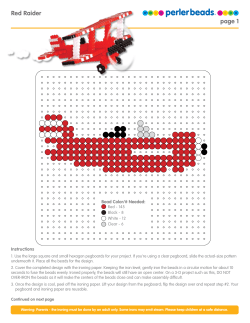
Morphology of polysaccharide beads and films for environmental
Morphology of polysaccharide beads and films for environmental and biomedical applications Mei Li1, Sarah Ziem2, Gisela Buschle-Diller1 1Department of Polymer and Fiber Engineering, Auburn University, Auburn, AL 2University of Applied Sciences, Reutlingen, Germany Introduction Experimental Methods Results and Discussion – Surface Morphology Polysaccharides are inexpensive and readily available worldwide from Additional filler, active agricultural and forest biomass[1]. These biopolymers can be formed into Polysaccharide solution compound, or drug hydrogels, beads, films and coatings and even into fibers and show incredible Beads were formed bywith cellulose Alginate versatility and potential for environmental and biomedical applications. They adding dissolved polysaccharide are used in food, cosmetic and pharmaceutical products as thickeners, into a crosslinking solution, films emulsifiers, binders, stabilizers and for drug encapsulation/delivery. by using a knife with defined Freshly made pectin beads Depending on the nature of their functional groups, they exhibit excellent distance to a glass plate. sorption capabilities. They can act as sorbents or as reservoirs for release of compounds. Especially useful are polysaccharide compounds with Crosslinking agent predictable and controlled swelling and discharge behavior. External and internal surfaces are important factors for the sorption capacity of a compound. Extensive studies were conducted using SEM to observe differences in surface morphology as it relates to composition and product formation. Pectin beads had smooth surfaces and a compact internal structure with small pores when air-dried (A), and rough external and flaky internal surfaces when freeze-dried (B). The addition of xanthan yields beads with cracked surfaces (C). B A C Project Goals The goal of the current work is to create a portfolio of polysaccharide beads and hydrogels with a wide variety of morphologies, porosities and other properties useful for filtering, delivering of active compounds, clean-up of oil and chemical spills, and similar applications. Alginate, pectin, chitosan, xanthan, and carrageenan are some of the basic polysaccharides currently being investigated and modified to serve a specific purpose in form of beads, hydrogels or electrospun into nanowebs. Their swelling ratios in water and aqueous solutions of selected drugs or dyes are being studied and their mechanical properties evaluated in relationship to their composition. The potential regeneration and reuse of the sorbent material is also being considered for highest efficiency in their respective application. The focus of the work presented here is mainly on product morphology and surface characteristics. In an aqueous environment some of these polysaccharides are polycationic, others polyanionic or neutral depending on the pH value. Alginate, obtained from brown algae and bacteria, for example, is a linear copolymer consisting of (1-4)-linked β-D-mannuronic acid (M) and α-L-guluronic acid (G) residues at [1,2] different ratios and distribution along the chains. Due to their acidic functional groups, alginate as well as xanthan can be very useful for binding of cationic compounds. Carrageenan from red seaweed contains ester-sulfate [1] groups. Crosslinking of these anionic polysaccharides can be achieved with 2+ 2+ positive ions to form films or beads, such as Ca and Zn or composite gels with a polycation, such as chitosan. HO O OH O O ONa ONa ONa HO O O HO O OH O OH O (MMM..) HO (GGG..) OH O HO HO O O HO OH O O HO OH CH2 OH CH2 OH CH2OH O O O OH OH O OH O Starch OH n Bead sizes: Composition and formation conditions had a large influence on the average diameter of beads and their surface morphology. Beads containing solid fillers, such as starch granules, were clearly bigger than their plain counterparts. All beads were rather large when freshly made, but never regained the same diameter after air or freeze drying. Pectin beads were irregular in shape and hard to crush, while alginate and xanthan formed perfectly round beads. Carrageenan beads showed the largest variation in bead sizes. They were the least stable of all samples upon drying.[3-5] w/v 2% alginate & starch 1% 1318.62 ±129.0 3% 1514.29 ±67.9 5% 2028.28 ±96.5 10% 2096.51 ±72.1 w/v 5% pectin 2% ZnAc wet 4271.16 ±32.4 air-dry 1710.16 ±3.8 2% alginate & cellulose 1275.91 ±69.3 1446.03 ±40.8 1857.46 ±61.2 2147.49 ±65.6 5% pectin 10% ZnAc 3984.94 ±21.9 1786.62 ±6.9 Electrospun fibers from alginate Pectin segment ...... OH OH O Surface Characteristics of Beads and Films COOCH3 O O HO crosslinking agents. Fillers or active compounds for release (drugs, dyes) were incorporated during the coagulation process. [3,4] Beads, films or fibers were formed and their morphology observed under the scanning electron microscope (SEM, Zeiss EVO 50). Bead diameter and size distribution were recorded Results and Discussion Beaddrying. formation after air or–freeze Swelling ratios and differences in sorption behavior were evaluated. Wet carrageenan beads and fibers NH2 COOCH3 O HO OH OH n O Chitosan O O HO NH2 COOH OH ONa n OH NH2 ...... (MGMG..) O m OH Alginate sodium OH O O ONa O HO O Wet carrageenan beads and Alginate wasaqueous dissolved in distilled water (2% w/v), pectin at 4 and 5% (w/v), fiber (2% solution) 1M and obtained xanthaninat 2%potassium (w/v) at room temperature; carrageenan was stirred into 1 Mchloride KCl solution at 70˚C. CaCl2, ZnCl2 and zinc acetate (ZnAc) served as Average diameter (mm) of beads with differing compositions OH O O Freshly made alginate-based beads OH 2.2 % alginate, electrospun Electrospinning of natural biopolymers, for example, alginate, can be difficult.[6] These nanofibers were electrospun from glycerol solution onto a metal target sprayed with CaCl2 solution 2.2 % alginate, electrospun and air-dried. Bead made from pectin Interior of pectin bead Beads form from pectin (5%) (5%), and crosslinked with (5%) containing 5% ascorbic and xanthan (2%) in ZnAc solution (10%); air-dried 5% ZnAc solution (air-dried) acid (freeze-dried) The surface of films made from xanthan solution differed from those of the beads (D). Alginate beads (E, F) were modified by adding solid fillers which changed they sorption behavior and impacted their surface morphology. D E F Film made from aqueous Beads from alginate (2%) Beads from alginate (2%) xanthan solution (2%) and and starch (10%) in CaCl2 and cellulose (10%) in CaCl2 solution; air-dried solution; air-dried ZnAc (5%); air-dried Conclusions Biopolymers from renewable resources, such as polysaccharides, offer endless opportunities for creating a toolbox of versatile materials. Beads, gels, fibers and films can be loaded with active compounds or used to absorb undesirable pollutants. Their surface characteristics and their porous system, among other factors, play an important role for their efficiency as environmental or biomedical devices and can be tailored by composition and method of synthesis. References 1. Habibi, Y., Lucia, L. (eds.): Polysaccharide Building Blocks. A Sustainable Approach to the Development of Renewable Biomaterials, Wiley, NY, 2012. 2. Mike, R., et al., Nanostructure of Calcium Alginate Aerogels Obtained from Multistep Solvent Exchange Route, Longmuir, 2008, 12547-12552 3. Li, M., Buschle-Diller, G., Polymeric functionalized beads from alginate for targeted release of auxin into water, 249th ACS Nat. Meeting & Exposition in Denver, CO, March 22-26, 2015. 4. Ziem, S., Li, M., Buschle-Diller, G., Drug delivery systems based on anionic polysaccharides, 247th ACS Nat. Meeting & Exposition, Dallas, TX, March 15-19, 2014. 5. Li, M., Buschle-Diller, G. Alginate - a promising polysaccharide for controlled contaminant removal from waste water, 247th ACS National Meeting & Exposition, Dallas, TX, March 15-19, 2014. 6. Alongi, R., Skinner, C., Hamilton, S.K., Buschle-Diller, G., Electrospinning of Biopolymer Network Structures, 247th ACS Nat. Meeting & Exposition, Dallas, TX, March 15-19, 2014.
© Copyright 2026