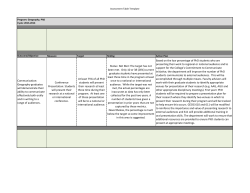
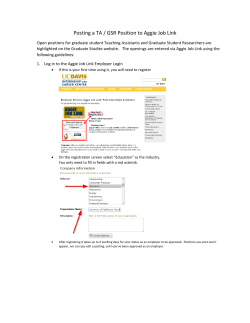
TURKU CENTRE FOR BIOTECHNOLOGY REPORT 2013
TURUN BIOTEKNIIKAN KESKUS ÅBO BIOTEKNIKCENTRUM TURKU CENTRE FOR BIOTECHNOLOGY TURKU CENTRE FOR BIOTECHNOLOGY REPORT 2013 TURUN BIOTEKNIIKAN KESKUS Tykistökatu 6 A P.O.BOX 123 FI 20521 Turku, Finland Tel: +358 2 333 8603, Fax +358 2 251 8808 Annual Report 2013 Turku Centre for Biotechnology Published by: Turku Centre for Biotechnology P.O. Box 123, FI-20521 Turku, Finland Tel. int. +358-2-333 8603, Fax int. +358-2-251 8808 http://www.btk.fi Editorial Board Riitta Lahesmaa (Chair) Daniel Abankwa Jane Zhi Chen Eleanor Coffey Garry Corthals Michael Courtney Laura Elo John Eriksson Attila Gyenesei David Hawkins Jyrki Heino Johanna Ivaska Panu Jaakkola Marko Kallio Linnéa Linko Harri Lähdesmäki Matti Nykter Matthias Nees Tassos Papageorgiou Jeroen Powels Cecilia Sahlgren Lea Sistonen Juha Strandén Mikael Wasberg Jukka Westermarck Andrey Zavialov Photographs: Photographs: KUV@TEHDAS Roni Lehti, Photograph archives of the Centre for Biotechnology. Front and back cover image: Reima Nurmikko. Painosalama Oy, Turku 2014 ISSN 1237-5217 CONTENTS Contents......................................................................................1 Organization.................................................................................2 Chairman’s Foreword ...................................................................3 From the Director .........................................................................4 Year 2013 in a Nutshell.................................................................5 Funding and Statistics .................................................................9 Publications 2013.......................................................................12 Personnel 2013..........................................................................19 The Finnish Microarray and Sequencing Centre..........................23 Cell Imaging Core.......................................................................27 Proteomics Facility......................................................................29 Protein Crystallography Core Facility ..........................................32 Bioinformatics Core....................................................................34 Virus Vector Facility.....................................................................36 Mechanisms and Biosensors of GTPases...................................38 Lymphocytes and Inflammation..................................................43 Protein Kinase Regulation of Brain Development and Disease....45 Translational Proteomics.............................................................51 Organisation of Neuronal Signaling Pathways.............................53 Computational Biomedicine........................................................57 Cytoskeletal and Survival Signaling.............................................60 Epigenomics...............................................................................67 Cell Adhesion and Cancer..........................................................69 Hypoxia in Cell Survival...............................................................73 Mitosis and Drug Discovery........................................................77 Molecular Systems Immunology and Stem Cell Biology..............79 Computational Systems Biology.................................................84 Cell Culture Models for Tumor Cell Invasion and Epithelial Plasticity.....................................................................................86 Computational Biology...............................................................90 Systems Medicine......................................................................92 Protein Crystallography...............................................................96 Integrin Activity in Disease........................................................102 Cell Fate...................................................................................106 Regulation and Function of Heat Shock Transcription Factors..111 Cancer Cell Signaling................................................................115 Adenosine Deaminases............................................................118 PhD Defences..........................................................................122 Life Outside the Lab.................................................................124 ORGANIZATION CHAIRMAN’S FOREWORD Board of Trustees 2013 Turku Centre for Biotechnology (CBT) is celebrating its 25th anniversary in the year 2014. When the decision to establish CBT was made in late ‘80s Turku looked very different to today. Before the first Science Park building, DataCity, was built in 1988 Kupittaa was an industrial area with warehouses, a large cattle yard belonging to a slaughterhouse and a sausage factory. A tall grain elevator dominated the scenery. The present day buildings with modern laboratories form quite a big contrast to that old picture and pronounce the restructuring of Finnish industry. Chairman HEINO Jyrki, Professor, University of Turku, Department of Biochemistry and Food Chemistry, Scientific Director, BioCity Turku Vice-chairman ERIKSSON John, Professor, Åbo Akademi University, Department of Biology Secretary LAHESMAA Riitta, Professor, Director, Turku Centre for Biotechnology Assistant Secretary ALANKO Satu, Coordinator, Turku Centre for Biotechnology and BioCity Turku Members HAAPALINNA Antti, Vice President, Research, R&D, Orion Corporation ORION PHARMA JALKANEN Sirpa, Professor, University of Turku, Department of Medical Microbiology and Immunology JOHNSON Mark, Professor, Åbo Akademi University, Department of Biosciences RÖNNEMAA Tapani, Professor, University of Turku, Dean of the Faculty of Medicine, SAARI Markku, Research coordinator, Turku Centre for Biotechnology SAVILAHTI Harri, Professor, University of Turku, Department of Biology SUOMINEN Kalle-Antti, Professor, Vice-Rector of University of Turku TÖRNQUIST Kid, Professor, Åbo Akademi University, Department of Biosciences WILLFÖR Stefan, Professor, Åbo Akademi University, Department of Chemical Engineering Vice-members FARDIM Pedro, Professor, Åbo Akademi University, Department of Chemical Engineering HUOVINEN Pentti, Professor, University of Turku, Department of Medical Microbiology and Immunology LAHTI Reijo, Professor, University of Turku, Dean of the Faculty of Mathematics and Natural Sciences PRIMMER Craig, Professor, University of Turku, Department of Biology ROKKA Anne, Research coordinator, Turku Centre for Biotechnology SCHLEUTKER Johanna, Professor, University of Turku, Department of Medical Biochemistry and Genetics SLOTTE J. Peter, Professor, Åbo Akademi University, Department of Biosciences VUORELA Pia, Professor, Åbo Akademi University, Department of Biosciences 2 During the past 25 years CBT has seen the ups and downs of Finnish life science research and high-tech. The ‘90s brought at first a deep economic depression but later a big increase in Finnish research and development funding. In life sciences it meant significant improvements in the infrastructure, the training of graduate students and the quality of science. The positive and optimistic atmosphere also initiated many translational projects, e.g. in drug development. Some of them have very recently achieved their goals. In the 2000s the development has been slower and in the global scale the relative impact of Finnish science has constantly decreased. When writing this, Finland’s economy has not grown for three years, which together with the implication of a full-cost model by the research funding agencies has significantly reduced the number of funded projects and weakened the future prospects of many scientists, especially the younger group leaders. Still many positive things have happened. The Academy of Finland has plans to increase its funding for research infrastructures and Turku researchers have actively participated in the establishment of European infrastructure networks. We can expect that also in the future Turku campus area will have up-to-date equipment and technologies available for life science research. CBT has during the past 25 years played a central role in the development of technology platforms and the important work will also continue in the future. Turku scientists have also been very successful in the tough national competition for the Academy of Finland Centres of Excellence and Academy Professor positions. The good quality of science can also be seen in the number of high-impact papers published by Turku researchers. From year 2004 the BioCity Turku organization has given the Elias Tillandz -prize for the best publication in Turku and the competition is clearly getting harder and harder every year. Many of the winners have worked in CBT, which tells that in addition to being a home for many important core laboratories, CBT has been able to provide good environment for scientific research. I want to congratulate and thank the director of CBT, its researchers and all personnel for the hard and successful work done during the past quarter of century and also to wish them the best of luck for the next 25 years. Jyrki Heino, MD, PhD, Professor of Biochemistry Scientific Director of the BioCity Turku and Chairman of the Board of the Turku Centre for Biotechnology 3 FROM THE DIRECTOR YEAR 2013 IN A NUTSHELL Our Centre had a productive and eventful year 2013. We once again exceeded our pre-agreed goals with altogether 10 PhDs completed and 68 publications produced during 2013. We also raised 6,2 M € in external competitive funding from the Academy of Finland, TEKES, Cancer Society of Finland, The Sigrid Jusélius Foundation, Juvenile Diabetes Research Foundation, EU and others. RESEARCH AND EDUCATION We recruited two new talented group leaders: Jeroen Powels, PhD, was appointed an Academy Fellow, a highly competitive position at the Academy of Finland, and Laura Elo-Uhlgrén got a competitive Juvenile Diabetes Research Foundation career development fiveyear grant to set up her independent group at the Centre. Riikka Lund was awarded the prestigious Millennium Distinction Award by Technology Academy Finland. Jyrki Heino’s and Riitta Lahesmaa’s groups shared the Tillandz prize for the best publications of the Biocity Community in 2012. Finnish Microarray and Sequencing Centre I am proud of our core facilities that continued to excel within the Biocenter Finland infrastructure network and, based on evaluation by external international experts, were able to raise significant external funding to further develop the services. The impact of Biocenter Finland networks is extraordinary as all the scientists working in Finland have full access to our cutting-edge technologies. Importantly, University of Turku decided to continue funding all CBT personnel who participate to Biocenter Finland core facilities. Likewise, Åbo Akademi University will continue to support these activities. We carried out a survey on well-being of our personnel and working groups were established to follow up and make proposals for improving practices facilitating well-being at work. All our group leaders have been encouraged to participate in an “Academic leadership” training program organized by the University of Turku. Based on our initiative, the program was organized for the first time in English and we are grateful for the University of Turku for this important contribution! This year, in 2014 CBT is celebrating its 25th Anniversary. During this time a large number of international scientists have been recruited, hundreds of young scientists have been trained and exciting research has been carried out. The state-of-the art core facilities and technology platforms have been established and continuously evaluated and updated to serve hundreds of users. Thus our key mission to promote cutting edge research has been fulfilled in many ways. CBT was established to centralize expensive technologies and equipment and to bring resources of the two universities in Turku together to enable cost efficient use of resources. This concept has been evaluated for many times during the years and without exception the CBT concept has always been fully endorsed and supported. Today, due to the current weak economic situation it is even more important to continue to make full and efficient use of our limited resources. Having sailed through storms and winds of change our CBT ship is now again getting wind in its sails and we are continuing full speed ahead! Thank you all CBT scientists and personnel – you are awesome! DEVELOPMENT OF INFRASTRUCTURE, RESEARCH SERVICES AND CORE FACILITIES • Altogether 6867 samples and 1256 plates were processed by FMSC for 276 different projects • Our services were used by 82 local, 20 other domestic, 6 foreign and 5 non-academic research groups or units • FMSC contributed into 41 publications • FMSC provided 12 lectures for undergraduate student or researchers, 4 public seminars or workshops and several tours into the FMSC facilities Proteomics and Mass spectrometry Laboratory • Petri Kouvonen returned from post-doctoral studies with R. Aebersold , Zürich • Dorota Muth-Pawlak finished her 2-year post. • Several courses and workshops organized at CBT: (1) Proteins at work course, (2) Basic proteomics course, (3) SRM workshop and (4) two Skyline workshops. • Organized 7th Summer School ‘Mass Spectrometry in Biotechnology and Medicine’ in Dubrovnik Cell Imaging Core • CIC continued as a key player in Light Microscopy of the Biocenter Finland National Infrastructure Network • Ketlin Adel was appointed as a full-time project engineer looking after flow cytometry • The Laser TIRF 3 from Zeiss was installed • Procurement procedures commenced for acquisition of a high-throughput flow cytometer commenced • CIC participated in the purchase of the IncuCyte ZOOM for live content imaging • CIC served ~300 users during 2013 from ~100 research groups. 12% of our user base comprised external users. • CIC provided over 100 hours teaching at national and international events. • The popular Lost in Imaging webinar series continued. Riitta Riitta Lahesmaa, MD, PhD, Professor Director Turku Centre for Biotechnology University of Turku and Åbo Akademi University 4 5 Viral vector facility • The viral vector facility overhauled its service charging system and started to provide user training as a charged service • Usage of the Biosafety Level 2 lab was added to the Asimov electronic reservation system to facilitate access campuswide • A new filtration system was purchased for the flow cytometry unit according to the guidelines of the Board for Gene Technology to enable safe sample analysis Bioinformatics Core Quality Assurance Unit • Organized courses for the university on (1) quality assurance and metrology and (2) how to assure the reliability of your laboratory test results • Individual training for graduate and post-graduate students • Supervising MSc- and PhD-theses • QA inspections for the Central Animal Laboratory and Forensic Medicine in GLP quality system • Internal audits of CBT • Linnéa Linko is a member in the Advisory Commission for Metrology and the chairman in its Education group as well as a member of The Eurachem Education and Training Working Group • High-throughput bioinformatics facility (HTB) analyzed data for 28 projects (21 local, 6 domestic, 1 international). • HTB continued the development of data analysis pipelines for various next-generation sequencing applications including RNA-seq, DNA methylation and targeted enrichment resequencing. •HTB started the work for setting up pipelines for metagenomics data analysis to support 16s rRNA and whole genome metagenome sequencing technologies. • New commercial software tools were acquired to support local users. Protein crystallography facility • Participation in several courses (Medical Biochemistry, TERBIO, Protein Crystallography and Structural Genomics’, Master’s degree program in Autonomous University of Barcelona) with lectures and demonstrations. Our course ‘Basic X-ray crystallography techniques: How to solve a protein structure’ was held in February-March. Several demonstrations to university and secondary school students. A 4-day course on protein complexes was organized together with the Proteomics facility. • Participation to several calls of Biocenter Finland, Finnish Roadmap and Infrastucture. Participation in a successful bid for a Finnish National Affiliated Center of INSTRUCT-Europe • The new X-ray generator run smoothly throughout the year. Several data sets were collected and structures deposited. The Oxford cryosystem was repumped several times owing to vacuum problems making a future replacement with a newer model an urgent matter. • New projects at various stages were initiated in collaboration with other groups in Finland and abroad. • All major crystallographic programs were kept updated to latest versions. 6 7 FUNDING AND STATISTICS PhD and MSc Theses PhD Theses (p. 12) Name Supervisor Site besides CBT Soile Tuomela Riitta Lahesmaa UTU, Institute of Biomedicine, Department of Medical Biochemistry and Genetics Nelly Rahkonen Riitta Lahesmaa, Riikka Lund UTU, Institute of Biomedicine, Department of Medical Microbiology and Immunology Minna Kyläniemi Riitta Lahesmaa UTU, Institute of Biomedicine, Department of Medical Biochemistry and Genetics Gunilla Högnäs Johanna Ivaska UTU, Institute of Biomedicine, Department of Medical Biochemistry and Genetics Antti Arjonen Johanna Ivaska UTU, Institute of Biomedicine, Department of Medical Biochemistry and Genetics Artur Padzik Eleanor Coffey ÅA, Department of Biosciences Anni Laine Jukka Westermarck UTU, Department of Pathology Mari Björkman Matthias Nees, Olli Kallioniemi UTU, Institute of Biomedicine, Department of Pharmacology Saima E. Ferraris John Eriksson ÅA, Department of Biosciences Tomoko Asaoka John Eriksson ÅA, Department of Biosciences Sources of funding received by Centre for Biotechnology in 2013 (12.6 Million €) External funding 2006-2013 MSc Theses Krista Maurinen Riitta Lahesmaa, Nelly University of Turku, Faculty of Rahkonen Medicine, Health Biosciences Johanna Myllyviita Riitta Lahesmaa, Minna Kyläniemi UTU, Department of Biochemistry Patrik Hollós Eleanor Coffey ÅA, Department of Biosciences Dani Flinkman Eleanor Coffey UTU, Department of Biochemistry Petra Miikkulainen Panu Jaakkola, Heidi Högel UTU, Institute of Biomedicine, Neeraj Prabhakar Cecilia Sahlgren ÅA, Department of Biosciences Sara Sarinko Cecilia Sahlgren ÅA, Department of Biosciences Rasmus Niemi Cecilia Sahlgren ÅA, Department of Biosciences Daniel Antfolk Cecilia Sahlgren ÅA, Department of Biosciences Iris Lahdeniemi Cecilia Sahlgren, Diana Toivola ÅA, Department of Biosciences Harri Santa Laura Elo, Olli Nevalainen UTU, Department of Information Technology Maria Jaakkola Laura Elo, Marko Mäkelä UTU, Department of Mathematics and Statistics Emine Lundsten Lea Sistonen ÅA, Department of Biosciences Alejandro Da Silva Lea Sistonen ÅA, Department of Biosciences Erik Niemelä John Eriksson ÅA, Department of Biosciences 8 9 Number of graduates 2006-2013 18 16 14 12 10 MSc 8 PhD 6 4 2 0 2006 2007 2008 2009 2010 2011 2012 2013 Publication impact factors 9 publications with IF > 10 19 publications with IF 5-10 40 publications with IF < 5 Citations each year to CBT publications From left to right. Upper row: Tuula Suvanto, Miina Nurmi, Riitta Lahesmaa, Pasi Viljakainen, Taina Kalevo-Mattila, Marjo Hakkarainen, Juha Strandén, Elina Pietilä, Susanna Pyökäri and Inga Pukonen. Bottom row: Petri Vahakoski, Mikael Wasberg, Tiina Lummevuo, Markku Saari, Sarita Heinonen, Linnéa Linko, Jouko Sandholm, Anne Lahdenperä and Mårten Hedman. 10 11 PUBLICATIONS 2013 PhD Theses 2013 1. Soile Tuomela: System-level characterization of TH2 cell development and immune cell responses to ZnO and TiO2 nanoparticles, University of Turku, p. 90. 6. Dirihan S, Terho P, Helander M, Saikkonen K (2013) Efficient analysis of ploidy levels in plant evolutionary ecology. Caryologia 66:251-256. IF 0.6 2. Nelly Rahkonen: Regulation of self-renewal and detection of karyotypic changes of pluripotent human embryonic stem cells. University of Turku, p. 60. 7. Eerola K, Nordlund W, Virtanen S, Dickens AM, Mattila M, Ruohonen ST, Chua SC, Jr., Wardlaw SL, Savontaus M, Savontaus E (2013) Lentivirus-Mediated alpha-MelanocyteStimulating Hormone Overexpression in the Hypothalamus Decreases Diet Induced Obesity in Mice. J Neuroendocrinol 25:1298-1307. IF 3.3 3. Minna Kyläniemi: Regulation of lymphocyte response in vitro and in vivo. University of Turku, p. 78. 4. Gunilla Högnäs: Integrins in Tumorigenesis and Cancer Cell Invasion. University of Turku, p. 104. 5. Antti Arjonen: Integrins on the move. University of Turku, p. 77. 6. Artur Padzik: JNK, a versatile regulator of kinesin-1 transport and cytoskeleton dynamics. Åbo Akademi University, p. 119. 7. Anni Laine: The Role of an Oncoprotein CIP2A in Breast Cancer, University of Turku, p. 138. 8. Saima E. Ferraris: Cellular responses to stress and kinase signaling activation: apoptosis and differentiation, Åbo Akademi University, p. 87. 9. Tomoko Asaoka: Regulation of cell fate by c-FLIP phosphorylation. Åbo Akademi University, p. 108. 10.Björkman, Mari: Identification of Epigenetic Targets in Prostate Cancer for Therapeutic Development. University of Turku. p. 62. Publications 2013 1. Abankwa D, Millard SM, Martel N, Choong CS, Yang M, Butler LM, Buchanan G, Tilley WD, Ueki N, Hayman MJ, Leong GM (2013) Ski-interacting protein (SKIP) interacts with androgen receptor in the nucleus and modulates androgen-dependent transcription. BMC Biochem. 14(1):10. IF 4.2 2. Battula P, Dubnovitsky AP, Papageorgiou AC (2013) Structural basis of L-phosphoserine binding to Bacillus alcalophilus phosphoserine aminotransferase. Acta Crystallogr Sect D-Biol Crystallogr 69:804-811. IF 14.1 3. Bouvard D, Pouwels J, De Franceschi N, Ivaska J (2013) Integrin inactivators: balancing cellular functions in vitro and in vivo. Nat Rev Mol Cell Biol 14:430-442. IF 37.2 4. Chen Z, Lonnberg T, Lahesmaa R (2013) Holistic Systems Biology Approaches to Molecular Mechanisms of Human Helper T Cell Differentiation to Functionally Distinct Subsets. Scand J Immunol 78:172-180. IF 2.2 12 5. Costa P, Scales TME, Ivaska J, Parsons M (2013) IntegrinSpecific Control of Focal Adhesion Kinase and RhoA Regulates Membrane Protrusion and Invasion. PLoS One 8:e74659. IF 3.7 8. Gardberg M, Kaipio K, Lehtinen L, Mikkonen P, Heuser VD, Talvinen K, Iljin K, Kampf C, Uhlen M, Grenman R, Koivisto M, Carpen O (2013) FHOD1, a Formin Upregulated in Epithelial-Mesenchymal Transition, Participates in Cancer Cell Migration and Invasion. PLoS One 8:e74923. IF 3.7 9. Haikarainen T, Frioux C, Zhnag LQ, Li DC, Papageorgiou AC (2013) Crystal structure and biochemical characterization of a manganese superoxide dismutase from Chaetomium thermophilum. Biochim Biophys Acta 1844:422-429. IF 3.8 10.Haikarainen T, Loimaranta V, Prieto-Lopez C, Battula P, Finne J, Papageorgiou AC (2013) Expression, purification and crystallization of the C-terminal LRR domain of Streptococcus pyogenes protein 0843. Acta Crystallogr F-Struct Biol Cryst Commun 69:559-561. IF 0.5 11.Hamalisto S, Pouwels J, de Franceschi N, Saari M, Ivarsson Y, Zimmermann P, Brech A, Stenmark H, Ivaska J (2013) A ZO-1/alpha 5 beta 1-Integrin Complex Regulates Cytokinesis Downstream of PKC epsilon in NCI-H460 Cells Plated on Fibronectin. PLoS One 8:e70696. IF 3.7 12.Hawkins RD, Larjo A, Tripathi SK, Wagner U, Luu Y, Lönnberg T, Raghav SK, Lee LK, Lund R, Ren B, Lähdesmäki H, Lahesmaa R. Global Chromatin State Analysis Reveals Lineage-Specific Enhancers during the Initiation of Human T helper 1 and T helper 2 Cell Polarization. Immunity. 2013, 38:1271-84. IF 19.8 13.Hognas G, Hamalisto S, Rilla K, Laine JO, Vilkki V, Murumagi A, Edgren H, Kallioniemi O, Ivaska J (2013) Aneuploidy facilitates oncogenic transformation via specific genetic alterations, including Twist2 upregulation. Carcinogenesis 34:2000-2009. IF 5.6 14.Jaakkola PM, Rantanen K (2013) The regulation, localization, and functions of oxygen-sensing prolyl hydroxylase PHD3. Biol Chem 394:449-457. IF 2.7 15.Jaaskelainen AE, Seppala S, Kakko T, Jaakkola U, Kallio J (2013) Systemic treatment with neuropeptide Y receptor Y1-antagonist enhances atherosclerosis and stimulates IL12 expression in ApoE deficient mice. Neuropeptides 47:6773. IF 2.1 13 16.Jin S, Mutvei AP, Chivukula IV, Andersson ER, Ramskold D, Sandberg R, Lee KL, Kronqvist P, Mamaeva V, Ostling P, Mpindi J, Kallioniemi O, Screpanti I, Poellinger L, Sahlgren C, Lendahl U (2013) Non-canonical Notch signaling activates IL-6/JAK/STAT signaling in breast tumor cells and is controlled by p53 and IKK alpha/IKKb. Oncogene 32:48924902. IF 7.3 17.Junttila S, Laiho A, Gyenesei A, Rudd S (2013) Whole transcriptome characterization of the effects of dehydration and rehydration on Cladonia rangiferina, the grey reindeer lichen. BMC Genomics 14:870-2164-14-870. IF 4.4 18.Kahara J, Lahdesmaki H (2013) Evaluating a linear k-mer model for protein-DNA interactions using high-throughput SELEX data. BMC Bioinformatics 14:S2. IF 3.0 19.Kalhori V, Kemppainen K, Asghar MY, Bergelin N, Jaakkola P, Tornquist K (2013) Sphingosine-1-Phosphate as a Regulator of Hypoxia-Induced Factor-1 alpha in Thyroid Follicular Carcinoma Cells. PLoS One 8:e66189. IF 3.7 20.Kallio A, Elo LL (2013) Optimizing Detection of Transcription Factor-Binding Sites in ChIP-seq Experiments. Methods Mol Biol 1038:181-91. IF 1.3 21.Kamalainen A, Viswanathan J, Natunen T, Helisalmi S, Kauppinen T, Pikkarainen M, Pursiheimo J, Alafuzoff I, Kivipelto M, Haapasalo A, Soininen H, Herukka S, Hiltunen M (2013) GRN Variant rs5848 Reduces Plasma and Brain Levels of Granulin in Alzheimer’s Disease Patients. J Alzheimers Dis 33:23-27. IF 4.2 22.Khanna A, Kauko O, Bockelman C, Laine A, Schreck I, Partanen JI, Szwajda A, Bormann S, Bilgen T, Helenius M, Pokharel YR, Pimanda J, Russel MR, Haglund C, Cole KA, Klefstrom J, Aittokallio T, Weiss C, Ristimaki A, Visakorpi T, Westermarck J (2013) Chk1 Targeting Reactivates PP2A Tumor Suppressor Activity in Cancer Cells. Cancer Res 73:6757-6769. IF 8.6 23.Khanna A, Pimanda JE, Westermarck J (2013) Cancerous Inhibitor of Protein Phosphatase 2A, an Emerging Human Oncoprotein and a Potential Cancer Therapy Target. Cancer Res 73:6548-6553. IF 8.6 24.Knuuti J, Saraste A, Kallio M, Minn H. (2013) Is cardiac magnetic resonance imaging causing DNA damage? Eur Heart J. 34(30):2337-9. IF 14.1 25.Koch S, Scifo E, Rokka A, Trippner P, Lindfors M, Korhonen R, Corthals GL, Virtanen I, Lalowski M, Tyynela J (2013) Cathepsin D deficiency induces cytoskeletal changes and affects cell migration pathways in the brain. Neurobiol Dis 50:107-119. IF 5.6 26.Kong L, Aho K, Granberg K, Lund R, Jarvenpaa L, Seppala J, Gokhale P, Leinonen K, Hahne L, Makela J, Laurila K, Pukkila H, Narva E, Yli-Harja O, Andrews PW, Nykter M, Lahesmaa R, Roos C, Autio R (2013) ESTOOLS Data@ Hand: human stem cell gene expression resource. Nat Methods 10:814-815. IF 23.6 14 27.Kong L, Tuomela S, Hahne L, Ahlfors H, Yli-Harja O, Fadeel B, Lahesmaa R, Autio R (2013) NanoMiner - Integrative Human Transcriptomics Data Resource for Nanoparticle Research. PLoS One 8:e68414. IF 3.7 28.Korhonen JT, Olkkonen VM, Lahesmaa R, Puolakkainen M (2013) ABC-cassette transporter 1 (ABCA1) expression in epithelial cells in Chlamydia pneumoniae infection. Microb Pathog 61-62:57-61. IF 2.0 29.Lahti L, Torrente A, Elo LL, Brazma A, Rung J (2013) A fully scalable online pre-processing algorithm for short oligonucleotide microarray atlases. Nucleic Acids Res 41:e110 IF 8.3 30.Laiho A, Kotaja N, Gyenesei A, Sironen A (2013) Transcriptome Profiling of the Murine Testis during the First Wave of Spermatogenesis. PLoS One 8:e61558. IF 3.7 31.Laine A, Sihto H, Come C, Rosenfeldt MT, Zwolinska A, Niemela M, Khanna A, Chan EK, Kahari V, KellokumpuLehtinen P, Sansom OJ, Evan GI, Junttila MR, Ryan KM, Marine J, Joensuu H, Westermarck J (2013) Senescence Sensitivity of Breast Cancer Cells Is Defined by Positive Feedback Loop between CIP2A and E2F1. Cancer Discov 3:182-197. IF 10.1 32.Lehtimaki S, Lahesmaa R (2013) Regulatory T Cells Control Immune Responses through Their Non-Redundant Tissue Specific Features. Front Immunol 4:294. 33.Lehtinen L, Ketola K, Makela R, Mpindi J, Viitala M, Kallioniemi O, Iljin K (2013) High-throughput RNAi screening for novel modulators of vimentin expression identifies MTHFD2 as a regulator of breast cancer cell migration and invasion. Oncotarget 4:48-63. IF 6.6 34.Li L, Ginet V, Liu X, Vergun O, Tuittila M, Mathieu M, Bonny C, Puyal J, Truttmann AC, Courtney MJ (2013) The nNOSp38MAPK Pathway Is Mediated by NOS1AP during Neuronal Death. J Neurosci 33:8185-8201. IF 6.9 35.Lonnberg T, Chen Z, Lahesmaa R (2013) From a genecentric to whole-proteome view of differentiation of T helper cell subsets. Brief Funct Genomics 12:471-482. IF 4.2 36.Lonnberg T, Yetukuri L, Seppanen-Laakso T, Lahesmaa R, Oresic M (2013) T-cell activation induces selective changes of cellular lipidome. Front Biosci (Elite Ed) 5:558-573. IF 3.5 37.Lund RJ, Emani MR, Barbaric I, Kivinen V, Jones M, Baker D, Gokhale P, Nykter M, Lahesmaa R, Andrews PW (2013) Karyotypically abnormal human ESCs are sensitive to HDAC inhibitors and show altered regulation of genes linked to cancers and neurological diseases. Stem Cell Res 11:10221036. IF 4.5 38.Mamaeva V, Sahlgren C, Linden M (2013) Mesoporous silica nanoparticles in medicine-Recent advances. Adv Drug Deliv Rev 65:689-702. IF 12.9 15 39.Mohazab L, Koivisto L, Jiang G, Kytomaki L, Haapasalo M, Owen GR, Wiebe C, Xie Y, Heikinheimo K, Yoshida T, Smith CE, Heino J, Haekkinen L, McKee MD, Larjava H (2013) Critical role for alpha v beta 6 integrin in enamel biomineralization. J Cell Sci 126:732-744. IF 5.9 49.Pouwels J, De Franceschi N, Rantakari P, Auvinen K, Karikoski M, Mattila E, Potter C, Sundberg JP, Hogg N, Gahmberg CG, Salmi M, Ivaska J (2013) SHARPIN Regulates Uropod Detachment in Migrating Lymphocytes. Cell Reports 5:619-628. IF 7.3 40.Najumudeen AK, Kohnke M, Solman M, Alexandrov K, Abankwa D (2013) Cellular FRET-Biosensors to Detect Membrane Targeting Inhibitors of N-Myristoylated Proteins. PLoS One 8:e66425. IF 3.7 50.Prabhakar N, Nareoja T, von Haartman E, Sen Karaman D, Jiang H, Koho S, Dolenko TA, Hanninen PE, Vlasov DI, Ralchenko VG, Hosomi S, Vlasov II, Sahlgren C, Rosenholm JM (2013) Core-shell designs of photoluminescent nanodiamonds with porous silica coatings for bioimaging and drug delivery II: application. Nanoscale 5:3713-3722. IF 6.2 41.Narva E, Pursiheimo J, Laiho A, Rahkonen N, Emani MR, Viitala M, Laurila K, Sahla R, Lund R, Lahdesmaki H, Jaakkola P, Lahesmaa R (2013) Continuous Hypoxic Culturing of Human Embryonic Stem Cells Enhances SSEA-3 and MYC Levels. PLoS One 8:e78847. IF 3.7 42.Narvi E, Jaakkola K, Winsel S, Oetken-Lindholm C, Halonen P, Kallio L, Kallio MJ (2013) Altered TUBB3 expression contributes to the epothilone response of mitotic cells. Br J Cancer 108:82-90. IF 5.3 43.Natunen T, Martiskainen H, Sarajarvi T, Helisalmi S, Pursiheimo J, Viswanathan J, Laitinen M, Makinen P, Kauppinen T, Rauramaa T, Leinonen V, Alafuzoff I, Haapasalo A, Soininen H, Hiltunen M (2013) Effects of NR1H3 Genetic Variation on the Expression of Liver X Receptor alpha and the Progression of Alzheimer’s Disease. PLoS One 8:e80700. IF 3.7 44.Natunen T, Parrado AR, Helisalmi S, Pursiheimo J, Sarajarvi T, Makinen P, Kurkinen KMA, Mullin K, Alafuzoff I, Haapasalo A, Bertram L, Soininen H, Tanzi RE, Hiltunen M (2013) Elucidation of the BACE1 Regulating Factor GGA3 in Alzheimer’s Disease. J Alzheimers Dis 37:217-232. IF 4.2 45.Nikula T, Mykkanen J, Simell O, Lahesmaa R (2013) Genome-wide comparison of two RNA-stabilizing reagents for transcriptional profiling of peripheral blood. Transl Res 161:181-188. IF 3.5 46.Nwaru BI, Takkinen H, Niemela O, Kaila M, Erkkola M, Ahonen S, Haapala A, Kenward MG, Pekkanen J, Lahesmaa R, Kere J, Simell O, Veijola R, Ilonen J, Hyoty H, Knip M, Virtanen SM (2013) Timing of infant feeding in relation to childhood asthma and allergic diseases. J Allergy Clin Immunol 131:78-86. IF 12.0 47.Nwaru BI, Takkinen H-, Niemela O, Kaila M, Erkkola M, Ahonen S, Tuomi H, Haapala A-, Kenward MG, Pekkanen J, Lahesmaa R, Kere J, Simell O, Veijola R, Ilonen J, Hyoty H, Knip M, Virtanen SM (2013) Introduction of complementary foods in infancy and atopic sensitization at the age of 5 years: timing and food diversity in a Finnish birth cohort. Allergy 68:507-516. IF 5.9 48.Peuhu E, Paul P, Remes M, Holmbom T, Eklund P, Sjoholm R, Eriksson JE (2013) The antitumor lignan Nortrachelogenin sensitizes prostate cancer cells to TRAIL-induced cell death by inhibition of the Akt pathway and growth factor signaling. Biochem Pharmacol 86:571-583. IF 4.6 16 51.Rantanen K, Pursiheimo J, Hogel H, Miikkulainen P, Sundstrom J, Jaakkola PM (2013) p62/SQSTM1 regulates cellular oxygen sensing by attenuating PHD3 activity through aggregate sequestration and enhanced degradation. J Cell Sci 126:1144-1154. IF 5.9 52.Sainio A, Nyman M, Lund R, Vuorikoski S, Bostrom P, Laato M, Bostrom PJ, Jarvelainen H (2013) Lack of Decorin Expression by Human Bladder Cancer Cells Offers New Tools in the Therapy of Urothelial Malignancies. PLoS One 8:e76190. IF 3.7 53.Salmela A, Pouwels J, Maki-Jouppila J, Kohonen P, Toivonen P, Kallio L, Kallio M (2013) Novel pyrimidine-2,4diamine derivative suppresses the cell viability and spindle assembly checkpoint activity by targeting Aurora kinases. Carcinogenesis 34:436-445. IF 5.6 54.Santos HM, Kouvonen P, Capelo J, Corthals GL (2013) Ontarget ultrasonic digestion of proteins. Proteomics 13:14231427. IF 4.1 55.Sarek G, Ma L, Enback J, Jarviluoma A, Moreau P, Haas J, Gessain A, Koskinen PJ, Laakkonen P, Ojala PM (2013) Kaposi’s sarcoma herpesvirus lytic replication compromises apoptotic response to p53 reactivation in virus-induced lymphomas. Oncogene 32:1091-1098. IF 7.3 56.Tahvanainen J, Kylaniemi MK, Kanduri K, Gupta B, Lahteenmaki H, Kallonen T, Rajavuori A, Rasool O, Koskinen PJ, Rao KVS, Lahdesmaki H, Lahesmaa R (2013) Proviral Integration Site for Moloney Murine Leukemia Virus (PIM) Kinases Promote Human T Helper 1 Cell Differentiation. J Biol Chem 288:3048-3058. IF 4.6 57.Teittinen KJ, Laiho A, Uusimaki A, Pursiheimo J, Gyenesei A, Lohi O (2013) Expression of small nucleolar RNAs in leukemic cells. Cell Oncol 36:55-63. IF 2.4 58.Tuomela J, Sandholm J, Kauppila JH, Lehenkari P, Harris KW, Selander KS (2013) Chloroquine has tumor-inhibitory and tumor-promoting effects in triple-negative breast cancer. Oncol Lett. Dec;6(6):1665-1672. IF 0.2 59.Tuomela S, Autio R, Buerki-Thurnherr T, Arslan O, Kunzmann A, Andersson-Willman B, Wick P, Mathur S, Scheynius A, Krug HF, Fadeel B, Lahesmaa R (2013) Gene Expression Profiling of Immune-Competent Human Cells Exposed to 17 Engineered Zinc Oxide or Titanium Dioxide Nanoparticles. PLoS One 8:e68415. IF 3.7 60.Tuomela S, Lahesmaa R (2013) Early T helper cell programming of gene expression in human. Semin Immunol 25:282-290. IF 5.9 61.Tyagarajan SK, Ghosh H, Yevenes GE, Imanishi SY, Zeilhofer HU, Gerrits B, Fritschy J (2013) Extracellular Signal-regulated Kinase and Glycogen Synthase Kinase 3 beta Regulate Gephyrin Postsynaptic Aggregation and GABAergic Synaptic Function in a Calpain-dependent Mechanism. J Biol Chem 288:9634-9647. IF 4.8 62.Velasquez EV, Rios M, Elena Ortiz M, Lizama C, Nunez E, Abramovich D, Orge F, Oliva B, Orellana R, Villalon M, Moreno RD, Tesone M, Rokka A, Corthals G, Croxatto HB, Parborell F, Owen GI (2013) Concanavalin-A Induces Granulosa Cell Death and Inhibits FSH-Mediated Follicular Growth and Ovarian Maturation in Female Rats. Endocrinology 154:1885-1896. IF 4.7 63.Vihervaara A, Sergelius C, Vasara J, Blom MAH, Elsing AN, Roos-Mattjus P, Sistonen L (2013) Transcriptional response to stress in the dynamic chromatin environment of cycling and mitotic cells. Proc Natl Acad Sci U S A 110:E3388-E3397. IF 9.7 64.Vuoristo S, Toivonen S, Weltner J, Mikkola M, Ustinov J, Trokovic R, Palgi J, Lund R, Tuuri T, Otonkoski T (2013) A Novel Feeder-Free Culture System for Human Pluripotent Stem Cell Culture and Induced Pluripotent Stem Cell Derivation. PLoS One 8. IF 3.7 65.Wei W, Gyenesei A, Semple CAM, Haley CS (2013) Properties of Local Interactions and Their Potential Value in Complementing Genome-Wide Association Studies. PLoS One 8:e71203. IF 3.7 66.Westermarck J, Ivaska J, Corthals GL (2013) Identification of Protein Interactions Involved in Cellular Signaling. Mol Cell Proteomics 12:1752-1763. IF 7.2 67.Wittig R, Rosenholm JM, von Haartman E, Hemming J, Genze F, Bergman L, Simmet T, Lindén M, Sahlgren C. Active targeting of mesoporous silica drug carriers enhances gamma-secretase inhibitor efficacy in an in vivo model for breast cancer. Nanomedicine (Lond) (2013). IF 5.3 68.Ylilauri M, Mattila E, Nurminen EM, Kapyla J, Niinivehmas SP, Maatta JA, Pentikainen U, Ivaska J, Pentikainen OT (2013) Molecular mechanism of T-cell protein tyrosine phosphatase (TCPTP) activation by mitoxantrone. BBAProteins and Proteomics 1834:1988-1997. IF 3.7 PERSONNEL 2013 Administration LAHESMAA Riitta, Director, Professor, Group Leader COFFEY Eleanor, Vice-Director, Adjunct Professor, Group Leader ALANKO Satu, Coordinator GRÖNROOS Sirkku, Administrative Planning Officer HIRVENSALO Eva, Clerical Official (on leave) JASMAVAARA Aila, Clerical Official LAHDENPERÄ Anne, Coordinator LUMMEVUO Tiina, Clerical Official SUVANTO Tuula, Project Secretary BioCity Turku HEINO Jyrki, Biocity Turku Scientific Director, Professor HEINO Ilona, Student ALANKO Satu, Coordinator Technical Staff HEDMAN Mårten, Systems Manager KORPIRANTA Virpi, Instrument Maintenance STRANDÉN Juha, Laboratory Engineer VAHAKOSKI Petri, Systems Manager VILJAKAINEN Pasi, Senior Technician VUORI Hannele, Instrument Maintenance WASBERG Mikael, Laboratory Manager Finnish Microarray and Sequencing Centre FEZAZI Bogata, Laboratory Technician GYENESEI Attila, Head of bioinformatics GHIMIRE Bishwa, IT Designer HAWKINS David, Group Leader LUND Riikka, Head of Laboratory PURSIHEIMO Juha-Pekka, Senior Scientist JUNNI Päivi, Laboratory Technician JUNTTILA Sini, Project Engineer KAUKO Leni, Researcher KONKI Mikko, Research Assistant KYTÖMÄKI Leena, Project Engineer LAIHO Asta, Acting head of bioinformatics MÄKELÄ Ville-Veikko, Research Technician NURMI Miina, Laboratory Technician PIETILÄ Sami, IT-designer RISSANEN Oso, Laboratory Technician SOIDINSALO Pasi, Undergraduate student VENHO Reija, Laboratory Technician VIRTANEN Eveliina, Project Engineer VUORIKOSKI Sanna, Researcher Cell Imaging Core COFFEY Eleanor, Adjunct Professor, Head of the Cell Imaging Core KANKAANPÄÄ Pasi, Coordinator of the Cell Imaging Core (January–June) ADEL Ketlin, Project Engineer 18 SANDHOLM Jouko, Research Coordinator SAARI Markku, Research Coordinator PALANI Senthil, CIC Part-time Technical Support Proteomics Facility CORTHALS Garry, Group Leader, Head of Proteomics HAAPANIEMI Pekka, Laboratory Technician HEINONEN Arttu, Laboratory Engineer IMANISHI Susumu, Senior Scientist KOUVONEN Petri, Senior Scientist MUTH-PAWLAK Dorotha, Senior Scientist ROKKA Anne, Senior Scientist Protein Crystallography Core PAPAGEORGIOU Tassos, Group Leader, Adjunct Professor HEDMAN Mårten, Systems Manager STRANDÉN Juha, Laboratory Engineer VAHAKOSKI Petri, Systems Manager VILJAKAINEN Pasi, Senior Technician Bioinformatics core DENESSIOUK Konstantin, Group Leader (Structural Bioinformatics) GYENESEI Attila, Senior Scientist (High-throughput Bioinformatics) CHOUHAN Bhanupratap Singh, Graduate Student GHIMIRE Bishwa, Undergraduate Student JUNTTILA Sini, Graduate Student KYTÖMÄKI Leena, Project Engineer LAIHO Asta, Project Engineer PIETILÄ Sami, Technical Team Officer Virus Vector facility COFFEY Eleanor, Group Leader, Coordinator ADEL Ketlin, Laboratory Technician LINKO Linnéa, Quality Assurance Officer Mechanisms and Biosensors of GTPases ABANKWA Daniel, Group Leader, Academy of Finland Research Fellow BLAZEVICS Olga, Post-doctoral Fellow GUZMAN Camilo, Post-doctoral Fellow LIGABUE Alessio, Post-doctoral Fellow NAJUMUDEEN Arafath, Graduate Student OETKEN-LINDHOLM Christina, Postdoctoral Fellow SILJAMÄKI Elina, Post-doctoral Fellow SOLMAN Maja, Graduate Student Lymphocyts and Inflammation CHEN Zhi Jane, MD, PhD, Group Leader, Academy of Finland Research Fellow CSENDES Brigitta, Graduate Student KHAN Mohd Moin, Graduate Student MOHAMMAD Imran Ahammad, Undergraduate Student 19 Protein Kinase Regulation of Brain Development and Disease COFFEY Eleanor, Group Leader, Academy of Finland Research Fellow DESHPANDE Prasannakumar, Graduate Student FLINKMAN Dani, Graduate Student FREEMANTLE Erika, Post-Doctoral Fellow HOLLOS Patrik, Graduate Student KOMULAINEN Emilia, Graduate Student MARCHISELLA, Francesca, Graduate Student MISIN Olavi, Undergraduate Student MOHAMMAD Hasan, Graduate Student PADZIK Artur, Post-Doctoral Fellow PYÖKÄRI Susanna, Laboratory Manager VARIDAKI, Artemis, Graduate Student ZDROJEWSKA Justyna, Graduate Student Translational Proteomics CORTHALS Garry, Group Leader, Head of Proteomics HAAPANIEMI Pekka, Laboratory Technician HEINONEN Arttu, Laboratory Engineer IMANISHI Susumu, Senior Scientist KOTTAHACHCHI Darshana, Graduate Student KOUVONEN Petri, Senior Scientist LUKASH Tanya, Senior Scientist MUTH-PAWLAK Dorotha, Senior Scientist NEES Susanne, Coordinator NGUYEN Mimi, Senior Scientist ROKKA Anne, Senior Scientist SUNI Veronika, Graduate Student VEHMAS Anni, Graduate Student Organisation of Neuronal Signaling Pathways COURTNEY Michael, Group Leader, Professor LI Lili, Graduate Student MARTINSSON Peter, Post-doctoral Fellow MELERO FERNANDEZ-DE MERA Raquel NÄRVÄINEN Jarkko, Integration Specialist SEPPÄNEN Aila, Laboratory Technician VERGUN Olga, Post-doctoral Fellow WANG Xijun, Graduate Student Computational Biomedicine ELO Laura, Group Leader, Adjunct Professor AFZAL Saira, Graduate Student CHAKROBORTY Deepankar, Undergraduate student GHIMIRE Bishwa, Graduate Student JAAKKOLA Maria, Graduate Student KOSKINEN Anna, Undergraduate Student LAAJALA Teemu Daniel, Graduate Student LAIHO Asta, Graduate Student LE THI THANH An, Post-doctoral Fellow 20 LEHTINEN Oona, Undergraduate student MAHMOUDIAN Mehrad, Undergraduate student PURSIHEIMO Anna, Graduate Student RANNIKKO Sami, Undergraduate Student SANTOS Rafael, Undergraduate student SEYEDNASROLLAH Fatemehsadat, Undergraduate Student SINGARAVELU Kalaimathy, Graduate Student SUOMI Tomi, Graduate Student Cytoskeletal and Survival Signaling ERIKSSON John, Group Leader, Professor CHENG Fang, Post-doctoral Fellow GULLMETS Josef, Graduate Student HYDER Claire, Post-doctoral Fellow JOKO Alia, Graduate Student LINDQVIST Julia, Graduate Student LINDSTRÖM Michelle, Undergraduate Student MOGOLLON Isabel, Undergraduate Student MOHANASUNDARAM Ponnuswamy, Graduate Student NIEMELÄ Erik, Graduate Student ISONIEMI Kimmo, Graduate Student JOKO Alia, Graduate Student PAUL Preethy, Post-doctoral Fellow PYLVÄNÄINEN Joanna, Graduate Student RAJENDRAN Senthil Kumar, Postdoctoral Fellow SAARENTO Helena, Research Associate TORVALDSON Elin, Graduate student WIKSTRÖM Vilhelm, Undergraduate Student WISTBACKA Num, Undergraduate Student ÖRN Fanny, Undergraduate Student Epigenomics HAWKINS David, Group Leader PASUMARTHY Kalyan Kumar, Postdoctoral Fellow VALENSISI Cristina, Post-doctoral Fellow Cell Adhesion and Cancer IVASKA Johanna, Professor, Group Leader ALANKO Jonna, Graduate Student ARJONEN Antti, Post-doctoral Fellow DE FRANCESCHI Nicola, Graduate Student GEORGIADOU Maria, Post-doctoral Fellow KAUKONEN Riina, Graduate Student LAASOLA Petra, Technician LILJA Johanna, Undergraduate Student NÄRVÄ Elisa, Post-doctoral Fellow PEUHU Emilia, Post-doctoral Fellow SAARI Markku, Research assistant SAHGAL Pranshu, Graduate Student SIIVONEN Jenni, Technician VIRTAKOIVU Reetta, Graduate Student Hypoxia in Cell Survival JAAKKOLA Panu, Group Leader HEIKKINEN Pekka, Graduate Student HÖGEL Heidi, Graduate Student KALEVO-MATTILA Taina, Technician MIIKKULAINEN Petra, Graduate Student RANTANEN Krista, Post-doctoral Fellow SILÉN Jonna, Graduate Student Mitosis and drug discovery KALLIO Marko, Group Leader, Adjunct Professor LAINE Leena, Post-doctoral Fellow MÄKI-JOUPPILA Jenni, Graduate Student NARVI Elli, Post-doctoral Fellow PRUIKKONEN, Sofia, Graduate Student TAMBE Mahesh, Graduate Student Molecular Systems Immunology and Stem Cell Biology LAHESMAA Riitta, Director, Professor, Group Leader BHOSALE Santosh, Graduate Student DIRASANTHA Obaiah, Undergraduate Student EDELMAN Sanna, Post-doctoral Fellow GODLETT David R., Visiting Professor HAKKARAINEN Marjo, Laboratory Technician HEINONEN Mirkka, Graduate Student HEINONEN Sarita, Laboratory Technician HURME Antti, Undergraduate Student HÄMÄLISTÖ Saara, Post-doctoral Fellow JALONEN Jussi, Undergraduate Student JUNNI Päivi, Laboratory Technician KALLIONPÄÄ Henna, Graduate Student KANDURI Kartiek, Graduate Student KHAN MOHN Moin, Graduate Student KOSOLA Sakari, Undergraduate Student LAAJALA Essi, Graduate Student LAHDENPERÄ Anne, Laboratory Manager LEHTIMÄKI Sari, Post-doctoral Fellow LIETZEN Niina, Post-doctoral Fellow LUND Riikka, Senior Scientist MOULDER Robert, Senior Scientist NGYEN Elizabeth, Post-doctoral Fellow PIETILÄ Elina, Laboratory Technician RAHKONEN Nelly, Post-doctoral Fellow RAO Anjana, Visiting Professor RAO Kanury, Visiting Professor RASOOL Omid, Adjunct Professor, Senior Scientist REDDY Maheswara Emani, Postdoctoral Fellow SALMI Jussi, Senior scientist SALO Verna, Graduate Student STOCKINGER Brigitta, Visiting Professor STUBB Aki, Undergraduate Student TRIPATHI Subhash, Graduate Student TUOMELA Soile, Post-doctoral Fellow ULLAH Ubaid, Post-doctoral Fellow VIITALA Miro, Undergraduate Student WIJMENGA Cisca, Visiting Professor ÖLING Viveka, Post-doctoral Fellow Quality Assurance Unit LINKO Linnéa, Group Leader, Adjunct Professor Computational Systems Biology LÄHDESMÄKI Harri, Group Leader, Professor BANAFSHEH Khakipoor, Undergraduate Student BASAK Eraslan, Undergraduate Student GÖKCEN Eraslan, Undergraduate Student INTOSALMI Jukka, Post-doctoral Fellow KANDURI Kartiek, Graduate Student KÄHÄRÄ Juhani, Undergraduate Student LAAJALA Essi, Graduate Student LARJO Antti, Graduate Student MALONZO Maia, Graduate Student MANNERSTRÖM Henrik, Graduate Student NOUSIAINEN Kari, Graduate Student OSMALA Maria, Graduate Student RAUTIO Sini, Graduate Student SOMANI Juhi, Graduate Student VATANEN Tommi, Graduate Student ÄIJÖ Tarmo, Graduate Student Cell Culture Models For Tumor Cell Invasion and Epithelial Platicity NEES Matthias, Group Leader AHONEN Ilmari, Graduate Student HÄRMÄ Ville, Post-doctoral Fellow MISHRA Mrinal, Undergraduate Student ROBINSON Sean, Graduate Student SCHUKOV Hannu-Pekka, Undergraduate Student TOIVONEN Pauliina, Laboratory technician TORISEVA Mervi, Post-doctoral Fellow VIRTANEN Johannes, Laboratory technician ÅKERFELT Malin, Post-doctoral Fellow Computational Biology NYKTER Matti, Group Leader ANNALA Matti, Graduate Student GRANBERG Kirsi, Post-doctoral Fellow HÄYRYNEN Sergei, Undergraduate Student KESSELI Juha, Post-doctoral Fellow KIVINUMMI Kati, Post-doctoral Fellow KYTÖLÄ Ville,Undergraduate Student LAAKSONEN Maria, Undergraduate Student LEHTINEN Birgitta, Undergraduate Student LIUKSIALA Thomas, Undergraduate Student RANTAPERO Tommi, Graduate Student RUUSUVUORI Pekka, Post-doctoral Fellow TABARO Francesco, Graduate Student YLIPÄÄ Antti, Graduate Student 21 Systems Medicine OREŠICˇ Matej, Group Leader Tuulia Hyötyläinen, Senior Scientist Protein Crystallography PAPAGEORGIOU Tassos, Group Leader, Adjunct Professor AMODA Adeleke, Undergraduate Student BATTULA Pradeep, Graduate Student BHADARI Sagar, MSc Student LASCORZ Marta, Visiting Scientist MULETA Abdi, Graduate Student POUDEL Nirmal, MSc Student SEID Amin, MSc Student SONKAR Kirti, Visiting Scientist SUBEDI Bishwa, Graduate Student Integrin activity in disease POUWELS Jeroen, Group Leader, Adjunct Professor KHAN Meraj Hasan, Graduate Student SKALDIN Maksym, Graduate Student Cell fate SAHLGREN Cecilia, Group Leader ANTFOLK Daniel, Graduate Student ANTILA Christian, Graduate Student LANDOR Sebastian, Graduate Student LERCHE Martina, Undergraduate Student MAMAEVA Veronika, Post-doctoral Fellow NIEMI Rasmus, Graduate Student NIINIMAKI Jenni, Undergraduate Student PARAMANOV Valeriy, Graduate Student PRABHAKAR Neeraj, Graduate Student RÅTTS Natalie, Laboratory Technician SJÖQVIST Marika, Graduate Student Regulation and Function of Heat Shock Transcription Factors SISTONEN Lea, Group Leader, Professor ASPELIN Camilla, Graduate Student BERGMAN Heidi, Graduate Student BJÖRK Johanna, Post-doctoral Fellow BLOM Malin, Undergraduate Student BUDZYNSKI Marek, Graduate Student DA SILVA Alejandro, Undergraduate Student ELSING Alexandra, Graduate Student ESTRADA Marianna, Undergraduate Student HENRIKSSON Eva, Senior Scientist HIMANEN Samu, Undergraduate Student JENSEN Maria, Undergraduate Student JOUTSEN Jenny, Graduate Student LUNDSTEN Emine, Graduate Student LUOTO Jens, Undergraduate Student LUSTIG Heidi, Undergraduate Student ORASNIEMI Satu, Undergraduate Student PUUSTINEN Mikael, Undergraduate Student ROOS-MATTJUS Pia, Senior Scientist SAARENTO Helena, Research Assistant SANDQVIST Anton, Post-doctoral Fellow VAINIO Petra, Graduate Student VIHERVAARA Anniina, Graduate Student Cancer Cell Signaling WESTERMARCK Jukka, Group Leader, Professor ARSIOLA Tiina, Head of Laboratory KALEVO-MATTILA Taina, Laboratory Technician KAUKO Otto, Graduate Student KAUR Amanpreet, Graduate Student LAINE Anni, Post-doctoral Fellow LIPSANEN Anna, Post-doctoral Fellow OKKERI Juha, Post-doctoral Fellow PUKONEN Inga, Laboratory Technician RUPP Christian, Post-doctoral Fellow SITTIG Eleonora, Graduate Student QIAO Xi, Graduate Student Adenosine Deaminases ZAVIALOV Andrey, Group Leader, Academy of Finland Research Fellow LIU Chengquian, Graduate Student MUKIIENKO Yuliia, Graduate Student RAI Balwant, Graduate Student SKALDIN Maksym, Graduate Student THE FINNISH MICROARRAY AND SEQUENCING CENTRE http://fmsc.btk.fi Contact details: Turku Centre for Biotechnology, BioCity, Tykistökatu 6A, P.O. Box 123, FIN-2050 Turku, Finland. Tel. +358-2-333 7697 Fax +358-2-251 8808. Email: [email protected] Heads/Coordinator Prof. Riitta Lahesmaa Dr. Attila Gyenesei (Head of bioinformatics) MSc Asta Laiho (Acting head of bioinformatics) Dr. Riikka Lund (Head of laboratory) Dr. Juha-Pekka Pursiheimo Technical Team Bishwa Ghimire, Bogata Fezazi, Päivi Junni, Sini Junttila, Leni Kauko, Leena Kytömäki, Ville-Veikko Mäkelä, Miina Nurmi, Sami Pietilä, Oso Rissanen, Reija Venho, Eveliina Virtanen, Sanna Vuorikoski, Pasi Soidinsalo (undergraduate student), Mikko Konki (undergraduate student) Steering Committee: Prof. Olli Carpén, Chair (University of Turku), Prof. Eva-Mari Aro (University of Turku), Prof. Klaus Elenius (University of Turku), Prof. Riitta Lahesmaa (University of Turku), Prof. Tarja Laitinen (University of Turku), Prof. Harri Lähdesmäki (University of Turku, Aalto University), Prof. Craig Primmer (University of Turku), Prof. Harri Savilahti (University of Turku), Prof. Lea Sistonen (Åbo Akademi University), Prof. Stina Syrjänen (University of Turku) Core facility description: The Finnish Microarray and Sequencing Centre (FMSC) is an internationally recognized Functional Genomics Core Facility that provides state-of-the-art research technologies and services in the areas of genomics, epigenomics, transcriptomics and bioinformatics for the Finnish and international scientific community. The main services include next-generation sequencing (NGS) and microarray based services mainly focusing on gene expression and its regulation as well as on epigenetics. We also provide quantitative Real-Time PCR and traditional DNA sequencing services. Our services cover all the steps from experimental planning and design to sample processing and data analysis. The Centre also regularly organizes courses, symposia and training for its users. Seminars and practical courses are held frequently to facilitate knowledge transfer within the field. Since 2010 FMSC has been a key member in the Biocenter Finland national infrastructure program. According to the division of tasks within the Genome-Wide Methods network, our Centre focuses on developing technologies in the areas of gene expression and 22 23 its regulation; one of the key goals in the Centre is to develop and implement advanced techniques and provide services for epigenomic applications. Funding: Biocenter Finland Academy of Finland University of Turku Åbo Akademi University Users: FMSC has provided genomics and transcriptomics services for over a decade, and thereby has gained a large customer base. FMSC processes annually thousands of microarray and NGS samples. In 2013 the Centre carried out 87 projects on NGS and microarray platforms from 49 research groups and analysed more than 2000 samples. The QPCR service ran 1256 plates and was used by 41 research groups. The Centre’s Sanger sequencing service analysed 4827 samples for 88 research projects. With our contribution, many papers were published last year in high-quality journals, such as Nature Immunology and Nature Methods. Publications with FMSC contributions and co-authors in 20112013 Publications FMSC contributions FMSC co-authors 2013 29 12 2012 46 13 2011 36 2 From left to right. Front row: Päivi Junni, Eveliina Virtanen, Miina Nurmi, Bishwa Ghimire, Leena Kytömäki and Sini Junttila. Back row: Mikko Konki, Ville-Veikko Mäkelä, Asta Laiho, Riikka Lund, Sanna Vuorikoski, Pasi Soidinsalo and Oso Rissanen. 24 25 CELL IMAGING CORE http://www.btk.fi/cell-imaging/ Cell Imaging Core Personnel: Director: Eleanor Coffey, PhD (Pasi Kankaanpää, MSc., stand-in January-June 2013) Address: Turku Centre for Biotechnology BioCity, Tykistokatu 6, P.O. Box 123, FI-20520 Turku, Finland. Tel: +358-2-3338605 Email: [email protected] Personnel: Research Coordinators: Jouko Sandholm, MSc, Markku Saari, MSc Project engineer: Ketlin Adel CIC technical support: Senthil Palani, MSc CIC partner technical support at Åbo Akademi: Jari Korhonen, MSc Steering Committee: Prof. Olli Carpén, MD, PhD, University of Turku; Prof. John Eriksson, PhD, Åbo Akademi University; Prof. Jyrki Heino, MD, PhD, University of Turku; Prof. Pekka Hänninen, PhD, University of Turku; Prof. Sirpa Jalkanen, MD, PhD, University of Turku; Prof. Riitta Lahesmaa, MD, PhD, University of Turku; Prof. Olli Lassila, MD, PhD; Prof. Matti Poutanen, PhD, University of Turku; Prof. Lea Sistonen, PhD, Åbo Akademi University; Prof. Kid Törnquist, PhD, Åbo Akademi University. Core facility description: The Cell Imaging Core (CIC) provides state-of-the-art cell imaging and flow cytometry instruments and services to researchers nation-wide, our customer base deriving largely from the Biocity Turku campus area. We provide open access services that benefit academic and industrial users alike. Our technology platform hosts state-of-the-art instruments for microscopy and flow cytometry, with expertise covering a broad range of imaging and flow cytometry modalities. From left to right, back row: Senthil Palani, Jari Korhonen, Ketlin Adel, Jouko Sandholm, Markku Saari. front row: Pasi Kankaanpää and Eleanor Coffey. 26 The Cell Imaging Core offers one-to-one training and consultation on instrument operation, experimental design and image analysis. We contribute to ongoing formal education by organizing training courses and workshops, while at once evaluating new advances in hardware and software tools. During 2013, our teaching efforts totaled more than 100 hours, including teaching at the Turku Bioimaging summer school. In addition, the Lost in Imaging webinar series continued for the third consecutive year. Our goal is to provide instrumentation and know-how that supports the research efforts of the BioCity Turku campus, while at once we broaden the scope of this research by introducing new technological and analysis solutions to the community. CIC staff have developed free-access Flowing Software for flow cytometry (www.flowingsoftware.com), and BioImageXD (www. bioimagexd.net) for high-end optical image data visualization and analysis. Areas of special expertise include fluorescence lifetime imaging microscopy (FLIM), live cell imaging, high-sensitivity 27 confocal imaging, fluorescence correlation spectroscopy (FCS), laser-capture microdissection, fluorescence assisted cell sorting (FACS), automated high-throughput flow cytometry and atomic force microscopy. PROTEOMICS FACILITY During 2013, CIC acquired a total internal reflection (TIRF) microscope (TIRF 3, Carl Zeiss) for high resolution imaging. We continued our collaboration agreements with other major owners of light microscopes in BioCity Turku, providing reservation infrastructure and centralized advertising for bio-imaging instruments campus-wide through cooperation agreements. These include multi-photon super-resolution imaging devices, slide scanning, live content imaging, atomic force microscopy and calcium imaging. This cooperation has enabled us to broaden the range of services that can be made available to researchers in a centralized manner. Director: Garry Corthals, PhD (2005 – July 2014). Turku Centre for Biotechnology, BioCity, Tykistökatu 6A, 20520 Turku, Finland. Tel. +358-2-333 8604, Fax. +358-2-2158808. E-mail: [email protected] During 2013, CIC actively continued to participate in the national Finnish BioImaging organization, and in the extensive Euro-BioImaging organization, which is currently under construction phase. Recent statistics show that CIC has grown to become the largest and most actively used light microscopic imaging facility and flow cytometry unit in Finland. In 2013, our services (including collaboration instruments) were used by approximately 300 people from 100 research groups, 12% of whom were “external users” (from outside of the University of Turku and Åbo Akademi University). We look forward to continued expansion of infrastructure and services available in the coming years, in anticipation of the successful completion of the EuroBioimaging The cell imaging core is best contacted by our designated email addresses and phone numbers: [email protected] (044-923 1356) and [email protected] (044-923 1322). We warmly thank our funders, users and collaborators for a most productive 2013. Major Instrumentation: • Microscopy: Zeiss LSM-780, Zeiss-510-META, StereoLumar V12, Lambert FLIM, Zeiss P.A.L.M. LCM, TIRF 3 Zeiss, Leica TCS SP5 Matrix. • Flow cytometry: BD-FACS Aria II Cell Sorter, BD-LSRII, BD-FACS Caliber, BD-FACScan. Funding: Biocenter Finland, the University of Turku, Åbo Akademi University, the Ministry of Social Affairs and Health. 28 http://www.btk.fi/proteomics Personnel: Senior scientists: Anne Rokka, PhD; Dorota Muth-Pawlak, PhD; Susumu Imanishi, PhD; Petri Kouvonen, PhD, Laboratory Engineer: Arttu Heinonen, MSc; Technician: Pekka Haapaniemi, MSc Steering Committee: Prof. Eva-Mari Aro (University of Turku), Dr. Eleanor Coffey (Åbo Akademi University), Prof. John Eriksson (Åbo Akademi University), Prof. Jyrki Heino (University of Turku), Prof. Riitta Lahesmaa (CBT), Prof. Matti Poutanen (University of Turku), Prof. Craig Primmer (University of Turku), Prof. Jukka Westermarck (CBT) and Prof. Johanna Ivaska (VTT & CBT) General description: The Turku Proteomics Facility is engaged in the development and application of proteomics and mass spectral methods in key areas of life science research. The Facility has developed a wide basis of operation and expertise in quantitative proteomics, posttranslational modification analysis, biological mass spectrometry and allied bioinformatic procedures. The mission of the Facility is to advance mass spectral methods and instrumentation to meet the needs in molecular biotechnology and medicine. Our goals are to identify new areas appropriate for mass spectrometry in biological sciences and to develop new approaches involving mass spectrometry, to apply cuttingedge mass spectrometry to tackle critical questions in biological sciences, and train students, post-doctoral fellows and practicing scientists in the use of mass spectrometry and encourage its wide and appropriate use. The facility receives funding locally through the University of Turku and nationally from Biocentre Finland. Nationally, the facility spearheads mass spectrometric services and training and in quantitative analysis of proteins and proteomes, and analyses of PTMs. Consequently major focuses for the facility have been the development of cutting-edge implementation of emerging technologies and methods in these fields. Furthermore the facility has developed a suite of methods specifically aimed at the analysis of tissues and proximal fluids, and is currently ideally suited to engage in clinical research collaborations and ongoing developments with the national and local Biobanks. 29 Analytical services: The facility provides mass spectrometry-based analytical services and offers researches access to sophisticated instrumentation that enables high-content proteome measurements. Moreover the facility encourages its users to learn and co-develop MS methods, thereby increasing the ocal knowledge base, users and community of scientists versed in proteomics and mass spectrometry. A full representation of our services in 2013 were as follows: • Proteome-wide analysis of cells, tissues and fluids is available in all life sciences. Several integrated fractionation techniques have been developed to provide deep proteome coverage from exquisite sample amounts. • Quantitative proteomics – analysis of proteomes following labelling with reagents such as iTRAQ, or using our in-house developed workflows for label-free analysis using MS1 or MS2 data. • Label-free quantitation for clinical studies – we have established a framework for label-free quantitative analysis, particularly useful for large-scale clinical studies. • Targeted quantitation by selected reaction monitoring (M/ SRM) – sensitive quantitative measurements of specific sets of proteins (up to 100) from complex samples. useful for HTP validation and large cohort studies. • Post-translational modifications – a long standing history with phosphorylation analysis, both in terms of enrichment and validation. • Biological mass spectrometry – various analytical measurements for protein, peptide and small molecules, mass determination and peptide and protein purity determination are offered. • Bioinformatics – in all areas of proteomics. Additionally ties have been strengthened with the group of Laura Elo who assists in computational and statistical analyses. Major mass spectrometry instrumentation: 1. LTQ Orbitrap Velos Pro with ETD; 2. Q Exactive; 3. TSQ Vantage; and 4. Q-Star Elite Funding: University of Turku, Åbo Akademi University, Biocenter Finland, the Systems Biology Research Program, European Cooperation in Science and Technology (e-COST) and Seventh Framework Programme (FP7). Users: The Turku Proteomics Facility assists costumers from national and international universities, research institutes and companies in their scientific objectives. From left to right: Anne Rokka, Pekka Haapaniemi, Dorota Muth-Pawlak, Susumu Imanishi and Garry Corthals. 30 31 PROTEIN CRYSTALLOGRAPHY CORE FACILITY http://www.btk.fi/crystallography/ Head: Anastassios C. Papageorgiou, PhD, Adjunct Professor in Biochemistry and Structural Biology Turku Centre for Biotechnology, BioCity, Tykistökatu 6A, FI-20521 Turku, Finland. Tel. +358-2-3338012, Fax +358-2-3338000. E-mail: [email protected] Technical Team: Technical support: Juha Strandén, Pasi Viljakainen. Computational support: Petri Vahakoski, Mårten Hedman form of collaborative efforts or as services. Protein Crystallography requires a multi-disciplinary approach and we are especially interested in bringing together expertise from various groups in order to better understand the structure-function relationship of biological macromolecules in key biological processes. Funding: Systems Biology research program, Biocenter Finland, University of Turku Users Main users include groups from UTU and ÅA as listed in http:// www.sci.utu.fi/projects/biokemia/bioxlabs/. Each group has at least three other collaborations. Steering committee: Jyrki Heino, Professor, Department of Biochemistry and Food Chemistry, University of Turku; Reijo Lahti, Professor, Department of Biochemistry and Food Chemistry, University of Turku; Tiina Salminen, Senior lecturer, Department of Biochemistry, Åbo Akademi University; BioXlabs-Turku Description of the Facility X-ray crystallography is a proven technique for detailed structurefunction studies of biological macromolecules. The Protein Crystallography Core Facility at CBT uses state-of-the-art equipment to determine the crystal structures of various proteins and their complexes. The Facility consists of an X-ray generator (Rigaku MicroMax 007 HF), Mar345 imaging plate detector, Varimax optics, a Cryostream Cooler (Oxford Cryosystems) and several computers running under Linux operating systems for heavy duty calculations. The Facility has several workstations to run a variety of molecular graphics software (COOT, CCP4mg, PyMol, Chimera, O, XtalView, Grasp), modeling and docking programs (MODELLER, Hex, Discovery Studio, ROSETTA), and various crystallographic packages (HKL, XDS, CNS, CCP4, SHELX, SOLVE, SHARP, PHENIX) for data processing, analysis, phasing and refinement. The Facility has long expertise in all steps of a crystal structure determination: protein purification, crystallization, data collection (both in-house and in synchrotron radiation sources), data processing, phase determination, refinement and detailed analysis of the final structure. Incubators at different temperatures (4° C, 16 °C and 23 °C) for crystallization set-ups and a number of various commercial screens for establishing initial crystallization conditions are available. In addition, we can provide homology modeling services and design of mutants for functional studies as well as ab initio predictions of protein structures. There is regular access to synchrotrons (e.g. ESRF, DIAMOND, DESY) and we have recently started to explore in situ diffraction options. Since protein crystallography requires highly pure protein preparations, we can offer full support and consultation on protein purification strategies apart from the services in structure determination and modeling. The Facility is able to undertake research projects for academic groups and companies, either in the 32 The X-ray generator and imaging plate detector. Diffraction image recorded in the facility. 33 BIOINFORMATICS CORE http://www.btk.fi/bioinformatics Contact information: Turku Centre for Biotechnology, BioCity, Tykistökatu 6B, FI-20521 Turku, Finland. Tel. +358-2-333 8634, Fax +358-2-251 8808. Email: [email protected] Funding: Biocenter Finland University of Turku Åbo Akademi University Users: The Bioinformatics core has users from Finnish universities, biocenters and research institutes in the field of biosciences. Heads/Coordinators Dr. Konstantin Denessiouk (Structural Bioinformatics) Dr. Attila Gyenesei (Head of Bioinformatics), MSc Asta Laiho (Acting Head of Bioinformatics) Technical Team: Bhanupratap Singh Chouhan, Sini Junttila, Asta Laiho, Leena Kytömäki, Bishwa Ghimire, Sami Pietilä Core facility description: The Bioinformatics Core at the Turku Centre for Biotechnology is divided into Structural Bioinformatics and High-throughput Bioinformatics facilities. The main goal of the Structural Bioinformatics facility is to apply methods and techniques of bioinformatics to study biological macromolecules, their interactions and function. We work in close co-operation with experimental groups and are able to provide structure-related analysis and prediction in different biological systems. The core works closely with the CSC Finnish IT Center for Science, the Finnish national supercomputing centre and the Structural Bioinformatics Laboratory at the Åbo Akademi University. High-throughput bioinformatics facility complements experimental genomics and transcriptomics by storing, analysing and integrating data. The core provides services in the analysis of microarray and deep sequencing data. The team takes advantage of in-house robust super-computing facility and state-of-the-art software. Team members are engaged in the ongoing development of advanced analysis tools and research on generating novel approaches for the analysis of high-throughput data sets. The main services of the Bioinformatics Core are: • Experimental design consultation • Data analysis of various microarray and deep sequencing data types • Data analysis education and training • Computer-based analysis of protein-protein and proteinligand interactions • Computer-aided prediction and intelligent molecular modeling and design • Computer-based ligand docking Analysis and prediction of effects of molecular recognition and mutations on protein function From left to right: Sami Pietilä, Asta Laiho, Leena Kytömäki, Bishwa Ghimire and Sini Junttila. 34 35 VIRUS VECTOR FACILITY http://www.btk.fi/viral-vectors Head of the Unit Eleanor Coffey, PhD, Turku Center for Biotechnology BioCity, Tykistokatu 6, P.O. Box 123, FI-20520, Turku, Finland. Consultant on Retroviruses: Jukka Westermarck, MD, PhD Consultant on Adenoviruses: Mikko Savantaus, MD, PhD Consultant on Lenti Vectors: Jari Heikkilä Consultant on Herpes Simplex Virus: Veijo Hukkanen Personnel Laboratory Technician: Ketlin Adel Quality Assurance: Linnéa Linko The Virus Vector Facility has over ten years of experience producing viral vectors for local and national research groups. We have participated since 2010 in the national infrastructure network on Viral Gene Transfer, funded by Biocenter Finland. Our primary function is to facilitate the use of viral vectors by local researchers and researchers in other parts of Finland. To this end, the virus vector facility • propagates adenoviruses expressing genes of interest, as a research service for customers • produces high-titer lenti vectors using customer plasmids • provides a range of quality assurance checks of viral prep yield and activity as a service •maintains a fully equipped bio-safety level-2 lab for researchers wishing to produce their own vectors • supplies working protocols and one-to-one training on production and safe handling of adeno and lenti vectors as a service to customers •coordinates a network of local experts from whom consultation on design of viral vectors can be sought The virus vector facility has a national, long-standing customer base providing services to researchers in the universities of Turku, Oulu and Helsinki and to a lesser degree to biotech companies. In addition to customer service, our infrastructure is used by 50 local researchers that produce adenoviruses, adeno-associated virus, retroviruses and lentiviral vectors for their research. These vector tools are used to obtain high efficiency gene transfer in difficult to transfect cells such as primary cultures of T lymphocytes and neurons and for in vivo cancer studies. Use of viral gene transfer for gene knockdown including stable knockdown studies is also popular. To build on local expertise in gene transfer technologies, the Virus Vector Facility networks with experts in viral vector design. Thus a number of local experts on retroviruses and alpha-viruses are available for consultation on vector design, production and concentration. Turku Viral Vector Facility follows without exception the safety guidelines of the Geenitekniikan Lautakunta. From left to right: Anna Lipsanen, Ketlin Adel, Eleanor Coffey and Linnéa Linko. 36 37 MECHANISMS AND BIOSENSORS OF GTPASES http://www.btk.fi/research/research-groups/abankwa/ Principal investigator: Daniel Abankwa, PhD, Docent (Adjunct Professor) at Åbo Akademi University, Academy of Finland Research Fellow. Tel. +358-2-3336969, Fax. +358-2-3338000. Email: [email protected] Biography: Daniel Abankwa (b. 1972) graduated in Chemistry (Dipl. Chem.) from the Georg-August University in Göttingen in 1997 and received his PhD in Molecular Neurobiology from the Heinrich-Heine University Düsseldorf (2001). In 2002, he joined Prof. Horst Vogel at the EPFL in Lausanne as a postdoc working with fluorescence methods to study membrane proteins. In 2006, he went to the Institute for Molecular Bioscience in Brisbane, Australia with an advanced research fellowship from the Swiss National Science Foundation to join the group of Prof. John Hancock. Since then his work is focused on mechanisms of the nanoscale organisation (nanoclustering) of small GTPases in the plasma membrane and its critical impact on signalling. He discovered a novel switch III-region in H-ras, which directs the reorientation of the protein on the membrane. In 2008 he joined Prof. Kirill Alexandrov as a junior group leader at the same institute, heading projects on Rho protein membrane anchorage, Rab nanoclustering and a chemical screening project to identify nanoclustering and lipid transferase inhibitors. In July 2010, Daniel joined the Turku Centre for Biotechnology. In June 2011 he became docent/ adjunct professor at Åbo Akademi University and since September 2011 he is holding an Academy of Finland Research Fellowship. Our research focuses on understanding the nanoscale organisation of small GTPases, in particular oncogenic Ras. Like many signalling proteins, Ras is cytoplasmically anchored to the plasma membrane, where it is highly concentrated within nanometer regions (nanocluster). Nanoclustering renders the associated signalling system more efficient. We aim at understanding and targeting this signalling architecture, by studying the determinants in the structure of Ras (e.g. the orientation-switch III) and of nanocluster modulating proteins. We employ quantitative fluorescence microscopic techniques, computational modelling, tailored fluorescence biosensor assays for compound and siRNA screening, molecular cell biological approaches, as well as tumour models to identify new nanocluster modulators as cancer biomarkers and targets. Research Questions: • Which are the molecular and structural determinants of the GTPase nanoscale organisation (nanoclustering) on the membrane? • Can we pharmacologically interfere with specific nanoclusters or nanocluster modulatory proteins? •In which physiological processes is nanoclustering particularly significant? • Do other membrane anchored signalling proteins also exploit nanoclustering for robust signal transmission? Personnel: Post-doctoral Fellows: Olga Blazevics, PhD; Camilo Guzman, PhD; Alessio Ligabue, PhD; Christina Oetken-Lindholm, PhD; Elina Siljamäki, PhD; Graduate students: Arafath Kaja Najumudeen, MSc; Maja Solman, MSc Description of the project Cancer is among the most common causes of deaths in humans. It is caused by the accumulation of mutations in genes, which ultimately lead to uncontrolled cell growth and spreading. The small GTPase Ras is highly mutated in human cancers, where it drives as a constitutively active protein cell transformation. However, several approaches to block oncogenic Ras have failed in the past 25 years, which earned it the premature status of an undrugable protein. In the wake of personalized medicine, which aims at targeting several components that are misregulated, interest in inhibiting oncogenic Ras as one of the cancer hallmarks has recently been reprioritized. Major funding bodies (e.g. NIH-NCI oncogene initiative) and companies worldwide currently support intensive research programs for anti-Ras drugs. 38 Ras orientation-switch III mutations affect nanoclustering and signalling. (i) A conformational equilibrium of Ras on the membrane is guided by a novel switch III. (ii) We showed that orientation-switch III mutants differentially interact with galectin-1, a nanocluster modulator. This leads to specific nanoclustering responses (Guzman C 2014, JBC). Nanoclusters, not free Ras, are sites of effector recruitment. Thus signalling output follows nanoclustering. This critical mechanism has escaped classical biochemical studies. Funding: The Academy of Finland, EU 7th framework (Marie-Curie grant), Cancer Society Finland, Biocenter Finland, Sigrid-Juselius Foundation 39 From left to right: Daniel Abankwa, Olga Blaževitš, Rounik Mazumdar, Camillo Guzman, Mideksa Yonatan Gebremariam, Christina Oetken-Lindholm, Arafath Najumudeen, Alessio Ligabue, Maja Solman and Elina Siljamäki. Collaborators: Dr. Christian Eggeling (Max-Planck Institute Göttingen, Germany), Dr. Harri Härmä (University of Turku), Dr. Jessica Rosenholm (Åbo Akademi University), Prof. Jukka Westermarck (Turku Centre for Biotechnology), Prof. Mike Waters (Institute for Molecular Bioscience, Australia), Prof. Alemayehu Gorfe and Prof. John Hancock (UT Medical School Houston, USA), Prof. Kirill Alexandrov (Institute for Molecular Bioscience, Australia), Prof. Johanna Ivaska (VTT, Turku Centre for Biotechnology), Prof. Robert Parton (Institute for Molecular Bioscience, Australia), Dr. Krishnaraj Rajalingam (University of Frankfurt, Germany), Katarzyna Blazewska (Lodz University of Technology, Poland) Selected Publications: Guzmán, C., Bagga, M., Kaur, A., Westermarck, J., and Abankwa, D. (2014) ColonyArea: An ImageJ Plugin to Automatically Quantify Colony Formation in Clonogenic Assays. PLoS ONE 9, e92444 Guzmán, C., Solman, M., Ligabue, A., Blazevitš, O., Andrade, D. M., Reymond, L., Eggeling, C., and Abankwa, D. (2014) The efficacy of Raf kinase recruitment to the GTPase H-ras depends on H-ras membrane conformer specific nanoclustering. Journal of Biological Chemistry [EPub ahead of print] Najumudeen, A. K., Köhnke, M., Solman, M., Alexandrov, K., and Abankwa, D. (2013) Cellular FRET-Biosensors to Detect Membrane Targeting Inhibitors of N-Myristoylated Proteins. PLoS ONE 8, e66425 Köhnke, M., Schmitt, S., Ariotti, N., Piggott, A. M., Parton, R. G., Lacey, E., Capon, R. J., Alexandrov, K., and Abankwa, D. (2012) Design and Application of In Vivo FRET Biosensors to Identify Protein Prenylation and Nanoclustering Inhibitors. Chemistry & Biology 19, 866–874 Sinha, B., Köster, D., Ruez, R., Gonnord, P., Bastiani, M., Abankwa, D., Stan, R. V., Butler-Browne, G., Vedie, B., Johannes, L., Morone, N., Parton, R. G., Raposo, G., Sens, P., Lamaze, C., and Nassoy, P. (2011) Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144, 402–413 Abankwa, D., Gorfe, A. A., Inder, K., and Hancock, J. F. (2010) Ras membrane orientation and nanodomain localization generate isoform diversity. Proceedings of the National Academy of Sciences 107, 1130–1135 Abankwa, D., Hanzal-Bayer, M. F., Ariotti, N., Plowman, S. J., Gorfe, A. A., Parton, R. G., McCammon, J. A., and Hancock, J. F. (2008) A novel switch region regulates H-ras membrane orientation and signal output. EMBO J 27, 727–735 Hill, M. M., Bastiani, M., Luetterforst, R., Kirkham, M., Kirkham, A., Nixon, S. J., Walser, P. J., Abankwa, D., Oorschot, V. M. J., Martin, S., Hancock, J. F., and Parton, R. G. (2008) PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 132, 113–124 40 41 LYMPHOCYTES AND INFLAMMATION Principal investigator: Zhi Jane Chen, MD; PhD, Academy of Finland Research Fellow. Turku Centre for Biotechnology, Åbo Akademi and University of Turku, Tykistökatu 6, FI-20520, Turku, Finland, Tel. +358-2-3338028. Email: [email protected] or [email protected] Biography: Zhi Jane Chen graduated from Beijing Medical University (Collage of Medicine, Peking University), China. She carried out her Ph.D study at the Turku Centre for Biotechnology and received Ph.D from Faculty of Medicine, University of Turku in 2004. During 2005-2007, she was a post-doctoral fellow in Dr. John O’Shea’s laboratory at the NIH, USA. She received a Post-doctoral Fellowship from the Academy of Finland and worked in the Department of Cell Biology and Anatomy, Faculty of Medicine, University of Turku in 2008-2010. In 2011, she joined Prof. Riitta Lahesmaa’s laboratory as a senior scientist. Since September 2012, she is holding an Academy of Finland Research Fellow position. Personnel: Graduate students: Khan Mohd Moin, MSc (co-supervision with Prof. Riitta Lahesmaa), Csendes Brigitta, MSc, Undergraduate student: Mohammad Imran Ahammad Description of the project Inflammatory and autoimmune diseases underlie a vast variety of human diseases. The immune system is often involved with these disorders. We focus on understanding the role of the relatively newly identified subsets of CD4+ T cells, Th17 and iTreg in regulating the inflammatory/auto-immune axis. We study Th17 and iTreg differentiation at multiple levels from mouse and human and integrate the results to build a comprehensive view of the processes. We are particularly interested in the study of the regulation and interactions between the immune and hormonal responses and how the interactions contribute to pathogenesis of immunemediated diseases. The results of our studies are expected to gain new insight into the molecular mechanisms of lymphocyte differentiation to Th17 and iTreg lineages. This in turn will be important for the development therapeutic strategies that will facilitate rational modulation of the immune response. Funding: The Academy of Finland From left to right: Brigitta Csendes, Zhi Jane Chen and Imran Ahammad Mohammad. 42 Collaborators: Prof. Riitta Lahesmaa: Director, Turku Centre for Biotechnology. Dr. John O’Shea: Scientific Director, Intramural Research Program, 43 NIAMS, NIH, USA. Dr. Wendy Watford: Assistant Professor, University of Georgia, College of Veterinary Medicine, USA. Dr. Diana Toivola: Docent, Åbo Akademi University. Dr. Harri Lähdesmäki, Professor, Aalto University PROTEIN KINASE REGULATION OF BRAIN DEVELOPMENT AND DISEASE http://www.btk.fi/research/research-groups/coffey/ Selected Publications: Lönnberg T, Chen Z, Lahesmaa R. From a gene-centric to wholeproteome view of differentiation of T helper cell subsets. Brief Funct Genomics. 2013 Nov;12(6):471-82. Chen Z, Lönnberg T, Lahesmaa R. Holistic systems biology approaches to molecular mechanisms of human helper T cell differentiation to functionally distinct subsets. Scand J Immunol. 2013 Aug;78(2):172-80. Tuomela S, Salo V, Tripathi SK, Chen Z, Laurila K, Gupta B, Aijö T, Oikari L, Stockinger B, Lähdesmäki H, Lahesmaa R. Identification of early gene expression changes during human Th17 cell differentiation. Blood. 2012 Jun 7;119(23):e151-60. Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ.: Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006 May 23;103(21):8137-42. Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007 Mar;26(3):371-81. Chen Z, Tato CM, Muul L, Laurence A, O’Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007 Sep;56(9):2936-46. Chen Z, Buki K, Vääräniemi J, Gu G, Väänänen H.K., The critical role of IL-34 in osteoclastogenesis. PLoS One. 2011 Apr 8;6(4):e18689. Chen Z, Laurence A, O’Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007 Dec;19(6):400-8. Chen Z, O’Shea JJ. Regulation of IL-17 production in human lymphocytes. Cytokine. 2008 Feb;41(2):71-8. Chen Z, O’Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol Res. 2008;41(2):87-102 Jiang JK, Ghoreschi K, Deflorian F, Chen Z, Perreira M, Pesu M, Smith J, Nguyen DT, Liu EH, Leister W, Costanzi S, O’Shea JJ, Thomas CJ. Examining the chirality, conformation and selective kinase inhibition of 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d] pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile (CP690,550). J Med Chem. 2008 Dec 25;51(24):8012-8. Chen Z, Lund R, Aittokallio T, Kosonen M, Nevalainen O, Lahesmaa R. Identification of novel IL-4/Stat6-regulated genes in T lymphocytes. J Immunol. 2003 Oct 1;171(7):3627-35. 44 Principal investigator: Eleanor Coffey, PhD, Adjunct Prof., Turku Centre for Biotechnology, Åbo Akademi and Turku University, BioCity, Tykistokatu 6B, FI-20521 Turku, Finland. Tel. +358-2-3338605, Fax. +358-2-3338000. Email: [email protected] Biography: Eleanor Coffey graduated from Trinity College Dublin in 1990 and received her PhD from the University of Dundee in 1994. She was awarded a Wellcome Trust fellowship to carry out post-doctoral research in Prof. Karl Åkerman’s laboratory from 1994-1997. In 1997 she founded the Neuronal Signalling group at Åbo Akademi University and in 2000 joined Turku Centre for Biotechnology as a group leader in molecular and cellular biology. In addition to running a research group, she directs the Cell Imaging and Viral Vector Facility at the Center. She coordinates a Marie Curie International Training Network r´BIRTH; Brain Imaging Return to Health, involving 9 academic and industrial partners around Europe. Personnel: Graduate students: Justyna Zdrojewska, MSc, Emilia Komulainen, MSc, Hasan Mohammed, MSc., Prasannakumar Deshpande, MSc, Patrik Hollos, MSc., Dani Flinkman, Francesca Marchisella, MSc., Artemis Varidaki, MSc. Undergraduate student: Olavi Misin. Lab Manager: Susanna Pyökari. Description of the project Neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease as well as stroke are characterised by the irreversible loss of nerve cell function. These diseases for which no cure is known are among the most costly to society. The protein kinase JNK is recognised as a critical player in stroke and neurodegeneration. However exactly how this family of kinases mediates cell death in the brain remains largely unknown. Although targeting of JNK for drug-based therapy is already underway, our understanding of the physiological function of JNK in the brain is in its infancy. A major challenge for signal transduction therapy is to selectively target the pathological function of signalling molecules without interfering with important physiological roles. To achieve this, our lab established a proteomics-based screen to identify protein kinase substrates and thereby broaden our understanding of kinase function. While we have used this methodology to successfully identify both novel and known substrates for JNK, p38 and PIM kinases (collaboration with Päivi Koskinen), among others (collaboration Erwin Wagner), the main focus of our research is to elucidate the molecular mechanism of JNK and JNK targets in the brain. Identification of novel JNK targets such as SCG10 and MAP2, 45 as well as others under study, has highlighted a critical role for JNK in maintaining microtubule homeostasis and subsequently regulating axodendritic architecture and nerve cell movement. Identification of the JNK phosphorylation site on kinesin-1 helped characterize a role for JNK in regulation of fast axonal transport in neurons. We combine biochemical, proteomic, cell biology and imaging methods with neuronal and organotypic cultures as well as transgenic mice to validate kinase targets and elucidate their function. In collaboration with Laurent Nyguen, we have established methods to track radial migration of neurons in the developing telencephalon using 4D imaging. In addition, we are examining dendrite and spine morphology in Jnk1-/- brains using lucifer yellow iontophoretic loading followed by quantitative 3D image analysis. An important finding from our lab is the compartmentalization of JNK function in neurons into physiological and pathological pools residing in the cytoplasm and nucleus respectively. By using compartmenttargeted peptide inhibitors of JNK, we have shown that nuclear JNK activity is critical for neuronal death in response to trophic deprivation (neuronal death that occurs during brain development) and excitotoxic stimuli (neuronal death that occurs during epilepsy, stroke and is contributory in neurodegenerative disorders). To explore the therapeutic potential of compartmental targeted JNK inhibitors, we are utilizing targeted inhibitors of JNK (prepared in our lab) and targeted FRET reporters of compartmental JNK activity, modified in our lab to investigate the potential therapeutic benefit of targeted kinase inhibition for treatment of excitotoxicity. Interestingly, although JNK is highly localised to the cytoplasm in neurons, we find that cytosolic JNK does not to these particular death mechanisms in neurons of the central nervous system. Instead, JNK plays a critical role in corticogenesis, being required to control the duration of two critical steps during formation of the cortex, i.e. multipolar stage transition and radial migration. This function of JNK is mediated by SCG10 and is independent of nuclear JNK activity. As an extension of this study on brain development, we are now examining regulation of neurogenesis in brain in the context of depressive disorders and anxiety. This is as a part of Marie Curie International Training Network, involving molecular neuroscience, patient imaging and imaging tool development. We have in recent years switched attention to Parkinson’s disease kinases LRRK2 and PINK1. To gain better understanding of the molecular underpinnings of Parkinson’s disease, we are carrying out phosphoproteomcis screens in collaboration with Peter James’s lab. Gain of function mutations in LRRK2 are the most common cause of familial Parkinson’s disease. Yet, substrates for LRRK2 have remained elusive and the disease mechanism is unknown. In collaboration with Peter James’s lab, we are searching for LRRK2 targets in brain using a shot-gun approach. We hope that in the long run this will enhance our understanding of Parkinson’s pathology and contribute tools that can be used for earlier clinical diagnosis. Funding: Åbo Akademi University, EU FP7 Marie Curie, The Academy of Finland, Biocenter Finland, Turku University Biomedical Sciences Graduate School. 46 Collaborators: Peter James (University of Lund), Kristen Verhey (University of Michigan), Casper Hoogenraad (University of Utrecht). Selected Publications: 1. Coffey, E.T. (2014) Nuclear and cytosolic JNK signalling. Nature Reviews Neuroscience. in press 2. Zdrojewska, J., Coffey, E.T. (2014) The impact of JNK on neuronal migration. Adv Exp Med Biol. 2014;800:37-57. 3. Jonsdottir, K., Zhang, H., Jhagroe, D., Skaland, I., Slewa, A., Björkblom, B., Coffey, E. T., Gudlaugsson, E., Smaaland, R., Janssen, E. A., and Baak, J. P. (2012) The prognostic value of MARCKS-like 1 in lymph node-negative breast cancer. Breast Cancer Res Treat 135, 381-390 4. Bjorkblom, B., Padzik, A., Mohammad, H., Westerlund, N., Komulainen, E., Hollos, P., Parviainen, L., Papageorgiou, A. C., Iljin, K., Kallioniemi, O., Kallajoki, M., Courtney, M. J., Magard, M., James, P., and Coffey, E. T. (2012) c-Jun N-terminal kinase phosphorylation of MARCKSL1 determines actin stability. Mol Cell Biol 32, 3513-3526 5. Westerlund, N., Zdrojewska, J., Padzik, A., Komulainen, E., Björkblom, B., Rannikko, E., Tararuk, T., Garcia-Frigola, C., Sandholm, J., Nguyen, L., Kallunki, T., Courtney, M. J., and Coffey, E. T. (2011) Phosphorylation of SCG10/stathmin-2 determines multipolar stage exit and neuronal migration rate. Nat Neurosci 14, 305-313 6. Mai, A., Veltel, S., Pellinen, T., Padzik, A., Coffey, E., Marjomäki, V., and Ivaska, J. (2011) Competitive binding of Rab21 and p120RasGAP to integrins regulates receptor traffic and migration. J Cell Biol 194, 291-306 7. Matlawska-Wasowska, K., Finn, R., Mustel, A., O’Byrne, C. P., Baird, A. W., Coffey, E. T., and Boyd, A. (2010) The Vibrio parahaemolyticus Type III Secretion Systems manipulate host cell MAPK for critical steps in pathogenesis. BMC Microbiol 10, 329 8. Uusi-Oukari, M., Kontturi, L. S., Coffey, E. T., and Kallinen, S. A. (2010) AMPAR signaling mediating GABA(A)R delta subunit up-regulation in cultured mouse cerebellar granule cells. Neurochem Int 57, 136-142 9. Podkowa, M., Zhao, X., Chow, C. W., Coffey, E. T., Davis, R. J., and Attisano, L. (2010) Microtubule stabilization by bone morphogenetic protein receptor-mediated scaffolding of c-Jun N-terminal kinase promotes dendrite formation. Mol Cell Biol 30, 2241-2250 10. Morfini, G. A., You, Y. M., Pollema, S. L., Kaminska, A., Liu, K., Yoshioka, K., Björkblom, B., Coffey, E. T., Bagnato, C., Han, D., Huang, C. F., Banker, G., Pigino, G., and Brady, S. T. (2009) Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci 12, 864-871 11. Waetzig, V., Wacker, U., Haeusgen, W., Björkblom, B., Courtney, M. J., Coffey, E. T., and Herdegen, T. (2009) Concurrent 47 protective and destructive signaling of JNK2 in neuroblastoma cells. Cell Signal 21, 873-880 13. Naumanen, T., Johansen, L. D., Coffey, E. T., and Kallunki, T. (2008) Loss-of-function of IKAP/ELP1: could neuronal migration defect underlie familial dysautonomia? Cell Adh Migr 2, 236-239 From left to right: Hasan Mohammad, Susanna Pyökäri, Patrik Hollós, Eleanor Coffey, Prasanna Kumar Desphande, Erika Freemantle, Dani Flinkman, Justyna Zdrojewska and Artur Padzik. 14. Björkblom, B., Vainio, J. C., Hongisto, V., Herdegen, T., Courtney, M. J., and Coffey, E. T. (2008) All JNKs can kill, but nuclear localization is critical for neuronal death. J Biol Chem 283, 19704-19713 15. Hongisto, V., Vainio, J. C., Thompson, R., Courtney, M. J., and Coffey, E. T. (2008) The Wnt pool of glycogen synthase kinase 3beta is critical for trophic-deprivation-induced neuronal death. Mol Cell Biol 28, 1515-1527 16. Johansen, L. D., Naumanen, T., Knudsen, A., Westerlund, N., Gromova, I., Junttila, M., Nielsen, C., Bøttzauw, T., Tolkovsky, A., Westermarck, J., Coffey, E. T., Jäättelä, M., and Kallunki, T. (2008) IKAP localizes to membrane ruffles with filamin A and regulates actin cytoskeleton organization and cell migration. J Cell Sci 121, 854-864 17. Westerlund, N., Zdrojewska, J., Courtney, M. J., and Coffey, E. T. (2008) Superior cervical ganglion-10 protein as a molecular effector of c-Jun N-terminal kinase 1: implications for the therapeutic targeting of Jun N-terminal kinase in nerve regeneration. Expert Opin Ther Targets 12, 31-43 18. Semenova, M. M., Mäki-Hokkonen, A. M., Cao, J., Komarovski, V., Forsberg, K. M., Koistinaho, M., Coffey, E. T., and Courtney, M. J. (2007) Rho mediates calcium-dependent activation of p38alpha and subsequent excitotoxic cell death. Nat Neurosci 10, 436-443 19. Tararuk, T., Ostman, N., Li, W., Björkblom, B., Padzik, A., Zdrojewska, J., Hongisto, V., Herdegen, T., Konopka, W., Courtney, M. J., and Coffey, E. T. (2006) JNK1 phosphorylation of SCG10 determines microtubule dynamics and axodendritic length. J Cell Biol 173, 265-277 20. Björkblom, B., Ostman, N., Hongisto, V., Komarovski, V., Filén, J. J., Nyman, T. A., Kallunki, T., Courtney, M. J., and Coffey, E. T. (2005) Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubuleassociated protein 2 as an effector. J Neurosci 25, 6350-6361 21. Ha, H. Y., Cho, I. H., Lee, K. W., Song, J. Y., Kim, K. S., Yu, Y. M., Lee, J. K., Song, J. S., Yang, S. D., Shin, H. S., and Han, P. L. (2005) The axon guidance defect of the telencephalic commissures of the JSAP1-deficient brain was partially rescued by the transgenic expression of JIP1. Dev Biol 277, 184-199 22. Cao, J., Semenova, M. M., Solovyan, V. T., Han, J., Coffey, E. T., and Courtney, M. J. (2004) Distinct requirements for p38alpha and c-Jun N-terminal kinase stress-activated protein kinases in different forms of apoptotic neuronal death. J Biol Chem 279, 35903-35913 23. Hongisto, V., Smeds, N., Brecht, S., Herdegen, T., Courtney, M. J., and Coffey, E. T. (2003) Lithium blocks the c-Jun stress response and protects neurons via its action on glycogen synthase kinase 3. Mol Cell Biol 23, 6027-6036 48 49 TRANSLATIONAL PROTEOMICS http://www.btk.fi/research/research-groups/corthals/ Principal investigator: Garry Corthals, PhD Turku Centre for Biotechnology, BioCity, Tykistökatu 6, FI-20521 Turku, Finland. Tel. +358-2-333 8889, Fax. +358-2-2158808. E-mail: [email protected]. Biography: Garry Corthals received his PhD in 1997 and has since worked in the field of biomedical proteomics. He has held positions at the Medical School, University of Washington, Seattle, the Garvan Institute for Medical Research, Sydney, and Geneva University Hospital and Medical Faculty. He is now leads a research group in Translational Proteomics at the Turku Centre for Biotechnology that focuses on developing and applying proteomics methods to improve personalized therapies and to understand protein level changes related to diseases primarily through the use of mass spectrometry and bioinformatics. Amongst his publications and books is the first book that appeared on Biomedical Applications in Proteomics. Besides the research group Dr Corthals is the Director of the Turku Proteomics Facility, is Chair of the Finnish Proteomics and Metabolomics technology platform, the Nordic Quantitative Proteomics network of research schools, the Nordic Signals research network and the Nordic MS imaging network and Chairs the new Developments Committee of the European Proteomics Association (EuPA). His is also co-chair of the pan European Imaging MS network. Personnel: Senior scientists: Anne Rokka, PhD; Dorota Muth-Pawlak, PhD; Mimi Nguyen, PhD; Susumu Imanishi, PhD; Tanya Lukash, PhD; Petri Kouvonen, PhD (visiting ETH Zurich 2011-2013) Graduate students: Anni Vehmas, Veronika Suni, Darshana Kottahachchi (visiting); Laboratory engineer: Arttu Heinonen; Technician: Pekka Haapaniemi; Coordinator: Susanne Nees. Apprentice: Nikolai Huwa. Description of the project: Our group’s focus is to develop and apply powerful proteomics tools to be used in translational and systems biology based projects, where technological developments are driven by biological questions. Of particular interest to our group are endometriosis, epilepsy and prostate cancer, as well as several others biomedical projects including the development of methods for quantitative proteomics and phosphorylation analysis driven by our group or through collaborative research. The group of researchers involved in our work has a diverse set of skills, ranging from chemistry and biochemistry, to clinical backgrounds, to computational scientists and mathematicians, reflecting a multidisciplinary environment. All of our research essentially evolves around applications in mass spectrometry (MS), which, over the past two decades MS, has emerged as the method of choice to discover, measure and characterize proteins and protein networks in biological systems. Left to right: Dorotha Muth-Pawlak, Eeva Rainio, Anne Rokka, Garry Corthals, Pekka Haapaniemi, Nikolai Huwa, Susumu Imanishi, Susanne Nees and Elisabeth Nguyen. 50 For the analysis of tissues we are interested in defining and measuring changes of proteins and peptides, which of these have an impact on 51 their microenvironment, which enter body fluids such as the blood system, and ultimately which impact on disease progression or reflect a disease state. We therefore require methods that enable highly sensitive identification and quantitation of proteins in tissues and body fluids. Measurement of proteins in tissues and tissue-substructures is pursued analysis of minute amounts of cryosectioned tissues, that ultimately enable exquisite detailing of the molecular components of cellular substructures, adding important molecular detail for regions of interest. The quantitative aspect of these measurements focuses on measuring protein change in tissues. To this end we are investigating novel computational methods that enable quantitative measurements of proteins in tissues. Another of interest for the group is the identification and quantitation of phosphopeptides and proteins. Again we have a two-tiered approach where we are developing both laboratory procedures as well as computational methods. Our recent observations have focused the on the use of planar surfaces that act as an enrichment and analytical platform for phosphopeptide analysis, paving the way for array based analyses. Our computational methods in phosphorylation analysis focus on increasing the speed and validation of phosphorylation analysis – nowadays seen as a bottleneck delaying true HTP phosphorylation analysis. Additionally we are developing several bioinformatics tools that allow the efficient investigation of proteomics workflows in the laboratory. Funding: The Systems Biology Research Program, Turku Centre for Computer Science Graduate Programme (TUCS), The National Graduate School in Informational and Structural Biology (ISB), the University of Turku, COST. Collaborators: Johanna Ivaska, Jukka Westermarck, Tiina Pakula (VTT), Laura Ruohonen (VTT), Laura Elo, Tuula Nyman, Thomas Kietzmann and Hannu Larjava. Selected Publications: Westermarck J, Ivaska J, Corthals GL. (2013) Identification of Protein Interactions Involved in Cellular Signalling. Mol Cell Proteomics. 12(7):1752-63 Santos HM, Kouvonen P, Capelo JL, Corthals GL. (2013) On-target ultrasonic digestion of proteins. Proteomics. 13(9):1423-7 Velasquez EV, Ríos M, Ortiz ME, Lizama C, Nuñez E, Abramovich D, Orge F, Oliva B, Orellana R, Villalon M, Moreno RD, Tesone M, Rokka A, Corthals G, Croxatto HB, Parborell F, Owen GI. (2013) Concanavalin-A induces granulosa cell death and inhibits FSH mediated follicular growth and ovarian maturation in female rats. Endocrinology. 154(5):1885-96. Tyagarajan SK, Ghosh H, Yevenes GE, Imanishi SY, Zeilhofer HU, Gerrits B, Fritschy JM. (2013) Extracellular Signal-regulated Kinase and Glycogen Synthase Kinase 3β Regulate Gephyrin Postsynaptic Aggregation and GABAergic Synaptic Function in a Calpain-dependent Mechanism. J Biol Chem. 288(14):9634-47 Koch S, Scifo E, Rokka A, Trippner P, Lindfors M, Korhonen R, Corthals G, Virtanen I, Lalowski M and Tyynelä J. (2013) Cathepsin D deficiency induces cytoskeletal changes and affects cell migration pathways in the brain. Neurobiol Dis. 50:107-119 52 ORGANISATION OF NEURONAL SIGNALING PATHWAYS www.uef.fi/aivi/neuro/signalling Principal investigator: Michael Courtney, PhD, Affiliated Group Leader at BTK, Professor of Cell Signaling at UEF. Molecular Signaling Laboratory, Department of Neurobiology, A.I. Virtanen Institute, University of Eastern Finland, P.O. Box1627, Neulaniementie 2, FIN-70211 Kuopio, Finland. Tel. +358 40 355 3663. Email: [email protected] Facility page: www.uef.fi/aivi/muic Biography: Michael Courtney (b. 1967) graduated from University of Cambridge in 1988 (B.A.), and the University of Dundee in 1991 (Ph.D). Postdoctoral Fellowships from the Royal Society, Wellcome Trust, Academy of Finland and Sigrid Jusélius Foundation supported his quantitative imaging development and application activities from 1992 in Prof. Karl Åkerman’s laboratory in Åbo Akademi University, Turku. After group leader positions at BTK from 1998, he was appointed from 2000 to a position at the A.I. Virtanen Institute, Kuopio and from 2006 to BTK. He has been affiliated with the Cell Imaging Core in Turku since its inception, and established and is director of the Multimodal Imaging Unit at Kuopio University, now the University of Eastern Finland. He was appointed to an Academy of Finland Researcher post from 2003-2008, and Professor of Cell Signaling at the University of Eastern Finland from 2008. Personnel: Post-doctoral Fellows: Peter Martinsson, PhD, Raquel MeleroFernandez de Mera, PhD, Olga Vergun, PhD; Graduate students: Lili Li, MSc, Uma Thanigaiarasu, MSc, Xijun Wang, MSc Technician Aila Seppänen; MUIC (Imaging Facility) Integration Specialist Jarkko Närväinen, MSc Description of the project: Disease states place cells under stressful conditions. Signaling pathways involving protein kinases p38MAPK, JNK and others are widely accepted to play a significant role in cell death in and outside the nervous system, they also contribute to development, differentiation, and even survival and proliferation. Thus drugs developed to directly target stress activated protein kinases may be of only limited use. To exploit the pathways for the development of novel neuroprotective drugs, we must elucidate the mechanisms that organise these pathways into pools with neurodegenerative or physiological functions within the complex architecture of neuronal cells. Only then can we begin to elective eliminate neurodegenerative activities of these pathways, which have been implicated neurodegenerative disease such as in Alzheimer’s disease and conditions such as cerebral ischaemia or stroke, increasingly major causes of death, disability and socioeconomic impact in society. 53 In recent years we focused on signaling pathways downstream of the NMDA receptor as they have immense translational potential, being implicated in chronic pain, depression and psychosis in addition to the neurodegenerative conditions mentioned above. The NMDA receptor GluN subunits form complexes with receptor scaffold PSD95 and nNOS leading to activation of neuronal p38 MAPK. These pathways have now demonstrated clinical potential as a drug targeting the GluN:PSD95 interaction is the first to succeed in a clinical trial for stroke. We showed that the interaction between PSD95 and nNOS is targetable by a peptide-competition approach, which can limit neurodegeneration via p38MAPK signaling in ex vivo cell culture models (Cao et al., 2005). The Lai lab and others developed small molecule inhibitors targeting this interaction and demonstrated in vivo efficacy using models of both neuropathic pain and cerebral ischaemia. A systematic analysis of the neuroprotective nNOS sequence we derived earlier (Cao et al., 2005) has now revealed an unexpected requirement for recruitment by nNOS of the protein NOS1AP (Li et al., 12013), which is encoded by a gene implicated in schizophrenia, sudden cardiac death and diabetic complications. We found this recruitment is also druggable. Furthermore NOS1AP binds MKK3, an activator of p38, the MAPK already implicated in neurodegeneration, depression and chronic pain. We have therefore embarked on a new project that combines the mapping of interaction surfaces, molecular modelling and in silico screening, systematic RNAi screens, cell-based interaction screens and high-throughput microscopy and other approaches. The aims are, amongst others, to develop a panel of inhibitory strategies for consideration in the wide range of NMDA receptorassociated disorders as well as derive genome-wide relevance of novel functional interactions. To this end we have established new collaborations to better address the translational value of this approach in the possible treatment of neuropathic pain, depression and schizophrenia as well as neurodegeneration (collaborations with Andrea Hohmann, Yvonne Lai, Andrew Harkin, Jari Tiihonen, Jari Koistinaho). To assist in these investigations we have continued to develop genetically-encoded optical reporters and actuators (optogenetics tools), in part via consortium projects of the Academy of Finland photonics programme. Previous activity in this area led to the development of novel imaging probes of cell death signalling (e.g. Hellwig et al., 2008; D’Orsi et al., 2012) and we have participated in the ROSIm consortium (coordinator Rashid Giniatullin, UEF) to facilitate the generation of new imaging reporters of reactiveoxygen species. Meanwhile, we coordinate the PhotoON consortium to develop a new optogenetic cassette compatible with regulatory peptide sequences we have been developing (see above), to facilitate the light-driven modulation of signaling pathways. As proof of principle we have initially selected the JNK pathway for optogenetic regulation (reported in Melero et al., 2013, SFN abstract 676.20; Hollos et al., 2013, SFN abstract 873.15), using the high affinity JNK-interacting sequence “JBD”, derived from the JIP class of MAPK scaffold proteins which can be used as a selective targetable inhibitor to discriminate the roles of distinct compartmentalized pools of kinase (e.g. Björkblom et al., 2005, 2008; Tararuk et al., 2006; Westerlund et al., 2011). 54 In addition we continue to maintain and develop the Multimodal Imaging Core facility at the University of Eastern Finland (www.uef. fi/aivi/muic), an open-access facility which provides instrumentation including widefield, confocal and TIRF microscopy as well as highthroughput microscopy and sample preparation facilities. Recent activities have focused on integration between 8/96/384 channel pipetting robot and plate reader/automated microscope, addition of high-speed sCMOS/LED-based imaging options and repair of the exisiting BD pathway 855-TwisterII-Cytomat Cell incubator system. Funding: The Photonics Programme of the Academy of Finland, the EU th 7 framework project “MEMOLOAD”, The University of Eastern Finland Innovative Research Initiatives, The Doctoral Programme in Molecular Medicine and the European Union 7th Framework Initial Training Network “ReBIRTH”. Collaborators: Andrea Hohmann and Yvonne Lai (University of Indiana Bloomington), Tibor Harkany and Jari Tiihonen (Karolinska Institute, Sweden), Jochen Prehn and Andrew Harkin (Trinity College Dublin), Eleanor Coffey and Tassos Papageorgiou (BTK, Åbo Akademi and University of Turku), Antti Poso, Jari Koistinaho and Rashid Giniatullin (University of Eastern Finland) and Anita Truttmann (University of Lausanne). Selected Publications: Tortoriello G, Morris CV, Alpar A, Fuzik J, Shirran SL, Calvigioni D, Keimpema E, Botting CH, Reinecke K, Herdegen T, Courtney M, Hurd YL, Harkany T. (2014) Miswiring the brain: Δ9tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 33(7):668-85. Li L-L, Ginet V, Liu X, Vergun O, Tuittila M, Mathieu M, Bonny C, Puyal J, Truttmann AC, Courtney MJ. (2013) The nNOSp38MAPK pathway is mediated by NOS1AP during neuronal death. J Neurosci. 33(19):8185-201. Björkblom B, Padzik A, Mohammad H, Westerlund N, Komulainen E, Hollos P, Parviainen L, Papageorgiou AC, Iljin K, Kallioniemi O, Kallajoki M, Courtney MJ, Mågård M, James P, Coffey ET. (2012) c-Jun N-terminal kinase phosphorylation of MARCKSL1 determines actin stability and migration in neurons and in cancer cells. Mol Cell Biol. 32(17):3513-26. D’Orsi B, Bonner H, Tuffy LP, Düssmann H, Woods I, Courtney MJ, Ward MW, Prehn JH (2012) Calpains Are Downstream Effectors of bax-Dependent Excitotoxic Apoptosis. J Neurosci. 32:1847-58. Westerlund N, Zdrojewska J, Padzik A, Komulainen E, Björkblom B, Rannikko E, Tararuk T, Garcia-Frigola C, Sandholm J, Nguyen L, Kallunki T, Courtney MJ, Coffey ET (2011) Phosphorylation of SCG10/stathmin-2 determines multipolar stage exit and neuronal migration rate. Nat Neurosci. 14:305-13. Yang H, Courtney MJ, Martinsson P, Manahan-Vaughan D (2011) LTD is enhanced, depotentiation is inhibited and LTP is unaffected by the application of a selective JNK inhibitor to the hippocampus of freely behaving rats. Eur J Neurosci.,33:1647-55. 55 Hellwig CT, Kohler BF, Lehtivarjo AK, Dussmann H, Courtney MJ, Prehn JH, Rehm M (2008) Real-time analysis of TRAIL/ CHXinduced caspase activities during apoptosis initiation. J Biol Chem. 283, 21676-85. COMPUTATIONAL BIOMEDICINE Björkblom B, Vainio JC, Hongisto V, Herdegen T, Courtney MJ, Coffey ET (2008) All JNKs can kill but nuclear localization is critical for neuronal death. J Biol Chem. 283, 19704-19713. Principal investigator: Laura Elo, PhD, Adjunct Professor in Biomathematics, Turku Centre for Biotechnology, and Department of Mathematics and Statistics, University of Turku, Tykistökatu 6A, FI-20521 Turku, Finland. Tel. +358-2-3338009, Fax. +358-2-2518808. E-mail: [email protected]. Hongisto V, Vainio JC, Thompson R, Courtney MJ, Coffey ET (2008) The Wnt pool of GSK-3β is critical for trophic deprivation induced neuronal death. Mol Cell Biol. 28, 1515-1527. Westerlund N, Zdrojewska J, Courtney MJ, Coffey ET (2008) SCG10 as a molecular effector of JNK1: Implications for the therapeutic targeting of JNK in nerve regeneration. Expert Opin Ther Targets, 12, 1-13. Semenova MM, Mäki-Hokkonen AM, Cao J, Komarovski V, Forsberg KM, Koistinaho M, Coffey ET, Courtney MJ (2007) Rho mediates calcium-dependent activation of p38α and subsequent excitotoxic cell death. Nat Neurosci. 10, 436-443. Tararuk T, Ostman N, Li W, Björkblom B, Padzik A, Zdrojewska J, Hongisto V, Herdegen T, Konopka W, Courtney MJ, Coffey ET (2006) JNK1 phosphorylation of SCG10 determines microtubule dynamics and axodendritic length. J Cell Biol. 173, 265-277. Björkblom B, Ostman N, Hongisto V, Komarovski V, Filén JJ, Nyman TA, Kallunki T, Courtney MJ, Coffey ET (2005) Constitutively active cytoplasmic JNK1 is a dominant regulator of dendritic architecture; role of MAP2 as an effector. J Neurosci. 25, 63506361. Cao J, Viholainen JI, Dart C, Warwick HK, Leyland ML, Courtney MJ (2005) The nNOS-PSD95 interface - a target for inhibition of excitotoxic p38 stress-activated protein kinase activation and cell death. J Cell Biol. 168, 117-126. Cao J, Semenova MM, Solovyan VT, Han J, Coffey ET, Courtney MJ (2004) Distinct requirements for p38α and JNK stress-activated protein kinases in different forms of apoptotic neuronal death. J Biol. Chem. 279, 35903-35913. Solovyan VT, Bezvenyuk ZA, Salminen A, Austin CA, Courtney MJ (2002) The role of topoisomerase II beta in the excision of DNA loop domains during apoptosis. J Biol Chem. 277, 2145821467. Coffey ET, Smiciene G, Hongisto V, Cao J, Brecht S, Herdegen T, Courtney MJ (2002) JNK2/3 is specifically activated by stress, mediating c-Jun activation, in the presence of constitutive JNK1 activity in cerebellar neurons. J Neurosci. 22, 4335-4345. Coffey ET, Hongisto V, Dickens M, Davis RJ, Courtney MJ (2000) Dual Roles for c-Jun N-terminal kinase in developmental and stress responses in cerebellar granule neurons. J Neurosci. 20, 7602-7613. Courtney MJ, Åkerman KEO, Coffey ET (1997) Neurotrophins protect cultured cerebellar granule neurons against the early phase of cell death by a two-component mechanism. J Neurosci. 17, 4201-4211. 56 http://www.btk.fi/research/research-groups/elo/ Biography: Laura Elo received her PhD in Applied Mathematics from the University of Turku in 2007. In 2008 she received a Post-doctoral Fellowship from the Academy of Finland. During that time, she did post-doctoral work with Prof. Riitta Lahesmaa, Molecular Immunology Group, Turku Centre for Biotechnology, and with Prof. Benno Schwikowski, Systems Biology Unit, Institut Pasteur, Paris, France. In 2009, she became also a principal investigator in the Data Mining and Modelling Group at University of Turku. Since 2011 she is an Adjunct Professor in Biomathematics at University of Turku. In 2013, she was awarded the highly competitive 5-year Career Development Award from the Juvenile Diabetes Research Foundation JDRF from the US and established an independent research group of Computational Biomedicine at University of Turku. Personnel: Post-doctoral Fellows: An Le Thi Thanh, PhD Graduate students: Saira Afzal, MSc, Bishwa Ghimire, MSc, Maria Jaakkola, MSc, Teemu Daniel Laajala, MSc, Asta Laiho, MSc, Anna Pursiheimo, MSc, Kalaimathy Singaravelu, MSc, Tomi Suomi, MSc Undergraduate students: Deepankar Chakroborty, B.Sc., Anna Koskinen, B.Sc., Oona Lehtinen, B.Sc., Mehrad Mahmoudian, B.Sc., Sami Rannikko, B.Sc., Rafael Santos, B.Sc., Fatemehsadat Seyednasrollah, B.Sc. Description of the project: We develop computational data analysis tools and mathematical modelling methods for biomedical research in close collaboration with experimental and clinical groups. A specific focus is on transforming high-dimensional molecular and clinical data into biomedical knowledge. While modern high-throughput biotechnologies, such as deep sequencing and mass-spectrometry-based proteomics, enable large-scale measurements of molecular events in health and disease, the experimental data alone are not sufficient for understanding the complex disease processes. Therefore, the goal of our research is to enable robust and reproducible interpretation of the data. Building on our previous computational, statistical and networkbased studies, we aim at establishing a computational framework that allows optimized integration and analysis of large-scale clinical and molecular data at multiple levels as well as heterogeneity between individuals. The ultimate goal is to improve the diagnosis, prognosis and treatment of complex diseases, such as diabetes 57 and cancer, by combining computational, experimental and clinical expertise. Funding: JDRF, The Academy of Finland, Päivikki and Sakari Sohlberg Foundation, Yrjö Jahnsson Foundation, The Finnish Cultural Foundation, The Finnish Cancer Foundation, The Finnish Funding Agency for Technology and Innovation (Tekes), University of Turku Graduate School (UTUGS), Turku Systems Biology Research Programme, Turku University Foundation Selected collaborators: Garry Corthals (Turku Centre for Biotechnology), David Goodlett (University of Maryland School of Pharmacy, Baltimore and Turku Centre for Biotechnology), Heikki Hyöty (University of Tampere), Panu Jaakkola (Turku University Hospital), Eija Korpelainen (CSC), Riitta Lahesmaa (Turku Centre for Biotechnology), Tarja Laitinen (Turku University Hospital and University of Turku), Olli Nevalainen (University of Turku), Howard Petrie (Scripps Florida), Tapio Salakoski (University of Turku), Benno Schwikowski (Institut Pasteur, Paris), Olli Simell (DIPP), Lucy Walker (University College London) pathways characterizes children with prediabetes in genomewide gene expression profiling. J Autoimmun. 35: 70-76. [IF 8.145] 8. Elo LL, Järvenpää H, Tuomela S, Raghav S, Ahlfors H, Laurila K, Gupta B, Lund RJ, Tahvanainen J, Hawkins RD, Oresic M, Lähdesmäki H, Rasool O, Rao KV, Aittokallio T, Lahesmaa R (2010). Genome-wide profiling of interleukin-4 and STAT6 transcription factor regulation of human Th2 cell programming. Immunity 32: 852-862. [IF 19.795] 9. Laajala E, Aittokallio T, Lahesmaa R, Elo LL (2009). Probelevel estimation improves the detection of differential splicing in Affymetrix exon array studies. Genome Biol. 10: R77. [IF 10.288] 10.Laajala TD, Raghav S, Tuomela S, Lahesmaa R, Aittokallio T, Elo LL (2009). A practical comparison of methods for detecting transcription factor binding sites in ChIP-seq experiments. BMC Genomics 10:618. [IF 4.397] Selected Publications: 1. Kallionpää H*, Elo LL*, Laajala E*, Mykkänen J, RicañoPonce I, Vaarma M, Laajala TD, Hyöty H, Ilonen J, Veijola R, Simell T, Wijmenga C, Knip M, Lähdesmäki H, Simell O and Lahesmaa R. Innate immune activity is detected prior to seroconversion in children with HLA-conferred type 1 diabetes susceptibility. To appear in Diabetes. (*equal contribution) [IF 7.895] 2. Seyednasrollah F, Laiho A, Elo LL. Comparison of methods for detecting differential expression in RNA-seq studies. To appear in Briefings in Bioinformatics. [IF 5.298] 3. Elo LL and Schwikowski B (2013) Analysis of Time-Resolved Gene Expression Measurements Across Individuals. PLoS ONE 8(12): e82340. [IF 3.730] 4. Lahti L, Torrente A, Elo LL, Brazma A, Rung J (2013). A fully scalable online pre-processing algorithm for short oligonucleotide microarray atlases. Nucleic Acids Res. 41: e110. [IF 8.278] 5. Elo LL, Schwikowski B (2012). Mining proteomic data for biomedical research. Invited review. WIREs Data Mining Knowl Discov. 2: 1-13. [IF 1.422] 6. Elo LL, Kallio A, Laajala TD, Hawkins RD, Korpelainen E, Aittokallio T (2012). Optimized detection of transcription factor binding sites in ChIP-seq experiments. Nucleic Acids Res. 40: e1. [IF 8.278] 7. Elo LL, Mykkänen J, Nikula T, Järvenpää H, Simell S, Aittokallio T, Hyöty H, Ilonen J, Veijola R, Simell T, Knip M, Simell O, Lahesmaa R (2010). Early suppression of immune response 58 From left to right: Maria Jaakkola, Deepankar Chakroborty, Fatemehsadat Seyednasrollah, Laura Elo, Teemu Daniel Laajala, Tomi Suomi, Anna Pursiheimo and An Le Thi Thanh. 59 CYTOSKELETAL AND SURVIVAL SIGNALING Principal Investigator: John E. Eriksson, PhD, Professor. Deptartment of Biology, Åbo Akademi University, FI-20520 Turku, Finland. Tel. int. + 358–2–215 3313. Laboratory address: Turku Centre for Biotechnology, BioCity, Tykistökatu 6B, P.O. Box 123, FIN-20521 Turku, Finland. Tel. int. + 358–2–333 8036, Fax int. +358–2–333 8000. E-mail: [email protected] Biography: John E. Eriksson (b. 1957) received his PhD at the Åbo Akademi University in 1990. He was a post-doctoral fellow at Northwestern University in the laboratory of Prof. Robert D. Goldman during 1990-1993 (Fogarty International Fellowship from the National Institutes of Health 1991-1993). In November 1993 he joined the Centre for Biotechnology as a Senior Research Fellow in Cell Biology. In 1999 he was appointed as Professor of Zoology at the Department of Biology, University of Turku. In 2006 he was appointed as Professor of Cell Biology at the Department of Biology, Åbo Akademi University and became Head of Cell Biology at the department in 2007. He is also the Chair of Turku BioImaging and Chair of the Biocenter Finland Imaging Infrastructure Network. Personnel: Post-doctoral Fellows: Fang Cheng, MD-PhD, Claire Hyder, PhD, Senthil Kumar, PhD, Preethy Paul, PhD, Graduate students: Josef Gullmets, MSc, Kimmo Isoniemi, MSc, Julia Lindqvist, MSc, Ponnuswamy Mohanasundaram, MSc, Erik Niemelä, MSc, Elin Torvaldson, MSc, Joanna Pylvänäinen, MSc, MSc, Alia Joko, MSc, Undegraduate students: Isabelle Mogollon, Michelle Lindström, Vilhelm Wikström, Num Wistbacka, Fanny Örn. Laboratory Technician: Helena Saarento. Description of the Project: Post-translational modifications (PTMs) modulate the activity of most eukaryotic proteins and are responsible for producing highly complex proteomes from relatively simple genomes. We use a selection of signaling networks that represent the core of our expertise to identify PTM targets and interactions when a cell is embarking upon fate-determining responses, such as activating transcriptional or post-translational defense and survival mechanisms or triggering death machineries. Our main models are apoptotic, stress-mediated, and cytoskeletal signaling and we are also interested in their interrelationship. By exploring the interactions between these completely different signaling modes, we hope to advance our understanding how critical intracellular signals are processed and integrated. We are especially interested in the interaction between death receptor, stress, and survival signaling. Early on, we observed that 60 growth signaling through the mitogen-activated kinase (MAPK/ ERK) pathway has a dominant inhibiting effect on apoptosis induced by death receptors (Fas, TRAIL, and TNF receptors) and have shown that this mode of regulation has ramifications both in regulating death receptor responses of recently activated T-cells and in the resistance of certain tumor cell lines to death receptor stimulation. On the other hand death receptors are also able to activate survival signals, both MAPK/ERK and NF-kB and stress signaling facilitates death receptor-mediated apoptosis in a independently of heat shock protein expression. The survival of cells is, therefore, determined by a continuum between these signaling modalities. An example of a signaling hub protein that affects the survival in all of the above signaling modes is c-FLIP, which is a specific inhibitor of death receptor signaling. Targeted FLIP degradation by ubiquitylation is responsible for the sensitization to death receptor signals following heat stress and during differentiation erythroid cells. We have found a PKCalpha/beta-mediated signaling module that regulates the turnover FLIP by an isoform and phosphorylation site-specific mechanism. These findings help understanding the regulation of death receptor responses during stress, fever, or inflammation, as well as during cell growth and differentiationrelated processes. Intermediate filaments (IFs) are major cytoskeletal proteins important for ultrastructural organization and protection against various mechanical and other types of stresses. We have established that intermediate filaments are important signaling determinants, a question that relates to how the organization of the cytoskeleton will affect different signaling modules. By employing the interactions of different IFs (keratin 8/18, vimentin, nestin) with their signaling partners as models, we have elucidated the relationship between the cytoskeletal structure and the signaling state of the cell, and how this relationship will affect cell differentiation, growth, and survival. We observed that IFs act as general scaffolds for signaling proteins, and have focused on the association of IFs with JNKs, Cdk5, PKC isoforms, 14-3-3, and surface adhesion molecules. Our findings include vimentin and nestin as regulators of adhesion, migration and invasion, showing that the IFs are form highly dynamic anchoring structures that are involved in organizing the surface molecules crucial for migration and invasion. Another topical highlight includes the discovery of nestin as regulator of Cdk5 signaling, forming a scaffold and rheostat for the Cdk5/ p35 signaling complex during the differentiation of muscle cells and in apoptosis of neuronal cells. We have also determined the roles of specific PTMs on nuclear lamins in regulating their turnover and organization. Collaborators: The studies on apoptosis-related signaling are done in collaboration with Ralph Budd (Univ. of Vermont), Marion McFarlane (Univ. of Leicester) and Lea Sistonen (Turku Centre for Biotechnology). The studies on IF-related signaling functions are carried out as a collaboration with Robert Goldman and Karen Ridge (Northwestern Univ., Chicago, USA), Johanna Ivaska (Univ. of Turku), and Thomas Magin (Univ. of Leipzig, German). 61 Funding: The Academy of Finland, TEKES, the European Union, the Finnish Cancer Organizations, the Sigrid Jusélius Foundation, the W. M. Keck Foundation, and the Åbo Akademi Foundation. Selected Publications: Hyder C.L., Lazaro G., Pylvänäinen J.W., Roberts M.W., Rosenberg S.M. & Eriksson J.E. (2014) Nestin regulates prostate cancer cell invasion by influencing FAK and integrin localisation and functions. J. Cell Sci., in press (Epub Mar 7, 2014). Kochin V., Shimi T., Torvaldson E., Adam S.A., Goldman A., Pack C.G., Melo-Cardenas J., Imanishi S.Y., Goldman R.D. & Eriksson J.E. (2014) Interphase phosphorylation of lamin A. J. Cell Sci., in press (Epub Apr 16, 2014). Paul P., Rajendran S.K., Peuhu E., Alshatwi A.A., Akbarsha M.A., Hietanen S. & Eriksson J.E. (2014) Novel action modality of the diterpenoid anisomelic acid causes depletion of E6 and E7 viral oncoproteins in HPV-transformed cervical carcinoma cells. Biochem. Pharmacol. 89:171-84. Peuhu E., Paul P., Remes M., Holmbom T., Eklund P., Sjöholm R., Eriksson J.E. (2013) The antitumor lignan Nortrachelogenin sensitizes prostate cancer cells to TRAIL-induced cell death by inhibition of the Akt pathway and growth factor signaling. Biochem. Pharmacol. 86:571-83. Ferraris S.E., Isoniemi K., Torvaldson E., Anckar J., Westermarck J. & Eriksson J.E. (2012). Nucleolar AATF regulates c-Junmediated apoptosis. Mol. Biol. Cell. 23: 4323-32. Karaman D.S., Desai D., Senthilkumar R., Johansson E.M., Råtts N., Odén M., Eriksson J.E., Sahlgren C., Toivola D.M. & Rosenholm J.M. (2012). Shape engineering vs organic modification of inorganic nanoparticles as a tool for enhancing cellular internalization. Nanoscale Res Lett. 7: 358 Mohseni, P., Sung, H.K., Murphy, A.J., Laliberte ,C.L., Pallari, H-M., Henkelman, M., Georgiou, J., Xie G., Quaggin, S.E., Thorner, P.S., Eriksson, J.E. & Nagy, A. (2011). Nestin is not essential for development of the CNS but required for dispersion of acetylcholine receptor clusters at the area of neuromuscular junctions. J. Neurosci. 31: 11547-52. Pallari H.M., Lindqvist J., Torvaldson E., Ferraris S.E., He T., Sahlgren C. & Eriksson J.E. (2011). Nestin as a regulator of Cdk5 in differentiating myoblasts. Mol. Biol. Cell 22: 1539-49. Toivonen H.T., Meinander A., Asaoka T., Westerlund M., Pettersson F., Mikhailov A., Eriksson J.E. & Saxen H. (2011).Modeling reveals that dynamic regulation of c-FLIP levels determines cellto-cell distribution of CD95-mediated apoptosis. J. Biol. Chem. 286: 18375-82. Yang J., Dominguez B., de Winter F., Gould T.W., Eriksson J.E. & Lee K.F. (2011). Nestin negatively regulates postsynaptic differentiation of the neuromuscular synapse. Nat. Neurosci. 14: 324-330. 62 Asaoka T., Kaunisto A. & Eriksson J.E. (2011). Regulation of cell death by c-FLIP phosphorylation. Adv. Exp. Med. Biol. 691: 625-30. Peuhu E., Kaunisto A., Laihia J.K., Leino L. & Eriksson J.E. (2010). Molecular targets for the protodynamic action of cis-urocanic acid in human bladder carcinoma cells. BMC Cancer. 10:521. Blom T., Bergelin N., Meinander A., Löf C., Slotte J.P., Eriksson J.E., Törnquist K. (2010). An autocrine sphingosine-1-phosphate signaling loop enhances NF-kappaB-activation and survival. BMC Cell Biol. 11: 45. Rosenholm J.M., Peuhu E., Bate-Eya L.T., Eriksson J.E., Sahlgren C. & Lindén M. (2010). Cancer-cell-specific induction of apoptosis using mesoporous silica nanoparticles as drug-delivery vectors. Small 6:1234-1241. Blomster H.A., Imanishi S.Y., Siimes J., Kastu J., Morrice N.A., Eriksson J.E. & Sistonen L. (2010). In vivo identification of sumoylation sites by a signature tag and cysteine-targeted affinity purification. J. Biol. Chem. 285:19324-9 de Thonel A., Ferraris S.E., Pallari H.M., Imanishi S.Y., Kochin V., Hosokawa T., Hisanaga S., Sahlgren C. & Eriksson J.E. (2010). Protein kinase Czeta regulates Cdk5/p25 signaling during myogenesis. Mol. Biol. Cell 21:1423-1434. Shen W.J., Patel S., Eriksson J.E., Kraemer F.B. (2010). Vimentin is a functional partner of hormone sensitive lipase and facilitates lipolysis. J. Proteome Res. 9:1786-1794. Peuhu E., Rivero-Müller A., Stykki H., Torvaldson E., Holmbom T., Eklund P., Unkila M., Sjöholm R. & Eriksson J.E. (2010). Inhibition of Akt signaling by the lignan matairesinol sensitizes prostate cancer cells to TRAIL-induced apoptosis. Oncogene 29:898-908. Imanishi S.Y., Kouvonen P., Smått J.H., Heikkilä M., Peuhu E., Mikhailov A., Ritala M., Lindén M., Corthals G.L. & Eriksson J.E. (2009). Phosphopeptide enrichment with stable spatial coordination on a titanium dioxide coated glass slide. Rapid Commun. Mass Spectrom. 23:3661-3667. Rosenholm J.M., Peuhu E., Eriksson J.E., Sahlgren C. & Lindén M. (2009). Targeted intracellular delivery of hydrophobic agents using mesoporous hybrid silica nanoparticles as carrier systems. Nano Lett. 9:3308-3311. Eriksson J.E., Dechat T., Grin B., Helfand B., Mendez M., Pallari H.M., Goldman R.D. (2009). Introducing intermediate filaments: from discovery to disease. J. Clin. Invest. 119:1763-1771 (review). Rosenholm J., Meinander A. Peuhu E., Niemi R., Eriksson J.E., Sahlgren C. & Lindén M. (2009). Selective uptake of porous silica nanoparticles by cancer cells. Amer. Chem. Soc. 27:197-206. Kaunisto A, Kochin V, Asaoka T, Mikhailov A, Poukkula M, Meinander A. & Eriksson JE. (2009). PKC-mediated phosphorylation regulates c-FLIP ubiquitylation and stability. Cell Death Differ.16:1215-26. 63 From left to right: Michelle Lindström, Carolyn Alia Joko, Fanny Örn, Senthil Rajendran, Ponnuswamy Mohana Sundaram, Kimmo Isoniemi, Fang Chen, Erik Niemelä, Helena Saarento, Claire Hyder, Vilhelm Wikström, Preethy Paul, Num Wistbacka, Elnaz Fazeli, Josef Gulmets and John Eriksson. Mikhailov A., Sokolovskaya A., Yegutkin G.G., Amdahl H., West A., Yagita H., Lahesmaa R., Thompson L.F., Jalkanen S., Blokhin D. & Eriksson J.E. (2008). CD73 participates in cellular multiresistance program and protects against TRAIL-induced apoptosis. J. Immunol. 181: 464-75. Meinander, A., Söderström, T.S., Kaunisto, A., Poukkula, M., Sistonen, L. and Eriksson, J.E. (2007) Fever-like hyperthermia controls T-lymphocyte persistence by inducing degradation of c-FLIPshort. J. Immunol. 178: 3944-53. Imanishi S.Y., Kochin V., Ferraris S.E., deThonel A., Pallari H-M., Corthals G.L. & Eriksson J.E. (2007). Reference-facilitated phosphoproteomics: fast and reliable phosphopeptide validation by mikro-LC-ESI-Q-TOF MS/MS. Mol. Cell. Proteomics 6: 13801391. Nieminen, M., Henttinen, T., Merinen, M., Marttila-Ichihara, F., Eriksson, J.E. and Jalkanen S. (2006) Vimentin function in lymphocyte adhesion and transcellular migration. Nat. Cell Biol. 8: 156-162. Kochin, V., Imanishi S.Y. and Eriksson, J.E. (2006) Fast track to a phosphoprotein sketch – MALDI-TOF characterization of TLCbased tryptic phosphopeptide maps at femtomolar detection sensitivity. Proteomics 6: 5676-82. Sahlgren, C.M., Pallari, H-P., He, T., Chou, Y-H., Goldman, R.D. and Eriksson, J.E. (2006) An essential role of a nestin scaffold for regulation of Cdk5/p35 signaling in oxidant-induced death of neuronal progenitor cells. EMBO J. 25: 4808-4819. Imanishi, S.Y., Kochin, V. and Eriksson, J.E. (2006) Optimization of phosphopeptide elution conditions in immobilized Fe(III) affinity chromatography. Proteomics 7: 174-176. Pallari, H.M. and Eriksson, J.E. (2006) Intermediate filaments as signaling platforms. Science STKE. 19: pe53 (review). Söderström, T.S., Nyberg, S., Nieminen, M.I. and Eriksson, J.E. (2005) CD95 capping is ROCK-dependent and dispensable for apoptosis. J. Cell Sci. 118: 2211-2223. Poukkula, M., Kaunisto, A., Hietakangas, V., Denessiouk, K., Katajamäki, T., Johnson, M.J., Sistonen, L. and Eriksson, J.E. (2005) Rapid turnover of c-FLIPshort is determined by its unique C-terminal tail. J. Biol. Chem. 280: 27345-27355. Goswami, A., Burikhanov, R., de Thonel, A., Fujita, N., Goswami, M., Zhao, Y., Eriksson, J.E., Tsuruo, T. and Rangnekar, V.M. (2005). Binding and phosphorylation of Par-4 by Akt is essential for cancer cell survival. (2005) Mol. Cell. 20: 33-44. Eriksson, J.E., He, T., Trejo-Skalli, A.V., Härmälä-Brasken, A.S., Hellman, J., Chou, Y.H. and Goldman, R.D. (2004) Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J. Cell Sci. 117:919-32. 64 65 EPIGENOMICS http://www.btk.fi/research/research-groups/hawkins/ Principal Investigator: David Hawkins, PhD, Turku Centre for Biotechnology, Biocity, 5th floor, Tykistökatu 6A, FI-20520, Finland. Tel. +358-2-3338094, Fax. +358-2-3338000. Email: [email protected]. Personnel: Post-doctoral Fellows: Kalyan Kumar Pasumarthy, PhD, Cristina Valensisi, PhD Description of the project: Epigenomics includes histone tail modifications, DNA methylation and noncoding RNAs. These factors are closely linked to transcriptional regulation, and provide unique signatures of cellular identity. The epigenome exhibits remarkable cellular specificities and is likely critical in defining unique cell populations such stem cells. Using next-generation sequencing and computational technologies, we are investigating how the epigenome plays a role in pluri- and multipotency of stem cells. We are also investigating the transcriptional regulation and unique signatures of cellular differentiation. Funding: Academy of Finland Collaborators: Riitta Lahesmaa, Turku Centre for Biotechnology. Harri Lähdesmäki, Aalto University. Riikka Lund, Turku Centre for Biotechnology. Saara Laitinen, Finnish Red Cross Blood Service. Timo Otonkoski, University of Helsinki Selected Publications: Hawkins RD*, Larjo A, Tripathi SK, Wagner U, Luu Y, Lonneberg T, Raghav S, Lee LK, Lund R, Ren B, Lähdesmäki H*, Lahesmaa R*. (2013). Global Chromatin State Analysis Reveals Lineage-Specific Enhancers During the Initiation of Human T helper 1 and T helper 2 Polarization. Immunity. 38(6):1271-1284. *Co-Corresponding Author. Xie W, Schultz MD, Lister R, Hou Z, Rajagopal N, Ray P, Whitaker JW, Tian S, Hawkins RD, Leung D, et al. (2013). Epigenomic Analysis of Multilineage Differentiation of Human Embryonic Stem Cells. Cell. 153(5):1134-1148. Hon G., Hawkins, R.D., Caballero O.L., Lo C., Lister R., Pelizzola M., Valsesia A., Ye Z., Kuan S., Edsall L.E., Camargo A.A., Stevenson B.J., Ecker J.R., Bafna V., Strausberg R.L., Simpson A.J. And Ren B. (2012) Global DNA hypomethylation coupled to repressive chromatin domain formation and gene silencing in breast cancer. Genome Res. 22: 246-258. Elo L.L., Kallio A., Laajala T.D., Hawkins R.D., Korpelainen E. and Aittokallio T. (2012) Optimized detection of transcription factor binding sites in ChIP-seq experiments. Nucl. Acids Res. 40: e1. Left to right: Cristina Valensisi and Kalyan Pasumarthy. 66 67 Hawkins R.D†., Hon G.C†., Yang C., Antosiewicz J.E., Lee L.K., Ngo Q.M., Klugman S., Ching K.A., Edsall L.E., Kuan S., Yu P., Liu H., Zhang X., Green R.D., Lobanenkov V.V., Stewart R., Thomson J.A. and Ren B. (2011) Dynamic chromatin states in human ES cells reveal potential regulatory sequences and genes involved in pluripotency. Cell Research. 21: 1393-1409. †Equal contribution of work. Lister R†., Pelizzola M†., Kida Y.S., Hawkins R.D., Nery J.R., Hon G., Antosiewicz-Bourget J., O’Malley R., Castanon R., Klugman S., Downes M., Yu R., Stewart R., Ren B., Thomas J.A., Evans R.M. and Ecker JR. (2011) Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 471: 68-73. †Equal contribution of work. Egelhofer T.A†., Minoda A†., Klugman S., Kolasinska-Zwierz P., Alekseyenko A.A., Gadel S., Gorchakov A.A., Gu T., Kharchenko P.V., Kuan S., Latorre I., Linder-Basso D., Luu Y., Ngo Q., Rechtsteiner A., Riddle N.C., Schwartz Y.B., Vielle A., Elgin S.C.R., Kuroda M.I., Park P.J., Pirrotta V., Ren B., Ahringer J., Strome S., Karpen G^., Hawkins R.D^. and Lieb J.D^. (2011) Assessment of histone-modification antibody quality. Nat. Struct. Mol. Biol. 18: 91-93. †Equal contribution of work; ^Co-corresponding Authors. Harris R.A., Wang T., Coarfa C., Nagarajan R.P., Hong C., Downey S.L., Johnson B.E., Fouse S.D., Delaney A., Zhao Y., Olshen A., Ballinger T., Zhou X., Forsberg K.J., Gu J., Echipare L., O’Geen H., Lister R., Pelizzola M., Xi Y., Epstein C.B., Bernstein B.E., Hawkins R.D., Ren B., Chung W.Y., Gu H., Bock C., Gnirke A., Zhang M.Q., Haussler D., Ecker J.R., Li W., Farnham P.J., Waterland R.A., Meissner A., Marra M.A., Hirst M., Milosavljevic A. and Costello J.F. (2010) Comparison of sequencing-based methods to profile DNA methylation and identification of monoallelic epigenetic modifications. Nat. Biotechnology. 28(10), 852-862. Elo L.L†., Järvenpää H†., Tuomela S†., Raghav S†., Ahlfors H., Laurila K., Gupta B., Lund R,J., Tahvanainen J., Hawkins R,D., Oresic M., Lähdesmäki H., Rasool O., Rao K,V., Aittokallio T. and Lahesmaa R. (2010) Genome-wide profiling of interleukin-4 and STAT6 transcription factor regulation of human Th2 cell programming. Immunity. 32: 852-862. Hawkins R.D†., Hon G.C†. and Ren B. (2010) Next-Generation Genomics: An Integrative Approach. Nat. Rev. Genetics. 11: 476-486. Hawkins R.,D†., Hon G.C†., Lee L.K., Ngo Q., Lister R., Pelizzola M., Kuan S., Edsall L.E., Ye Z., Espinoza C., AntosiewiczBourget J., Agarwahl S., Shen L., Ruotti V., Wang W., Stewart R., Thomson J.A., Ecker J.R. and Ren B. (2010) Distinct epigenomic landscapes of pluripotent and lineage-committed human cells. Cell Stem Cell. 6: 279-491. Lister R†., Pelizzola M†., Dowen R.H., Hawkins R.D., Hon G.C., Tonti-Filippini J., Nery J.R., Lee L.K., Edsall L.E., AntosiewiczBourget J., Ruotti V., Elwell A., Hernandez A., Stewart R., Millar A.H., Thomson J.A., Ren B. and Ecker J.R. (2009) Human DNA methylomes at single-base resolution reveal widespread cellspecific epigenetic signatures. Nature. 462: 315-322. Heintzman N.D†., Hon G†., Hawkins R.D†., Kheradpour P., Ching K.A., Stuart R.K., Harp L.F., Ching C.W., Liu H., Zhang X., Green R.D., Crawford G.E., Kellis M. and Ren B. (2009) Histone modifications at human enhancers reflect global cell-typespecific gene expression. Nature. 459: 108-112. 68 CELL ADHESION AND CANCER http://www.btk.fi/research/research-groups/ivaska/ Principal investigator: Johanna Ivaska, Professor, PhD, Turku Centre for Biotechnology, Tykistökatu 6, FI-20520 Turku, Finland; Phone: + 358 2 333 7954; FAX: + 358 2 2518808, email: [email protected] Biography: Johanna Ivaska (b. 1972) received her MSc in Biochemistry in 1995 and PhD in 2000 from the University of Turku. In 2000 she received a Post-doctoral Fellowship from the Academy of Finland. In 2001 she received the EMBO Long Term Fellowship. She was a post-doctoral fellow at Cancer Research UK LIR in Prof. Peter Parker’s laboratory during 2000-2003. She returned to Finland in 2003 and joined VTT Medical Biotechnology and University of Turku Centre for Biotechnology as senior research fellow of the Academy of Finland and established her own research group. She was selected as a member of the EMBO Young Investigator program for 2007-2009. She was nominated professor of Molecular Cell Biology at University of Turku for 2008-2014. Personnel: Post-doctoral Fellows: Antti Arjonen, PhD, Maria Georgiadou PhD, Elisa Närvä, PhD, Emilia Peuhu, PhD, Graduate students: Jonna Alanko, MSc, Nicola De Franceschi, MSc, Riina Kaukonen, MSc, Pranshu Sahgal, B.Sc., Reetta Virtakoivu, MSc, Undergraduate students: Johanna Lilja, Research assistant: Markku Saari, M.Sc (CIC, part-time), Technicians: Jenni Siivonen, Petra Laasola Description of the project We investigate the relationship between cell adhesion and cancer. Migration and cell proliferation are critically regulated by physical adhesion of cells to each other and to their non-cellular surroundings (i.e. extracellular matrix) mediated by a family of adhesion receptors called integrins. To unravel the cellular pathways regulated by integrins and to begin to understand the mechanisms involved we have performed genome-wide screens to identify integrin-binding intracellular proteins and regulators of integrin activity to gain novel insight into integrin signaling and traffic in cancer cells. Based on these findings we are currently actively investigating these topics: 1) regulation of integrin activity by SHARPIN in cell migration, development and cancer. 2) Cooperation between integrins and receptor-tyrosine kinases like Met. 3) Integrin endo/exocytic traffic in cancer cell invasion. 4) The functional role of vimentin and adhesion in EMT. With all these projects we aim to understand adhesion regulated signaling and the biological function of integrin membrane traffic in human malignancies. 69 Funding: ERC Starting Grant, Academy of Finland, Sigrid Juselius Foundation, Finnish Cancer Organizations, EMBO Selected Publications: Pouwels, J., De Franceschi, N., Rantakari, P., Auvinen, K., Karikoski, M., Mattila, E., Potter, C., Sundberg, J.P., Hogg, N., Gahmberg, C.G., Salmi, M. and Ivaska, J. (2013) SHARPIN regulates uropod detachment in migrating lymphocytes. Cell Rep. 2013 5:619-628. Bouvard, D., Pouwels, J., De Franceschi, N. and Ivaska, J. (2013) Integrin inactivators: balancing cellular functions in vitro and in vivo. Nat. Rev. Mol. Cell Biol. 14:430-442. Högnäs, G., Hämälistö, S., Rilla, K., Laine, J.O., Vilkki, V., Murumägi, A., Edgren, H., Kallioniemi, O. and Ivaska J. (2013) Aneuploidy facilitates oncogenic transformation via specific genetic alterations, including Twist2 upregulation. Carcinogenesis. 34:2000-2009. PKCε Regulation of an α5 Integrin-ZO-1 Complex Controls Lamellae Formation in Migrating Cancer Cells. Sci. Sign., 2 (77): ra32. Pellinen T., Tuomi, S., Arjonen, A., Wolf, M., Edgren, H., Meyer, H., Grosse, R., Kitzing, T., Rantala, JK., Kallioniemi O., Fässler, R., Kallio, M., and Ivaska, J. (2008) Integrin traffic regulated by Rab21 is necessary for cytokinesis. Dev. Cell, 15:371-385. Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA, Ivaska J. (2006) Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J. Cell Biol. 2006 173:767-80. Mattila E., Pellinen, T., Nevo, J., Vuoriluoto, K. Arjonen, A. and Ivaska, J (2005) Negative regulation of EGFR signalling via integrin α1β1mediated activation of protein tyrosine phosphatase TCPTP. Nat. Cell Biol. 7: 78-85. Arjonen, A., Alanko, J., Veltel, S., Ivaska, J. (2012) Distinct Recycling of Active and Inactive β1 Integrins. Traffic 13:610-625. Pellinen, T., Rantala, J.K., Arjonen, A., Mpindi, J-P., Kallioniemi, O. and Ivaska, J. (2012) A functional genetic screen reveals new regulators of β1-integrin activity. J Cell Sci. 125:649-661. Virtakoivu, R., Pellinen, T., Rantala, J.K., Perälä, M. and Ivaska, J. (2012) Distinct roles of AKT isoforms in regulating β1-integrin activity, migration and invasion in prostate cancer. Mol. Biol. Cell. 17:3357-3369. Högnäs, G., Tuomi, S., Veltel, S., Mattila, E., Murumägi, A., Edgren, H., Kallioniemi, O. and Ivaska, J. (2012) Cytokinesis failure due to derailed integrin traffic induces aneuploidy and oncogenic transformation in vitro and in vivo. Oncogene 31:3597-3606. Vuoriluoto, K., Haugen, H., Kiviluoto, S., Mpindi, J-P, Nevo, J., Gjerdrum, C., Lorens, J.B. and Ivaska, J. (2011) Vimentin regulates EMT induction and migration by governing Axl expression in breast cancer. Oncogene 30:1436-1448. Rantala, J.K., Pouwels, J., Pellinen, T., Veltel, S., Laasola, P., Potter, C., Duffy, T., Sundberg, J.P., Askari, J.A.-. Humphries, M., Kallioniemi, O., Parsons, M., Salmi, M. and Ivaska, J. (2011) Sharpin is an endogenous inhibitor of beta1-integrin activation. Nat. Cell Biol. 13:1315-1324. Mai, A., Veltel, S., Pellinen, T., Padzik, A., Coffey, E., Marjomäki, V. and Ivaska, J. (2011) Competitive binding of Rab21 and p120RasGAP to integrins regulates receptor trafficking in migrating cancer cells. J. Cell Biol. 194:291-306. Nevo, J., Mai, A., Tuomi, S., Pellinen, T., Pentikäinen, O.T., Heikkilä, P., Lundin, J., Joensuu, H., Bono, P. and Ivaska, J. (2010) Mammary derived growth inhibitor (MDGI) interacts with integrin α-subunits and suppresses integrin activity and invasion. Oncogene 29:6452-6463. Tuomi, S., Mai, A., Nevo, J., Laine, JO, Vilkki, V., Öhman, TJ., Gahmberg, CG., Parker, PJ. and Ivaska, J. (2009) 70 From left to right. Back row: Ghaffar Muharram, Riina Kaukonen, Maria Georgiadou, Markku Saari, Nicola De Franceschi, Elisa Närvä, Reetta Virtakoivu, Antti Arjonen and Pranshu Sahgal, Lilja. Front row: Jonna Alanko, Petra Laasola and Johanna Ivaska. 71 HYPOXIA IN CELL SURVIVAL Principal investigator: Panu Jaakkola, MD, PhD, Turku Centre for Biotechnology, Biocity, Tykistökatu 6B, P.O. Box 123, FIN-20521, Turku, Finland, Tel. +358 2 3338566, Fax. +358 2 3338000, E-mail: [email protected] Biography: Panu Jaakkola (b. 1965) received his MD in 1992 and PhD in 1998 at the University of Turku. In 1999 he received a Junior Fellowship from the Academy of Finland. He was a post-doctoral fellow at the University of Oxford in Prof. Peter Ratcliffe’s laboratory during 1999-2001. He joined the Turku Centre for Biotechnology in the fall 2001. In 2002-2007 he worked as a fellow of the Academy of Finland. Currently he is appointed as a senior research fellow by the medical faculty and is also a consultant at the department of medical oncology and radiation therapy at Turku university hospital. Personnel: Post-doctoral Fellow: Krista Rantanen, (PhD) Graduate students: Heidi Högel, (MSc), Petra Miikkulainen, (MSc), Jonna Silen, (MSc), Pekka Heikkinen, (MSc), Undergraduate student: Olli Metsälä, Technician: Taina Kalevo-Mattila Description of the project: Hypoxia (reduced O2 tension) is the main tissue damaging factor in normal tissue. In contrast, tumours use hypoxia as a growthpromoting factor. During ischemic assaults such as strokes, hypoxia activates apoptosis and leads to severe tissue damage. During cancer progression hypoxia causes inhibition of apoptosis and enhances tumour aggressiveness and metastasis. In keeping with this, it has been known for much of the past century that hypoxia causes resistance cancer treatments -both to chemotherapy and radiotherapy -and leads to poor prognosis. The aim of the project is to reveal mechanisms by which hypoxia regulates survival decisions in cancer progression. The reduced oxygen is sensed by a family of enzymes called the HIF prolyl hydroxylases (PHD1-3). Under normoxia the hypoxiainducible factor (HIF) is hydroxylated by PHDs at critical proline residues. This leads to ubiquitylation and proteosomal destruction of HIF by von Hippel-Lindau tumour suppressor (pVHL). Under hypoxic conditions the hydroxylation ceases and HIF is stabilised. HIF then exerts its effects by activation of some 200 genes. These have key functions in glucose homeostasis, angiogenesis, as well as cell survival and metastasis formation. HIF however, can only partially explain the effects of hypoxia on cell survival and cancer progression From left to right: Panu Jaakkola, Petra Miikkulainen, Krista Rantanen, Heidi Högel, Meraj Khan and Taina Kalevo-Mattila. 72 Our studies have revealed novel and separate functions for two PHD isoforms (PHD2 and -3) in regulating cell growth, survival and regulation of apoptosis. For example, we have shown that the PHD3 isoform selectively regulates cell cycle progression 73 under hypoxia by regulating the expression of p27kip1, a cell cycle controlling kinase inhibitor. Moreover, we have demonstrated a strong interplay between the oxygen sensing and autophagy pathways for example through p62/SQSTM1. Besides studying several aspects of molecular and cellular biology of the hydroxylases, we study the clinical importance of these factors having a particular interest in renal clear cell and other carcinoma progression. Funding: Sigrid Juselius Foundation, Finnish Cancer Unions. Turku University Hospital (EVO), Turku University Foundation Collaborators: Peter Ratcliffe and Chris Pugh (Oxford University, UK), Eric Metzen (Luebeck University, Germany), Heikki Minn (PET Centre, Turku University Hospital) Selected Publications: Rantanen K., Pursiheimo J., Högel H., Miikkulainen P., Sundström J. and, Jaakkola P.M. (2012). p62/SQSTM1 regulates hypoxia response by attenuating normoxic PHD3 activity through aggregate sequestration and enhanced degradation. J Cell Sci. Jan 23. squamous cell carcinoma associates with tumor aggressiveness. Clin Cancer Res 12(4):1080-1087 Marxsen, J. H., Stengel, P., Doege, K., Heikkinen, P., Jokilehto, T., Wagner, T., Jelkmann, W., Jaakkola, P., and Metzen, E. (2004) Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases. Biochem J 381, 761 Jaakkola, P., Mole, D. R., Tian, Y. M., Wilson, M.I., Gielbert, J., Gaskell, S.J., Kriegsheim, Av, Hebestreit, H.F., Mukherji, M., Schofield, C.J., Maxwell, P.H., Pugh, C.W., Ratcliffe, P.J. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. (2001) Science 292; 468-72. Epstein, A.C.R., Gleadle, J.M., McNeill, L.A., Hewitson, K.S., O’Rourke, J., Mole, D.R., Mukherji, M., Metzen, E., Wilson, M.I., Dhanda, A., Tian, Y.-M., Masson, N., Hamilton, D.L., Jaakkola, P., Barstead, R., Hodgkin, J., Maxwell, P.H., Pugh, C.W., Schofield, C.J., Ratcliffe, P.J. C.elegans EGL-9 and mammalian homologues define a family of dioxygenases that regulate HIF through prolyl hydroxylation. (2001) Cell 107; 4354. Högel H., Rantanen K., Jokilehto T., Grenman R. and, Jaakkola P.M. (2011). Prolyl hydroxylase PHD3 enhances the hypoxic survival and G1 to S transition of carcinoma cells. PloS One 6(11):e27112 Jokilehto T., Jaakkola P.M. (2010) The role of HIF prolyl hydroxylases in tumor growth. J Cell Mol Med. 14(4):758-70. Heikkinen P., Nummela M., Kähäri V.M. and Jaakkola P.M. (2010). Hypoxia converts Smad7 from tumor suppressor into tumor promoter. Cancer Res., 70(14):5984-93 Heikkinen P.T., Nummela M., Leivonen S.K., Westermarck J., Hill C.S., Kähäri V.-M., Jaakkola P.M. (2010). Hypoxia activated Smad3-specific dephosphorylation by PP2A. (2010). J Biol. Chem., 285(6):3740-9. Epub 2009 Dec 1. Jokilehto T., Högel H., Heikkinen, P., Rantanen K., Elenius, K., Sundström J., Jaakkola P.M. (2010). Retention of prolyl hydroxylase PHD2 in the cytoplasm prevents PHD2-induced anchorage-independent carcinoma cell growth. Exp. Cell Res. 316(7):1169-78 Pursiheimo J., Rantanen K., Heikkinen P.T., Johansen T., Jaakkola P.M. (2009). Hypoxia-activated autophagy accelerates degradation of SQSTM1/p62. Oncogene, 28(3):334-344. Rantanen K., Pursiheimo J., Högel H., Himanen V., Metzen E., Jaakkola P.M. (2008) Prolyl Hydroxylase PHD3 Activates Oxygen-dependent Protein Aggregation. Mol Biol Cell 19(5): 2231-40. Jokilehto, T., Rantanen, K., Luukkaa, M., Grenman, R., Minn, H., Kronqvist, P., Jaakkola P.M. (2006). Overexpression and nuclear translocation of HIF prolyl hydroxylase PHD2 in head and neck 74 75 MITOSIS AND DRUG DISCOVERY Principal investigator: Marko Kallio, PhD Docent, Principal Scientist and Team Leader, Affiliated Group Leader at CBT, VTT Biotechnology for Health and Wellbeing, Itäinen Pitkäkatu 4C, FI-20521, Turku,Finland and Turku Centre for Biotechnology, BioCity, Tykistökatu 6B, FI-20521 Turku, Finland. Tel. +358-(50)-4097725, Fax +358(0)20-7222840, E-mail: [email protected], [email protected] Biography: Marko Kallio (b. 1967) graduated in Genetics from University of Turku in 1992 and received his PhD degree from Department of Human Genetics at University of Turku 1996. During his early career Dr. Kallio was in three laboratories; 1996-98 as a Post-doctoral Fellow in the laboratory of Prof. Gary Gorbsky (Univ. Virginia, USA), 19982000 as a Senior Post-doctoral Fellow in the laboratories of Prof. John Eriksson and Prof. Lea Sistonen (Univ. Turku, Finland) and 2000-2003 as an Assistant Research Professor at the University of Oklahoma HSC, USA. In early 2004, Dr. Kallio moved back to Finland and has since been a Team leader at VTT Biotechnology for Health and Wellbeing, a research institute affiliated with the University of Turku. Personnel: Post-doctoral Fellows: Leena Laine, PhD, Elli Narvi, PhD, Graduate students: Jenni Mäki-Jouppila, MSc, MSc, Mahesh Tambe, MSc Sofia Pruikkonen Description of the projects: The Mitosis and Drug Discovery Team investigates mechanisms of cell division in somatic cells and in meiotic systems. Study of cell division errors may help to explain origin of genomic instability and can lead to discovery of novel therapeutic possibilities and diagnostics opportunities in the fight against cancer. We are especially interested of conditions that suppress cancer cell’s viability as a consequence of premature inactivation of the spindle assembly checkpoint (SAC), a conserved signalling pathway which monitors the fidelity of mitosis. In our main projects, we are working to validate the mechanism of action of our putative anti-Hec1 compounds and SAC targeting miRNAs that effectively perturb normal mitosis and trigger cancer cell killing in cell culture assays. Funding: The Academy of Finland, VTT Technical Research Centre of Finland, DRDP and TuDMM Graduate Schools, Bayer Schering Pharma AG, The Finnish Cancer Organisations, The Cultural Foundation of Finland From left to right: Leena Laine, Elli Narvi, Mahesh Tambe, Jenni Mäki-Jouppila and Marko Kallio. 76 77 Collaborators: Gary Gorbsky (OMRF, Oklahoma USA), Todd Stukenberg (Univ. Virginia, USA), Lauri Aaltonen (Biomedicum Helsinki), Lea Sistonen (Turku Centre for Biotechnology), Pirkko Härkönen (Univ. Turku), Klaus Elenius (Univ. Turku). MOLECULAR SYSTEMS IMMUNOLOGY AND STEM CELL BIOLOGY http://www.btk.fi/research/research-groups/lahesmaa/ Selected Publications: Salmela AL, Pouwels J, Mäki-Jouppila J, Kohonen P, Toivonen P, Kallio L, and Kallio M. (2013) Novel pyrimidine-2,4-diamine derivative suppresses the cell viability and spindle assembly checkpoint activity by targeting Aurora kinases. Carcinogenesis 34: 436-45. Narvi E, Jaakkola K, Winsel S, Oetken-Lindholm C, Halonen P, Kallio L, Kallio MJ. (2013) Altered TUBB3 expression contributes to the epothilone response of mitotic cells. Br. J. Cancer 108: 82-90. Salmela AL, Kallio MJ. (2013) Mitosis as an anti-cancer drug target. Chromosoma 122: 431-49. Nilsson EM, Brokken LJ, Narvi E, Kallio MJ, Härkönen PL. (2012) Identification of fibroblast growth factor-8b target genes associated with early and late cell cycle events in breast cancer cells. Mol. Cell Endocrinol. 358: 104-15 Salmela AL, Pouwels J, Kukkonen-Macchi A, Waris S, Toivonen P, Jaakkola K, Mäki-Jouppila J, Kallio L, Kallio MJ. (2012) The flavonoid eupatorin inactivates the mitotic checkpoint leading to polyploidy and apoptosis. Exp. Cell Res. 318: 578-92 Niittymäki I, Gylfe A, Laine L, Laakso M, Lehtonen HJ, Kondelin J, Tolvanen J, Nousiainen K, Pouwels J, Järvinen H, Nuorva K, Mecklin JP, Mäkinen M, Ristimäki A, Ørntoft TF, Hautaniemi S, Karhu A, Kallio MJ, Aaltonen LA. (2011) High frequency of TTK mutations in microsatellite-unstable colorectal cancer and evaluation of their effect on spindle assembly checkpoint. Carcinogenesis 32: 305-11. Vuoriluoto M, Laine LJ, Saviranta P, Pouwels J, Kallio MJ. (2011) Spatio-temporal composition of the mitotic Chromosomal Passenger Complex detected using in situ proximity ligation assay. Mol. Oncol. 5: 105-11. Kukkonen-Macchi A, Sicora O, Kaczynska K, Oetken-Lindholm C, Pouwels J, Laine L, and Kallio MJ. (2011) Loss of p38gamma MAPK induces pleiotropic mitotic defects and massive cell death. J. Cell Sci. 124: 216-27. Principal investigator: Riitta Lahesmaa, Professor, Director. Turku Centre for Biotechnology, BioCity, Tykistökatu 6A, FI-20521 Turku, Finland. Tel +358-2-333 8601, Fax +358-2-2518808. E-mail: riitta.lahesmaa [at] btk.fi. Biography: Riitta Lahesmaa received her MD in 1984 and PhD in 1987 from the University of Turku, and was appointed Docent in Immunology in 1990. She was a post-doctoral fellow at Stanford University Medical Center with Professor Lawrence Steinman during the years 1990-1993 (NIH Fogarty Fellowship). In 1994 she moved to Syntex Research Institute (later Roche Bioscience) in Palo Alto, California. As a Principal Scientist she focused on lymphocyte signaling and drug discovery with state-of-the-art functional genomics tools. In 1998 she was appointed Director of Turku Centre for Biotechnology. In 2009 she carried out research in Professor Anjana Rao’s laboratory in Immune Disease Institute, Harvard Medical School, Boston. She also directs BioCity Turku Research Program “Turku Centre for Systems Biology” since 2000. Personnel: Laboratory Manager: Anne Lahdenperä, PhD Visiting Professors/Scientists: Professor David Goodlett, Finland Distinguished Professor, University of Baltimore, U.S., Professor Anjana Rao, La Jolla Institute for Allergy & Immunology, La Jolla, U.S., Dr. Kanury Rao, International Centre for Genetic Engineering and Biotechnology, New Delhi, India, Dr. Brigitta Stockinger, National Institute for Medical Research, London, U.K., Professor Cisca Wijmenga, University of Groningen, The Netherlands Senior Scientists: Riikka Lund, PhD, Adjunct Professor, Robert Moulder, PhD, Omid Rasool, PhD, Adjunct Professor, Jussi Salmi, PhD Post-doctoral Fellows: Sanna Edelman, PhD, Maheswara Reddy Emani, PhD, Saara Hämälistö, PhD, Sari Lehtimäki, PhD, Niina Lietzen, PhD, Elizabeth Ngyen, PhD, Nelly Rahkonen, PhD, Soile Tuomela, PhD, Ubaid Ullah, PhD, Viveka Öling, PhD Graduate students: Santosh Bhosale, MSc, Mirkka Heinonen, MSc, Henna Kallionpää, MSc, Kartiek Kanduri, MSc, Mohd Moin Khan, MSc, Essi Laajala, MSc (tech), Verna Salo, MSc, Subhash Tripathi, M.Tech, MSc Technicians: Marjo Hakkarainen, Sarita Heinonen, Päivi Junni, Elina Pietilä Undergraduate students: Obaiah Dirasantha, Antti Hurme, Jussi Jalonen, Sakari Kosola, Aki Stubb, Miro Viitala 78 79 Description of the project Molecular mechanisms of T cell activation and differentiation to functionally distinct subsets. These studies are carried out in the Academy of Finland Centre of Excellence on Molecular Systems Immunology and Physiology, where we are responsible for Molecular Systems Immunology research. Immune-mediated diseases such as type 1 diabetes, rheumatoid arthritis, asthma and allergies result from abnormal immune response. T lymphocytes that orchestrate the immune response can differentiate into functionally distinct lineages to combat infection and disease. The correct response to cytokines and a controlled balance of T lymphocyte populations are critical for the immune system and for the avoidance of autoimmune disorders. Dissecting pathways and regulatory networks leading to the development of T helper 1 (Th1), Th2, Th17 or regulatory T cells (Treg) is essential to understand the pathogenesis of allergy and inflammatory diseases. Th2 cytokines lead to a series of inflammatory processes characteristic for asthma and other atopic diseases whereas Th1 and Th17 cells play a role in the pathogenesis of autoimmune diseases (e.g. type I diabetes). Treg cells have an important role in inhibiting all these T effector cell functions. Detailed analysis of upstream T cell Receptor (TCR)/key cytokine receptor induced differentiation will increase our understanding of these processes central for human health and disease and provide novel insights into new therapeutic interventions. The research highlight of the year was to discover a novel mechanism regulating the immune response that can contribute to the susceptibility for autoimmune diseases (Hawkins et al. 2013). By combining state-ofthe art techniques, next-generation deep sequencing and computational data mining we found new epigenetic factors that regulate lymphocyte function. Regulatory regions of the genes studied displayed variations (single nucleotide polymorphisms or SNPs) that have been associated with predisposition to autoimmune diseases such as type 1 diabetes, rheumatoid arthritis and inflammatory bowel disease. Such variations were able to influence the binding of transcription factors that regulate gene expression. These discoveries provide new insights into and basis for the study of emergent mechanisms of immune-mediated diseases. Our results have led to novel hypotheses on the key factors involved in human Th cell differentiation (reviewed in Chen et al. 2013, Lehtimäki & Lahesmaa 2013, Lönnberg et al. 2013 and Tuomela & Lahesmaa 2013). Our key goal is to elucidate their functions further and to understand how human T cell response can be modulated. Type 1 diabetes (T1D) is the most common metabolic-endocrine disorder in children in western countries and the annual incidence of T1D in Finland is record high. In almost all children, progression to clinical T1D is associated with the presence of β cell specific autoantibodies. Clinical T1D occurs when a substantial proportion of the β cells have been destroyed or impaired in their function. At this point T1D patient is dependent on a daily insulin substitution for the rest of his/her life and there is a high risk of developing acute and long-term complications. Development of early diagnostics would enable early therapy and possibly preventive treatments resulting in a significant reduction in the health care costs. To investigate the genes and molecular pathways in the pathogenesis of T1D we performed genome-wide transcriptomics analysis on a unique series of prospective whole-blood RNA samples from atrisk children collected in the Finnish Type 1 Diabetes Prediction and Prevention (DIPP) study Kallionpää et al, Diabetes, 2014). We studied 28 autoantibody-positive children, out of which 22 progressed to clinical disease. Collectively the samples covered the time span from before the development of autoantibodies (seroconversion) through the 80 diagnosis of diabetes. Healthy controls matched for date and place of birth, gender and HLA-DQB1 susceptibility were selected for each case. Additionally, we genotyped the study subjects to identify potential genetic variants associated with the observed transcriptional signatures. Genes and pathways related to innate immunity functions, such as the type 1 interferon response, were active and interferon response factors (IRFs) were identified as central mediators of the interferon-related transcriptional changes. Importantly, this signature was detected already before the type 1 diabetes associated autoantibodies were detected. Together, the data provide a unique starting point for new hypotheses explaining type 1 diabetes biology. Human embryonic stem cells (hESC) have a unique capacity to differentiate to any type of cell or tissue providing an enormous potential for therapeutic applications (Lund et al. 2012). Our results indicate that it is essential to monitor stem cell lines carefully to minimize the risk of malignancies in stem cell therapies. (Närvä et al. 2010, Hussein S, et al. 2011). Our goal is to elucidate the molecular mechanisms regulating self renewal and pluripotency of hESC and induced pluripotent stem cells (iPS). We have identified novel genes and signaling pathways characteristic for the pluripotent hESC and iPS cells based on a genome wide transcriptome analyses of hESC. This resulted in the discovery of a RNA binding protein L1TD1 selectively expressed in stem cells and required for hESC renewal (Närvä et al. 2012) and a data base (Kong et al. 2013) to facilitate stem cell research. We also aim at understanding the role of L1TD1 and its regulatory network in human cancer. Funding: The Academy of Finland – including Academy of Finland Centre of Excellence in Molecular Systems Immunology and Physiology Research, The National Technology Agency of Finland (TEKES), JDRF, The Sigrid Jusélius Foundation, The Finnish Cancer Organizations, Turku University Hospital Fund, Graduate Schools (TuBS, ISB, Turku Doctoral Programme of Molecular Medicine), University of Turku, Åbo Akademi University, European Research Council, EU 7th framework projects “SYBILLA”, “DIABIMMUNE”, “NANOMMUNE”, “PEVNET”, “Nanosolutions”, EraSysBioPlus. Key Collaborators: Ruedi Aebersold & Matthias Gstaiger (ETZ, Zürich, Swizerland), Reija Autio (Tampere University of Technology ), Christopher Burge (MIT, Cambridge, MA, USA), Sanjeev Galande (IISER, Pune, India), Heikki Hyöty (U. Tampere), Mikael Knip (U. Helsinki), Harri Lähdesmäki (Aalto University, CBT), David Goodlett (University of Washington, Seattle, WA, USA and a FiDiPro in CBT) , Matej Oresic (CBT and Steno Diabetes Center), Anjana Rao (La Jolla Institute for Allergy and Immunology, San Diego, CA, USA and visiting professor at CBT), Kanury V.S. Rao (ICGEB, New Delhi, India and visiting professor at CBT), Bing Ren (Ludwig Institute for Cancer Research, University of California, San Diego, USA), Jorma Toppari and Olli Simell (U. Turku), Brigitta Stockinger (NIMR, London, UK and visiting professor at CBT), Thomas Tushl (Rockefeller University, New York, NY, USA), Cisca Wijmenga (University of Groningen, The Netherlands and visiting professor at CBT) Selected Recent Publications: Ahlfors H, Limaye A, Elo LL, Tuomela S, Burute M, Gottimukkala K, Notani D, Rasool O, Galande S, Lahesmaa R. SATB1 dictates expression of multiple genes including IL-5 involved in human T helper cell differentiation. Blood. 2010 Sep 2;116(9):1443-53. 81 82 From left to right. Front row: Essi Laajala, Moin Mohd Khan, Robert Moulder, Elina Pietilä, Henna Kallionpää, Sarita Heinonen, Riitta Lahesmaa, Maheswara Reddy Emani, Elizabeth Ngyen, Obaiah Dirasantha. Second row: Anne Lahdenperä, Marjo Hakkarainen, Nelly Rahkonen, Sanna Edelman, Mirkka Heinonen, Kartiek Kanduri, Omid Rasool, Jussi Jalonen, Sari Lehtimäki, Ubaid Ullah, Subash Tripathi, Riikka Lund, Jussi Salmi and Jane Chen Zhi. Third row: Verna Salo, Santosh Boshale and Miro Viitala. Chen Z, Lönnberg T, Lahesmaa R. Holistic systems biology approaches to molecular mechanisms of human helper T cell differentiation to functionally distinct subsets. Scand J Immunol. 2013, 78:172-80. Elo LL#, Järvenpää H#, Tuomela S#, Raghav S#, Ahlfors H, Laurila K, Gupta B, Lund RJ, Tahvanainen J, Hawkins RD, Orešicˇ; M, Lähdesmäki H, Rasool O, Rao KVS*, Aittokallio T*, Lahesmaa R. Genome-wide profiling of interleukin-4 and STAT6 transcription factor regulation of human Th2 cell programming. Immunity. 2010 Jun 25;32(6):852-62. #, * Equal contribution. Hawkins, R. D., Larjo, A., Tripathi, S. K., Wagner, U., Luu, Y., Lönnberg, T., Raghav, S. K., Lee, L. K., Lund, R., Ren, B., Lähdesmäki, H., and Lahesmaa R. (2013) Global chromatin state analysis reveals lineagespecific enhancers during the initiation of human T helper 1 and T helper 2 cell polarization. Immunity. 38, 1271-1284 Hussein S, Batada N, Vuoristo S, Autio R, Närvä E, Ng S, Hämäläinen R, Olsson C, Lundin K, Mikkola M, Trokovic R, Peitz M, Brüstle O, Alitalo K, Lahesmaa R, Nagy A #, Otonkoski T# Increased mutation load is associated with reprogramming of human somatic cells .#.Equal contribution. Nature, (2011) 471:58-62. Kallionpää H, Elo LL, Laajala E, Mykkänen J, Ricaño-Ponce I, Vaarma M, Laajala TD, Hyöty H, Ilonen J, Veijola R, Simell T, Wijmenga C, Knip M, Lähdesmäki H, Simell O, Lahesmaa R. Innate immune activity is detected prior to seroconversion in children with HLA-conferred type 1 diabetes susceptibility. Diabetes. 2014 Feb 18. [Epub ahead of print] Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J, Laiho A, Tahiliani M, Sommer CA, Mostoslavsky G, Lahesmaa R, Orkin SH, Rodig SJ, Daley GQ, Rao A. Tet1 and tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011, 8:200-13. Kong, L., Aho, K. L., Granberg, K., Lund, R., Järvenpää, L., Seppälä, J., Gokhale, P., Leinonen, K., Hahne, L., Mäkelä, J., Laurila, K., Pukkila, H., Närvä, E., Yli-Harja, O., Andrews, P. W., Nykter, M., Lahesmaa, R., Roos, C., and Autio, R. (2013) ESTOOLS Data@Hand: human stem cell gene expression resource. Nat Methods. 10, 814-815 Lehtimäki, S., and Lahesmaa, R. (2013) Regulatory T Cells Control Immune Responses through Their Non-Redundant Tissue Specific Features. Front Immunol. 4:294. Lund RJ, Närvä E, Lahesmaa R. Genetic and epigenetic stability of human pluripotent stem cells. Nat Rev Genet. 2012 Sep 11. doi: 10.1038/ nrg3271. Review. Lönnberg, T., Chen, Z., and Lahesmaa, R. (2013) From a gene-centric to whole-proteome view of differentiation of T helper cell subsets. Brief Funct Genomics. 12, 471-482 Närvä E, Autio R, Rahkonen N, Kong L, Harrison N, Kitsberg D, Borghese L, Itskovitz-Eldor J, Rasool O, Dvorak P, Hovatta O, Otonkoski T, Tuuri T, Cui W, Brüstle O, Baker D, Maltby E, Moore HD, Benvenisty N, Andrews PW, Yli-Harja O & Lahesmaa R. High resolution genome wide DNA analysis on a large panel of Human Embryonic Stem Cell lines reveals novel genomic changes associated with cultureand affecting gene expression. Nat Biotechnol. 2010 Apr;28(4):371-7. O’Shea JJ, Lahesmaa R, Vahedi G, Laurence A, Kanno Y. Genomic views of STAT function in CD4+ T helper cell differentiation. Nat Rev Immunol. 2011 Apr;11(4):239-50. Review. Roncagalli R, Hauri S, Fiore F, Liang Y, Chen Z, Sansoni A, Kanduri K, Joly R, Malzac A, Lähdesmäki H, Lahesmaa R, Yamasaki S, Saito T, Malissen M, Aebersold R, Gstaiger M, Malissen B. Quantitative proteomics analysis of signalosome dynamics in primary T cells identifies the surface receptor CD6 as a Lat adaptor-independent TCR signaling hub. Nat Immunol. 2014, 15:384-92. Tahvanainen J, Kallonen T, Lähteenmäki H, Heiskanen KM, Westermarck J, Rao KV, Lahesmaa R. PRELI is a mitochondrial regulator of human primary T helper cell apoptosis, STAT6 and Th2 cell differentiation. Blood. 2009 Feb 5;113(6):1268-77. Epub 2008 Oct 22 Tuomela S, Salo V, Tripathi SK, Chen Z, Laurila K, Gupta B, Äijö T, Oikari L, Stockinger B, Lähdesmäki H, Lahesmaa R. Identification of early gene expression changes during human Th17 celldifferentiation. Blood. 119: e151-160, 2012. Tuomela, S., and Lahesmaa, R. (2013) Early T helper cell programming of gene expression in human. Semin Immunol. 25, 282-290 83 COMPUTATIONAL SYSTEMS BIOLOGY http://users.ics.aalto.fi/harrila/ Principal investigator: Harri Lähdesmäki, D.Sc. (Tech), Assistant Professor (tenure track), Academy Research Fellow Contact information: Aalto University School of Science, Department of Information and Computer Science, PO Box 15400, FI-00076 Aalto, Finland. Tel. +358 9 47001, Fax. +358 9 470 23277, E-mail: [email protected]. Biography: Harri Lähdesmäki (b. 1977) graduated in bionformatics from Tampere University of Technology in 2005. Personnel: Post-doctoral Fellows: Jukka Intosalmi Graduate students: Kartiek Kanduri, Laajala Essi, Antti Larjo, Maia Malonzo, Henrik Mannerström, Kari Nousiainen, Maria Osmala, Rautio Sini, Somani Juhi, Vatanen Tommi, Tarmo Äijö Undergraduate students: Eraslan Basak, Eraslan Gökcen, Khakipoor Banafsheh, Kähärä Juhani Description of the project: We use computational techniques to model and understand molecular regulatory mechanisms and their role in health and disease. We focus on developing statistical modeling and machine learning methods to understand transcriptional, posttranscriptional and epigenetic regulatory mechanisms, protein signaling pathways, and effects of mutations on regulatory mechanisms. We also develop methods for biological sequence analysis, combining heterogeneous biological information sources and analyzing high-throughput measurement data, such as deepsequencing and microarray measurements. Research projects are carried out in close collaboration with experimental groups, and we collaborate on molecular immunology, stem cell, cancer and type 1 diabetes systems biology research projects. Funding: Academy of Finland, EU FP7, EraSysBio+, Tekes, Aalto University, Emil Aaltonen Foundation, FICS and TISE graduate schools. Collaborators: Prof. Riitta Lahesmaa (University of Turku), Prof. Matej Orešicˇ (VTT Technical Research Centre of Finland), Prof. Mikael Knip (University of Helsinki), Prof. Olli Simell (Hospital District of Southwest Finland) Selected publications from 2013: Hawkins RD*, Larjo A*, Tripathi SK*, Wagner U, Luu Y, Lönnberg T, Raghav SK, Lee LK, Lund R, Ren B, Lähdesmäki H, Lahesmaa 84 R, Global chromatin state analysis reveals lineage-specific enhancers during the initiation of human T helper 1 and T helper 2 cell polarization, Immunity, Vol. 38, No. 6, pp. 1271-1284, 2013. Närvä E, Pursiheimo J-P, Laiho A, Rahkonen N, Emania MR, Viitala M, Laurila K, Sahla R, Lund R, Lähdesmäki H, Jaakkola P and Lahesmaa R, Continuous hypoxic culturing of human embryonic stem cells enhances Ssea-3 and Myc levels, PLoS ONE, Vol. 8, No. 11, e78847, 2013. Kähärä J, Lähdesmäki H, Evaluating a linear k-mer model for protein-DNA interactions using high-throughput SELEX data, BMC Bioinformatics, 14(Suppl 10):S2, 2013. Ko M, An J, Bandukwala HS, Chavez L, Äijö T, Pastor WA, Segal MF, Li H, Koh KP, Lähdesmäki, Hogan PG, Aravind L, Rao A, Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX,” Nature, Vol. 497, No. 7447, pp. 122-126, 2013. Äijö T, Granberg K, Lähdesmäki H, Sorad: A systems biology approach to predict and modulate dynamic signaling pathway response from phosphoproteome time-course measurements, Bioinformatics, Vol. 29, No. 10, pp. 1283-1291, 2013. Weirauch MT, Cote A, Norel R, Annala M, Zhao Y, Riley TJ, SaezRodriguez J, Cokelaer T, Vedenko A, Talukder S, DREAM5 consortium, Bussemaker HJ, Morris QD, Bulyk ML, Stolovitzky G, Hughes TR, Evaluation of methods for modeling transcription factor sequence specificity, Nature Biotechnology, Vol. 31, No. 2, pp. 126-134, 2013. Tahvanainen J, Kyläniemi MK, Kanduri K, Gupta B, Lähteenmäki H, Kallonen T, Rajavuori A, Rasool O, Koskinen PJ, Rao KVS, Lähdesmäki H, Lahesmaa R, Proviral integration site for Moloney murine leukemia virus (PIM) kinases promote human T helper 1 cell differentiation, The Journal of Biological Chemistry, Vol. 288, No. 5, pp. 3048-3058, 2013. Kanduri C, Ukkola-Vuoti L, Oikkonen J, Buck G, Blancher C, Raijas P, Karma K, Lähdesmäki H, Järvelä I, The genome wide landscape of copy number variations in the isolated Finnish population: the MUSGEN study provides evidence for a founder effect, European Journal of Human Genetics, Vol. 288, No. 5, pp. 3048-3058, 2013. Ukkola-Vuoti L, Kanduri C, Oikkonen J, Buck G, Blancher C, Raijas P, Karma K, Lähdesmäki H, Järvelä I, Genome-wide copy number variation analysis in extended families and unrelated individuals characterized for musical aptitude and creativity in music, PLoS ONE, Vol. 8, No. 2, e56356, 2013. Lehmusvaara S, Erkkilä T, Urbanucci A, Jalava S, Seppälä J, Kaipia A, Kujala P, Lähdesmäki H, Tammela TLJ and Visakorpi T, Goserelin and bicalutamide treatments alter the expression of microRNAs in prostate, The Prostate, Vol. 73, No. 1, pp. 101-112, 2013. Larjo A, Lähdesmäki H, Active learning for Bayesian network models of biological networks using structure priors, In IEEE International Workshop on Genomic Signal Processing and Statistics, Houston, TX, USA, November 17-19, 2013. 85 CELL CULTURE MODELS FOR TUMOR CELL INVASION AND EPITHELIAL PLASTICITY Principal investigator: Matthias Nees, PhD, Docent for Genetics University of Turku, VTT Medical Biotechnology. Itäinen Pitkäkatu 4C, FI-20520 Turku, Finland Tel. +358-40-8314 839, Fax. +358-2 2840 Biography: Matthias Nees (b. 1966) graduated from the University of Heidelberg, Germany in 1993 for work in the field of head & neck cancers. He received his PhD in 1997 from the German Cancer Research Center in Heidelberg, for work on Human Papillomaviruses (HPV). He did post-doctoral research at the National Institutes of Health (NCI, 1997-2001), and EMBL/ Heidelberg University (2002 - 2005). He is currently a principal investigator at VTT Medical Biotechnology, and a team leader at CBT. Personnel: Post-doctoral Fellows: Ville Härmä, Malin Åkerfelt, Mervi Toriseva. Graduate students: Ilmari Ahonen, Sean Robinson (with CEA France). Technicians: Pauliina Toivonen, Johannes Virtanen Undergraduate students: Mrinal Mishra, Hannu-Pekka Schukov Description of the projects: Over the past years, our group (located at VTT, Pharmacity) has systematically established a phenotypic platform that was specifically designed for phenotypic drug discovery, based on the morphology of multicellular organoid structures that form in organotypic, three-dimensional (3D) cell- and tissue culture. We focus mainly on prostate, breast, bladder and ovarian carcinomas, and utilized these models in both academic and custom research applications. In close collaboration with Turku University Hospital (TYKS) and the Turku Prostate Cancer Consortium (TPCC), we also establish primary cell cultures of urological cancers for personalized medicine and patient-specific drug testing. Furthermore, we have developed a novel 3D co-culture platform that combines tumour cells with stromal and cancer-associated fibroblasts (CAFs). These recapitulate critical aspects of complex tumor biology. Our goal is to faithfully recapitulate the complex histology and texture, epithelial differentiation, extracellular matrix (ECM) and microenvironment (TME) of human cancer tissues, but also their often extreme heterogeneity and dynamic. This includes tumor cell motility and invasion, parameters likely related to metastasis and aggressive cancer progression. Our 3D culture 86 and co-culture platform is standardized and miniaturized, allowing medium scale throughput for high content screens. Assays can be performed in both 96- and 384-well plates, using plate-based high-content readers (IncuCyte, PE Operetta) for rapid biometric readout, or confocal spinning-disc microscopy (Zeiss Axiovert) for more detailed imaging. Microscopic imaging can be either performed in real-time, live cell settings (using fluorochromes, stable markers like GFP and DsRed or reactive dyes), or using endpoint markers (e.g. antibody stains, immune fluorescence). In both cases, the readout is of morphometric nature and utilizes the phenotypes of multicellular spheroid structures as a basis for functional evaluation of anti-cancer drugs, siRNAs, and other treatments. Morphological changes in shape, size and texture of tumour organoids have been demonstrated by us and others to correlate with tumour histology, progression, and pathological grading. In particular, dynamic phenotypic changes (such as tumour cell invasion) are predictive for patient outcome. For this purpose, we have developed the AMIDA automated image analysis software package that allows rapid segmentation of large numbers of confocal image stacks, together with statistical & machine learning solutions for the subsequent data normalization and interpretation. Scientifically, we mainly address the mechanisms of tumour cell plasticity, and the molecular pathways involved in the loss of tissue organization versus epithelial differentiation. Both the TME and ECM play decisive roles in the regulation of tumour spheroid morphology, local invasion into the ECM, and tumour cell motility. Even well-differentiated structures are often of only metastable nature, and can spontaneously progress to form highly invasive structures. We have explored the functional role of proteases, G-protein coupled receptors and down-stream signalling pathways in this decision-making process, which could be a prerequisite for the formation of local or distant metastases. Morphological switches may also transform collective into single-cell (amoeboid) patterns of invasion, or epithelial versus mesenchymal invasion processes. Funding: The Academy of Finland (consortium project with Medisiina and FIMM Helsinki), VTT, University of Turku, EU Innovative Medicines Initiative (IMI), EU 7th framework, K. Albin Johansson Foundation Collaborators: List your key collaborators as follows: Lea Sistonen (Åbo Akademi University), Pirkko Härkönen (U. Turku), Olli Kallioniemi and Tuomas Mirtti (FIMM), Varda Rotter and Moshe Oren (Weizmann Institute of Science, Rehovot/Israel), Markku Kallajoki, Pekka Taimen and Peter Boström (University Hospital Turku), Julia Schueler (Oncotest GmbH Heidelberg), and others. 87 Selected Publications: Härmä V, Schukov HP, Happonen A, Ahonen I, Virtanen J, Siitari H, Åkerfelt M, Lötjönen J, Nees M: Quantification of Dynamic Morphological Drug Responses in 3D Organotypic Cell Cultures by Automated Image Analysis PLOS ONE 08 May 2014 | 10.1371/journal.pone.0096426 Björkman M., Östling P., Härmä V., Virtanen J., Mpindi J.P., Rantala J., Mirtti T., Vesterinen T., Lundin M., Sankila A., Rannikko A., Kaivanto E., Kohonen P., Kallioniemi O., Nees M.: Systematic knockdown of epigenetic enzymes identifies a novel histone demethylase PHF8 overexpressed in prostate cancer with an impact on cell proliferation, migration and invasion. Oncogene. 2012 Jul 19;31(29):3444-56. Härmä V., Knuuttila M., Virtanen J., Mirtti T., Kohonen P., Kovanen P., Happonen A., Kaewphan S., Ahonen I., Kallioniemi O., Grafström R., Lötjönen J., Nees M.: Lysophosphatidic acid and sphingosine-1-phosphate promote morphogenesis and block invasion of prostate cancer cells in three-dimensional organotypic models. Oncogene. 2012 Apr 19;31(16):207589. From left to right: : Mrinal Mishra, Mervi Toriseva, Malin Åkerfelt, Pauliina Toivonen, XX, YY, Mathias Nees, ZZ and QQ. Härmä V., Virtanen J., Mäkelä R., Happonen A., Mpindi J.P., Knuuttila M., Kohonen P., Lötjönen J., Kallioniemi O., Nees M.: A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS One. 2010 May 3;5(5):e10431. Björkman M., Rantala J., Nees M., Kallioniemi O.: Epigenetics of prostate cancer and the prospect of identification of novel drug targets by RNAi screening of epigenetic enzymes. Epigenomics. 2010 Oct;2(5):683-9. Rantala J.K., Mäkelä R., Aaltola A.R., Laasola P., Mpindi J.P., Nees M., Saviranta P., Kallioniemi O.: A cell spot microarray method for production of high density siRNA transfection microarrays. BMC Genomics. 2011 Mar 28;12:162. 88 89 COMPUTATIONAL BIOLOGY http://www.uta.fi/ibt/institute/research/nykter/ Principal investigator: Matti Nykter, D. Sc. (Tech), Professor. Affiliated Group Leader at CBT, Institute of Biosciences and Medical Technology, University of Tampere Biokatu 8 (Finn-Medi 2), 33520 Tampere, Finland Tel. +358-40-8490651. Email: [email protected] Biography: Matti Nykter (b. 1978) received the degree of Master of Science (Engineering) with Distinction in information technology in 2002 and the degree of Doctor of Science (Technology) in signal processing in 2006 from Tampere University of Technology, Tampere, Finland. He has worked as a visiting researcher at The University of Texas M. D. Anderson Cancer Center in Houston, Texas, USA in 20042005, and as a post-doctoral research at the Institute for Systems Biology, Seattle, USA during 2007-2009. From 2010 till 2012 he was a group leader at the Department of Signal Processing at Tampere University of Technology. From beginning of 2013 he is a professor of bioinformatics at the Institute of Biosciences and Medical Technology, University of Tampere. His research interests are focused on development and application of computational methodologies to understand the mechanisms of gene regulation in context of disease related dysregulation Personnel: Post-doctoral Fellows: Kati Kivinummi, PhD, Kirsi Granberg, PhD, Juha Kesseli, D.Sc., Pekka Ruusuvuori, D.Sc. Graduate students: Antti Ylipää, Matti Annala, Tommi Rantapero, Francesco Tabaro Undergraduate students: Thomas Liuksiala, Sergei Häyrynen, Ville Kytölä, Maria Laaksonen, Birgitta Lehtinen. Description of the project The Computational Biology groups uses systems biology methodology to study biology. Our research is rooted in high throughput measurement data from genomic and transcriptomic levels. We develop and apply computational tools and mathematical modelling to understand the biosystems. Research activities of our laboratory range from theoretical biology to experimental work. Theoretical work is focused on the fundamental principles of biological systems, such as the information processing and the effect of structural constrains to dynamics. Applied research is focused on cancer research as well as on immunology and cellular differentiation. Main research directions are currently related to cancer research. We are using deep sequencing the characterize the cancer genome of prostate cancer and glioma. We have identified novel oncogenic mechanisms that are currently ongoing functional validation. Another key project is related to understanding cell differentiation. We have integrated a collection of over three thousand gene arrays, measured from 166 normal cell types. 90 Based on novel data integration and data analysis methodology, we are studying the gene networks that give raise to different cell types and apply computational approach to uncover recipes for cell type reprogramming experiments. Funding: The Academy of Finland, Finnish Funding Agency for Technology and Innovation (Tekes), Tampere University of Technology, Tampere Doctoral Programme in Information Science and Engineering, Graduate School in Electronics, Telecommunications and Automation, Emil Aaltonen Foundation, Sigrid Juselius Foundation. Collaborators: Wei Zhang (University of Texas MD Anderson Cancer Center), Ilya Shmulevich (Institute for Systems Biology), Tapio Visakorpi (University of Tampere), Riitta Lahesmaa (Turku Centre for Biotechnology), Johanna Schleutker (University of Turku), Hannu Haapasalo (Tampere University Hospital), Harri Lähdesmäki (Aalto University), Merja Heinäniemi (University of Eastern Finland), Olli YliHarja (Tampere University of Technology). Selected Publications: Liuksiala T, Teittinen KJ, Granberg K, Heinäniemi M, Annala M, Mäki M, Nykter M, Lohi O. Overexpression of SNORD114-3 marks acute promyelocytic leukemia. Leukemia 2014 ;28(1)233-6 Heinäniemi M, Nykter M, Kramer R, Wienecke-Baldacchino A, Sinkkonen L, Zhou JX, Kreisberg R, Kauffman SA, Huang S, Shmulevich I. Gene-pair expression signatures reveal lineage control. Nat Methods 2013 ;10(6)577-83 Parker B.C., Annala M., Cogdell D., Granberg K., Sun Y., Ji P., Gumin J., Zheng H., Hu L., Li X., Yli-Harja O., Haapasalo H., Visakorpi T., Liu X., Liu C.-G., Sawaya R., Fuller G.N., Chen K., Lang F.L., Nykter M., and Zhang W. (2013) FGFR3-TACC3 fusion escapes miR-99a regulation and promotes tumorigenesis in glioblastoma. Journal of Clinical Investigations, 123(2). Moore L.M., Kivinen V., Liu Y., Annala M., Cogdell D., Liu X., Liu C.G., Sawaya R., Yli-Harja O., Shmulevich I., Fuller G.N., Zhang W., Nykter M. (2012) Transcriptome and Small RNA Deep Sequencing Reveals Deregulation of miRNA Biogenesis in Human Glioma. J Pathol 229(3)449-59. Holmes K.M., Annala M., Chua C.Y., Dunlap S.M., Liu Y., Hugen N., Moore L.M., Cogdell D., Hu L., Nykter M., Hess K., Fuller G.N., Zhang W. (2012) Insulin-like growth factor-binding protein 2-driven glioma progression is prevented by blocking a clinically significant integrin, integrin-linked kinase, and NF-κB network. Proc Natl Acad Sci USA 109(9):3475-3480 Yang J., Ylipää A., Sun Y., Zheng H., Chen K., Nykter M., Trent J., Ratner N., Lev D.C. and Zhang W. (2011) Genomic and molecular characterization of malignant peripheral nerve sheath tumor identifies the IGF1R pathway as a primary target for treatment. Clin. Cancer Res . 17: 7563-7573. 91 SYSTEMS MEDICINE http://www.btk.fi/research/affiliated-groups/oresic-matej-systemsbiology/ Principal investigator: Matej Orešicˇ, PhD, Steno Diabetes Center, Niels Steensens Vej 2, 2820 Gentofte, Denmark. Phone: +358-44-972-6094 Email: [email protected] Biography: Matej Orešicˇ holds a PhD in biophysics from Cornell University. Between 2003 and 2014 he led the research in domains of quantitative biology and bioinformatics at VTT Technical Research Centre of Finland (Espoo, Finland), where he was a Research Professor in Systems Biology and Bioinformatics. In 2014 he joined Steno Diabetes Center in Gentofte/Denmark as principal investigator, where he leads a newly established department of systems medicine. Prior to joining VTT, Dr. Orešicˇ was a head of computational biology and modeling at Boston-based Beyond Genomics, Inc. and bioinformatician at LION Bioscience Research in Cambridge/MA. Personnel: Seniors scientists: Tuulia Hyötyläinen, PhD (co-PI) Description of the project Our main research area is systems medicine, particularly metabolomics applications in biomedical research and related integrative bioinformatics. Specifically, we are particularly interested in the identification of disease vulnerabilities associated with different metabolic phenotypes and the underlying mechanisms linking these vulnerabilities with the development of specific disorders or their co-morbidities. Such in depth understanding of the metabolic phenotypes in health and disease is crucial if one is to implement personalized medicine. We also initiated the popular MZmine open source project, leading to popular software for metabolomics data processing. Funding: List sources of funding as follows: The Academy of Finland, EU 7th framework, Juvenile Diabetes Research Foundation (JDRF), GENFL Head Health Challenge I. Collaborators: Mikael Knip (University of Helsinki), Riitta Lahesmaa (University of Turku), Olli Simell (University of Turku), Harri Lähdesmäki (Aalto University), Antonio Vidal-Puig (Cambridge University), Fredrik Bäckhed (Gothenburg University), David Menon (Cambridge University), Olli Tenovuo (University of Turku), Kirsi Pietiläinen (University of Helsinki), Jaana Suvisaari (National Institute for Health and Welfare), Jaakko Kaprio (University of Helsinki). 92 Selected Publications: Bondia-Pons I, Maukonen J, Mattila I, Rissanen A, Saarela M, Kaprio J, Hakkarainen A, Lundbom J, Lundbom N, Hyötyläinen T, Pietiläinen KH, Orešicˇ M , Metabolome and faecal microbiota in monozygotic twin pairs discordant for weight- a Big Mac challenge, FASEB J. (2014). doi: 10.1096/fj.14-250167 La Torre D, Seppänen-Laakso T, Larsson HE, Hyötyläinen T, Ivarsson SA, Lernmark Å, Orešicˇ M , the DiPiS study group, Decreased cord-blood phospholipids in young age at onset type 1 diabetes, Diabetes 62, 3951-3956 (2013). Orešicˇ M , Hyötyläinen T, Kotronen A, Gopalacharyulu P, Nygren H, Arola J, Castillo S, Mattila I, Hakkarainen A, Borra R JH, Honka MJ, Verrijken A, Francque S, Iozzo P, Leivonen M, Jaser N, Juuti A, Sørensen TIA, Nuutila P, Van Gaal L, Yki-Järvinen H, Prediction of non-alcoholic fatty liver disease and liver fat content by serum molecular lipids, Diabetologia 56, 2266-2274 (2013). Orešicˇ M , Gopalacharyulu P, Mykkänen J, Lietzen N, Mäkinen M, Nygren H, Simell S, Simell V, Hyöty H, Veijola R, Ilonen J, SysiAho M, Knip M, Hyötyläinen T, Simell O, Cord serum lipidome in prediction of islet autoimmunity and type 1 diabetes, Diabetes 62, 3268-3274 (2013). Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall H-U, Bamberg K, Angelin B, Hyötyläinen T, Orešicˇ M , Bäckhed F, Gut microbiota regulates bile acid metabolism by reducing the levels of taurobetamuricholic acid, a naturally occurring FXR antagonist, Cell Metab. 17, 225–235 (2013). Hyötyläinen T, Mattila I, Wiedmer S, Koivuniemi A, Taskinen M-R, Yki-Järvinen H, Orešicˇ M , Metabolomic analysis of polar metabolites in lipoprotein fractions identifies lipoprotein-specific metabolic profiles and their association with insulin resistance, Mol. Biosyst. 8, 2559-2565 (2012). Orešicˇ M , Seppänen-Laakso T, Sun D, Tang J, Therman S, Viehman R, Mustonen U, van Erp TGM, Hyötyläinen T, Thompson P, Toga AW, Huttunen MO, Suvisaari J, Kaprio J, Lönnqvist J, Cannon TD, Phospholipids and insulin resistance in psychosis: a lipidomics study of twin pairs discordant for schizophrenia, Genome Med. 4, e1 (2012). Orešicˇ M , Hyötyläinen T, Herukka S-K, Sysi-Aho M, Mattila I, Seppänan-Laakso T, Julkunen V, Gopalacharyulu PV, Hallikainen M, Koikkalainen J, Kivipelto M, Helisalmi S, Lötjönen J, Soininen H, Metabolome in progression to Alzheimer’s disease, Transl. Psychiatry 1, e57 (2011). (Commentary in Alzheimer Research Forum) (Commentary in Neurology Today) Sysi-Aho M, Ermolov A, Gopalacharyulu PV, Tripathi A, SeppänenLaakso T, Maukonen J, Mattila I, Ruohonen ST, Vähätalo L, Yetukuri L, Härkönen T, Lindfors E, Nikkilä J, Ilonen J, Simell O, Saarela M, Knip M, Kaski S, Savontaus E, Orešicˇ M , Metabolic regulation in progression to autoimmune diabetes, PLoS Comp. Biol. 7 (10), e1002257 (2011). Pietiläinen K, Róg T, Seppänen-Laakso T, Virtue S, Gopalacharyulu P, Tang J, Rodriguez-Cuenca S, Maciejewski A, Naukkarinen J, Rissanen A, Ruskeepää A-L, Niemelä P, Velagapudi V, Castillo S, Nygren H, Hyötyläinen T, Kaprio J, Yki-Järvinen H, Vattulainen 93 I, Vidal-Puig A, Orešicˇ M , Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans, PLoS Biol. 9(6), e1000623 (2011). Orešicˇ M, Hänninen V, Vidal-Puig A, Lipidomics: a new window to biomedical frontiers, Trends Biotechnol. 26(12), 647-652 (2008). Orešicˇ M , Tang J, Seppänen-Laakso T, Mattila I, Saarni SE, Saarni SI, Lönnqvist J, Sysi-Aho M, Hyötyläinen T, Perälä J, Suvisaari J, Metabolome in schizophrenia and other psychotic disorders: a general population-based study, Genome Med. 3, e19 (2011). Nikkilä J, Sysi-Aho M, Ermolov A, Seppänen-Laakso T, Simell O, Kaski S, Orešicˇ M , Gender dependent progression of systemic metabolic states in early childhood, Mol. Syst. Biol. 4, e197 (2008). Hilvo M, Denkert C, Lehtinen L, Müller B, Brockmöller S, Seppänen-Laakso T, Budczies J, Bucher E, Yetukuri L, Castillo S, Berg E, Nygren H, Sysi-Aho M, Griffin JL, Fiehn O, Loibl S, Richter-Ehrenstein C, Radke C, Hyötyläinen T, Kallioniemi O, Iljin K, Orešicˇ M, Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression, Cancer Res. 71, 3236-3245 (2011). Pluskal T, Castillo S, Villar-Briones A, Orešicˇ M , MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data, BMC Bioinformatics 11, 395 (2010). Westerbacka J, Kotronen A, Fielding BA, Wahren J, Hodson L, Perttilä J, Seppänen-Laakso T, Suortti T, Arola J, Hultcrantz R, Castillo S, Olkkonen VM, Frayn KN, Orešicˇ M , Yki-Järvinen H, Splanchnic balance of free fatty acids, endocannabinoids and lipids in subjects with NAFLD, Gastroenterology 139, 19611971 (2010). Yetukuri L, Söderlund S, Koivuniemi A, Seppänen-Laakso T, Niemelä PS, Hyvönen M, Taskinen M-R, Vattulainen I, Jauhiainen M, Orešicˇ M , Composition and lipid spatial distribution of High Density Lipoprotein particles in subjects with low and high HDL-cholesterol, J. Lipid Res. 51, 23412351 (2010). Yetukuri L, Katajamaa M, Medina-Gomez G, Seppänen-Laakso T, Vidal Puig A, M. Orešicˇ M , Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis, BMC Systems Biology 1, e12 (2007). Laaksonen R, Katajamaa M, Päivä H, Sysi-Aho M, Saarinen L, Junni P, Lütjohann D, Smet J, Van Coster R, Seppänen-Laakso T, Lehtimäki T, Soini J, Orešicˇ M , A systems biology strategy reveals biological pathways and plasma biomarker candidates for potentially toxic statin induced changes in muscle, PLoS ONE 1(1): e97 (2006). Katajamaa M, Orešicˇ M , Processing methods for differential analysis of LC/MS profile data, BMC Bioinformatics 6:179 (2005). Orešicˇ M , Shalloway D, Specific Correlations between Relative Synonymous Codon Usage and Protein Secondary Structure, J. Mol. Biol. 281, 31-48 (1998). Orešicˇ M , Shalloway D, Hierarchical characterization of energy landscapes using Gaussian packet states, J. Chem. Phys. 101, 9844-9856 (1994). Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu PV, Yetukuri L, Islam S, Felin J, Perkins R, Borén J, Orešicˇ M, Backhed F, The gut microbiota modulates host energy and lipid metabolism in mice, J. Lipid Res. 51, 1101-1112 (2010). Orešicˇ M , Seppänen-Laakso T, Yetukuri L, Bäckhed F, Hänninen V, Gut microbiota affects lens and retinal lipid composition, Exp. Eye Res. 89, 604-607 (2009). Kotronen A, Velagapudi VR, Yetukuri L, Westerbacka J, Bergholm R, Ekroos K, Makkonen J, Taskinen M-R, Orešicˇ M , Yki-Järvinen H, Saturated fatty acids containing triacylglycerols are better markers of insulin resistance than total serum triacylglycerol concentrations, Diabetologia 52(4), 684-690 (2009). Orešicˇ M , Simell S, Sysi-Aho M, Näntö-Salonen K, SeppänenLaakso T, Parikka V, Katajamaa M, Hekkala A, Mattila I, Keskinen P, Yetukuri L, Reinikainen A, Lähde J, Suortti T, Hakalax J, Simell T, Hyöty H, Veijola R, Ilonen J, Lahesmaa R, Knip M, Simell O, Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes, J. Exp. Med. 205(13), 2975-2984 (2008). 94 95 PROTEIN CRYSTALLOGRAPHY Principal investigator: Anastassios C. Papageorgiou, PhD, Adjunct Professor in Biochemistry and Structural Biology Turku Centre for Biotechnology, BioCity, Tykistökatu 6A, FI-20521 Turku, Finland. Tel. +358-2-3338012, Fax +358-2-3338000. E-mail: [email protected] Biography: Tassos Papageorgiou obtained his PhD from the University of Athens in 1992. He was a post-doctoral fellow at the University of Oxford and University of Bath (UK). In May 2000, he joined the Centre for Biotechnology as senior scientist in protein crystallography. Personnel: Graduate students: Bishwa Subedi, Abdi Muleta, Pradeep Battula, MSc students: Amin Seid, Nirmal Poudel, Sagar Bhadari, Undergraduate students: Adeleke Amoda, Visiting scientist: Kirti Sonkar, Marta Lascorz Description of the project We use X-crystallography, molecular biology, and biophysical techniques to study the structure and function of biological molecules. One of our major projects has been the Dps family of proteins that are widely spread among procaryotes and responsible for protection against oxidative stress due to their ability to oxidize and store iron. Although Dps proteins are structurally similar to ferritins, they form a spherical shell of 12 subunits instead of 24 and have a different ferroxidase center compared to that of ferritins. Work on several new Dps proteins continued in order to understand better the iron core formation using X-ray crystallography, microcalorimetry, EXAFS, magnetization measurements, and Mössbauer spectroscopy. Small crystals of a Dps protein from the Lyme disease pathogen Borrelia burgdorferi were grown but they need further optimization. Work on newly identified bacterial adhesins continued during last year. With the increased resistance to antibiotics, adhesins have become an attractive therapeutic target in the fight against microbial diseases. Various constructs of two newly identified bacterial adhesins containing leucine-rich repeats were used for protein expression and purification. Small crystals were grown and are currently in optimization. Various data sets have been collected and attempts to determine the structures are underway. In addition, biochemical data and docking calculations are in progress to study the precise binding mechanism to receptors found on the membrane of host cells. Small angle X-ray experiments were carried out in ESRF Grenoble and revealed the overall shape of the molecules. A third adhesin with galabiosebinding activity was expressed and purified. Small crystals were found in various conditions but instability and oligomerization problems have prompted us to look at alternative constructs and homologues. 96 Studies on oxidative stress protection and detoxification mechanisms continued on human-rat chimeric glutathione transferases (GSTs) or mutants created through directed evolution approaches to produce new GSTs with altered specificity for new applications in biomedicine, environmental security, and agriculture. Crystals of human GST-A1 have been grown in our lab for use in structure-assisted drug design efforts. Docking calculations were carried out to study the binding of diphenylether herbicides in the active site. Using directed evolution approaches, new GSTs have been generated with altered specificity. Structural studies are currently in progress. In the theme of enzyme function and stability, we continued our work on PhaZ7, an extracellular depolymerase involved in the degradation of poly(R)-hydroxyalkanoates (PHAs), a group of biodegradable thermoplastic polyesters considered as substitutes for non-degradable plastics. Several mutants were generated by our collaborators and characterized for their ability to bind PHAs. Crystal structure determination has revealed a large conformational change that may play a role in the enzyme’s function. Binding of a tetramer analogue has suggested a possible substrate binding binding . Work on the Atu (acyclic terpene utilization) catabolic pathway found in P. Aeruginosa has continued using a combination of X-ray crystallography, biophysics, molecular biology, homology modelling, computational and bioinformatics tools. Atu enzymes are involved in the metabolisn of acyclic terpenes that possess a great potential in biotechnology, for example in the food, drink and pharmaceutical industry. Reprocessing of previously collected diffraction images gave a better data set extended to 2.15 Å. The structure is currently under rounds of refinement and extensive rebuilding. Docking calculations have been carried out to identify key residues for substrate binding. Recent reports implicating phosphoserine aminotransferase (PSAT), a vitamin B6 enzyme, in cancer and tuberculosis has led us to investigate closer the substrate binding mechanism of this enzyme for potential drug design efforts. In addition to the phosphoserine-bound PSAT structure determined to 1.5 Å, new structures in the presence of other substrates are currently being determined to high/atomic resolution. These studies will reveal the exact local conformational changes during the catalytic cycle of the enzyme. Funding University of Turku, Biocenter Finland, EU FP7 (access to synchrotrons), Biostruct-X, Cultural Foundation of Southwestern Finland, CIMO Collaborators Jukka Finne (University of Helsinki), Sauli Haataja (University of Turku), Vuokko Loimaranta (University of Turku), Dieter Jendrossek (University of Stuttgart), Nikos Labrou (Agricultural University of Athens), Li Duochuan (Shandong Agricultural University), Xin Li (Ohio State university), Maria Fillat (University of Zaragoza) 97 Haikarainen, T., Loimaranta, V., Prieto-Lopez, C., Battula, P., Finne, J., and Papageorgiou, A. C. (2013) Expression, purification and crystallization of the C-terminal LRR domain of Streptococcus pyogenes protein 0843. Acta Crystallogr Sect F Struct Biol Cryst Commun 69, 559–561 Battula, P., Dubnovitsky, A. P., and Papageorgiou, A. C. (2013) Structural basis of L-phosphoserine binding to Bacillus alcalophilus phosphoserine aminotransferase. Acta Crystallogr D Biol Crystallogr 69, 804–811 Chronopoulou, E. G., Papageorgiou, A. C., Markoglou, A., and Labrou, N. E. (2012) Inhibition of human glutathione transferases by pesticides: Development of a simple analytical assay for the quantification of pesticides in water. Journal of Molecular Catalysis. B, Enzymatic 81, 43–51 Björkblom, B., Padzik, A., Mohammad, H., Westerlund, N., Komulainen, E., Hollos, P., Parviainen, L., Papageorgiou, A. C., Iljin, K., Kallioniemi, O., Kallajoki, M., Courtney, M. J., Mågård, M., James, P., and Coffey, E. T. (2012) c-Jun N-Terminal Kinase Phosphorylation of MARCKSL1 Determines Actin Stability and Migration in Neurons and in Cancer Cells. Mol. Cell. Biol. 32, 3513–3526 Skopelitou, K., Dhavala, P., Papageorgiou, A. C., and Labrou, N. E. (2012) A glutathione transferase from Agrobacterium tumefaciens reveals a novel class of bacterial GST superfamily. PLoS ONE 7, e34263 Haikarainen, T., Paturi, P., Lindén, J., Haataja, S., Meyer-Klaucke, W., Finne, J., and Papageorgiou, A. C. (2011) Magnetic properties and structural characterization of iron oxide nanoparticles formed by Streptococcus suis Dpr and four mutants. J Biol Inorg Chem 16, 799–807 Haikarainen, T., Thanassoulas, A., Stavros, P., Nounesis, G., Haataja, S., and Papageorgiou, A. C. (2011) Structural and thermodynamic characterization of metal ion binding in Streptococcus suis Dpr. J Mol Biol 405, 448–460 Li, D.-C., Li, A.-N., and Papageorgiou, A. C. (2011) Cellulases from thermophilic fungi: Recent insights and biotechnological potential. Enzyme Research 2011, 1–9 Article ID 308730 Selected publications: Papageorgiou, A. C., and Mattsson, J. (2014) Protein structure validation and analysis with x-ray crystallography. Methods Mol Biol 1129, 397–421 Haikarainen, T., Frioux, C., Zhnag, L.-Q., Li, D.-C., and Papageorgiou, A. C. (2014) Crystal structure and biochemical characterization of a manganese superoxide dismutase from Chaetomium thermophilum. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 1844, 422–429 Jendrossek, D., Hermawan, S., Subedi, B., and Papageorgiou, A. C. (2013) Biochemical analysis and structure determination of Paucimonas lemoignei poly(3-hydroxybutyrate) (PHB) depolymerase PhaZ7 muteins reveal the PHB binding site and details of substrate-enzyme interactions. Mol Microbiol 90, 649– 664 98 Wakadkar, S., Zhang, L.-Q., Li, D.-C., Haikarainen, T., Dhavala, P., and Papageorgiou, A. C. (2010) Expression, purification and crystallization of Chaetomium thermophilum Cu,Zn superoxide dismutase. Acta Crystallogr Sect F Struct Biol Cryst Commun 66, 1089–1092 Haikarainen, T., Tsou, C.-C., Wu, J.-J., and Papageorgiou, A. C. (2010) Structural characterization and biological implications of di-zinc binding in the ferroxidase center of Streptococcus pyogenes Dpr. Biochem Biophys Res Commun 398, 361–365 Wakadkar, S., Hermawan, S., Jendrossek, D., and Papageorgiou, A. C. (2010) The structure of PhaZ7 at atomic (1.2 Å) resolution reveals details of the active site and suggests a substrate-binding mode. Acta Crystallogr Sect F Struct Biol Cryst Commun 66, 648–654 99 Axarli, I., Georgiadou, C., Dhavala, P., Papageorgiou, A. C., and Labrou, N. E. (2010) Investigation of the role of conserved residues Ser13, Asn48 and Pro49 in the catalytic mechanism of the tau class glutathione transferase from Glycine max. Biochim Biophys Acta 1804, 662–667 Labrou NE Papageorgiou AC Avramis VI (2010) Structure-function relationships and clinical applications of L-asparaginases. Curr Med Chem 17, 2183–2195 Haikarainen, T., and Papageorgiou, A. C. (2010) Dps-like proteins: structural and functional insights into a versatile protein family. Cell Mol Life Sci 67, 341–351 Haikarainen, T., Tsou, C.-C., Wu, J.-J., and Papageorgiou, A. C. (2010) Crystal structures of Streptococcus pyogenes Dpr reveal a dodecameric iron-binding protein with a ferroxidase site. J Biol Inorg Chem 15, 183–194 Melissis, S., Papageorgiou, A., Labrou, N. E., and Clonis, Y. D. (2010) Purification of M-MLVH-RT on a 9-Aminoethyladenine(1,6-diamine-hexane)-triazine Selected from a Combinatorial Library of dNTP-Mimetic Ligands. Journal of Chromatographic Science 48, 496–502 Dhavala, P., and Papageorgiou, A. C. (2009) Structure of Helicobacter pylori L-asparaginase at 1.4 A resolution. Acta Crystallogr D Biol Crystallogr 65, 1253–1261 Mitsiki, E., Papageorgiou, A. C., Iyer, S., Thiyagarajan, N., Prior, S. H., Sleep, D., Finnis, C., and Acharya, K. R. (2009) Structures of native human thymidine phosphorylase and in complex with 5-iodouracil. Biochem Biophys Res Commun 386, 666–670 Axarli, I., Dhavala, P., Papageorgiou, A. C., and Labrou, N. E. (2009) Crystal structure of Glycine max glutathione transferase in complex with glutathione: investigation of the mechanism operating by the Tau class glutathione transferases. Biochem J 422, 247–256 Axarli, I., Dhavala, P., Papageorgiou, A. C., and Labrou, N. E. (2009) Crystallographic and functional characterization of the fluorodifen-inducible glutathione transferase from Glycine max reveals an active site topography suited for diphenylether herbicides and a novel L-site. J Mol Biol 385, 984–1002 From left to right. Back row: Amin Seid, Abdi Muleta,Tassos Papageorgiou and Nirmal Poudel. Front row: Kirti Sonkar, Pradeep Battula and Sagar Bhadari. 100 101 INTEGRIN ACTIVITY IN DISEASE http://www.btk.fi/research/research-groups/pouwels/ Principal investigator: Jeroen Pouwels, Ph.D., Adjunct Professor of Biochemistry, Academy of Finland Research Fellow Turku Centre for Biotechnology Tykistökatu 6A FIN-20521 Turku, Finland phone: +358-440466088 Fax: +358-2-2158808. email: [email protected] Biography: Jeroen Pouwels (b. 1976) graduated from Wageningen University (The Netherlands) in 1999 (Molecular Sciences) and received his PhD from Wageningen University in 2004 (Plant Molecular Biology). He did one postdoc in the research team of Dr. Marko Kallio at VTT Medical Biotechnology in Turku (2004-2008). In 2009 he received an Academy of Finland Post-doctoral Fellowship for research in the team of Prof. Dr. Johanna Ivaska (Turku Centre for Biotechnology, VTT Medical Biotechnology), which was continued using a Finnish Cancer Institute Postdoc Grant. In 2013 he was awarded the title of Adjunct Professor of Biochemistry and he established the Integrin Activity in Disease research group at the Turku Centre for Biotechnology as an Academy of Finland Research Fellow. Personnel: Graduate students: Meraj Hasan Khan, MSc., Maksym Skaldin, MSc. Description of the project Regulation of integrin activity is fundamentally important during development and in many physiological processes in adults. Impaired regulation of integrin activation has been linked to bleeding disorders, skin blistering, immune-deficiencies, chronic inflammation, thrombosis and cancer. Integrins usually switch between an active and an inactive state. Activation of integrins, which leads to enhanced integrin-dependent signalling and strengthened local cell adhesion, can occur by outside-in or inside-out signalling. Outside-in signalling is triggered by binding to extracellular ligands like collagen or fibronectin, while inside-out signalling is mediated by binding of proteins to the intracellular region, which propagates conformational changes from the intracellular to the extracellular domains leading to an increase in affinity for ligands. Currently, members of two protein families, Kindlins and Talins, are known to activate integrins by binding to the cytoplasmic tail of betaintegrins. Even though molecules keeping integrins inactive or switching activated integrins back to inactive conformation are likely to be biologically as important as integrin activators for regulating the dynamic nature of integrin function, such molecules are not well characterized. Moreover, the cytoplasmic domains of integrin 102 alpha subunits are important in maintaining the integrin in an inactive conformation but the mechanisms involved have remained enigmatic. Very recently, a few proteins were shown to inhibit integrin activity by binding to the beta-integrin cytoplasmic tail and competing with Talin and Kindlin (DOK1 and Filamin). However, given the broad range of cellular functions regulated by the 24 different alpha-beta integrin heterodimers, it is very likely that more modulators of integrin activity remain to be discovered. To identify novel regulators of integrin activity we have utilized the cell spot microarray (CSMA), a high-throughput RNAi screen developed at VTT Medical Biotechnology (Pellinen et al., J. Cell Sc. 2012), and identified Sharpin as a novel inhibitor of integrin activity in vivo and in vitro (Rantala, Pouwels et al., Nature Cell Biology, 2011). This study opened a new paradigm in integrin regulation by showing that the dynamic switching between the inactive and active conformations is physiologically controlled in vivo by a protein interacting with the alpha-subunit cytoplasmic domain. Very recently we have also shown that Sharpin regulates lymphocyte function associated antigen-1 (LFA-1), an integrin present on leukocytes (Pouwels et al., Cell Reports, 2013). In these cells Sharpin mediates release of the cell rear (uropod) during cell migration and therefore plays an important role in leukocyte transmigration and homing in vivo. As Sharpin has oncogenic potential, Sharpin knockout mice show a psoriasis-like phenotype with chronic inflammation and proliferative dermatitis, and Sharpin plays a role in linear ubiquitination and regulation of the NF-κB pathway, understanding the exact mechanism of Sharpin-induced integrin inhibition is highly important, as well as finding potential other roles for this protein. The aim of the integrin activity in disease group is to characterize Sharpin and its integrin-inhibiting characteristics in more detail, both at the molecular level and how Sharpin could play a role in human diseases. This includes functional posttranslational modifications of Sharpin, as well as identification of proteins that functionally interact with Sharpin. Funding: The Academy of Finland. Collaborators: Prof. Johanna Ivaska (Turku Centre for Biotechnology, Turku, Finland), Dr. John Sundberg (The Jackson Laboratory, Bar Harbor, USA), Dr. Maddy Parsons (King’s College London Guy’s Campus, London, UK), Prof. Henning Walczak (University College London, Cancer Research UK, London, UK), Prof Marko Salmi (MediCity Research Laboratory, University of Turku, Turku, Finland). Selected Publications († Equal Contribution): Pouwels J†, De Franceschi N†, Rantakari P, Auvinen K, Karikoski M, Mattila E, Potter C, Sundberg JP, Hogg N, Gahmberg CG, Salmi M, Ivaska J. (2013) SHARPIN Regulates Uropod Detachment in Migrating Lymphocytes. Cell Rep. 5(3): 619-28. Hämälistö S†, Pouwels J†, De Franceschi N, Saari M, Ivarsson Y, Zimmermann P, Brech A, Stenmark H, Ivaska J. (2013) A ZO-1/ α5β1-integrin complex regulates cytokinesis downstream of 103 PKCε in NCI-H460 cells plated on fibronectin. PLOS ONE. 8(8): e70696. Bouvard D†, Pouwels J†, De Francesci N, Ivaska J. (2013) Integrin inactivators: balancing cellular functions in vitro and in vivo. Nature Reviews Mol. Cell Biol. 14(7): 430-42. Pouwels J, Nevo J, Pellinen T, Ylänne J and Ivaska J. (2012) Negative regulators of integrin activity. J Cell Sci. 125(Pt 14): 3271-80. Rantala JK†, Pouwels J†, Pellinen T, Veltel S, Mattila E, Laasola P, Potter CS, Duffy T, Sundberg JP, Kallioniemi O, Askari JA, Humphries M, Parsons M, Salmi M and Ivaska J. (2011) SHARPIN is an endogenous inhibitor of beta1-integrin activation. Nature Cell Biol. 13(11): 1315-1324. From left to right: Johanna Lilja, Jeroen Pouwels and Maksym Skaldin 104 105 CELL FATE Funding: The Academy of Finland, Åbo Akademi University, Centre of Excellence in Cell Stress and Molecular Aging, Abo Academi, Doctoral Network of Molecular Biosciences, EU 7th NotchIT, Turku Graduate school for Biomedical Sciences, Cancer Society of Finland, Sigrid Juselius Foundation, Marie Curie CIG FP7. Principal investigator: Cecilia Sahlgren, PhD, Academy Research Fellow, Turku Centre for Biotechnology, BioCity, Tykistökatu 6B, FI-20521 Turku, Finland. Tel. 358-2-3338611, Fax. +358-2-251 8808 E-mail: [email protected] Associate professor in biomedical engineering, University of Technology, The Netherlands, E-mail:[email protected] Eindhoven Biography: Cecilia Sahlgren received her PhD from Turku Centre of Biotechnology, Åbo Akademi University December 2002. She was appointed research fellow at the Department of Biology at Åbo Akademi University from 2003-2005. 2005-2007 she was a postdoctoral fellow in Prof. Urban Lendahls lab at the Department of Cell and Molecular Biology at the Karolinska Institute. 2008 she was appointed senior research fellow Åbo Akademi University. In 2009 she founded the Cell fate group at the Turku Centre for Biotechnology. She currently holds an Academy of Finland Research Fellow position. She has an affiliated position at the Eindhoven University of Technology as an associate professor in biomedical engineering Personnel: Post-doctoral Fellows: Veronika Mamaeva, MD, PhD. Graduate students: Marika Sjöqvist, M.Sc, Neeraj Prabhakar, MSc Sebastian Landor, M.Sc, Christian Antila, M.Sc, Daniel Antfolk, M.Sc, Rasmus Niemi, MSc, Valeriy Paramanov, MD. Laboratory Technician: Natalie Ratts. Undergraduate students: Jenni Niinimaki, B.Sc, Martina Lerche, B.Sc. Description of the project: We aim at understanding the basic molecular principles of signaling mechanisms regulating cell fate choices during stem cell differentiation, and how disturbances in these mechanisms link to cancer. Another important goal is to develop technology to specifically monitor and tune these signals at will in specific cell populations, in order to steer stem cell fate and curtail oncogenic activities. We are particularly interested in the role and regulation of the evolutionary conserved Notch signaling pathway, a key regulator of stem cell function and tumorigenesis. The main objectives of our research are to understand i) how the cellular microenvironment influences Notch signaling activities and how this impinges on cell identity, function and tumor progression, ii) how Notch signaling interlinks with other signaling and cellular mechanisms to fine tune and modulate the cellular response, iii) how intracellular temporal and spatial control of Notch signaling activities are achieved and to iv) develop technology platforms to regulate Notch signaling in cancer stem and regeernation and tools for bioimaging of cellular functions in vivo. 106 Collaborators: Prof. Urban Lendahl (Karolinska Institute), Prof. John Eriksson (Turku Centre for Biotechnology). Prof. Milos Pekny (Sahlgrenska Academy at Göteborg University), Ph.D Susumu Imanishi (Turku Centre for Biotechnology), Prof. Lea Sistonen (Turku Centre for Biotechnology). Dr.Tech Jessica Rosenholm (Laboratory for Physical Chemistry, Åbo Akademi University, Turku), Prof. Mika Linden (Dept of Chemistry, Ulm University, Germany), Prof Carlijn Bouten, Dept of biomedical engineering, Eindhoven University of Technology, Ass. Professor Patricia Dankers, Dept for Chemcial Biology, Eindhoven University of Technology. Selected Publications: Sjöqvist M, Antfolk D, Ferraris S, Rraklli V, Granqvist C, Antila C, Mutvei A, Imanishi SY, Holmberg J, Jin S, Eriksson JE, Lendahl U, and Sahlgren C. aPKC regulates Notch receptor routing and activity in a Notch signalling dependent manner. Cell Research, in press Böcking D, Wiltschka O, Niinimäki J, Shokry H, Brenner R, Lindén M & Sahlgren C (2014) Mesoporous silica nanoparticlebased substrates for cell directed delivery of Notch signalling modulators to control myoblast differentiation. Nanoscale 6: 1490–8 Mamaeva V, Sahlgren C* & Lindén M* (2013) Mesoporous silica nanoparticles in medicine--recent advances. Adv. Drug Deliv. Rev. 65: 689–702 *Equal contribution Wittig R, Rosenholm JM, von Haartman E, Hemming J, Genze F, Bergman L, Simmet T, Lindén M & Sahlgren C (2013) Active targeting of mesoporous silica drug carriers enhances γ-secretase inhibitor efficacy in an in vivo model for breast cancer. Nanomedicine (Lond). Available at: http://www.ncbi. nlm.nih.gov/pubmed/23898823 Jin S, Mutvei AP, Chivukula I V, Andersson ER, Ramsköld D, Sandberg R, Lee KL, Kronqvist P, Mamaeva V, Ostling P, Mpindi J-P, Kallioniemi O, Screpanti I, Poellinger L, Sahlgren C & Lendahl U (2013) Non-canonical Notch signaling activates IL-6/ JAK/STAT signaling in breast tumor cells and is controlled by p53 and IKKα/IKKβ. Oncogene 32: 4892–902 Prabhakar N, Näreoja T, von Haartman E, Karaman DŞ, Jiang H, Koho S, Dolenko TA, Hänninen PE, Vlasov DI, Ralchenko VG, Hosomi S, Vlasov II, Sahlgren C & Rosenholm JM* (2013) Coreshell designs of photoluminescent nanodiamonds with porous silica coatings for bioimaging and drug delivery II: application. Nanoscale 5: 3713–22 Wilhelmsson U, Faiz M, de Pablo Y*, Sjöqvist M,* Andersson D, Widestrand A, Potokar M, Stenovec M, Smith PLP, Shinjyo N, 107 Pekny T, Zorec R, Ståhlberg A, Pekna M, Sahlgren C & Pekny M (2012) Astrocytes negatively regulate neurogenesis through the Jagged1-mediated Notch pathway. Stem Cells 30: 2320–9 *Equal contribution Rosenholm JM, Mamaeva V, Sahlgren C* & Lindén M* (2012) Nanoparticles in targeted cancer therapy: mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine (Lond) 7: 111–20. *Equal contribution Landor SK-J, Mutvei AP, Mamaeva V, Jin S, Busk M, Borra R, Grönroos TJ, Kronqvist P, Lendahl U & Sahlgren C (2011) Hypoand hyperactivated Notch signaling induce a glycolytic switch through distinct mechanisms. Proc. Natl. Acad. Sci. U. S. A. 108: 18814–9 Mamaeva V, Rosenholm JM, Bate-Eya LT, Bergman L, Peuhu E, Duchanoy A, Fortelius LE, Landor S, Toivola DM, Lindén M & Sahlgren C (2011) Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of Notch signaling in cancer. Mol. Ther. 19: 1538–46 Rosenholm JM, Sahlgren C* & Lindén M* (2010b) Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles--opportunities & challenges. Nanoscale 2: 1870–83 *Equal contribution Rosenholm JM, Peuhu E, Bate-Eya LT, Eriksson JE, Sahlgren C* & Lindén M* (2010a) Cancer-cell-specific induction of apoptosis using mesoporous silica nanoparticles as drug-delivery vectors. Small 6: 1234–41 *Equal contribution Rosenholm JM, Peuhu E, Eriksson JE, Sahlgren C* & Lindén M* (2009b) Targeted intracellular delivery of hydrophobic agents using mesoporous hybrid silica nanoparticles as carrier systems. Nano Lett. 9: 3308–11 *Equal contribution Rosenholm JM, Meinander A, Peuhu E, Niemi R, Eriksson JE, Sahlgren C* & Lindén M* (2009a) Targeting of porous hybrid silica nanoparticles to cancer cells. ACS Nano 3: 197–206 *Equal contribution Sahlgren C, Gustafsson M V, Jin S, Poellinger L & Lendahl U (2008) Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl. Acad. Sci. U. S. A. 105: 6392–7 From left to right. Back row: Valeriy Paramonov, Ramsus Niemi, Martina Lerche and Marika Sjöqvist. Front row: Daniel Antfolk, Christian Antila and Sebastian Landor 108 109 From left to right: Marianna Estrada, Satu Orasniemi, Maria Jensen, Malin Blom, Jens Luoto, Jenny Joutsen, Camillla Aspelin, Samu Himanen, Emine Lundsten, Eva Henriksson, Lea Sistonen, Mikael Puustinen, Heidi Bergman and Alexandra Elsing. 110 REGULATION AND FUNCTION OF HEAT SHOCK TRANSCRIPTION FACTORS Principal Investigator: Lea Sistonen, PhD, Professor of Cell and Molecular Biology, Department of Biosciences, Åbo Akademi University. Laboratory address: Centre for Biotechnology, BioCity, Tykistökatu 6, P.O.BOX 123, FIN-20521 Turku, Finland. Tel. +358-2-333 8028, 215 3311; E-mail: [email protected], [email protected] Biography: Lea Sistonen (b. 1959) completed her undergraduate studies at Åbo Akademi University in 1984 and received her PhD from the University of Helsinki in 1990. She was a post-doctoral fellow at Northwestern University in Dr. Richard I. Morimoto’s laboratory in 1990-1993 (Fogarty International Fellowship 1991-1993). In November 1993 she joined the Centre for Biotechnology as a senior research fellow in molecular biology. In April 2000 she was appointed as Professor of Cell and Molecular Biology at Åbo Akademi University. During the 5-year period 2004-2009 she was Academy Professor, the Academy of Finland. Personnel: Senior scientists: Eva Henriksson, PhD, Pia Roos-Mattjus, PhD Post-doctoral Fellows: Johanna Björk, PhD, Anton Sandqvist, PhD Graduate students: Camilla Aspelin, MSc, Heidi Bergman, MSc, Marek Budzynski, MSc, Alexandra Elsing, MSc, Jenny Joutsen, MSc, Emine Lundsten, MSc, Petra Vainio, MSc, Anniina Vihervaara, MSc Research assistant: Helena Saarento, MSc Undergraduate students: Malin Blom, Alejandro Da Silva, Marianna Estrada, Samu Himanen, Maria Jensen, Heidi Lustig, Jens Luoto, Satu Orasniemi, Mikael Puustinen Description of the Project: The heat shock response is an evolutionarily well-conserved cellular defense mechanism against protein-damaging stresses, such as elevated temperatures, heavy metals, and viral and bacterial infections. The heat shock proteins (Hsps) function as molecular chaperones to protect cells by binding to partially denatured proteins, dissociating protein aggregates, and regulating the correct folding and intracellular translocation of newly synthesized polypeptides. Hsps are transcriptionally regulated by heat shock factors, HSFs (HSF1-4 in mammals). Although HSFs are best known as stress-inducible transcriptional regulators, they are also important for normal developmental processes. The repertoire of HSF targets expands well beyond the Hsps, and HSF functions span from the heat shock response to development, metabolism, 111 lifespan and disease, especially cancer and neurodegenerative disorders. Our objective is to unravel the molecular mechanisms by which the different members of the HSF family are regulated during normal development and under stressful conditions. In particular, we investigate the expression and activity of HSF1 and HSF2. We were the first to report that HSF2 forms a complex with HSF1 and regulates the heat shock response. Our studies on HSF1-HSF2 heterotrimers and their impact on various target genes are designed to elucidate the roles of HSFs in proteinmisfolding disorders, such as neurodegenerative diseases, as well as in aging and cancer progression. Most studies have focused on HSF1, but it is important to consider the existence of multiple HSFs and interactions between them, especially when searching for potential drugs to modify their expression and/or activity. We have found that HSF1 activity is primarily regulated by various post-translational modifications (PTMs), e.g. acetylation, phosphorylation and sumoylation. All these PTMs are induced by stress stimuli but their effects on HSF1 vary. Upon stress, HSF1 undergoes phosphorylation-dependent sumoylation within a bipartite motif, which we found in many transcriptional regulators and gave name PDSM (phosphorylation-dependent sumoylation motif). Stress-inducible hyperphosphorylation and sumoylation of HSF1 occur very rapidly, whereas acetylation of HSF1 increases gradually, indicating a role for acetylation in the attenuation phase of the HSF1 activity cycle. Our ultimate goal is to determine the PTM signatures for both HSF1 and HSF2. Using mouse spermatogenesis as a model system, we discovered an inverse correlation between the cell- and stagespecific wave-like expression patterns of HSF2 and a specific microRNA, miR-18, which is a member of the Oncomir-1/miR17∼92 cluster. We found that miR-18 represses the expression of HSF2 by directly targeting its 3’UTR, and using our in vivo method T-GIST (Transfection of Germ cells in Intact Seminiferous Tubules), we showed that inhibition of miR-18 in intact mouse seminiferous tubules leads to increased HSF2 protein levels and altered expression of HSF2 target genes. Our original finding that miR-18 regulates HSF2 activity in spermatogenesis links miR-18 to HSF2-mediated physiological processes and opens a whole new window of opportunities to elucidate the physiological and stress-related functions of HSF2, either alone or in conjunction with HSF1. In contrast to HSF1, which is a stable protein and evenly expressed in most tissues and cell types, the amount of HSF2 varies and correlates with its activity. We have demonstrated that the ubiquitin E3 ligase APC/C (anaphase-promoting complex/cyclosome) mediates ubiquitylation and degradation of HSF2 during the acute phase of the heat shock response. Subsequently, we expanded our studies to involve the stress effects on the cell cycle, adding a new dimension to the research field. To this end, our ChIP-sequencing studies, identifying HSF1 and HSF2 target sites in cycling and mitotic human cells under optimal growth conditions and upon exposure to acute heat 112 stress, highlight an orchestrated transcriptional program that controls a multitude of cellular processes in stressed cycling cells. Surprisingly, HSF1 was found to possess a limited capacity to interact with the condensed chromatin and induce transcription in mitotic cells, whereas HSF2 is capable of binding to several targets, indicating a specific impact of HSF2 on transcription throughout the cell cycle progression. Since HSF2 expression declines during mitosis in many cell types, and the HSF2 levels are further decreased in response to stress, we have now addressed the functional relevance of HSF2 in the regulation of cell survival in mitotic cells exposed to acute stress. Our results indicate that in cells where HSF2 is downregulated, both HSF1 and RNA polymerase II that are normally displaced from mitotic chromatin are able to access the target genes. Furthermore, HSF2-deficient cells display diminished mitotic abnormalities and increased survival upon acute heat stress, thereby providing a protective mechanism to mitotic cells that are highly vulnerable to stress. Funding: The Academy of Finland, the Sigrid Jusélius Foundation, the Finnish Cancer Organizations, the Magnus Ehrnrooth Foundation, Turku Doctoral Programme of Biomedical Sciences (TuBS), and Åbo Akademi University (Centre of Excellence in Cell Stress and Molecular Aging). Collaborators: Klaus Elenius, Susumu Imanishi, Noora Kotaja and Jorma Toppari (University of Turku), John Eriksson, Peter Slotte and Kid Törnquist (Åbo Akademi University), Marko Kallio and Matthias Nees (VTT Medical Biotechnology, Turku), Valérie Mezger (University of Paris Diderot, France), Rick Morimoto (Northwestern University, Evanston, IL, USA), Jorma Palvimo (University of Eastern Finland, Kuopio), Dennis Thiele (Duke University, Durham, NC, USA), Laszlo Vigh (Biological Research Center, Szeged, Hungary). Selected Publications: Elsing A.N., Aspelin C., Björk J.K., Bergman H.A., Himanen S.V., Kallio M.J., Roos-Mattjus P. and Sistonen L. (2014) Expression of HSF2 decreases in mitosis to enable stress-inducible transcription and cell survival. J. Cell Biol., in press. Vihervaara A., Sergelius C., Vasara J., Blom M.A.H., Elsing A.N., Roos-Mattjus P. and Sistonen L. (2013) Transcriptional response to stress in the dynamic chromatin environment of cycling and mitotic cells. Proc. Natl. Acad. Sci. USA 110: E3388-E3397. Sundvall M.*, Korhonen A.*, Vaparanta K., Anckar J., Halkilahti K., Salah Z., Aqeilan R.I., Palvimo J.J., Sistonen L. and Elenius K. (2012) Protein inhibitor of activated STAT3 (PIAS3) promotes sumoylation and nuclear sequestration of the intracellular domain of ErbB4. J. Biol. Chem. 287: 2321623226. 113 Anckar J. and Sistonen L. (2011) Regulation of HSF1 function in the heat shock response: implications in aging and disease. Annu. Rev. Biochem. 80: 1089-1115. Björk J.K. and Sistonen L. (2010) Regulation of the members of the mammalian heat shock factor family. FEBS J. 277: 4126-4139. Ahlskog J.K., Björk J.K., Elsing A.N., Aspelin C., Kallio M., Roos-Mattjus P. and Sistonen L. (2010) Anaphasepromoting complex/cyclosome participates in the acute response to protein-damaging stress. Mol. Cell. Biol. 30: 5608-5620. Åkerfelt M.*, Vihervaara A.*, Laiho A., Conter A., Christians E.C., Sistonen L. and Henriksson E. (2010) Heat shock transcription factor 1 localizes to sex chromatin during meiotic repression. J. Biol. Chem. 285: 34469-34476. Björk J.K.*, Sandqvist A.*, Elsing A.N., Kotaja N. and Sistonen L. (2010) miR-18, a member of OncomiR-1, targets heat shock transcription factor 2 in spermatogenesis. Development 137: 3177-3184. Åkerfelt M., Morimoto R.I. and Sistonen L. (2010) Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11: 545-555. Blomster H.A.*, Imanishi S.Y.*, Siimes J., Kastu J., Morrice N.A., Eriksson J.E. and Sistonen L. (2010) In vivo identification of sumoylation sites by a signature tag and cysteine-targeted affinity purification. J. Biol. Chem. 285: 19324-19329. Blomster H.A., Hietakangas V., Wu J., Kouvonen P., Hautaniemi S. and Sistonen L. (2009) Novel proteomics strategy brings insight into the prevalence of SUMO-2 target sites. Mol. Cell. Proteomics 8: 1382-1390. Westerheide S.D.*, Anckar J.*, Stevens S.M.Jr., Sistonen L. and Morimoto R.I. (2009) Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 323: 1063-1066. Sandqvist A., Björk J.K., Åkerfelt M., Chitikova Z., Grichine A., Vourc’h C., Jolly C., Salminen T.A., Nymalm Y. and Sistonen L. (2009) Heterotrimerization of heat-shock factors 1 and 2 provides a transcriptional switch in response to distinct stimuli. Mol. Biol. Cell 20: 1340-1347. Åkerfelt M.*, Henriksson E.*, Laiho A., Vihervaara A., Rautoma K., Kotaja N. and Sistonen L. (2008) Promoter ChIP-chip analysis in mouse testis reveals Y chromosome occupancy by HSF2. Proc. Natl. Acad. Sci. USA 105: 11224-11229. *equal contribution 114 CANCER CELL SIGNALING http://www.btk.fi/research/research-groups/westermarck/ Principal investigator: Jukka Westermarck, MD, PhD, Professor. Address: Turku Centre for Biotechnology, BioCity, Tykistökatu 6B, FIN-20251 Turku, Finland. Tel. +358-2-3338621, Fax +358-2-2158808. E-mail: [email protected]. Biography: Jukka Westermarck (b. 1969) received his MD in 1996 and PhD in 1998 at the University of Turku. He was a post-doctoral fellow at European Molecular Biology Laboratory in Heidelberg, Germany, in Dr. Dirk Bohmann´s laboratory during 1999-2001. He was an Academy of Finland senior scientist during 2002-2007. Between 2006-2009 he was appointed as a group leader at Institute of Medical Technology (IMT), University of Tampere, Finland. In 2008 he was appointed to a Research Professor position at the Finnish Cancer Institute, in 2009 to a Research Director position at Turku Centre for Biotechnology (leave of absence until 2014) and in 2011 to a part-time position as a Professor of Cancer Biology at Department of Pathology, University of Turku (until 2104). Personnel: Senior scientist: Jukka Westermarck, MD, PhD Post-doctoral Fellows: Anna Lipsanen, PhD, Juha Okkeri, PhD, Anni Laine, PhD, Christian Rupp, PhD Graduate students: Otto Kauko, MD, MSc, Amanpreet Kaur, MSc, Xi Qiao, MSc, Eleonora Sittig, MSc Technicians: Taina Kalevo-Mattila, Lab.Tech., Inga Pukonen, B.Eng. Head of Laboratory: Tiina Arsiola, PhD Description of the project: The general goal of our research group is to explore regulation and function of human tumor suppressor Protein Phosphatase 2A (PP2A) in human malignancies. Various PP2A complexes regulate activity of variety of critical signaling pathways in cancer by dephosphorylating phosphorylated serine/threonine residues in kinases, transcription factors and other signaling proteins. PP2A inhibition is one of requirements of human cell transformation and thereby understanding of the function and regulation of PP2A tumor suppressor activity is a fundamentally important question in cancer biology. In addition to genetic alterations in some of the PP2A complex components, PP2A activity is inhibited in human cancers by endogenous inhibitor proteins. Our laboratory recently identified CIP2A as a novel PP2A inhibitor protein, and was first to demonstrate cancer-promoting roles for both CIP2A and another PP2A inhibitor protein PME-1. In addition, PP2A inhibitor protein SET promotes activity of several oncogenic pathways and tumor growth. Expression of all of these PP2A inhibitor proteins shows strong association with tumor progression in several human cancer types and thus represents novel potential targets for cancer therapy. Most of the projects in our laboratory are focused on further understanding of CIP2A, PME-1 and SET as novel human oncoproteins, and their suitability as novel drug targets for cancer therapies. Moreover, as there is a clear need for novel genetic mouse models to study role of PP2A in cancer development and progression, and to model potential PP2A re- 115 From left to right: Xi Qiao, Christian Rupp, Amanpreet Kaur, Juha Okkeri, Anna Lipsanen, Jukka Westermarck, Taina Kalevo-Mattila, Otto Kauko, Tiina Arsiola, Anni Laine, Eleonora Sittig and Inga Pukonen. activating therapies, we are in the process of generating conditional mouse models targeting endogenous PP2A inhibitors. Funding: The Academy of Finland, Sigrid Juselius Foundation, Turku Graduate School of Biomedical Sciences, Cancer Research Foundation of Finland, Biocenter Finland, Foundation of the Finnish Cancer Institute. Collaborators: Rosalie Sears (Oregon Health and Science University), Owen Sansom (Beatson Institute for Cancer Research, Glasgow), Heikki Joensuu (Helsinki University Hospital), Tapio Visakorpi (University of Tampere), Juha Klefström (University of Helsinki), Tero Aittokallio (FIMM, Helsinki) Selected Publications: Khanna, A., Kauko, O., Böckelman, C., Laine, A., Schreck, I., Partanen, J.I., Szwadja, A., Bormann, S., Bilgen T, Helenius, M., Pokharel, Y.R., Pimanda, J., Russel, M.R., Haglund, C., Cole, K.A., Klefström, J., Aittokallio, T., Weiss, C., Ristimäki, A., Visakorpi, T. and Westermarck, J. (2013). Chk1 targeting reactivates PP2A tumor suppressor activity in cancer cells. Cancer Research 73(22): 6757-6769. Laine, A., Sihto, H., Come, C., Rosenfeldt, M., Zwolinska, A., Niemelä, M., Khanna, A., Chan, E.K., Kähäri, V.-M., Kellokumpu-Lehtinen, P.L., Sansom, O.J., Evan, G.I., Junttila, M.R., Ryan, K.M., Marine, J.-C., Joensuu, H. and Westermarck, J. (2013). Senescence sensitivity of breast cancer cells is defined by positive feedback loop between CIP2A and E2F1. Cancer Discovery 3: 182-197. Niemelä, M., Kauko, O., Sihto, H., Mpindi, J.-P., Nicorici, D., Pernilä, P., Kallioniemi, O.-P., Joensuu, H., Hautaniemi, S. and Westermarck, J. (2012). CIP2A signature reveals the MYC dependency of CIP2Aregulated phenotypes and its clinical association with breast cancer subtypes. Oncogene 31: 4266-4278. Ventelä, S., Mäkelä, J.-A., Kulmala, J., Westermarck, J. and Toppari, J. (2012). Identification and regulation of a stage-specific stem cell niche enriched by Nanog positive spermatogonial stem cells in the mouse testis. STEM CELLS 30: 1008-1020. Ventelä, S., Come, C., Mäkelä, J.-A., Hobbs, R.M., Mannermaa, L., Kallajoki, M., Chan, E.K., Pandolfi, P.P., Toppari, J. and Westermarck, J. (2012). CIP2A promotes proliferation of spermatogonial progenitor cells and spermatogenesis in mice. PLoS ONE 7: e33209. Come, C., Laine, A., Chanrion, M., Edgren, H., Mattila, E., Liu, X., Jonkers, J., Ivaska, J., Isola, J., Darbon, J.-M., Kallioniemi, O.-P., Thezenas, S. and Westermarck, J. (2009). CIP2A is associated with human breast cancer aggressivity. Clin. Cancer Res. 15: 5092-5100. Khanna, A., Böckelman, C., Hemmes, A., Junttila, M.R., Wiksten, J.-P., Lundin, P., Junnila, S., Murphy, D., Evan, G.I., Haglund, C., Westermarck, J.* and Ristimäki, A.* (2009). c-Myc-dependent regulation and prognostic role of CIP2A in gastric cancer. J. Natl. Cancer Inst. 101: 793-805. *equal contribution Puustinen, P., Junttila, M.R., Vanhatupa, S., Sablina, A.A., Hector, M.E., Teittinen, K., Raheem, O., Ketola, K., Lin, S., Kast, J., Haapasalo, H., Hahn, W.C. and Westermarck, J. (2009). PME-1 protects extracellular signal-regulated pathway activity from protein phosphatase 2A-mediated inactivation in human malignant glioma. Cancer Res. 69: 2870-2877. Westermarck, J. and Hahn, W.C. (2008). Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol. Med. 14: 152-160. Junttila, M.R., Li, S.-P. and Westermarck, J. (2008). Phosphatasemediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 22: 954-965. Junttila, M.R., Puustinen, P., Niemelä, M., Ahola, R., Arnold, H., Böttzauw, T., Ala-aho, R., Nielsen, C., Ivaska, J., Taya, Y., Lu, S.L., Li, S., Chan, E.K.L., Wang, X.-J., Grenman, R., Kast, J., Kallunki, T., Sears, R., Kähäri, V.-M. and Westermarck, J. (2007). CIP2A inhibits PP2A in human malignancies. Cell 130: 51–62. 116 117 ADENOSINE DEAMINASES Principal investigator: Andrey Zavialov, PhD, Finnish Academy Research Fellow (group leader), Turku Centre for Biotechnology, University of Turku, Tykistökatu 6, FI-20520,Turku, Finland, Tel. +358403776216, Fax. +358-2-3338000, Email: [email protected] Biography: Andrey Zavialov (b. 1975) has obtained his M.S. in Biotechnology from Russian Chemical Technology University (Moscow) and a PhD in Molecular Biology from Uppsala University (Sweden). Between 2005-2010 Dr Zavialov received his post-doctoral training in Immunology at Institute of Cellular and Molecular Pharmacology (France) and worked as a research scientist and an assistant research professor at A*-STAR’s Singapore Immunology Network (SIgN) and University of Hawaii at Manoa (U.S.A.). Dr. Zavialov is a recipient of the Harold M. Weintraub graduate student Award, EMBO and HFSP long-term fellowships. In 2011 he was selected as a Research Fellow of the Academy of Finland. Personnel: Graduate students: Maksym Skaldin (M.S.), Chengquian Liu (M.S.), Yuliia Mukiienko (MD), Balwant Rai (MFO) Description of the project: Two distinct enzymes of adenosine deaminase, ADA1 and ADA2, have been found in humans. Inherited mutations in ADA1 result in severe combined immunodeficiency (SCID). This observation led to extensive studies of the structure and function of this enzyme that have revealed its important role in lymphocyte activation. In contrast, the physiological role of ADA2 is unknown. ADA2 activity in serum is increased in various diseases in which monocytes/macrophages are activated. We have found that ADA2 is a heparin-binding protein. The enzyme was purified and identified as a member of a new class of adenosine deaminase related growth factors (ADGF). Biochemical data suggest that ADA2 may be active at sites of inflammation during hypoxia and in areas of tumor growth where the adenosine concentration is significantly elevated and the extracellular pH is low. We showed that ADA2 is secreted by monocytes undergoing differentiation into macrophages or dendritic cells, and that activated T cells are likely the main target for ADA2. The recently solved structure of ADA2 allows us to establish the role of unique ADA2 domains in the enzyme’s interaction with its specific receptor. The presence of two different ADAs in humans and their interaction with adenosine receptors, which may affect their function, has largely been ignored due to a lack of knowledge and a dearth of research teams performing systematic studies of adenosine receptors together with ADAs. At the same time, clinical studies have shown that ADA2 is a very specific biological marker for common and life-threatening diseases such as HIV, tuberculosis and breast cancer. Recently, our collaborators from the NIH and a group from Israel have identified patients with mutations in the ADA2 gene. It was shown that ADA2 concentration in the plasma of these patients is reduced more than 10-fold compared to 118 that in healthy subjects. Furthermore, these patients display multiple health problems, including early onset systemic inflammation, multiple ischemic strokes, and vasculitis. Interestingly, the symptoms of the ADA2-deficient patients are distinct from SCID patients with ADA1 deficiency. Although the absence of either functional ADA1 or ADA2 leads to disregulated immune function, the lack of one functional enzyme is not compensated for by the presence of the remaining enzyme, suggesting that ADA1 and ADA2 have distinct roles. Strikingly, in both reports, the patients that were homozygous for a common mutation in Gly47 had clear symptoms of polyarteritis nodosa vasculopathy (PAN). Therefore, this type of mutation in ADA2 would be the first known and well-characterized cause of PAN. Our studies will explore the possibility that ADA2 is an immunomodulatory protein, which may directly or indirectly affect immune responses against intracellular pathogens or tumor cell proliferation. Our goal is to establish the physiological role of ADA2 in inflammation and tumor immunity and to explore its therapeutic potential. Funding: The Academy of Finland; CIMO Collaborators: Dr. Ivona Aksentijevich (NIH/NHGRI, U.S.A.), Dr. Urpo Lamminmäki (University or Turku), Dr. Mikko Seppänen (University of Helsinki), Prof. Jose Parcel (Arnau de Vilanova University Hospital, Lleida, Spain), Dr. Anton Zavialov (University of Turku), Dr. Yuanan Lu (University of Hawaii, U.S.A.) Selected Publications: van Montfrans J, Zavialov A, Zhou Q. Mutant ADA2 in Vasculopathies. N Engl J Med. 371(5):478-481. Zhou, Q., Yang, D., Ombrello, A.K., Zavialov, A.V. at al. (2014). Intermittent Fever and Early-Onset Stroke Due to Mutations in ADA2. N Engl. J Med. 370: 911-20. Zavialov, A. V., X. Yu, D. Spillmann, G. Lauvau and A.V. Zavialov.2010. Structural basis for the growth factor activity of human adenosine deaminase ADA2. J Biol Chem 285:12367-12377. Zavialov, A. V., E. Gracia, N. Glaichenhaus, R. Franco, and G. Lauvau. 2010. Human adenosine deaminase 2 induces differentiation of monocytes into macrophages and stimulates proliferation of T helper cells and macrophages. J Leukoc Biol 88:279-290. Gao, N., A. V. Zavialov, M. Ehrenberg, and J. Frank. 2007. Specific interaction between EF-G and RRF and its implication for GTPdependent ribosome splitting into subunits. J Mol Biol 374:1345-1358. Gao, H., Z. Zhou, U. Rawat, C. Huang, L. Bouakaz, C. Wang, Z. Cheng, Y. Liu, A. Zavialov, R. Gursky, S. Sanyal, M. Ehrenberg, J. Frank, and H. Song. 2007. RF3 induces ribosomal conformational changes responsible for dissociation of class I release factors. Cell 129:929-941. Rawat, U., H. Gao, A. Zavialov, R. Gursky, M. Ehrenberg, and J. Frank. 2006. Interactions of the Release Factor RF1 with the Ribosome as Revealed by Cryo-EM. J Mol Biol 357:1144-1153. Hauryliuk, V., A. Zavialov, L. Kisselev, and M. Ehrenberg. 2006. Class-1 release factor eRF1 promotes GTP binding by class-2 release factor eRF3. Biochimie 88:747-757. 119 Zavialov, A. V., V. V. Hauryliuk, and M. Ehrenberg. 2005. Splitting of the posttermination ribosome into subunits by the concerted action of RRF and EF-G. Mol Cell 18:675-686. Zavialov, A. V., V. V. Hauryliuk, and M. Ehrenberg. 2005. Guaninenucleotide exchange on ribosome-bound elongation factor G initiates the translocation of tRNAs. J Biol 4:9. Zavialov, A. V., and A. Engstrom. 2005. Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity. Biochem J 391:51-57. Gao, N., A. V. Zavialov, W. Li, J. Sengupta, M. Valle, R. P. Gursky, M. Ehrenberg, and J. Frank. 2005. Mechanism for the disassembly of the posttermination complex inferred from cryo-EM studies. Mol Cell 18:663-674. Frank, J., J. Sengupta, H. Gao, W. Li, M. Valle, A. Zavialov, and M. Ehrenberg. 2005. The role of tRNA as a molecular spring in decoding, accommodation, and peptidyl transfer. FEBS Lett 579:959-962. Allen, G. S., A. Zavialov, R. Gursky, M. Ehrenberg, and J. Frank. 2005. The cryo-EM structure of a translation initiation complex from Escherichia coli. Cell 121:703-712. Zavialov, A. V., and M. Ehrenberg. 2003. Peptidyl-tRNA regulates the GTPase activity of translation factors. Cell 114:113-122. Valle, M., A. Zavialov, J. Sengupta, U. Rawat, M. Ehrenberg, and J. Frank. 2003. Locking and unlocking of ribosomal motions. Cell 114:123-134. Valle, M., A. Zavialov, W. Li, S. M. Stagg, J. Sengupta, R. C. Nielsen, P. Nissen, S. C. Harvey, M. Ehrenberg, and J. Frank. 2003. Incorporation of aminoacyl-tRNA into the ribosome as seen by cryo-electron microscopy. Nat Struct Biol 10:899-906. Rawat, U. B., A. V. Zavialov, J. Sengupta, M. Valle, R. A. Grassucci, J. Linde, B. Vestergaard, M. Ehrenberg, and J. Frank. 2003. A cryo-electron microscopic study of ribosome-bound termination factor RF2. Nature 421:87-90. Pedersen, K., A. V. Zavialov, M. Y. Pavlov, J. Elf, K. Gerdes, and M. Ehrenberg. 2003. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell 112:131-140. Mora, L., A. Zavialov, M. Ehrenberg, and R. H. Buckingham. 2003. Stop codon recognition and interactions with peptide release factor RF3 of truncated and chimeric RF1 and RF2 from Escherichia coli. Mol Microbiol 50:1467-1476. Klaholz, B. P., T. Pape, A. V. Zavialov, A. G. Myasnikov, E. V. Orlova, B. Vestergaard, M. Ehrenberg, and M. van Heel. 2003. Structure of the Escherichia coli ribosomal termination complex with release factor 2. Nature 421:90-94. Zavialov, A. V., L. Mora, R. H. Buckingham, and M. Ehrenberg. 2002. Release of peptide promoted by the GGQ motif of class 1 release factors regulates the GTPase activity of RF3. Mol Cell 10:789-798. Zavialov, A. V., R. H. Buckingham, and M. Ehrenberg. 2001. A posttermination ribosomal complex is the guanine nucleotide exchange factor for peptide release factor RF3. Cell 107:115-124. From left to right: Yuliia Mukiienko, Chengquian Liu and Andrey Zaviyalov. 120 121 PhD DEFENCES 122 123 LIFE OUTSIDE THE LAB 124 125 126 127 128 129 130
© Copyright 2026