equity attributable to the owners of the Company. In the case of medical biology companies, whether controlled de jure or de facto, minority interests of other shareholders, i.e. laboratory doctors, must be assessed based on the financial rights attached to their shares rather than voting rights. These shares of stock are entitled to a priority dividend, calculated on a formula defined in each company’s by-laws so long as their holders are professionally active in the company. However their rights to any surplus on liquidation (net assets) are strictly limited, which gives this portion a non-significant accounting value. Most by-laws of consolidated French companies call for two classes of shares. Class A shares are held by laboratory doctors associated in the SELs (Sociétés d’exercice liberal). They are awarded a priority dividend according to a formula worked out in the by-laws of each company and representing a profit sharing arrangement. They have this right 250 as long as they are professionally active in the company. They are not actually minority interests but rather a mechanism of compensation for the services such professionals render to the Group. Consequently dividends thereon are recognized as compensation expense in profit or loss in the period in which services giving rise to profit sharing are rendered. 3.1.2. Investments in associates (equity accounted investees) An associate is an entity over which the Group has significant influence and that is not a subsidiary. Significant influence is the power to participate in the financial and operating policy decisions of the investee but is not control over those policies. Investments in associates are accounted for using the equity method (equity accounted investees) and are recognised initially at cost. The Group’s investment includes goodwill identified on acquisition, net of any accumulated impairment losses. The consolidated financial statements include the Group’s share of the income and expenses and equity movements of equity accounted investees, after adjustments to align the accounting policies with those of the Group, from the date that significant influence commences until the date that significant influence ceases. 3.1.3. Interests in joint ventures A joint venture is a contractual arrangement whereby the Group and other parties undertake an economic activity that is subject to joint control (i.e. when the strategic financial and operating policy decisions relating to the activities of the joint venture require the unanimous consent of the parties sharing control). Jointly controlled entities are consolidated using the equity method in accordance with the option provided by IAS 31, Interests in Joint Ventures. 3.1.4. Transactions eliminated on consolidation Intra-group balances and transactions, and any internal income and expenses arising from intragroup transactions, are eliminated in preparing the consolidated financial statements. Unrealised gains arising from transactions with equity accounted investees are eliminated against the investment to the extent of the Group’s interest in the investee. Internal losses are eliminated in the same way as internal gains, but only to the extent that there is no evidence of impairment. 3.1.5. Business combinations For acquisitions on or after 1 January 2009, the Group applies IFRS 3 revised (2008) and measures goodwill as the difference between (a) the sum of (i) the fair value of the consideration transferred, (ii) the recognised amount of any non-controlling interest in the acquiree, (iii) the acquisition date fair value of any previously held interest in the acquiree, and (b) the net recognised amount (generally fair value) of the identifiable assets acquired and liabilities assumed, all measured as of the acquisition date. Consideration transferred includes the fair values of the assets transferred, liabilities incurred by the Group to the previous owners of the acquiree, and equity interests issued by the Group. It also includes the fair value of any contingent consideration. When this difference is negative (negative goodwill), a bargain purchase gain is recognized immediately in profit or loss. For business combinations that occurred since 2009, the Group measured any non-controlling interest in majority of cases at its proportionate interest in the identifiable net assets of the acquiree. Transaction costs that the Group incurs in connection with a business combination, such as finder’s fees, legal fees, due diligence fees, and other professional and consulting fees are expensed as incurred. If a business combination is achieved in stages, re-measurement of any previously held equity interest in the acquiree at its acquisition-date is performed at fair value with any resulting gain or loss recognized in the statement of earnings. A contingent liability of the acquiree assumed in a business combination is recognized only if such a liability represents a present obligation and arises from a past event, and its fair value can be measured reliably. 251 If consideration transferred include a contingent consideration (earn-out for example), it is recorded at fair value at acquisition date. For a contingent consideration recorded as financial instrument in the scope of IAS 39, subsequent fair value variations are recognized in statement of income. If a contingent consideration is classified as equity, it will not be remeasured. Acquisitions and disposal of non-controlling interests Acquisitions and/or disposal of non-controlling interests are accounted for as transactions with equity holders in their capacity as equity holders. Therefore no goodwill is recognized or derecognized as a result of such transactions. 3.2. Financial instruments Financial instruments include financial assets and financial liabilities. Financial assets comprise available-for-sale financial assets, loans and receivables carried at amortized cost including trade and other receivables, and financial assets measured at fair value through income, including derivative financial instruments. Financial liabilities include borrowings, trade and other payables, derivative financial instruments and other financial liabilities. 3.2.1. Non-derivative financial instruments Non-derivative financial instruments comprise investment in equity and debt securities, trade and other receivables, loans and borrowing at amortized cost and trade and other payables. The Group initially recognizes trade and other receivables on the date that they are originated. All other financial assets are recognized initially on the trade date at which the Group becomes a party to the contractual provisions of the instrument. Available-for-sale financial assets The Group’s investments in equity securities (generally the non-consolidated investments) and certain debt securities are classified as available-for-sale financial assets. These items are measured at fair value on initial recognition, which generally correspond to the acquisition cost plus any directly attributable transaction costs. Subsequent to initial recognition, they are measured at fair value and changes therein, other than impairment losses are recognised in other comprehensive income and presented within equity in the fair value reserve. When an investment is derecognised, the cumulative gain or loss in other comprehensive income is transferred to profit or loss. Loans and receivables at amortized cost Loans and receivables, including trade and other receivables are financial assets with fixed or determinable payments that are not quoted in an active market. Loans and receivables primarily include loans and advances to associates or non-consolidated companies, and guarantee deposits, are recognized initially at fair value, plus any directly attributable transaction costs. Subsequent to initial recognition, loans and receivables are measured at amortised cost using the effective interest rate method, less any impairment losses. On initial recognition, trade and other receivables are recorded at fair value, which generally corresponds to their nominal value. Impairment losses are recorded based on the estimated risk of non-recovery. Financial assets and liabilities are offset and the net amount presented in the statement of financial position when, and only when, the Group has a legal right to offset the amounts and intends either to settle on a net basis or to realise the asset and settle the liability simultaneously. Financial liabilities including trade and other liabilities Financial liabilities, such as loans and borrowings carried at amortized cost, trade and other payables are recognized initially at fair value. Subsequent to initial recognition, these financial liabilities are measured at amortised cost using the effective interest rate method. On initial 252 recognition, any issue or redemption premiums and discounts and issuing costs are added to/deducted from the nominal value of the borrowings concerned. These items are taken into account when calculating the effective interest rate and are therefore recorded in the consolidated income statement over the life of the borrowings using the amortized cost method. Financial liabilities are broken down into current and non-current liabilities in the consolidated statement of financial position. Current financial liabilities comprise: Financial liabilities with a settlement or maturity date within 12 months of the statement of financial position date Financial liabilities in respect of which the Group does not have an unconditional right to defer settlement for at least 12 months after the statement of financial position date 3.2.2. Derivative financial instruments, including hedge accounting The Group holds derivative financial instruments to hedge its interest rate risk exposures, for certain contracts the formal documentation of hedging relationship at inception has been prepared enabling hedge accounting according to IAS 39, whereas other instruments used in economic hedges have not been formally documented as hedging relationship therefore not qualifying for hedge accounting. Derivatives are recognised initially at fair value; attributable transaction costs are recognised in profit or loss as incurred. Subsequent to initial recognition, derivatives are measured at fair value, and changes therein are accounted for as described below. Cash flow hedges When a derivative is designated as the hedging instrument in a hedge of the variability in cash flows attributable to a particular risk associated with a recognised asset or liability or a highly probable forecast transaction that could affect profit or loss, the effective portion of changes in the fair value of the derivative is recognised in other comprehensive income and presented in the hedging reserve in equity. The amount recognised in other comprehensive income is removed and included in profit or loss in the same period as the hedged cash flows affect profit or loss under the same line item in the statement of comprehensive income as the hedged item. Any ineffective portion of changes in the fair value of the derivative is recognised immediately in profit or loss as financial income or expenses. On initial designation of the hedge, the Group formally documents the relationship between the hedging instrument and hedged item, including the risk management objectives and strategy in undertaking the hedge transaction, together with the methods that will be used to assess the effectiveness of the hedging relationship. The Group makes an assessment, both at the inception of the hedge relationship as well as on an ongoing basis, whether the hedging instruments are expected to be “highly effective” in offsetting the changes in the fair value or cash flows of the respective hedged items during the period for which the hedge is designated, and whether the actual results of each hedge are within a range of 80-125 percent. For a cash flow hedge of a forecast transaction, the transaction should be highly probable to occur and should present an exposure to variations in cash flows that could ultimately affect reported net income. If the hedging instrument no longer meets the criteria for hedge accounting, expires or is sold, terminated or exercised, then hedge accounting is discontinued prospectively. The cumulative gain or loss previously recognised in other comprehensive income and presented in the hedging reserve in equity remains there until the forecast transaction occurs. If the forecast transaction is no longer expected to occur, then the balance in other comprehensive income is recognised immediately in profit or loss. In other cases the amount recognised in other comprehensive income is transferred to profit or loss in the same period that the hedged item affects profit or loss. Other derivatives When a derivative financial instrument is not designated in a qualifying hedge relationship, all changes in its fair value are recognised immediately in profit or loss. 253 3.3. Cash and cash equivalent Cash and cash equivalents comprise cash on hand, bank current accounts, and other bank deposits and short term investments considered to be readily convertible into a known amount of cash and where the risk of a change in their value is deemed to be negligible based on the criteria set out in IAS 7. Bank overdrafts that are repayable on demand and form an integral part of Group’s cash management are recorded under “Short term borrowings” but included as a component of cash and cash equivalents for the purpose of the statement of cash flows. 3.4. Share capital Ordinary shares Ordinary shares are classified as equity. Incremental costs directly attributable to the issue of ordinary shares and share options are recognised as a deduction from equity, net of any tax effects. Repurchase of share capital (Treasury shares) Own equity instruments which are repurchased (treasury shares) are presented as a deduction from equity. The amount of the consideration paid, which includes directly attributable costs, net of any tax effects, is recognised as a deduction from equity. No gain or loss is recognized in the consolidated statement of income on the purchase, sale, issue or cancelation of the Group own equity, but the resulting surplus or deficit on the transaction is transferred to/from retained earnings. 3.5. Property, plant and equipment 3.5.1. Recognition and measurement Items of property, plant and equipment are measured at cost less accumulated depreciation and accumulated impairment losses. Cost includes expenditure that is directly attributable to the acquisition of the asset. Purchased software that is integral to the functionality of the related equipment is capitalised as part of that equipment. When parts of an item of property, plant and equipment have different useful lives or provide benefits in a different pattern, they are accounted for as separate items (major components) of property, plant and equipment, thus necessitating the use of different depreciation rates and methods. An item of property, plant and equipment is derecognised on disposal or when the asset is permanently withdrawn from use and no future economic benefits are expected. Gains and losses on disposal of an item of property, plant and equipment are determined by comparing the proceeds from disposal with the carrying amount of property, plant and equipment, and are recognised net within results from non-recurring activities in profit or loss. When revaluated assets are sold, the amounts included in the revaluation reserve are transferred to retained earnings. 3.5.2. Depreciation Depreciation is based on the depreciable amount, which is the cost of an asset, or other amount substituted for cost, less its residual value. The residual value is estimated to be nil at the end of the useful life, except for real estate in certain cases. Depreciation is recognized in profit or loss on a straight-line basis over the estimated useful lives of each part of an item of property, plant and equipment, since this most closely reflects the expected pattern of consumption of the future economic benefits embodied in the asset. Leased assets are depreciated over the shorter of the lease term and their useful lives unless it is reasonably certain that the Group will obtain ownership by the end of the lease term. Land is not depreciated. 254 The estimated useful lives for the current and comparative periods are as follows: buildings leasehold improvements & fixtures Laboratory & Office equipment fixtures and fittings Other 15-30 3-10 3-10 2-10 2-10 years years years years years Depreciation methods, useful lives and residual values are reviewed at each financial year-end and adjusted if appropriate. 3.6. Intangible assets 3.6.1. Goodwill Goodwill that arises upon the acquisition of subsidiaries, either through share deals or asset deals, is included in intangible assets. For the measurement of goodwill at initial recognition, see Note 6Acquisitions of subsidiaries Subsequent measurement Goodwill is measured at cost less accumulated impairment losses if any. In respect of equity accounted investees, the carrying amount of goodwill is included in the carrying amount of the investment, and an impairment loss on such an investment is not allocated to any asset, including goodwill, that forms part of the carrying amount of the equity accounted investee. 3.6.2. Other intangible assets Other intangible assets that are acquired by the Group and have finite useful lives are measured at cost less accumulated amortisation and accumulated impairment losses. Other intangible assets consist primarily of software and licenses. 3.6.3. Subsequent expenditure Subsequent expenditure is capitalised only when it increases the future economic benefits embodied in the specific asset to which it relates. All other expenditure is recognised in profit or loss as incurred. 3.6.4. Amortisation Amortisation is calculated over the cost of the asset, or other amount substituted for cost, less its residual value. Amortisation is recognised in profit or loss on a straight-line basis over the estimated useful lives of intangible assets, other than goodwill, from the date that they are available for use, since this most closely reflects the expected pattern of consumption of the future economic benefits embodied in the asset. The estimated useful lives for the current and comparative periods are as follows: Licenses 1-5 years Software 1-5 years Amortisation methods, useful lives and residual values are reviewed at each financial year-end and adjusted if appropriate. 3.7. Leased assets Leases in terms of which the Group assumes substantially all the risks and rewards of ownership are classified as finance leases. Upon initial recognition the leased asset is measured at an amount equal to the lower of its fair value and the present value of the minimum lease payments. Subsequent to initial recognition, the asset is accounted for in accordance with the accounting policy applicable to that asset. However, if there is no reasonable certainty that the Group will obtain ownership by the 255 end of the lease term, the finance lease assets are depreciated over the shorter of the estimated useful life of the asset and the lease term. The Group regularly reviews its contracts and arrangements to determine whether an arrangement is, or contains a lease. The analysis is based on the substance of the arrangement at inception date. If the Group believes the fulfillment of the arrangement is dependent on the use of a specific asset or assets and the arrangement conveys a right to use the asset, then the arrangement contains a lease and IAS 17 is applicable to the lease element. At inception, payments required by the arrangement are split into lease payments and payments related to other elements of such arrangement based on their relative fair values. The Group uses equipment for its medical analyses. The contracts in use for this activity stipulate that the equipment is put at disposal for free if the laboratory buys exclusively from the supplier chemical reagents for a certain indicative volume during the term of the contract. As stated before, despite the fact that these agreements are not in the legal form of a lease, the arrangements qualify as lease contracts and Labco applied the requirements of IAS 17 to the lease element whereby the payments required by the arrangement are split into lease payments and payments relating to the other elements of the arrangement based on their relative fair values. For these contracts that are classified as finance leases, the related assets have been recognised in the statement of financial position of the Group. Other leases are operating leases and the leased assets are not recognised in the Group’s statement of financial position. Operating lease payments are recognised as an expense in the consolidated statement of income. 3.8. Inventories Inventories consist of raw materials (“reagents”) and consumables and are measured at the lower of cost and net realisable value. The cost of inventories is based on the weighted average unit costs, and includes expenditure incurred in acquiring the inventories and other costs incurred in bringing them to their existing location and condition. Net realisable value is the estimated selling price in the ordinary course of business, less the estimated costs of completion and selling expenses. 3.9. Impairment 3.9.1. Financial assets (including receivables) A financial asset not carried at fair value through profit or loss is assessed at each reporting date to determine whether there is objective evidence that it is impaired. A financial asset is impaired if objective evidence indicates that a loss event has occurred after the initial recognition of the asset, and that the loss event had a negative effect on the estimated future cash flows of that asset. Objective evidence that financial assets (including equity securities) are impaired can include default or delinquency by a debtor, restructuring of an amount due to the Group on terms that the Group would not consider otherwise, indications that a debtor or issuer will enter bankruptcy, or the disappearance of an active market for a security. In addition, for an investment in an equity security, a significant (more than 30%) or prolonged decline in its fair value below its cost is objective evidence of impairment. 3.9.2. Non-financial assets The carrying amounts of the Group’s non-financial assets, but other than inventories and deferred tax assets, are reviewed at each reporting date to determine whether there is any indication of impairment. If any such indication exists, then the asset’s recoverable amount is estimated. For goodwill, and intangible assets that have indefinite useful lives or that are not yet available for use, the recoverable amount is estimated each year at the same date time during the year-end closing process, and additionally whenever there is an indication that such assets may be impaired. 256 The recoverable amount of an asset or cash-generating unit is the greater of its value in use and its fair value less costs to sell. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money, all the other risks specific to the assets being considered in the estimated future cash flows from the assets. Depending on the timely availability each year of long term business plans, future cash flows are either estimated based on the long term 5 year business plans approved by senior management or estimated based on the budget prepared for the following year and which are afterward extrapolated over the next 4 years consistently with the latest 5 years business plan, plus in any case the estimate of the terminal value using a perpetual growth rate. For the purpose of impairment testing, assets that cannot be tested individually are grouped together into the smallest group of assets that generates cash inflows from continuing use that are largely independent of the cash inflows of other assets or groups of assets (the “cash-generating unit, or CGU”). For the purposes of goodwill impairment testing, the lowest level at which goodwill is monitored for internal reporting purposes corresponds to the following geographical areas: France, Germany, Iberia, Italy, Belgium and United Kingdom. Goodwill acquired in a business combination is allocated to CGUs or groups of CGUs that are expected to benefit from the synergies of the combination. The Group’s corporate assets (Labco SA, Labco Belgium, Labco Finance) could not be allocated on a reasonable and consistent basis to each cash-generating units. As such, they are included in the group of cashgenerating units’ impairment test (global test). Local holdings are included in their respective country. An impairment loss is recognized if the carrying amount of an asset or its CGU exceeds its estimated recoverable amount. Impairment losses are recognized in profit or loss. Impairment losses recognized in respect of CGUs or groups of CGUs are allocated first to reduce the carrying amount of any goodwill allocated to the units or group of units, and then to reduce the carrying amounts of the other assets in the unit (group of units) on a pro rata basis. An impairment loss in respect of goodwill is not reversed. In respect of other assets, impairment losses recognized in prior periods are assessed at each reporting date for any indications that the loss has decreased or no longer exists. An impairment loss is reversed if there has been a change in the estimates used to determine the recoverable amount. An impairment loss is reversed only to the extent that the asset’s carrying amount does not exceed the carrying amount that would have been determined, net of depreciation or amortization, if no impairment loss had been recognized. Goodwill that forms part of the carrying amount of an investment in an associate is not recognised separately, and therefore is not tested for impairment separately. Instead, the entire amount of the investment in an associate is tested for impairment as a single asset when there is objective evidence that the investment in an associate may be impaired. Such impairment loss can be reversed if the recoverable amount subsequently increases. 3.10. Employee benefits 3.10.1. Short-term employee benefits Short-term employee benefit obligations are measured on an undiscounted basis and are expensed as the related service is provided. A liability is recognised for the amount expected to be paid under short-term cash bonus or profitsharing plans if the Group has a present legal or constructive obligation to pay this amount as a result of past service provided by the employee, and the obligation can be estimated reliably. 3.10.2. Long term employee benefits, including retirement agreements Depending on the laws and practices in force in the countries where Labco operates, Group companies have legal obligations in terms of pensions, early retirement payments and retirement 257 bonuses. Such obligations are generally defined State contribution plans, which costs are expensed based on the amount of contribution payable in the period. The Group is also concerned by other post-employment or post-retirement employee benefits which correspond to the legal retirement indemnity mainly applicable in France and Italy. Commitments at retirement date and other similar advantages essentially correspond to the retirement compensations due to employees when they retire. Their assessment is made on the basis of an actuarial calculation using the projected unit credit method and taking into account the rate of staff turnover and mortality rates which are determined based on official age tables and estimated future salary increase. Discount rates are determined by the reference to the yield at the measurement date on high-quality corporate bonds. In compliance with IAS 19 revised, actuarial gains and losses are recognized directly in other comprehensive income in equity and are not amortized in the Income Statement. 3.10.3. Termination benefits Termination benefits are recognised as an expense when the Group is committed demonstrably, without realistic possibility of withdrawal, to a formal detailed plan to either terminate employment before the normal retirement date, or to provide termination benefits as a result of an offer made to encourage voluntary redundancy. Termination benefits for voluntary redundancies are recognised as an expense if the Group has made an offer of voluntary redundancy, it is probable that the offer will be accepted, and the number of acceptances can be estimated reliably. If benefits are payable more than 12 months after the reporting period, then they are discounted to their present value. 3.10.4. Share-based payment transactions The grant date fair value of share-based payment awards granted to employees is recognised as an expense, with a corresponding increase in equity, over the period required for the employees unconditionally becoming entitled to the awards. The amount recognised as an expense is adjusted to reflect the number of awards for which the related service and non-market vesting conditions are expected to be met, such that the amount ultimately recognised as an expense is based on the number of awards that do meet the related service and non-market performance conditions at the vesting date. Share-based payment arrangements in which the Group receives goods or services as consideration for its own equity instruments are accounted for as equity-settled share-based payment transactions, regardless of how the equity instruments are obtained by the Group. 3.11. Provisions A provision is recognised if, as a result of a past event, the Group has a present legal or constructive obligation that can be estimated reliably, and it is probable that an outflow of economic benefits will be required to settle the obligation. Provisions giving rise to a cash outflow after more than one year are discounted if the impact is material. Discount rates reflect current assessments of the time value of money and risks that are specific to the liability and not included in expected cash flows. The unwinding of the discount is recognised as finance cost. 3.12. Revenue The Group earns revenues from medical analyses both routine and esoteric which are invoiced to insurance companies, hospitals, individuals, pharmacies, and National Heath entities. Revenue from medical analyses in the course of ordinary activities is measured at the fair value of the consideration received or receivable, net of returns, trade discounts and volume rebates. Revenue in connection with rendered services is recognized at the time the service is provided. Revenue is based on the net amount billed or billable if it can be estimated reliably. If it is probable that discounts will be granted and the amount can be measured reliably, then the discount is recognised as a reduction of revenue as the sales are recognised. 258 The process of estimating the ultimate collection of receivables associated with our clinical testing business involves significant assumptions and judgments. Billings for services reimbursed by thirdparty payers, including social security systems, are recorded as revenue net of allowances for differences between amounts billed and the estimated receipts from such payers. Adjustments to the allowances, based on actual receipts from the third-party payers, are recorded upon settlement as an adjustment to net revenue. Government payers Payments for clinical laboratory testing services made by the government are based on fee schedules set by governmental authorities. Collection of such receivables is normally a function of providing the complete and correct billing information within the various filing deadlines. Collection varies from country to country. Private insurers Reimbursements from private insurers are based on negotiated fee-for-service schedules and on capitated payment rates. Substantially all of the accounts receivable due from private insurers represent amounts billed under negotiated fee-for-service arrangements. We utilize a standard approach to establish allowances for doubtful accounts for such receivables, which considers the aging of the receivables, historical collection experience and other factors. Client payers Client payers include physicians, hospitals, employers and other commercial laboratories. Credit risk and ability to pay are more of a consideration for these payers than healthcare insurers and government payers. We utilize a standard approach to establish allowances for doubtful accounts for such receivables, which considers the aging of the receivables, as well as specific account reviews, historical collection experience and other factors. Patient receivables (individuals) Patients are billed based on established patient fee schedules, subject to any limitations on fees negotiated with healthcare insurers or physicians on behalf of their patients. Collection of receivables due from patients is subject to credit risk and ability of the patients to pay. We utilize a standard approach to establish allowances for doubtful accounts for such receivables, which considers the aging of the receivables, historical collection experience and other factors. Other income in revenue mainly corresponds to interests earned on operating receivables as well as income generated by activities not directly related to clinical diagnostics and screening services. 3.13. Lease payments Payments made under operating leases are recognised in profit or loss on a straight-line basis over the term of the lease. Lease incentives received are recognised as an integral part of the total lease expense, over the term of the lease. Minimum lease payments made under finance leases are apportioned between the finance expense and the reduction of the outstanding liability. The finance expense is allocated to each period during the lease term so as to produce a constant periodic rate of interest on the remaining balance of the liability (effective interest rate method). 3.14. Finance income and finance costs Finance income comprises interest income on funds invested (including available-for-sale financial assets), dividend income, gains on hedging instruments that are recognised in profit or loss, and foreign currency gains. Interest income is recognised as it accrues in profit or loss, using the effective 259 interest method. Dividend income is recognised in profit or loss on the date that the Group’s right to receive payment is established. Finance costs comprise of cost of net debt and other financial expenses. Cost of net debt includes interest expense on borrowings and financial leases, as well as expenses related to derivatives. Other financial expenses mainly include unwinding of the discount on provisions. Borrowing costs that are not directly attributable to the acquisition, construction or production of a qualifying asset are recognised in profit or loss using the effective interest method. Labco Group does not own any qualifying asset. 3.15. Income tax Income tax (income or expense) comprises current and deferred tax. Current tax and deferred tax are recognised in profit or loss except to the extent that it relates to a business combination, or items recognised directly in equity or in other comprehensive income. Current tax is the expected tax payable or receivable on the taxable income or loss for the year, using tax rates enacted or substantively enacted at the reporting date, and any adjustment to tax payable in respect of previous years. Deferred tax is recognised in respect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. Deferred tax is not recognised for the following temporary differences: the initial recognition of assets or liabilities in a transaction that is not a business combination and that affects neither accounting nor taxable profit or loss, and differences relating to investments in subsidiaries to the extent that it is probable that they will not reverse in the foreseeable future. In addition, deferred tax is not recognised for taxable temporary differences arising on the initial recognition of goodwill. Deferred tax is measured at the tax rates that are expected to be applied to temporary differences when they reverse, based on the laws that have been enacted or substantively enacted by the reporting date. Deferred tax assets and liabilities are offset if there is a legally enforceable right to offset current tax liabilities and assets, and they relate to income taxes levied by the same tax authority on the same taxable entity, or on different tax entities, but they intend to settle current tax liabilities and assets on a net basis or their tax assets and liabilities will be realised simultaneously. A deferred tax asset is recognised for unused tax losses, tax credits and deductible temporary differences, to the extent that it is probable that future taxable profits will be available against which they can be utilised. Deferred tax assets are reviewed at each reporting date and are reduced to the extent that it is no longer probable that the related tax benefit will be realised. Additional income taxes that arise from the distribution of dividends; are recognized at the same time that the liability to pay the related dividend is recognized; For French entities of the Group, the former Business Tax has been modified by a law enacted December 30, 2009. The Business Tax now consists of two components: “Cotisation Foncière des Entreprises (CFE)”,which is a tax on rental value of lands “Cotisation sur la Valeur Ajoutée des Entreprises (CVAE)”, which is a tax determined on added value as defined based on statutory accounts In accordance with the definition of income tax in IAS 12 and the definition of added value stipulated by article 1586 sexies of French Tax code, and by homogeneity with the treatment in other countries of taxes based on net aggregate of income and charges, Labco considers that the CVAE tax should be recorded as an income tax given its definition. 3.16. Results from operating activities, and net non-recurring expenses Results from operating activities correspond to the operating performance of the various activities performed by Labco Group. Results from operating activities before non-recurring activities is an 260 indicator used by the Group to present “a level of operational performance that can be used as part of an approach to forecast recurring performance”. In order to facilitate understanding of recurring operating performance, non-recurring expenses and income lines have been defined and include non-recurring, unusual, items that are clearly not related to recurring activities and of certain significance. Those non-recurring expenses and income consist of: Impairment and reversal of impairment on non-operational assets and liabilities Gains / losses on sale of assets Restructuring expenses and provisions for major litigations Perimeter effect including transaction costs for significant and unusual acquisitions (cancelled or realized as for realized acquisitions, costs are expensed according to IFRS 3 revised guidance implemented starting 2009 by Labco), as well as earn out variations of fair value subsequent to the 1 year window period. 3.17. Earnings per share The Group has not issued shares in a public market and is not in the process of doing so. Therefore the Group is not required to but has decided to present voluntarily basic and diluted earnings per share (EPS) data for its ordinary shares in accordance with IAS 33. Basic EPS is calculated by dividing the profit or loss attributable to ordinary shareholders of the Company by the weighted average number of ordinary shares outstanding during the period, adjusted for own shares held. Diluted EPS is determined by adjusting the profit or loss attributable to ordinary shareholders and the weighted average number of ordinary shares outstanding, adjusted for own shares held and, for the effects of all dilutive potential ordinary shares, which comprise convertible notes and warrants and free shares granted to employees. 3.18. Geographical information The Group has not issued shares in a public market and is not in the process of doing so. Therefore the Group is not required to disclose segment information as required by IFRS 8. However the Group has decided to disclose a breakdown of revenue by country provided in Note 7Geographical information Iberia corresponds to the aggregate of Portugal and Spain. 3.19. EBITDA (Earnings Before Interest, Taxes, Depreciation and Amortization) EBITDA is a non-Gaap measure but corresponds to an aggregate that is commonly used by stakeholders for analyzing the Group’s performance. EBITDA has been defined by the Group based on Results from operating activities before non-recurring activities restated for net depreciation, amortization and impairment, provisions and reversal. 3.20. Share based payment transactions and transaction costs for usual small size acquisition Labco presents in its operating result certain cost items on a specific line in order to help management and financial investors to better understand the Group’ economic performance because it identifies separately elements which are non operational and inherently difficult to predict due to their irregular nature, even if the costs are for a certain period not very significant. 3.21. Determination of fair values A number of the Group’s accounting policies and disclosures require the determination of fair value, for both financial and non-financial assets and liabilities. Fair values have been determined for measurement and/or disclosure purposes based on the following methods. When applicable, further information about the assumptions made in determining fair values is disclosed in the notes specific to that asset or liability. 261 3.21.1. Property, plant and equipment The fair value of property, plant and equipment recognised as a result of a business combination is based on market values. The market value of property is the estimated amount for which a property could be exchanged on the date of valuation between a willing buyer and a willing seller in an arm’s length transaction after proper marketing wherein the parties had each acted knowledgeably and willingly. The fair value of items of plant, equipment, fixtures and fittings is based on the market approach and cost approaches using quoted market prices for similar items when available and replacement cost when appropriate. 3.21.2. Trade and other receivables The fair value of trade and other receivables is estimated as the present value of future cash flows, discounted at the market rate of interest at the reporting date. The net carrying value is considered as a reasonable estimate of their fair value considering the short payment and settlement periods applied by Labco Group. This fair value is determined for disclosure purposes. 3.21.3. Derivatives The fair value of interest rate swaps is based on broker quotes. Those quotes are tested for reasonableness on an ad-hoc basis by discounting estimated future cash flows based on the terms and maturity of each contract and using market interest rates for a similar instrument at the measurement date. Fair values also reflect the credit risk of the instrument and include adjustments to take account of the credit risk of the Group entity and counterparty when appropriate. 3.21.4. Non-derivative financial liabilities Fair value, which is determined for disclosure purposes, is calculated based on the present value of future principal and interest cash flows, discounted at the market rate of interest at the reporting date. For finance leases the market rate of interest is determined by reference to similar lease agreements. 3.21.5. Share-based payment transactions The fair value of employee share options is generally measured using a binomial lattice model. Measurement inputs include share price on measurement date, exercise price of the instrument, expected volatility (based on weighted average historic volatility of similar quoted entities), weighted average expected life of the instruments (based on historical experience and general option holder behaviour), expected dividends, and the risk-free interest rate (based on government bonds). Service and non-market performance conditions attached to the transactions are not taken into account in determining fair value. 262 Financial risk management Note 4 4.1. Overview The Group has exposure to the following risks from its use of financial instruments: credit risk liquidity risk market risk This note presents information about the Group’s exposure to each of the above risks, the Group’s objectives, policies and processes for measuring and managing risk, and the Group’s management of capital. Further quantitative disclosures are included throughout these consolidated financial statements. 4.2. Risk management framework The Board of directors, previously Strategic Committee before the conversion into “société anonyme”, has overall responsibility for the establishment and oversight of the Group’s risk management framework. The Group’s risk management policies are established to identify and analyse the risks faced by the Group, to set appropriate risk limits and controls, and to monitor risks and adherence to limits. The Group Audit Committee oversees how management monitors compliance with the Group’s risk management policies and procedures. 4.3. Credit risk Credit risk is the risk of financial loss to the Group if a customer or counterparty to a financial instrument fails to meet its contractual obligations, and arises principally from the Group’s receivables from customers and investment securities. Detailed quantitative information on credit risk are provided in Note 20 Trade and other receivables. 4.3.1. Trade and other receivables The Group’s exposure to credit risk is influenced mainly by the individual characteristics of each customer. The Group has no significant concentrations of credit risks due to the large numbers of customers and individually immateriality of amounts due. The Group performs ongoing credit evaluations of its receivables and establishes an allowance for impairment that represents its estimate of incurred losses in respect of trade and other receivables. The main components of this allowance are a specific loss component that relates to individually significant exposures. 4.3.2. Investments and cash and cash equivalents The Group’s exposure to credit risk arises from default of the counterparty. The Group limits its exposure to credit risk by investing mainly in liquid securities with counterparties that have a high credit rating. Management actively monitors its investments and does not expect any counterparty to fail to meet its obligations. 263 4.4. Liquidity risk Liquidity risk is the risk that the Group will encounter difficulty in meeting the obligations associated with its financial liabilities that are settled by delivering cash or another financial asset. The Group’s approach to managing liquidity is to ensure, as far as possible, that it will always have sufficient liquidity to meet its liabilities when due, under both normal and stressed conditions, without incurring unacceptable losses or risking damage to the Group’s reputation. This planning considers the maturity of both its financial assets, and its projected cash flow from operations. Typically the Group ensures that it has sufficient cash on demand to meet expected operational expenses for a period of 60 days, including the servicing of financial obligations. In addition, the Group maintains a line of credit (Revolving Credit Facility) under which drawings could be made for financing acquisitions or for general financing purposes. Refer to the Note 23 Borrowings and other financial liabilities for a description of the main characteristics of our Revolving Credit Facility. Detailed quantitative information on liquidity risk are provided in Note 29 Financial instruments. 4.5. Market risk – interest rate risk Market risk is the risk that changes in market prices, such as interest rates, will affect the Group’s income or the value of its holdings of financial instruments. The objective of market risk management is to manage and control market risk exposures within acceptable parameters, while optimising the return. The Group’s exposure to the risk of changes in market interest rates relates primarily to the debt drawn on the revolving credit facility (RCF). Major part of our Group long term debt is at fixed rate, enabling to limit the impacts of market risks. Detailed quantitative information on interest rate risk are provided in Note 29 Financial instruments. 4.6. Operational risk Operational risk is the risk of direct or indirect loss arising from a wide variety of causes associated with the Group’s processes, personnel, technology and infrastructure, and from external factors other than credit, market and liquidity risks such as those arising from legal and regulatory requirements and generally accepted standards of corporate behaviour. Operational risks arise from all of the Group’s operations. The Group’s objective is to manage operational risk so as to balance the avoidance of financial losses and damage to the Group’s reputation with overall cost effectiveness and to avoid control procedures that restrict initiative and creativity or impair operational independence of laboratory managers in countries where regulations emphasize medical independence of laboratory managers. The primary responsibility for the development and implementation of controls to address operational risk is assigned to senior management within each business unit. This responsibility is supported by the development of overall Group standards for the management of operational risk in the following areas: compliance with regulatory and other legal requirements review regular accreditation procedures requirements for the periodic assessment of operational risks faced, and the adequacy of controls and procedures to address the risks identified requirements for the reporting of operational losses and proposed remedial action training and professional development 264 4.7. Ethical and business standards. Capital management The Board of directors’s policy is to maintain a strong capital base so as to maintain investor, creditor and market confidence and to sustain future development of the business. The Board of directors seeks to maintain a balance between the higher returns that might be possible with higher levels of borrowings and the advantages and security afforded by a sound capital position. There were no changes in the Group’s approach to capital management during the year. Neither Labco SA nor any of its subsidiaries are subject to externally imposed capital requirements. 265 Significant events Note 5 5.1. Acquisitions, business set-up, disposals and mergers 5.1.1. Acquisitions and business set-up Refer to Note 6 – Acquisition of subsidiaries for detailed information on acquisitions performed in 2012. Main acquisitions during the reporting period are shown below by country. iPP, our joint venture for developing business in the United Kingdom and consolidated under equity method, has started on June 1st 2012 the operation of the contract with Taunton and Somerset NHS Foundation Trust and Yeovil District Hospital NHS Foundation Trust. Through this contract, iPP delivers the full range of laboratory services to a Joint Venture operated by iPP and the Trusts whilst the clinical interpretation and clinical advice functions continue to be provided by the Trusts’ medical staff who remain employed by the NHS. The partnership and related contract will last for 20 years. Whilst the partnership currently focus on providing services to both Trusts, which provide healthcare for a population of 500,000 along with over 100 GP practices in the area, the joint venture has been deliberately structured in a way that allows other Trusts in the region to join the collaboration and benefit from investment in the new service and the wide range of innovations and service improvements it will deliver. As at December 31, 2012 iPP generates a turnover of 7,6 M£ (at 100%). 5.1.2. Disposals Labco management decided to dispose the non-core business entity (operating imaging business) Centro Diagnostico Missori Srl in Italy, owned at 50% by Labco Group and consolidated fully in the group financial statements. Centro Diagnostico Missori Srl classified as assets held for sale under IFRS 5 as at March 31, 2012, have been effectively disposed in June 2012 generating a consolidated gain on sale. 5.1.3. Mergers and legal reorganisation Labco Group has continued in 2012 to implement numerous mergers between French SELs, in order to reinforce, in compliance with French regulation, synergies actions by concentrating laboratories. Similar merger operations have been performed in Spain and Portugal, as well as finalization of the legal structure reorganisation in order to optimize the number of existing tax groups and enables to have only one tax group in Spain starting 2012, and one tax group in Portugal in 2014. 266 5.2. Legal conversion of Labco into a SA (“Société Anonyme”) and capital increases Legal conversion of Labco into a SA (“Société Anonyme”) On January 12, 2012, an extraordinary general meeting of Labco’s shareholders decided the conversion of Labco from the corporate form of SAS (“Société par actions simplifiée”) into a SA (“Société anonyme”). As a consequence, the corporate governance bodies have evolved. Under the French company law applicable to SA, Labco SA’s affairs are now managed by the board of directors (conseil d’administration), whose members are elected by our shareholders. Decisions are now taken within the board of directors under the rule of “one man - one vote”. Labco’s board of directors elected a Chairman (président du conseil d’administration), Andreas Gaddum, and appointed a General Manager (directeur général), Philippe Charrier. Capital increases On March 12, 2012, the board of directors recorded an increase in the share capital of our Company of €54 367 corresponding to 54 367 issued C shares deriving from exercise of financial investors warrants. Pursuant to the deliberations of the extraordinary shareholders’ meeting held on March 12, 2012, a capital increase of the Company of €24 748 796 has been carried out as of March 28, 2012 through the issuance at par of 24 748 796 shares with a par value of one euro each. The share capital was increased from €43 391 100 to €68 139 896. The preferential subscription rights have been maintained and the shares have been subscribed solely in cash. As of September 30, 2012 the costs directly related to the capital increase have been recorded as a deduction of issuance premium balances for an amount of 138 K€. On June 7, 2012, the board of directors recorded an increase in the share capital of our Company of €115 756 as a consequence of its decision taken on April 5, 2012, using the delegation of powers 267 granted by the general meeting of shareholders held on March 12, 2012, to increase the share capital and additional paid in capital of €1 034 859 by the issuance of 115 756 A shares at a subscription price of 8,94 € per share. On October 30, 2012, an extraordinary general meeting of Labco’s shareholders decided an increase in the share capital of our Company of €192 485 by the issuance of 192 485 A shares subscribed through contribution in kind. On December 6, 2012, the board of directors recorded an increase in the share capital of our Company of €11 185 as a consequence of its decision taken on October 4, 2012, using the delegation of powers granted by the general meeting of shareholders held on March 12, 2012, to increase the share capital and additional paid in capital of €99 994 by the issuance of 11 185 A shares at a subscription price of 8,94 € per share. In total, the share capital amounts as at December 31, 2012 to €68 459 322 5.3. Non-recurring restructuring plans Restructuring in Germany At year-end 2011, a restructuring of the laboratory MVZ Duisburg was implemented with a restructuring provision recorded for an amount of 0,8 M€. As at December 31, 2012, restructuring expenses have been recorded for 0,4 M€ and remaining provision amounts to 0,4 M€ mainly covering onerous renting contracts for unused sites. End 2012, German management decided to restructure fundamentally the Mittelhessen region in which Labco operates 3 labs by especially merging the activities of those labs (“Hessen merger”). Restructuring plan in Mittel-Hessen (Marburg/Giessen) consist of closing of labs, dismissal of employees, build up of a new platform and lastly merger of Marburg/Giessen. As a consequence, non-recurring restructuring expenses have been incurred in 2012 for 172 K€ and a restructuring provision of 199 K€ has been recorded as at December 31, 2012. To take into account new facts in Germany relating notably to the impact of the new federal quota system announced in December 2012 and the full impact of the remediation of the alleged fraudulent activities in MVZ Dillenburg (refer to the Note 27 Litigations and Contingent liabilities) Labco has performed an impairment test on the Cash Generating Unit Germany resulting in an impairment amounting to 36 M€ of goodwill allocated to Cash Generating Unit Germany. Refer to Note 13 Goodwill for a detailed explanation on impairment analysis. “Deep Dive” efficiency program in France In the context of additional price pressure in French biology, driven by the overall economic environment, Management has decided to launch a full and detailed review of French operations in order to restructure the French network by identifying productivity improvements through accelerated concentration and in-depth reorganization. This “Deep Dive” program into the French network took place throughout first semester 2012 and resulted in a human resources optimization plan including its associated implementation cost (severance packages). As a consequence, non-recurring restructuring expenses have been incurred in 2012 for 663 K€ and a restructuring provision of 115 K€ has been recorded as at December 31, 2012 for people notified before year-end. Further costs are expected in 2013 with the finalization of the “Deep Dive” efficiency program. Restructuring in Iberia (Portugal and Spain) At year-end 2011, a significant restructuring program was initiated both in Spain and Portugal. As a consequence, a restructuring provision was recorded for an amount of 2,2 M€. Some restructuring measures have been implemented in 2012 leading to use the provision for an amount of 1,1 M€. As at 268 December 31, 2012 the remaining restructuring provision amounts to 1,1 M€ for the residual measures to be implemented. 5.4. RCF Financing agreement amendments RCF Financing agreement amendments We have issued a “Request for Amendments” to Natixis, acting as Agent pursuant to the Revolving Credit Facility Agreement. The amendments consisted of an amendment of the “Leverage ratios”, an amendment of the “Incurrence Ratios” and a technical amendment. Please refer to Note 23 Borrowings and other financial liabilities for details of amended ratios. Natixis, acting as Agent pursuant to the Revolving Credit Facility Agreement, confirmed that the “request for Amendments” reached the consent of the Majority Lenders on April 13, 2012. The amendments entered into force immediately and had their first application for the calculation of the compliance certificates for the Quarter ending on March 31, 2012. 5.5. Strategic projects In May 2012, Labco management received indications of interest from potential buyers interested in acquiring Labco. In compliance with the terms of our shareholders’ agreement, our largest shareholder, 3i, appointed strategic advisors and organized an orderly sale process, which is currently ongoing. 3i has informed us that they have received non-binding offers from potential buyers who have expressed interest in acquiring a minority, a majority or all of our share capital. We understand that 3i is currently reviewing the offers received and at the same time considering the potential of stand-alone value creation for Labco. 3i intends to enter into more detailed discussions with one or more selected potential buyers. However, 3i may suspend or end the process, at any time. Moreover, Labco has incurred non-recurring expenses for an amount of 2.8 M€ related mainly to external advisors and lawyers for diligences work used internally for various strategic options. A sale of a controlling or significant stake in the Company may constitute a “Change of Control” under the Indenture and the Revolving Credit Facility agreement, and thereby trigger a requirement, unless waived by the holders of the Notes, that we offer to repurchase the Notes from holders at a price of 101% of their principal amount, plus accrued and unpaid interest. Additionally, a change of control under the Revolving Credit Facility Agreement, unless waived by the lenders, results in the cancellation of the commitments under the Revolving Credit Facility and all amounts outstanding under the Revolving Credit Facility would become due and payable. Refer to Note 34 Events after the reporting period for a description of the subsequent issuance on February 13, 2013 of Additional 8.5% Senior Secured Notes due 2018 issued for an aggregate nominal of 100 M€. 269 Note 6 Acquisitions of subsidiaries Business combination Main acquisitions during the 2011 reporting period are shown below by country. At the end of the 1 year window period, most goodwill have been confirmed with no significant changes apart from CIC group goodwill which has been decreased by 2.6m€. Main acquisitions during the reporting period are shown below by country: 270 All companies acquired earn revenues from medical analyses. Through these acquisitions the Group expects to reduce costs through economies of scale, and the goodwill thus represents the fair value of the expected synergies resulting from the acquisition. All amounts are provisional and subject to modification in the twelve months period following the acquisition date. The cumulative effect of acquisitions on the Group’s assets and liabilities on acquisition date corresponding to identifiable assets acquired and liabilities assumed for share deals acquisitions performed in 2012 as well as cumulative consideration transferred is presented below: The goodwill is attributable mainly to the synergies expected to be achieved from integrating the companies into the Group. On top of goodwill generated by share deals, Labco also made acquisitions of asset deals that generated an increase of goodwill amounting to 4,2 M€. 271 Note 7 Geographical information Detailed of revenue by country break down as follows: Iberia corresponds to the aggregate of Portugal and Spain. Note 8 Payroll related expenses Other personnel related costs include amongst other profit sharing, pensions expenses, travel expenses, fees for training of personnel, food allowances. Salaries and wages expenses include also the variable remuneration paid to biologists under various legal forms, either compensation paid as salary or fees or, mainly for French biologists, the priority dividends paid on the current year result. As explained in the basis of preparation section, the priority dividends to be paid to certain laboratory doctors after year-end are recognized as employee benefits expense and liability in the current year. Information about the share based payment transactions is included in note 25 - Share based payment schemes. 272 Other operating expenses Note 9 Other operating expenses include amongst other service charges relating to security and cleaning, marketing related expenses, storage costs. According to IFRS 3 revised the transactions costs related to acquired entities are recorded in the consolidated statement of income, as well as with transaction costs for abandoned deals. Given the non operational, irregular or non-recurring nature of these costs, they have been presented on a separate line of consolidated statement of income, and depending on the amount of costs incurred by transaction project, they are qualified as transaction costs for usual small size acquisitions recorded as other operating expenses, or they are qualified as transaction costs for significant and unusual transactions recorded as non recurring operating expenses in the line Perimeter effect The Group incurred acquisition-related costs of 1 626 K€ in 2012 (2011: 3 015 K€) for usual small size acquisition relating to external legal fees, due diligence costs and stamp taxes. Non recurring income and expenses Note 10 Restructuring expenses, provisions for major litigations, impairment and reversal of impairment on other non-operational assets and liabilities and impairment of goodwill mainly include in 2012 following expenses or provisions: 2,3 M€ of restructuring expenses mainly in relation with the restructuring schemes implemented in Spain, Portugal, Germany (Duisburg) and Belgium (MAB) announced at year-end 2011 and the finalization of the closure of Brussels headquarter. Those costs having been accrued, corresponding use of restructuring provisions have been recorded in the line Impairment and reversal of impairment on other non-operational assets and liabilities for 2,3 M€. 0,3 M€ of non-recurring expenses for the repurchase of the shares of a French laboratory owned by a biologist stopping to operate, already accrued as at December 31, 2011 with therefore corresponding reversal of the provisions being recorded in the line “Provisions, impairment losses and reversals on liabilities”. 273 0,8 M€ of restructuring expenses and provisions in relation with the “Deep dive” efficiency program implemented in France during first semester 2012 and 0,4 M€ of restructuring expenses and provisions for the 2012 restructuring scheme implemented in Germany (Mittelhessen merger). 1,7 M€ of major litigation against former vendors of Dillenburg for alleged fraudulent activities and related consequences 1,8 M€ of net non-recurring income related to the settlement indemnity to be received in context of the early termination of a clinic contract in France, net of estimated restructuring expenses. 1,5 M€ of IFRS 2 expenses as a consequence of the cancellation of the Restricted Stock Unit “Free shares 2011” scheme in Q2 2012. 2,8 M€ of non-recurring costs expensed mainly in relation to strategic projects, which corresponds principally to advisors fees. 36 M€ of impairment of goodwill allocated to Germany Cash Generating Unit as a consequence of annual impairment test. The Gains on sale of assets correspond mainly to consolidated gain on the sale of Centro Diagnostico Missori Srl. Restructuring expenses and provisions for major litigations mainly included in 2011 expenses relating to strategic refinancing projects (4,2 M€), net severance costs mainly in Iberia and Germany (5,8 M€) and non-recurring major litigations expenses and provisions mainly related to Germany (4,5 M€). Moreover an impairment of goodwill of 95 M€ allocated to Iberian Cash Generating Unit has been recorded. Perimeter effect corresponds to earn out variations of fair value subsequent to the 1 year window period for an amount of 386 K€ (2011: 422 K€) as well as earn out contracted on the acquisition of the CIC Group expensed for an amount of 2 329 K€. Note 11 Net finance costs Other financial expenses correspond mainly to unwinding of the discount on provisions and other financial charges like foreign exchanges gains and losses. As a consequence of the Refinancing operations in January 2011, and the early repayments of historical debts, a total of 26,1 M€ of one-off financial charges has been expensed in 2011 (write off of previous financing debt issuance costs and break up costs for early cancelation of previous financing and derivatives instruments). The interest expenses correspond now mainly to the 500 M€ Senior Secured Bonds at an effective interest rate of 9 %, the RCF interests expenses on the drawn part of the RCF and commitments fees and amortization of RCF debt issuance costs for the undrawn part of the Revolving Credit Facility. 274 Furthermore it includes some one-off costs related to the covenant amendments implemented in April 2012 for 0,6 M€. 275 Note 12 Income tax expenses Reconciliation of effective tax rate The Group has operations in various tax jurisdictions which have different tax laws and rates. Consequently, the effective tax rate on consolidated income may vary from year to year, according to the source of earnings. The Group has incurred losses amounting to 11,1 M€, for which no deferred tax asset has been recognized because the Group is to date not expecting future tax benefits which according to financial projections can be used to offset future taxable income in a timeframe of 5 years. It is mainly related to losses incurred by holdings. The non–deductible expenses correspond mainly to the effect of the restatements of priority dividends paid to French biologists that have been classified as remuneration expenses under IFRS according to a “substance over form” analysis. In fact priority dividends recorded as personnel expenses in the consolidated financial statements are not an expense recorded in local books, and as a consequence it will not generate any Tax deductibility. 276 Tax losses and tax credits not recognised as deferred tax assets amounted to 136 M€ as of December 31,2012, tax losses mainly originated in Germany and Iberia. 277 Note 13 Goodwill Impairment testing for cash-generating units containing goodwill For the purpose of impairment testing, goodwill is allocated to groups of cash-generating units defined at the level of a country, except for Iberia (including Spain and Portugal), which represent the lowest level within the Group at which the goodwill is monitored for internal management purposes. The aggregate carrying amounts of goodwill allocated to each group of unit and key assumptions of the impairment testing model are as follows: 278 The recoverable amount of each cash-generating unit was based on its value in use which was determined by discounting the future cash flows generated from the continuing use of the unit. The main assumptions on which the value in use of a cash generating unit is based are the discount rate and trends in volumes, prices and direct costs (inflation) over the period. The calculation of the value in use was based on the following key components: The Group’s 5 years business plans determined during Summer 2012 in context of strategic operations and rationalized with 2013 budget. Trends in volumes, prices and direct costs are based on past trends and on the future market outlook which include a certain level of uncertainties, especially in the current context of economic difficult environment in certain European countries. Given announcement of German EBM quotas (ie a capped percentage of the scheduled fees under the statutory health insurance (“EBM”)) for fourth quarter 2012 at 95,4% and at 89,2% for first quarter 2013, as well as a deterioration of our German activity in 2012, German local management updated its business plans in relation with 2013 budget. That updated business plan was used to perform the annual impairment test analysis on Germany cash generating unit. The cash flows projections for the years 2013 to 2016 include also: o Taxes impact by applying an average theoretical rate per country; o Working capital variance; o Capital expenditures corresponding in general to 2,5% of forecasted annual turnover. The terminal value is then calculated by discounting the forecast flows of the last year (2016) using a perpetual growth rate between 0,5% and 2% depending on the cash generating unit. This percentage is management’s best estimate of the expected market evolution based on an organic growth rate such as inflation. The discount rate is based on the Group’s weighted average cost of capital (WACC) including a leveraged beta, cost of debt and cost of equity (including market risk premium and size premium); Discount rates used are post-tax discount rates applied to post tax cash flows. Applying those rates result in value in use similar to those computed using pre-tax discount rates applied to pre-tax cash flows. (as requested by IAS 36). Result of annual impairment testing After having performed the annual impairment testing on goodwill, an impairment charge of 36 M€ has been recorded to partially write off goodwill allocated to the German cash generating unit, to take into account the announced German central federal EBM quotas and the consequences of the alleged fraudulent activities in our Dillenburg laboratory and the likely impact on Labco’s business. With regards to the assessment of value in use of the cash generating units, management believes that no reasonably possible change in any of the above key assumption would cause the carrying value of the unit to exceed materially its recoverable amount, except for German cash generating unit subject to impairment. By applying the sensitivity test to the discount rate and growing rate assumptions, it appears that an increase or a decrease of 100 basis point would not significantly change the conclusions of the impairment tests. In the case of Germany CGU, these sensitivity tests revealed that its goodwill would decrease by 3,5 M€ in the event of a 100 basis point decrease in the long term growth rate (meaning 279 a negative growth rate of 0,5%) or by 6,1 M€ in the event of a 100 basis point increase in the discount rate. 280 Note 14 Intangible assets The line “Other” corresponds mainly to the acquisition of the Fresenius contract by Labco UK, recorded partly as an advanced payment in non-current other receivables at December 31, 2011. Impairment testing on Software and Patents and other intangible assets As of December 31, 2012, the Group has assessed that there were no impairment indicators relating to software, patents and other intangible assets. 281 Note 15 Property, plant and equipment Property, plant and equipment at 31/12/2012 break down by country as follows: 282 Leased plant and machinery Included in the Property, Plant and equipment schedule are the finance lease plant and machinery: The leased plant and machinery mainly relate to the automats included in technical equipment used for medical analyses. The contracts in use for this activity stipulate that, if the laboratory buys exclusively from the supplier chemical reagents for a certain indicative volume during the term of the contract, the supplier, in return, puts an automat at the disposal of the Group for free during the contractual period (referred to as “pay per test” equipment”). These “put at disposal” schemes, although not under the legal form of a leasing agreement, correspond, in substance, to a lease agreement whereby the global price paid for the reagent includes the cost of the consumable and the rent/lease of the machine. As a consequence under IFRS, such agreements are analysed in accordance with IAS 17 on leases with respect to the transfer of majority of risks and rewards. A number of such contracts have been classified as finance leases. For these contracts, the relating finance lease assets and liabilities have been recognized on the balance sheet at the lower of the fair value of the asset and the present value of the minimum lease payment at inception of the contract. The assets are depreciated over the average lease term (60 months). 283 Note 16 Investments in associates The Group’s share of profit in its associates (equity accounted investees) for the year was (69) K€ (2011: (267) K€). The Group has mainly a 51% interests in the joint venture iPP, a 49% interest in a French biology laboratory (Val de Garonne), a 36% interest in an entity, Labo des Charentes, owned by Isolab as well as a 50% interest in a Spanish biology laboratory (Lab Dos Analisis) and a 30% interest in a Portuguese laboratory (Genetica Molecular Laboratorio SL). In 2012, Labco group acquired the controlling ownership of the 3 French biology laboratories historically recorded under equity method (Brigout, Degraef Pouliquen and Sèvre et loire biologie) Otherwise the Group owned interests between 10% and 50% in local Economic Interest Group (so called Société Civile de Moyens [SCM] in France and Consorzio in Italy), which corresponds to entity in which support functions are pooled and working for the Labco group labs but also other external entities. For those entities, the Group has significant influence but no control of the entities. In 2012 the Group receives dividends from its investments in equity accounted investees for an amount of 372 K€ (2011: 302 K€). Details of the Group’s associates at the end of the reporting period are as follows: 284 Summarized financial information for the main investments in associates is as follows (100% of amounts): 285 Note 17 Other non-current assets Non-current assets correspond mainly to deposits and guarantees provided to lessors for the renting of buildings and other premises, as well as non-current other receivables. For entities in which the Group has an ownership below 20% and no significant influence, they are not consolidated and the investments in those entities have been classified as available for sale financial assets pursuant to IAS 39 and, as such, recognized at fair value or historical value when fair value could not be reliably estimated. Unrealized gains and losses are taken directly to other comprehensive income, except for impairment losses that are recognized in the Income Statement. No unrealized gain or loss was recognized in 2012 and 2011. The principal equity investments in unconsolidated companies held by the Group are as follows: The entities Labco Corporate Assistance and Labco Services France owned at 100% by Labco SA are not consolidated as at December 2012 because they were just created and had no activity. Those entities will join in 2013 the Labco SA tax integration but will remain with no activity until Labco management decides otherwise. The entity Laboratorios Martinez Reig has been recently acquired in 2012 with no financial information available timely and a limited activity. 286 Note 18 Deferred tax assets and liabilities (a) Capitalized tax losses carried-forward represent French entities for 2,0 M€, Iberian entities for 2,4 M€, and Italian entities for 0,4 M€. The increase compared to 2011 is mainly explained by the activation of deferred tax assets on tax losses carried forward in Iberia for 2,1 M€ and in Labco SA for 2,1M€ after re-estimating the probability to use these losses within the next 5 years given changes in our forecasts following restructuring actions implemented cumulated with the impacts of the new Tax laws enacted in various countries. Note 19 Inventories There were no significant write-offs regarding inventories during 2011 and 2012. 287 Note 20 Trade receivables and other current assets The decrease in Trade receivables is mainly explained by an improvement of overdue collection in Spain compared to December 31, 2011 position with increased payment delay in Iberia due to economic context and increased payment delay of Public Services, partly compensated by acquisition effects and increase in activity in Belgium or in France. The Group’s exposure to credit and currency risks related to trade and other receivables is disclosed below. Credit risk Exposure to credit risk The carrying amount of financial assets represents the maximum credit exposure. The maximum exposure to credit risk at the reporting date was: Impairment losses The movement in the allowance for impairment in respect of loans and receivables during the year was as follows: 288 The Group has no significant concentrations of credit risk due to the large number of customers and individually non-significance of amounts due. The Group performs ongoing credit evaluations of its receivables. The actual write off relating to trade receivables as at 31 December 2012 relates mainly to several non-significant clients especially in Iberia and amounts to 1.6 M€. As at 31 December 2011 it amounts to 1,6 M€. Actual losses are however within management’s expectations. Given settlement received from Inami in Belgium in fourth quarter 2012, impairment losses of other receivables has been released (0.9 M€) and presented in the line “Other”. Based on historic default rates, the Group believes that, apart from the above, no additional impairment allowance is necessary in respect of trade receivables. At 31 December 2012, as for 2011, the Group does not have any collective impairments on its loans and receivables or its investments. Note 21 Cash and cash equivalents For the purposes of the consolidated statement of cash flows, cash and cash equivalents include cash on hand and in banks, net of outstanding bank overdrafts and cash equivalent. Cash and cash equivalents at the end of the reporting period as shown in the consolidated statement of cash flows can be reconciled to the related items in the consolidated statement of financial position as follows: Cash equivalents correspond, according to the categorization by hierarchy of fair values as stated by IFRS 7, to financial instrument of level 1. Revolving Credit Facility (“RCF”) covenants impose to keep a minimum cash balance of 20 M€ at each quarter end. Note 22 Capital and reserves attributable to owners of the parent Ordinary shares As at December 31, 2012 the authorised share capital comprised 68 459 322 shares. The shares have a par value of one euro (1 €), all shares being fully paid. The shares are denominated into five types, the holders of shares are entitled to the same rights to receive dividend, and are entitled to one vote per share at general meetings of shareholders of the Company. The share capital of Labco is divided into five types of shares, each held by a different category of shareholder: certain laboratory doctors from whom we acquired clinical laboratories (the “A Shareholders”); our shareholders known as “founders” (the “B Shareholders”); financial investors (the “C Shareholders”); and 289 other shareholders (the “D Shareholders” and the “Ordinary Shareholders”) such as our management, family members and estate planning entities of the laboratory doctors who are also our A Shareholders. Approximately 193 laboratory doctors of our Group are A Shareholders, holding together with some of their affiliates who are also A Shareholders, approximately 20,3% of the Company’s share capital. These laboratory doctors became shareholders of the Company by reinvesting a part of the purchase price we paid to acquire their laboratories. The aggregate shareholding of our A Shareholders and their affiliates (who are D Shareholders or Ordinary Shareholders) represents 30,9% of the Company’s share capital. The B Shareholders, representing the “founding” shareholders of our Group, are Eric Souêtre, Stéphane Chassaing, Luis Vieira and the shareholders from whom we acquired “General Lab S.A.” in 2007. Together, they hold approximately 17,6% of the Company’s share capital. The C Shareholders are financial investors who together hold approximately 41,4% of the Company’s share capital. To date, the investment vehicles managed by “3i” are, together, our largest shareholder, the latter having invested €115 million and holding approximately 17,8% of our share capital. “3i” is an international investor focused on private equity, infrastructure and debt management. Other financial investors include notably Viking Limited, a private equity firm that invested in Labco in 2004, CM-CIC Investissements, TCR Capital and Ixen Investissement. The D Shareholders and the Ordinary Shareholders, comprised of our management and the family members and estate planning entities of the laboratory doctors who are also our shareholders, hold the remaining approximately 20.6% of the Issuer’s share capital (i.e., approximately 19.6% of D shares and 0.9% of ordinary shares). The remaining part of the share capital, ie approximately 0,1%, is held by the Company itself as Treasury shares. Issuance of ordinary shares during the period On March 12, 2012, the board of directors recorded an increase in the share capital of our Company of €54 367 corresponding to 54 367 issued C shares deriving from exercise of financial investors warrants. The share capital is increased from €43 336 733 to €43 391 100. Pursuant to the deliberations of the extraordinary shareholders’ meeting held on March 12, 2012, a capital increase of the Company of €24 748 796 has been carried out as of March 28, 2012 through the issuance at par of 24 748 796 shares with a par value of one euro each. The share capital was increased from €43 391 100 to €68 139 896. The preferential subscription rights have been maintained and the shares have been subscribed solely in cash. As of June 30, 2012 the costs directly related to the capital increase have been recorded as a deduction of issuance premium balances for an amount of 138 K€. On June 7, 2012, the board of directors recorded an increase in the share capital and additional paid in capital of 1 035 K€ by the issuance of 115 756 A shares at a subscription price of 8.94 € per share. 290 On October 30, 2012, an extraordinary general meeting of Labco’s shareholders decided an increase in the share capital and additional paid in capital of 1 721 K€ by the issuance of 192 485 A shares at a subscription price of 8.94 € per share in order to remunerate the contribution in kind of remaining Isolab shares as agreed in the acquisition agreement dated February 2012. On December 6, 2012, the board of directors recorded an increase in the share capital of our Company of €11 185 as a consequence of its decision taken on October 4, 2012, using the delegation of powers granted by the general meeting of shareholders held on March 12, 2012, to increase the share capital and additional paid in capital of €99 994 by the issuance of 11 185 A shares at a subscription price of 8.94 € per share. In total, the share capital amounts as at December 31, 2012 to €68 459 322. Hedging reserve The hedging reserve comprises the effective portion of the cumulative net change in the fair value of cash flow hedging instruments related to hedged transactions that have not yet occurred. As a consequence of refinancing operations performed in January 2011, all cumulative fair value changes of cash flow hedging instruments recorded in other comprehensive Income have been recycled in profit & loss. Stock option plan reserve The stock option reserve comprises the employee expenses relating to the share-based payment plans of the Group. Actuarial gains and losses reserve The actuarial gains and losses reserve comprises the cumulative net change in actuarial gains and losses (due to discount rate and main actuarial assumptions) computed for the long term employee benefits valuation. Reserve for own shares The reserve for own shares comprises the costs of the Company’s shares held by the Group. As of December 31, 2011, the Group held 646 K€ of the Company’s shares, corresponding to 80 728 shares. Twenty one (21) treasury shares have been sold during the three months ended 31 March 2012 to twenty managers, two (2) have been repurchased, and as of December 31, 2012, the Group held 646 K€ of the Company’s shares, corresponding to 80 709 shares (80 707 A shares and 2 D shares) Dividends No dividends were declared and paid to the shareholders of Labco SA during 2011 and 2012. Equity Warrant schemes Labco has issued warrants to financial investors and key management personnel of our Group. Warrants Issued to Financial Investors During the year ended December 31, 2008, the Company issued three warrant schemes entitling the holders, upon exercise of the warrants to subscribe for other financial instruments or shares of the Company at a fixed price of €1 per share. The number of warrants that can be exercised depends on the ability of the Group to meet certain financial and operational targets. The exact terms of the exchange at the exercise dates were determined when the Company entered into the issuance agreements. Subject to the fulfillment of 291 their conditions, the warrants are exercisable at any time during a period of 15 years after their date of subscription. A total of 4 223 394 “ABSA C1” were issued, i.e., 4 223 394 Class C shares to which a total of 16 893 576 warrants of four different kinds were attached, providing subscription rights for a maximum of 4 113 531 new Class C shares at a price of €1 per share. A total of 80 878 shares were issued in 2009 by the exercise of 80 878 warrants attached to the “ABSA C1”, pursuant to their terms and conditions. A total of 4 223 394 “ABSA C2” were issued, i.e., 4 223 394 Class C shares to which a total of 8 446 788 warrants of two different kinds were attached, giving subscription rights for a maximum of 2 323 852 new Class C shares at a price of €1 per share. A total of 27 049 shares were issued in 2010 by the exercise of 27 049 warrants attached to the “ABSA C2”, pursuant to their terms and conditions. A total of 1 967 083 “BEABSA” were issued, i.e., 1 967 083 options giving subscription rights for a maximum of 2 097 145 “ABSA C3”, i.e., 2 097 145 Class C shares to which a total of 4 541 820 warrants of two different kinds could be attached, giving subscription rights for a maximum of 1 176 860 new Class C shares at a price of €1 per share. A total of 2 020 660 “ABSA C3” were issued in 2009 by exercise of the 1 967 083 “BEABSA”, pursuant to their terms and conditions. A total of 54 367 C shares were issued in 2012 by the exercise of 54 367 financial investors warrants. Subsequently on January 18, 2013, general meetings of holders of certain securities issued by Labco and an extraordinary general meeting of Labco’s shareholders decided to amend the terms and conditions of the ABSA C1, ABSA C2 and BEABSA in order to modify their conditions of exercise. Warrants Issued to Mezzanine Lenders Three additional warrant schemes were issued by the Company in July 2008. These warrants entitle the holders upon exercise of the instrument to buy one share of the Company at either a fixed price of €1 (mezzanine B and C) or €14,206582 (mezzanine A) per share. The warrants were granted at a price of €0,0001 per warrant. A total of 494 241 Senior Mezzanine warrants, split in 313 243 Senior Mezzanine A, 38 752 Senior Mezzanine B and 142 246 Senior Mezzanine C, were issued. A total of 6 018 shares were issued in 2010 by exercise of 6 018 Senior Mezzanine B warrants, pursuant to their terms and conditions. A total of 1 464 175 Junior Mezzanine warrants, consisting of 927 975 Junior Mezzanine A, 114 800 Junior Mezzanine B and 421 400 Junior Mezzanine C, were issued. A total of 17 829 shares were issued in 2010 by exercise of 17 829 Junior Mezzanine B warrants, pursuant to their terms and conditions. A total of 1 460 443 Junior additional Mezzanine warrants, consisting of 925 609 Junior Additional Mezzanine A, 114 508 Junior Additional Mezzanine B and 420 326 Junior Additional Mezzanine C, were issued. A total 17 784 shares were issued in 2010 by exercise of 17 784 Junior Additional Mezzanine B warrants, pursuant to their terms and conditions. No mezzanine lenders’ warrants were exercised in 2012. Subsequently on January 18, 2013, Labco entered into a settlement agreement with the Mezzanine lenders and the C Shareholders regarding the exercise conditions of certain Mezzanine warrants. 292 Borrowings and other financial liabilities Note 23 This note provides information about the contractual terms of the Group’s interest-bearing loans, borrowings, which are measured at amortized cost, and other financial liabilities. For more information about the Group’s exposure to interest rate, foreign currency and liquidity risk, see note 29 Financial Instruments. Labco performed a refinancing operation early 2011 that significantly modified the sources of fundings for Labco Group. On January 14, 2011, Labco SAS issued High Yield Senior Secured Notes (also, the “Bond”) due 2018 for 500M€ with international investors in London and with Deutsche Bank as Trustee. The main terms and conditions of the Notes are: Fixed interest rate amounting to 8.5%, with interests paid semi-annually in arrears. Maturity of the Notes is January 15, 2018 The Notes are guaranteed on a senior secured basis by certain subsidiaries, mainly through first ranking liens over the capital stock of certain subsidiaries and certain present and future intercompany loan receivables Under the Notes indentures, Labco will have to respect certain covenants mainly related to reporting and information requirement. The Notes have been listed on the Official List of the Irish Stock Exchange and admitted to trading on the Irish Stock Exchange. The Notes proceeds have been primarily used to repay the December 31, 2010 existing bank debt facilities as well as fees related to this operation. Fees directly linked to the debt issuance or linked to the strategic refinancing project amounted to approximately 16 M€ (excluding VAT) and following their 293 analysis have been either expensed or capitalized as debt issuance costs to be amortized over the Notes maturity using the effective interest rate method. At the same time end of January 2011, Labco SAS entered into a 135 M€ revolving credit facility under a syndicated Revolving Credit Facility Agreement (the “RCF”) with Credit Suisse, Deutsche Bank, Natixis and UBS as lead banks, and Natixis as agent. The main terms and conditions of the RCF are: Floating interest rate defined as Euribor + a margin of 4,5% per annum (margin that may reduced to 4.0% or 3.5% per annum by reference to a total net debt to adjusted EBITDA test); Maturity of the RCF is February 15th, 2017; Borrowings under the RCF will mandatory be used to finance part or all of purchase price and related costs of certain permitted acquisitions (as defined in the agreement), refinance existing indebtedness of entities acquired, finance restructuring costs in relation with acquisition, finance capital expenditure and fund working capital and other general corporate purposes of the Group, provided that the aggregate outstanding amounts of utilization under capital expenditure or working capital funding may not at any time exceed 50 M€; The RCF is guaranteed on a joint and several basis by certain subsidiaries qualified as Guarantors, mainly through first ranking liens over the capital stock of certain subsidiaries and certain present and future intercompany loan receivables. The RCF requires that the EBITDA of the Guarantors represent not less than 70% of consolidated EBITDA of the Group; The Revolving Credit Facility Agreement also requires Labco, each Borrower and each Guarantor (together, the “Obligors”) to observe certain customary affirmative and restrictive covenants, subject to certain exceptions, including: o covenants relating to financial information and accounting; o covenants relating to obtaining required authorizations; compliance with laws; compliance with environmental laws; information about environmental claims; payment of taxes; pari passu ranking of unsecured payment obligations; insurance; granting of access; intellectual property; compliance with financial assistance laws; guarantor coverage (as described above); publicity; limitations on disposals and reorganizations; additional restrictions on distribution of dividends; o no other financial indebtedness ranking senior to, or pari passu with, the RCF (other than additional Notes and, subject to lenders’ consent, additional revolving credit facilities); o restriction on incurring additional indebtedness subject to customary exceptions including up to (i) 25 M€ at any time for all members of the Group and (ii) 15 M€ at any time for all members of the Group other than the Borrowers and the Guarantors; and o restriction on incurring recourse factoring or similar arrangements in excess of 5.0 M€ in aggregate at any time. The Revolving Credit Facility Agreement also requires Labco to ensure compliance with some financial covenants detailed below. Fees directly linked to the debt issuance amounted to approximately 8.8 M€ (excluding VAT) and have been capitalized as debt issuance costs to be amortized over the RCF maturity using the effective interest rate method. An Intercreditor Deed Agreement has also been signed between, among others, the Obligors, Deutsche Bank AG, London Branch as Security Agent, Natixis as senior Agent, Deutsche Bank AG, London Branch as Senior Secured Notes Trustee, the Lenders (as Revolving Credit Facility Lenders), the Arranger (as Senior Arrangers), the Hedge Counterparties (each as defined in the Intercreditor Deed), and the Intra-Group Lenders (as defined in the Intercreditor Deed) to define securities and guarantees provided by certain subsidiaries in the context of Bond and RCF issuance. 294 As of December 31, 2012, the Group borrowings comprises A 500 M€ 8.5% Senior Secured Notes due 2018 net of debt issuance costs, with interests paid semi-annually in arrears; Some bilateral bank borrowings for a total of 15.8 M€; The Revolving Credit Facility (RCF) amounting 135 M€ entered into in January 2011 has been drawn as at December 31, 2012, for an amount of 41 M€ and debt issuance costs have been capitalized and amortized over RCF maturity. 8.5% Secured Senior Notes covenants Under the Notes indentures, Labco has to respect certain covenants mainly related to reporting and information requirement. Revolving Credit Facility (RCF) covenants The RCF includes certain financial covenants as defined in the agreements. leverage ratio tested quarterly (calculated as the ratio of consolidated total net debt at each quarter end to consolidated adjusted EBITDA for the 12 months ending on that quarter end), super senior gross leverage ratio tested quarterly (calculated as the ratio of total super senior gross debt at each quarter end to adjusted EBITDA for the 12 months ending on that quarter end); minimum cash balance of €20 million, tested quarterly; and operating capital expenditure for a financial year not to exceed 3.5% of consolidated group revenue Furthermore, Labco should also achieve an incurrence ratio when we are willing to draw on the RCF. The incurrence ratio is calculated as the ratio of pro forma the acquisitions consolidated total net debt to consolidated adjusted EBITDA for the immediate preceding period pro forma the effect of acquisitions. As stated in Note 5 Significant Events, we obtained an amendment of the “Leverage ratios”, an amendment of the “Incurrence Ratios” and a technical amendment. The amendments signed on April 13, 2012 entered into force immediately and have their first application for the calculation of the compliance certificates for the Quarter ending on March 31, 2012 Amended “Leverage Ratios” in clause 26.2(a) of the Revolving Credit Facility Agreement is as follows: Relevant Period Initial Ratio Amended Ratio Relevant Period expiring after 31 December 2011 but on or before 31 December 2012 5.25:1 5.75:1 Relevant Period expiring after 31 December 2012 but on or before 31 December 2013 5.00:1 5.75:1 295 Relevant Period expiring after 31 December 2013 but on or before 31 December 2014 4.75:1 5.50:1 Relevant Period expiring after 31 December 2014 but on or before 31 December 2015 4.50:1 5.25:1 Relevant Period expiring after 31 December 2015 4.25:1 5.00:1 Amended “Incurrence Ratios” in clause 27.14(c) of the Revolving Credit Facility Agreement is as follows: Relevant Period Initial Ratio Amended Ratio Relevant Period expiring after 31 December 2011 but on or before 31 December 2012 5.00:1 5.25:1 Relevant Period expiring after 31 December 2012 but on or before 31 December 2013 4.75:1 5.25:1 Relevant Period expiring after 31 December 2013 but on or before 31 December 2014 4.50:1 5.00:1 Relevant Period expiring after 31 December 2014 but on or before 31 December 2015 4.25:1 4.75:1 Relevant Period expiring after 31 December 2015 4.00:1 4.50:1 The Group requested also that the definition of EBITDA set forth in clause 26.1 (Financial definitions) of the Revolving Credit Facility Agreement be amended by adding a new paragraph (ix) under paragraph (a) of the EBITDA definition as follows: “before taking into account any VAT loss resulting from a corporate recharge”. As of December 31, 2012, Labco achieved the expected covenants ratio targets. Finance lease liabilities The Group has finance leases mainly for the technical equipment (refer to note 15 Property, plant and equipment). 296 Note 24 Employee benefit liabilities All of employees of the Group are covered by state pension and collective plans managed by third parties if required under local legislation. Those plans are defined contribution plans. In addition to these legal pension schemes, a provision is set up for post-employment benefits in France and Italy amounting to 8,2 M€ as of December 31, 2012 (6,2 M€ and 2,0 M€ thousand respectively). In Italy, there is a legal obligation (so called TFR) to pay 7,4% of the employee’s annual remuneration for every working year at the departure date. In France the principal assumptions used for the purposes of the actuarial valuations are as follows: The movements in the provision for post employment benefits in France and in Italy can be summarized as follows: The French post employment benefit and the portion of TFR before January 1st, 2007 accounted for as post employment benefit are not financed through external fundings. The cumulative net actuarial gains and losses recognized in OCI is detailed in the table Other comprehensive Income and amount to 302 K€ (2011: (578) K€). 297 Note 25 Share based payment schemes Warrants Issued to Management On March 24, 2005, the Company established two manager share warrant schemes (denominated “BSA 2005-1-1” and “BSA 2005-1-2”) that entitle key management personnel to subscribe for new shares of the Company, subject to the fulfillment of the conditions detailed in the corresponding issuance agreements. On June 7, 2006 (“BSA 2006-1-1”), March 13, 2007 (“BSA 2007-1-1”), December 30, 2007 (“BSA 2007-1-2”), March 5, 2008 (“BSA 2008-1-1”) and September 20, 2010 (“BSA 2010-1-1”), additional manager share warrant schemes were implemented in favor of key management personnel of the Group. Each share warrant gives the beneficiary the right to subscribe for one share of the Company at an exercise price determined on the allocation date, on predetermined graded vesting dates. The exercise conditions mainly depended on certain financial targets and, in some cases, include an employment condition. The meeting of the Strategic Committee of the Company held on April 23, 2008 established that the exercise conditions of the three categories of share warrants issued on June 7, 2006, March 13, 2007 and December 30, 2007 (BSA 2006-1-1, BSA 2007-1-1 and BSA 20071-2) were fulfilled, pursuant to their terms and conditions, so as to allow the holders to exercise such share warrants at any time. The general meeting of shareholders held on December 30, 2008 modified the terms and conditions of the two categories of share warrants issued on March 24, 2005 (BSA 2005-1-1 and BSA 2005-1-2) to remove all constraints in order to allow the holders to exercise such share warrants at any time. As a result of these decisions and the modifications to the schemes, the beneficiaries can exercise the share warrants granted by these five schemes at any time, within a period of ten years following the date of subscription. According to IFRS 1, the Group did not apply IFRS 2 to 2005, 2006 and 2007 share-based payment arrangements since they had vested at the date of transition. For the BSA 2008-1-1 scheme, the share warrants vest in 2009, 2010 and 2011. For the BSA 2010-1-1 scheme, the share warrants can be exercised only upon the sale or initial public offering of the Company. No warrants issued to management were exercised in 2012. On April 7, 2011, the Company established a Restricted Stock Unit Plan (denominated “Free Shares 2011”) that entitle key management personnel to obtain up to 150 000 free shares, subject to the fulfillment of the conditions detailed in the issuance agreement. IFRS 2 was applied to the Free Shares 2011 plan and those free shares will vest in case of sale or initial public offering of Labco. The fair value of the services received in return for free shares is based on the fair value of the free shares granted, measured using the same weighted average models of scenarios than for the “BSA 2010-11” plan given the vesting conditions are similar based on exit price per share in case of sale or initial public offering of Labco. No pre-vesting forfeiture has been considered given the limited number of people involved. The total fair value of “Free Shares 2011” plan has been estimated to 2.6 M€. The services received during 2012 (share-based payment expense) amounts to a total of 565 K€, 220 K€ for Restricted Stock Unit “Free Shares 2011” scheme Q1 2012 expense given the plan has been cancelled in Q2 2012 and 345 K€ for 2010 BSA schemes. Indeed, the 2008 BSA plan is now fully vested and the 2010 BSA schemes IFRS 2 expense has been revised due to the departure of certain beneficiaries. The Restricted Stock Unit scheme has been cancelled in Q2 2012 resulting in the residual IFRS 2 estimated charges initially spread over vesting period to be immediately recorded in consolidated statement of income for an amount of 1 537 K€ in non-recurring expenses and the whole amount of Fair Value reserve related to Restricted Stock Unit scheme of 3.5 M€ recycled in other consolidation reserves. The total amount of Fair Value reserves related to the stock options plans amounts to 3.5 M€ as at December 31, 2012 (4,0 M€ as at December 31, 2011). 298 Terms and conditions of the share option programs The terms and conditions relating to the grants of share warrants are as follows; all share warrants are to be settled by physical delivery of shares: Given the dilutive effect of March 12, 2012 capital increase at par value, all equity warrants holders have been granted a protection right against the dilutive effect. In fact, pursuant to the ninth resolution adopted by the general shareholders’ meeting held on March 12, 2012, holders of securities granting access to the share capital will be allowed to subscribe to new shares as of right, at the ratio of four new shares with a par value of one euro each for seven shares subscribed with their securities (seven shares subscribed by exercise of their securities granting access to the share capital giving the right to subscribe to four additional new shares at a subscription price of one euro per share). Fair value of the options granted during the period The fair value of the services received in return for share options is based on the fair value of the share options granted, measured using the Binomial model for 2008 scheme and using a weighted average models of scenarios for the “BSA 2010-1-1” scheme and “Free Shares 2011” plan with the following inputs: The Group recognized as share-based payment expense 345 K€ during the year (1 233 K€ in 2011) for 2010 schemes and 220 K€ for Free shares 2011 for Q1 2012 as well as 1 537 K€ in nonrecurring expenses following cancelation of the Free shares plan (878 K€ in 2011). The total amount of reserves related to the stock options plans amounts to 3 504 K€ (4 037 K€ in 2011). 299 Movements in share options during the year No options have been exercised in 2011 or in 2012. As a reminder, the protection right of equity warrants holders against the dilutive effect of March 12, 2012 capital increase enables them to subscribe in case of exercise of the warrants new shares at the ratio of four new shares with a par value of one euro each for seven shares subscribed with their securities. Note 26 Provisions Provision for litigation In the normal conduct of its business, the Group has legal suits relating to different matters (personnel, taxes, suppliers) with uncertainties about the amount or timing of the outflows. According to management and as confirmed by legal counsels, the recorded provision is considered to be sufficient to cover probable losses. The line “Other” corresponds mainly to the reversal of the provision for tax litigation recorded at year end 2011 for Labco SA tax reassessment for period 2008 – 2010 and actual expenses have been recorded for the same amount. The main provision (1.0 M€) for litigation is related to the major litigation with former vendors of Dillenburg for alleged fraudulent activities and the related consequences. Provisions for restructuring The provisions for restructuring correspond mainly to remaining provisions for restructuring in Iberia and Germany (Duisburg) recorded at year-end 2011 covering principally the severance payments and the remaining rental fees for the building to be abandoned as well as provisions set up in 2012 for restructuring plan Mittelhessen merger in Germany and Deep dive efficiency plan in France (refer to Note 5.3 Non-recurring restructuring plans). Other Provisions The other provisions correspond mainly to the provision for negative share of investments in associates recorded under equity method and the increase in 2012 is related to the shares of iPP net losses. 300 Note 27 Litigations and Contingent liabilities Group companies are involved in various legal proceedings arising in the ordinary course of business, including disputes concerning professional liability and employee related matters, as well as inquiries from governmental agencies and health insurance carriers regarding, among other things, billing issues or litigations with tax, social security and customs authorities. Provisions have been set aside for the probable costs, as estimated by the Group’s entities and their counsel, for the various litigations. We had a lawsuit against the seller of one of our clinical laboratories which, although not material to us, is referred to in other notes as the Duisburg litigation. We also summarize below the lawsuit against the seller of another clinical laboratories MVZ Dillenburg in a context of alleged fraudulent activities. MVZ Duisburg In 2009, one of our German subsidiaries, MVZ Labor Duisburg GmbH, brought an arbitral claim for breach of contract against the laboratory doctor from whom it had acquired a clinical laboratory in Duisburg. Following the acquisition, the laboratory lost one of its larger clients, a blood bank owned by the laboratory’s former owner and his wife. In the proceedings, MVZ Labor Duisburg GmbH claims that the loss of the contract constitutes a breach of the non-compete clause of the acquisition agreement. This case was settled in 2011. Pursuant to the settlement agreement, the defendant paid € 1 million to MVZ Labor Duisburg GmbH. MVZ Dillenburg In 2008, we acquired MVZ Medizinisches Fachlabor Dillenburg (“Dillenburg”), a German laboratory company. In April 2012, we discovered that the selling shareholders of Dillenburg had charged unlawful fees to the German Doctors’ Insurance Billing Organization (Kassen¨arztliche Vereinigung Hessen, or “KV Hessen”) and private health insurance companies. The new management promptly ended this practice upon its discovery. The actions of the selling shareholders are subject to an ongoing internal investigation. The new management has notified both the state prosecutor and KV Hessen of the fraud, has enabled the clarification of facts and is closely cooperating with the state prosecutor and KV Hessen who have initiated a criminal investigation against the selling shareholders of Dillenburg. We believe that the German Doctors’ Insurance Billing Organization and other private health insurance companies may commence reimbursement claims against Dillenburg to recover fees inappropriately charged. The fraudulent charging of fees could also result in a withdrawal of the license of Dillenburg. However, we believe this is unlikely, due to the fact that Labco was victim of a fraud when we acquired the shares of Dillenburg GmbH, because the sellers had practiced the fraudulent charging before the acquisition and because the new management of Dillenburg has promptly notified the state prosecutor and KV Hessen of the fraud. We are currently reviewing any other action we may commence against the selling shareholders of Dillenburg. As of December 31, 2012 we have also recorded a non-recurring litigation expense of €1.7 million in relation to this issue. No contingent liability has been identified for which it will be necessary to disclose information in the notes to the consolidated financial statements. Additionally, we operate in a regulated industry. As such, in the ordinary course of business, we are subject to national and local regulatory scrutiny, supervision and controls. Below is a summary of the challenges and proceedings brought against us by the French Ordre des pharmaciens and Ordre des médecins and of the litigation we brought against the Ordre des pharmaciens before the European Commission. 301 Ordre des Pharmaciens and Ordre des Médecins In France, the Ordre des pharmaciens and, to a lesser extent, the Ordre des médecins have challenged our organization and legal structure. In numerous instances, the Ordre des pharmaciens instituted disciplinary actions against our laboratories or laboratory doctors. In 2003, shortly after our creation, the Ordre des pharmaciens and the Ordre des médecins (together, the “Ordres” in a joint letter to Labco) expressed the view that Labco’s project of creating a network of laboratories contravened the principle of independence of laboratory doctors. In their joint letter, the Ordres singled out two aspects of Labco’s structure: (i) capital ownership and voting rights arrangements between the laboratory doctors working in each laboratory company and the rest of the Labco Group; and (ii) the ultimate ownership of a network of laboratories by a financial holding company not subject to the French regulations pertaining to clinical laboratories. In keeping with this initial position, the Ordre des pharmaciens, as well as in several instances the Ordre des médecins, have raised a number of objections to the organization of our French operations in the context of their administrative review of proposed changes in the articles of association or ownership structure of clinical laboratories. These objections were communicated to the French administrative authorities in charge of granting the administrative authorization necessary to operate our laboratories. All such authorizations were nevertheless eventually granted. Most of the objections raised by the Ordres dealt with the capital ownership structure of our French laboratory companies. The Ordres argued that ownership of a large majority of shares in a clinical laboratory by a person or entity other than the laboratory doctors working in that laboratory constituted a threat to the independence of such laboratory doctors—notwithstanding the fact that such laboratory doctors retained a majority of the voting rights at shareholders’ meetings, as required by French law. It also challenged the separation of voting rights from economic rights in such a manner. In addition, the Ordre des pharmaciens expressed the view that the supermajority voting provisions contained in the articles of association of our SELs for certain matters were incompatible with the principle of independence of laboratory doctors insofar as they took away from doctors practicing within the laboratory the final decision making power over a number of matters pertaining to the laboratory. Finally, the Ordre des pharmaciens challenged the formula set out in the articles of association of SELs for the distribution of Priority Dividends to those laboratory doctors who are also shareholders in their laboratory company. The Ordre des pharmaciens argued that such a formula, by limiting ex-ante the share of dividends to be distributed to laboratory doctors, was incompatible with the principle of the independence of laboratory doctors. Both the Ordre des pharmaciens and the Ordre des médecins have, in several cases, raised objections to the registration, based on one or more of the above grounds, of one of our SELs on their respective national registries. At the disciplinary level, the Ordre des pharmaciens introduced a number of actions against SELs in the Labco network, as well as against laboratory doctors practicing within these SELs. Several of these actions, some of which are still pending, directly challenge the capital ownership structure and the supermajority voting provisions contained in the articles of association of our French laboratory companies as a breach of the principle of independence of laboratory doctors. Labco has appealed decisions of the disciplinary body of the Ordre des pharmaciens in the highest French administrative court (Conseil d’Etat) and has won a number of cases on procedural grounds. No final decision has, however, been reached on the merits of these cases. The Ordre des pharmaciens has also regularly brought, or threatened to bring, legal actions against our laboratory companies for failing to timely file with it proposed changes in articles of association or capital ownership. Some of these actions are still pending. We have appealed some of these decisions and have won a number of such appeals on procedural grounds. In addition, certain of our laboratory doctors and laboratory companies have been disciplined for failing to maintain adequate health, safety and quality standards. Certain of these disciplinary procedures, brought on the grounds of a failure to meet filing requirements or to maintain adequate quality and safety standards, resulted in decisions to close, for periods ranging from one week to several months, several of our laboratories. In several instances, however, we successfully obtained from the responsible administrative authority a requisition order to prevent such closing, arguing the public need for access to local laboratory testing services. 302 In 2007, Labco filed a complaint with the European Commission, arguing that the Ordre des pharmaciens had inappropriately used the administrative and disciplinary powers granted to it to impede the development of free competition and the creation of groups of laboratories in the French clinical laboratories services market. On December 8, 2010, the Commission ruled against the Ordre des pharmaciens, finding that the Ordre des pharmaciens had (i) systematically targeted groups like Labco since 2003 with the aim of impeding their development and (ii) attempted to set minimum prices on the French market between September 2004 and September 2007 by seeking to impose minimum prices for the provision of clinical laboratory services, limiting the negotiation of discounts under contracts with large customers such as hospitals or insurance bodies. The Commission held that the Ordre des pharmaciens had breached the antitrust rules and provisions on restrictive business practices of the Treaty establishing the European Community by introducing such distortions to competition without adequate public health grounds to do so, and imposed a 5 M€ fine on the Ordre des pharmacien and its governing bodies. According to the press release issued by the European Commission commenting on this decision, the Commission’s ruling did not extend to an appreciation of the French laws regulating the clinical laboratories market, but was only directed at the behavior of the Ordre des pharmaciens. The Ordre des pharmaciens has since appealed this decision before the General Court of the European Communities. Pending a decision on appeal, aggrieved parties such as Labco are entitled to seek damages before French courts on the basis of the European Commission’s findings. 303 Note 28 Trade and other liabilities Payables relating to the acquisition of subsidiaries are as follows: Note 29 Financial instruments The Group’s principal financial instruments, other than derivatives, comprise bank loans and overdrafts, debentures, finance leases, trade payables, purchase contracts and loans granted. The main purpose of these financial instruments is to raise finance for the Group’s operations. The Group has various financial assets such as accounts receivables and cash and short-term deposits, which arise directly from its operations. The main risks arising from the Group’s financial instruments are credit risk, liquidity risk and interest rate risk. Under our current financing strategy, the Secured Senior Notes are 8,5% fixed rate, therefore we are only exposed to market risk arising from fluctuations in interest rates of our Revolving Credit Facility (the RCF) drawn as at December 31, 2012 for a net amount of 41 M€. We are not required to enter into hedging transactions or to use derivative financial instruments to mitigate the adverse effects of interest rate fluctuations pursuant to the RCF Agreement. We do not 304 enter into financial instruments for trading or speculative purposes. The derivatives existing as at December 31, 2010 have been early cancelled as a consequence of the refinancing operation early 2011. Labco has only one non-significant instrument for a laboratory in Belgium and is accounted for as trading derivatives (fair value through P&L). That derivative corresponds, according to the categorization by hierarchy of fair value as stated in IFRS 7, to level 2 financial instruments. Labco do not have any level 3 financial instruments. Due to the Group’s specific interest rate risk position and funding structure, risk management policies require to manage the cash flow volatility. Liquidity risk The Group monitors its risk to a shortage of funds using a systematic liquidity planning scheme. This scheme considers the maturity of its financial investments and assets and the projected cash flows from operations. Please refer to Note 23 Borrowings and other financial liabilities for a detail of maturities of financial indebtedness, as well as for a description of the covenants in place with the RCF agreement. Under these covenants, if the Group does not respect contractual requirements, it may result in early repayment clause being activated by syndicated borrowers. Revolving Credit Facility (“RCF”) covenants impose to keep a minimum cash balance of 20 M€ at each quarter end. The refinancing operation performed February 13, 2013 has subsequently modified the source of financing, please refer to Note 34 Events after the reporting period and to the prospective liquidity analysis presented in that paragraph. Interest rate risk At the reporting date the interest rate profile of the Group’s interest-bearing financial instruments was: The fixed rate financial liabilities correspond to 8.5% Senior Secured Notes, the finance leasing, the recourse factoring and the bilateral loan and borrowings, that are under fixed rate, whereas the variable rate instruments correspond to cash and cash equivalent for financial assets and to the RCF drawn and floating rates loan and borrowings, as well as bank overdrafts. 305 The other financial instruments of the Group that are not included in the above table are noninterest bearing and are therefore not subject to interest rate risk. Cash flow sensitivity analysis for variable rate instruments Given the refinancing operation performed February 13, 2013 resulting in the RCF being fully reimbursed (refer to note 34 Events after the reporting period), a change of 100 basis points in interest rates would have increased or decreased the annual interest rate charges by less than 0.1 M€ (2011: 2.4 M€). This analysis assumes that all other variables remain constant and financial income on financial assets is considered as not significant. Fair values The fair values of financial assets and liabilities, together that are not at fair value in the statement of financial position, are not significantly different from recorded carrying amounts. The basis for determining fair values is disclosed in note 3.21 Determination of fair values. 306 Note 30 Capital commitments and contingencies Operating lease and commercial commitments The Group has entered into commercial leases on certain motor vehicles and items of machinery. These leases have an average life of between two and five years with no renewal option included in the contracts. Furthermore Labco leases almost all of the properties where its labs are located. The lease in France is on a 3/6/9 year lease contracts and in Portugal and Spain, situation is such that Labco can exit leases at 6-12 months notice. Operating lease expense related to property amounted to 21.0 M€ in 2012 (2011: 19.1 M€) and equipment lease expense of around 8.7 M€ (2011: 7.8 M€). Finance lease and commercial commitments Reagents suppliers in certain instances provide the testing equipment free of charge to laboratories in exchange for exclusive purchasing commitments, including sometimes minimum volume commitments. Management believes minimum volume commitments for consumables are substantially below current volumes and therefore we do not consider these minimum purchase commitments to be material for us. As stated in Note 15 Property, Plant and Equipment, some of these contracts have been qualified as capital lease over an average duration of 5 years because the contracts have tacit renewal clauses, but no purchase options. Renewals are at the option of the specific entity that holds the leases. Future minimum lease payments under the finance leases are as follows: Off balance sheet commitments given and received As of December 31, 2012, the Group’s off-balance sheet commitments consist principally of guarantees given in the course of its investing and financing activities, especially securities given to secure the Notes and the RCF. 307 Indeed the obligations taken by Labco in the Senior Secured Notes indentures and by the borrowing entities, direct subsidiaries of Labco, according to the RCF agreement, have been guaranteed by commitments given by a certain number of Group entities, called Guarantors. Commitments given to the Security Agent are mainly: The initial Guarantors and Borrowers agreed to pledge the shares of certain subsidiaries they owned; If a Guarantor or Borrower acquires a subsidiary company which has an annual EBITDA equal to or greater than 750 K€, it agreed to pledge the shares of the said subsidiary company; If a company accedes to the RCF Agreement as a new Borrower, the said company must pledge the shares held in its direct subsidiaries which has an annual EBITDA equal to or greater than 750 K€; If a company accedes to the RCF Agreement as a new Guarantor, the said company must pledge the shares held in its direct subsidiaries which has an annual EBITDA equal to or greater than 1 M€; In any case, additional Borrowers and Guarantors agreed to pledge the long term intercompany loans receivables with other members of the Group they have or they could enter in the future arising under any intra group loan in excess of 1 M€. As at December 31, 2012, the Guarantors are the following entities: 308 The list of off-balance sheet commitments was as follows: Furthermore in the context of iPP starting the operations of the contract with Taunton and Somerset NHS Foundation Trust and Yeovil District Hospital NHS Foundation Trust, Labco has issued a parent company guarantee to the benefit of the Trusts according to which it guarantees the performance of the operating obligations of iPP under the outsourcing contract and for a period lasting over contract period plus two years in general. Labco has also issued similarly a parent company guarantee to the benefit of Southwest Pathology Services in relation to the performance of the supply chain agreement contracted between iPP and Southwest Pathology Services. 309 310 311 312 313 Note 31 Earnings per share Computation of weighted average number of actions for basic and diluted earnings per share: 314 Note 32 Related party transactions Transactions with key management personnel Key executive management compensation With the exception of the standing independent members of the board of directors, members of the board of directors and the executive committee receive no compensation for their services on either of these committees. The independent member of the board of director, Daniel Bour, received in 2012 a compensation of €40 000 per year. Certain members of the board of directors (Philippe Charrier, Andreas Gaddum, Stéphane Chassaing, Albert Sumarroca, Philippe Dauchy, Philippe Sellem, Xavier Merlen, Thierry Mathieu, Gilles Meshaka) and the executive committee (Philippe Charrier, Albert Sumarroca, Luis Vieira, Etienne Couelle, Oliver Harzer, Santiago Valor, Philippe Cailly, Andrew Geoffrey Searle and Vincent Marcel) are, or were in 2012, compensated for certain other services they render to our Group. Such remuneration is paid to them (or to professional companies wholly owned by them) by way of a fixed annual salary (or fees) and an annual bonus. In 2011, the executive committee was enlarged with members starting during second semester 2011 and exceptional bonuses have also been paid to certain key management personnel in relation with refinancing operations. The aggregate remuneration paid to or accrued on behalf of members of the board of directors and the executive committee for such other services was approximately 5.8 M€ for the year ended December 31, 2012 (2011: 6.9 M€). (i) : Post-employment benefits are not significant and correspond only for the few members concerned to French legal post-employment benefits due to employees as described in note 24 Employee benefit liabilities. (ii) : Restricted Stock Unit “Free Shares 2011” scheme to the benefit of certain senior management members has been canceled in Q2 2012 resulting in the residual IFRS 2 estimated charges initially spread over vesting period to be immediately recorded in consolidated statement of income for an amount of 1 537 K€ as described in note 25. Furthermore the 2008 BSA plan is now fully vested and no senior management members have any remaining 2010 BSA. Other related party transactions with key management members Secondment Agreement with 3i Gestion SA Labco SAS and 3i Europe plc, an affiliate of 3i, entered in the past into a secondment agreement pursuant to which Mr. Etienne Couelle was seconded to Labco. The contract terminated on October 1st, 2011 with Etienne Couelle being hired by Labco. In 2011, 3i France was paid 220 K€ under that contract. Legal Services We have engaged Maryse Coppet Souêtre, a partner in the law firm Cabinet Coppet Sprl, to provide us with various legal services. Mrs. Coppet Souêtre was the wife of Mr. Eric Souêtre, one of 315 our founding shareholders and a member of the board of directors. We believe that Mrs. Coppet Souêtre’s legal services are negotiated on an arm’s length basis. Our payments to Cabinet Coppet Sprl amounts to nil as at December 31, 2012 (2011: 0.2 M€). Other related party transactions Balances and transactions between Labco SA and its subsidiaries, which are related parties of Labco SA, have been eliminated on consolidation and are not disclosed in this note. A number of associates accounted for under the equity method incur expenses for certain subsidiaries of the Group. These operating expenses are recharged to the relevant subsidiaries. The net operating expenses that have been recharged by the associates accounted amounted to nil in 2012 (2011: 856 K€). All transactions and outstanding balances with the related parties during the year are priced on an arm’s length basis. None of the balances is secured. 316 Note 33 Group entities Subsidiaries of Labco Deutschland group are consolidated in the financial statements herewith presented. In accordance with § 246B of German commercial laws (HGB), these entities may benefit from the exemption of publishing financial report and consolidated financial statements pursuant to German GAAP. Subsidiaries that have opted for this exemption are listed below. The consolidation scope at December 31, 2012 and December 31, 2011 was established as follows: EM: Equity Method / FC: Global integration / NC: Not consolidated 317 EM: Equity Method / FC: Global integration / NC: Not consolidated 318 EM: Equity Method / FC: Global integration / NC: Not consolidated 319 EM: Equity Method / FC: Global integration / NC: Not consolidated * Companies which are sub-consolidated in Questao 320 Note 34 Events after the reporting period Additional 8,5% Senior Secured Notes due 2018 issued for an aggregate nominal of 100 M€ Labco S.A. issued on February 13, 2013 €100 million in aggregate principal amount of its 8.5% Senior Secured Notes due 2018, which constitutes an add-on of, and form a single class with, Labco’s existing 500 M€ 8.5% Senior Secured Notes due 2018. The Additional Senior Secured Notes will mature on January 15, 2018. The gross proceeds of the issuance amounts to 103 M€, ie meaning a yield of 7.75% and will be used to repay the outstanding amounts borrowed under Labco’s revolving credit facility, pay the costs, fees and expenses in relation to the issuance transaction and for general corporate purposes. As a consequence Labco revolving credit facility amounting to 135 M€ will be fully reimbursed but not canceled and the unused portion of the proceeds of the Additional Senior Secured Notes will remain as cash and cash equivalent on our balance sheet. Prospective liquidity analysis at refinancing date taking into account balances for other financial debts and trade payables as at December 31, 2012 is as follows (a) Revolving Credit Facility amounting 135 M€ has been fully reimbursed with the proceed of the additional Notes issuance (RCF was drawn for an amount of 67 M€ as at additional Notes issuance date ie February 13, 2013). Amounts to be paid are estimated at refinancing date as the commitments fees paid on undrawn facility until early 2017 with a rate corresponding to 45% of margin (margin being estimated at 4.5%). Revolving Credit Facility will be however drawn for financing future acquisitions as soon as the excess cash obtained from additional €100 million Notes issuance would have been used. (b) Other financial debt corresponds to other loans and borrowings, finance lease liabilities, accrued HYB interests to be paid in January 2013 and factoring debt. Disposal of assets and acquisitions Some acquisitions have been performed in February and March 2013 for a total consideration of 5,2 M€. It corresponds mainly to 3 entities, or asset deals in France and to the acquisition of the 25% minority interests in the entity Hauts de Garonne. Detailed information on entities acquired could not be disclosed as requested by IFRS 3 (2008) given the recent closings and the time necessary to obtain accounts on closing date. Evolution of Dillenburg litigation Labco S.A. and its subsidiaries, Labco Deutschland and MVZ Medizinisches Fachlabor Dillenburg GmbH, have signed a settlement agreement on March 27, 2013 with the former vendors of our laboratory in Dillenburg in the context of the major litigation initiated given the alleged fraudulent activities, and related consequences. That agreement stipulates the payment of a total indemnity of 5,5 M€ by the former vendors of Dillenburg and the waiver of the litigations they had initiated against Labco and its subsidiaries. 321 Note 35 Auditor fees The amount of the signatory statutory auditors fees for the consolidated financial statements of Labco for the year 2012 for all the consolidated companies, where they are appointed is broken down as follow.. (a) including fees related to issuance of letters of comfort for the 100 M€ additional bond issuance operation 322 Financial Year ended December 31, 2013 Contents CONSOLIDATED STATEMENT OF INCOME 324 CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME 325 CONSOLIDATED STATEMENT OF FINANCIAL POSITION 326 CONSOLIDATED STATEMENT OF CHANGES IN EQUITY 327 CONSOLIDATED STATEMENT OF CASH FLOWS NOTE 1 NOTE 2 NOTE 3 NOTE 4 NOTE 5 NOTE 6 NOTE 7 NOTE 8 NOTE 9 NOTE 10 NOTE 11 NOTE 12 NOTE 13 NOTE 14 NOTE 15 NOTE 16 NOTE 17 NOTE 18 NOTE 19 NOTE 20 NOTE 21 NOTE 22 NOTE 23 NOTE 24 NOTE 25 NOTE 26 NOTE 27 NOTE 28 NOTE 29 NOTE 30 NOTE 31 NOTE 32 NOTE 33 NOTE 34 NOTE 35 NOTE 36 328 REPORTING ENTITY 329 BASIS OF PREPARATION 329 SIGNIFICANT ACCOUNTING POLICIES 333 FINANCIAL RISK MANAGEMENT 347 SIGNIFICANT EVENTS 350 ACQUISITIONS OF SUBSIDIARIES 353 SEGMENT INFORMATION 355 PAYROLL RELATED EXPENSES 356 OTHER OPERATING EXPENSES 357 NON RECURRING INCOME AND EXPENSES 357 NET FINANCE COSTS 358 INCOME TAX EXPENSES 359 GOODWILL 361 INTANGIBLE ASSETS 364 PROPERTY, PLANT AND EQUIPMENT 365 INVESTMENTS IN ASSOCIATES 367 OTHER NON-CURRENT ASSETS 369 DEFERRED TAX ASSETS AND LIABILITIES 370 INVENTORIES 370 TRADE RECEIVABLES AND OTHER CURRENT ASSETS 371 CASH AND CASH EQUIVALENTS 372 CAPITAL AND RESERVES ATTRIBUTABLE TO OWNERS OF THE PARENT 372 BORROWINGS AND OTHER FINANCIAL LIABILITIES 375 EMPLOYEE BENEFIT LIABILITIES 380 SHARE BASED PAYMENT SCHEMES 381 PROVISIONS 383 LITIGATIONS AND CONTINGENT LIABILITIES 384 TRADE AND OTHER LIABILITIES 386 FINANCIAL INSTRUMENTS 386 CAPITAL COMMITMENTS AND CONTINGENCIES 390 EARNINGS PER SHARE 397 RELATED PARTY TRANSACTIONS 398 ASSETS AND LIABILITIES HELD FOR SALE AND DISCONTINUED OPERATIONS 400 GROUP ENTITIES 402 EVENTS AFTER THE REPORTING PERIOD 405 AUDITOR FEES 405 323 Consolidated statement of income For the year ended 31 December 2013 324 Consolidated statement of comprehensive income For the year ended 31 December 2013 The accompanying notes are an integral part of the financial statements 325 Consolidated statement of financial position As at 31 December 2013 The accompanying notes are an integral part of the financial statements 326 Consolidated statement of changes in equity As at 31 December 2013 As at 31 December 2012 The accompanying notes are an integral part of these consolidated financial statements. 327 Consolidated statement of cash flows For the year ended 31 December 2013 The accompanying notes are an integral part of these consolidated financial statements. 328 Notes to the consolidated financial statements for the year ended 31 December 2013 Reporting entity Note 1 The parent company of Labco Group is Labco SA (the “Company”), which is a limited liability company incorporated and registered in France. The address of the Company’s registered office is 60 - 62, rue de Hauteville, 75010, Paris, France. The consolidated financial statements of the Company as at and for the year ended 31 December 2013 comprise the Company and its subsidiaries (together referred to as the “Group” and individually as “Group entities”) and the Group’s interest in associates. The Group primarily is involved in clinical diagnostics testing and screening services mainly in France, Spain, Portugal, Italy, Belgium, the United Kingdom and Switzerland and also provides clinical laboratory testing services to customers in Latin America, the Middle East and North Africa. Basis of preparation Note 2 2.1. Statement of compliance The consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (IFRSs), as adopted by the European Union (EU) and IFRS as published by IASB effective as at December 31, 2013. As a reminder, the Group’s consolidated financial statements have been prepared for the first time in 2010 in accordance with IFRSs, the opening IFRS balance sheet being prepared as of January 1, 2009. The accounting policies retained are the same as those used in preparing the consolidated financial statements at 31 December 2012, except for The Standards and Interpretations adopted by the European Union applicable as from 1 January 2013, which have no significant effect on the consolidated financial statements of the Group Certain Standards and Interpretations adopted by the European Union not mandatorily applicable as from 1 January 2013 but for which the Group has elected to implement early adoption and which have limited impact on the consolidated financial statements of the Group: o IFRS 10 – Consolidated Financial Statements, IFRS 11 – Joint Arrangements and IFRS 12 – Disclosures of Interests in Other Entities, as well as the resulting revised IAS 27 and IAS 28 The consolidated financial statements were authorised for issue by the Board of directors on March 31, 2014. 2.2. IFRS basis adopted 2.2.1. Standards, amendments and interpretations effective as of January 1, 2013 The Group’s consolidated financial statements comply with the amendments to published standards and interpretations which came into effect on January 1. 2013 and have been adopted by the European Union. The following amendments and interpretations are mandatorily applicable as of January 1. 2013: Amendment to IAS 1 –Presentation of Items of Other Comprehensive Income Amendment to IAS 19 – Employee Benefits These amendments were already early implemented by Labco for the annual consolidated financial statements as at 31 December 2012. 329 • IFRS 13 – Fair Value Measurement This standard is applicable prospectively and has no effect on the fair value currently measured by the Group. Amendment to IFRS 7 – Disclosures – Offsetting Financial Assets and Financial Liabilities Amendment to IAS 12 – Deferred Tax: Recovery of Underlying Assets These amendments have no significant impact on the Group consolidated financial statements. 2.2.2. Standards, amendments and interpretations not mandatorily applicable as of January 1, 2013 The Group has elected to early adopt the following standards or amendments whose application is not mandatory as of January 1, 2013: IFRS 10 Consolidated Financial Statements IFRS 10 replaces the requirements and guidance in IAS 27 relating to consolidated financial statements. This standard introduces a single consolidation framework for all types of investee entities, and gives a new definition of control. According to IFRS 10, an investor controls an investee when the investor is exposed, or has rights, to variable returns from its involvement with the investee and has the current ability to affect those returns through its power over the investee. The retrospective application of the standard on the Group’s consolidation scope has no effect on the Group’s comparative information reported in financial statements as at December 31, 2013. IFRS 11 Joint Arrangements IFRS 11, Joint Arrangements replaces IAS 31 Interests in joint venture and especially abolishes the proportionate integration method. Labco was in a joint arrangement through its subsidiary iPP in the United Kingdom, the joint venture with Sodexo. The retrospective application of the standard has no effect on the Group’s comparative information presentation because the jointly controlled entity iPP was already under IAS 31 recorded under equity method. Indeed the option, proposed by IAS 31, of using equity method for jointly controlled entity was chosen by Labco in 2011. IFRS 12, Disclosures of interest in other entities IFRS 12, Disclosures of interest in other entities, integrates and makes consistent the disclosure requirements for interests in subsidiaries, joint arrangements, associates, and unconsolidated structured entities and present those requirements in a single IFRS. 2.2.3. New standards, amendments and interpretations not applicable as of January 1. 2013 A number of new standards, amendments to standards and interpretations are not yet effective for the year ended 31 December 2013, and have not been applied in preparing these consolidated financial statements. IFRS 9 – Financial Instruments: Classification and Measurement Amendments to IFRS 7 – Financial Instruments: Disclosures and Amendments to IAS 32 - Financial Instruments: Presentation Amendments to IAS 36 – Recoverable Amount Disclosures for Non-Financial Assets Amendments to IAS 39 – Novation of Derivatives and Continuation of Hedge Accounting 330 IFRIC 21 – Levies The Group is currently reviewing these standards, amendments and interpretations to assess their possible effect on its financial information. 2.2.4. Summary of options used on the first time adoption of IFRS As a first time adopter in 2010, the opening IFRS balance sheet has been prepared as of January 1, 2009 (i.e. date of transition to IFRS) using IFRSs as adopted by the European Union effective December 31, 2010. In accordance with IFRS 1, the Group has elected to use the following main exemptions for the preparation of its first IFRS financial statements: business combinations that occurred before the date of transition to IFRS are not retrospectively restated in accordance with IFRS 3 – Business Combinations; the long term employee benefits have been fully recorded; for the share based payment transactions, only the 2008 scheme has been restated according to IFRS 2, and financial instruments held have all been classified as financial assets available for sale at the date of transition, with the exception of liabilities and receivables and trade receivables. 2.3. Basis of measurement The consolidated financial statements have been prepared on the historical cost basis except for the following items in the statement of financial position: derivative financial instruments are measured at fair value 2.4. certain long term financial assets are measured at fair value Functional and presentation currency These consolidated financial statements are presented in euro, which is the Company’s functional currency. All financial information presented in euro has been rounded to the nearest thousand. 2.5. Use of estimates and judgments The preparation of the consolidated financial statements in conformity with IFRSs requires management to make judgments, estimates and assumptions that affect the application of account
© Copyright 2026