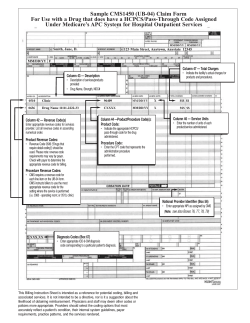
PDF Version - InsideHealthPolicy.com
InsideHealthPolicy.com’s Inside CMS exclusive news on the most powerful agency in health care Vol. 17, No. 51 - December 18, 2014 Industry Looks At 21st Century Cures To Set Stage For PDUFA VI Representatives from the pharmaceutical and biotechnology industries said Friday (Dec. 12) they expect work on the bipartisan 21st Century Cures initiative this spring to be a staging ground for negotiations over the sixth iteration of the Prescription Drug User Fee Act (PDUFA) in 2016 and 2017. Industry leaders also said they expected the biotechnology industry to enter the negotiations with FDA as an equal partner with the pharmaceutical industry, speaking on a PDUFA VI panel at an IIRsponsored FDA/CMS Summit in Washington. Brian Harvey, vice president of Global Regulatory Affairs for Pfizer, echoed the sentiments of other panelists saying that 21st Century Cures will be a “dry run” for PDUFA. “What better way to really sort of formulate your ideas and vet them to make sure they really hold water than to have to do it that much earlier,” Harvey said. “I’m very optimistic that something may come out of 21st Century Cures, but even for those things that don’t make it through the process we’re that much further along for PDUFA VI.” The 21st Century Cures initiative is a bipartisan effort spurred by House Energy and Commerce Chair Fred Upton (R-MI) and Ranking Minority Member Diana DeGette (D-CO) that is expected to include a broad spectrum of provisions aimed at spurring development of new drugs continued on page 10 Spending Bill Bashes RACs, Hits CMS And OMHA Over Appeals Backlog Lawmakers in their report accompanying the recently passed fiscal 2015 spending bill blast CMS’ Recovery Audit contractor program for harming providers and contributing to the backlog at the third level of Medicare appeals. But the American Coalition for Healthcare Claims Integrity, which represents RACs, slammed appropriators for being sympathetic to providers, who the RACs allege are incorrectly billing Medicare, and say the report amounts to the hospital industry using lawmakers to bully CMS over the RAC program. The appropriations report states that CMS needs to educate providers on reducing errors, develop ways to reduce the backlog at the Office of Medicare continued on page 12 Appropriators Ask HHS To Study 340B Drug Program, Hep C Rx Costs Congress’ massive spending deal includes two provisions related to the debate over drug prices — one on the 340B drug discount program and one on hepatitis C drug prices. The report accompanying the legislation urges HHS to investigate whether the most vulnerable patients benefit from the 340B program, and it asks CMS to study the prevalence of hepatitis and the cost of treating the sickest patients. The 340B program, administered by HHS’ Health Resources and Services Administration, makes drug makers discount the price of outpatient drugs for certain hospitals and other health care providers. Safety net hospitals and drug makers are battling over the scope of the program, as HRSA drafts continued on page 13 ICD-10 Delay Still A Possibility Though Not Included In Latest Spending Bill House Energy & Commerce Chair Fred Upton (R-MI) and House Rules Committee Chair Pete Sessions (R-TX) plan to hold a hearing next year on the health care industry’s preparations for ICD-10, after a further ICD-10 delay was considered but not included in Congress’ latest spending bill. Still, those following the issue say the idea of delaying implementation of the new Medicare code set could pop up again next year as lawmakers consider what to do about the flawed Medicare physician payment formula. Sessions raised the idea of again delaying implementation of ICD-10 as part of the “CRomnibus” spending package released late Tuesday (Dec. 9), according to some following the issue, but the delay was ultimately not continued on page 14 McClellan Suggests Including Drug Spending In ACOs By Condition CMS could use quality measures to make accountable care organizations responsible for Part D drug spending on a per-condition, Brookings Institution senior fellow Mark McClellan said. The former CMS chief also said at an FDA/CMS Summit on Thursday (Dec. 11) that inefficiencies in the health care system must be eliminated if Medicaid programs and other insurers are going to pay for specialty drugs. McClellan said the price of drugs should be viewed in the context of the costs of services that drugs avoid by keeping people out the hospital, or, in the case of Gilead’s hepatitis C drugs, by curing many people who otherwise would have cost the health care system over decades. However, he noted that Medicaid programs must stay within their budgets and said education and other public health programs already are being squeezed out by the cost of Sovaldi and Harvoni. State officials probably see states getting more value out of incremental increases in spending on early childhood education, he said, so biomedical companies must make drugs more valuable by targeting medications to the right people and helping those people stick to their regimens. One way to do that is for ACOs to take more responsibility for drug spending, McClellan suggested. Part B drugs already fall under ACOs because they’re physician-administered, but Part D drugs do not. Although CMS recently raised the idea of ACOs combining with drug plans and Medicaid and accepting global payments, some healthcare consultants believe high-priced specialty drugs might scare ACOs away from accepting the risk of drug spending. Most ACOs already have a difficult enough time accepting the risk of medical costs, they say. McClellan noted that medication adherence is poor for certain conditions, such as diabetes, and ACOs could partner with drug makers in those areas to cut costs, McClellan said. He noted that in some instances drug companies have accepted contracts that only pay them when a drug works. Drug makers understand their products best so they should be the ones to target the right populations and improve medication adherence, McClellan added. He said that Sovaldi and Harvoni are probably bad examples of a need for medication adherence programs because these drugs already have high adherence rates. CMS could use the performance metrics that it uses to judge the quality of care to steer ACOs toward accepting the risk of drug spending in areas where medication adherence would do the most good, McClellan suggested. CMS on Oct. 2 sought advice on including drug spending in ACOs as part of a request for information on health plan innovation concepts. A CMS spokesperson said the responses to that FRI are not yet publicly available — responses were due Nov. 3. An ACO consultant said several ideas have been floated for including drug spending in ACOs. However, CMS’ information request was aimed at demonstrations, which are run separately from the Medicare Shared Savings Program, and the recent MSSP proposed rule does not mention including Part D in that program, according to an analyst who is studying the proposal. — John Wilkerson In omnibus spending report... Lawmakers To CMS: Justify Home Health Face-to-Face Requirements Lawmakers are asking CMS to justify its use of face-to-face requirements for home health care and explain how the provisions are impacting Medicare spending, laying out the request as part of the report accompanying the fiscal 2015 spending bill. The report also asks CMS to analyze home health rebasing, which the industry has strongly opposed. The House and Senate report, which accompanied the recently passed 2015 omnibus appropriations bill, says that CMS as part of its fiscal 2016 budget request should “quantify and explain how the policy directing physicians to conduct face-to-face certifications for home health care has prevented fraud, increased access to health care, and impacted costs to the Medicare and Medicaid programs.” The report also says CMS should include ways to simplify the provider documentation for face-to-face encounters as Hot Documents Now Available on InsideHealthPolicy.com The following new documents are available on InsideHealthPolicy.com, our new online health news service. Subscribers to InsideHealthPolicy.com also have access to hundreds of other health-related documents, daily news updates, and a searchable archive of back issues. CMS Issues Model Notices For Employers Objecting To Contraceptive Coverage Senate Aging Committee Recommends Ways To Boost Generic Drug Use In Medicare HHS Announces State Innovation Models (SIM) Award Round 2 Recipients Energy And Commerce Dems Report Details Potential District-By-District Impact If Admin Loses King v. Burwell Not an online subscriber? Inside CMS subscribers can get a free, two-week trial to InsideHealthPolicy.com by calling 1-800-424-9068 or signing up at http://www.insidehealthpolicy.com/health_signup.asp. 2 INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 part of its fiscal 2016 budget request. Industry has viewed the Affordable Care Act’s face-to-face requirements as flawed and unclear. As part of the face-to-face documentation, a physician narrative was required to describe a patient’s clinical conditions and why a beneficiary needs home health care. The National Association for Home Care and Hospice sued CMS over the face-to-face requirements and physician narrative earlier this year because of confusion around what the narrative required. Home health providers said they were seeing claims denied because of documentation problems over which they had no control. In the home health final pay rule, CMS eliminated the physician narrative requirement from the face-to-face documentation following the lawsuit. The changes are set to go into effect in 2015. HHS, in a recent report on the agency’s finances, also said that the face-to-face denials were driving up Medicare’s improper payment rates. “HHS believes clarifying the face-to-face requirements will lead to a decrease in these errors and improve provider compliance with regulatory requirements, while continuing to strengthen the integrity of the Medicare program,” HHS’ financial report says. Bill Dombi, vice president for law at the National Association for Home Care and Hospice, noted the bi-partisan, bicameral support behind the report’s comments on face-to-face issues, and said that support backs up the industry’s concerns. some lawmakers have also recently pushed CMS to consider a settlement for home health denials that have occurred because of face-to-face documentation issues. The Partnership for Quality Home Healthcare also thanked lawmakers for drawing attention to both face-toface issues and concerns about home health cuts. “Our community welcomes the home health language in this budget agreement as an important step towards rebasing and regulatory relief which will stabilize access for the 3.5 million home-bound Medicare beneficiaries who depend on skilled home healthcare services,” Partnership CEO Eric Berger said in a statement. The report also says that CMS should quickly provide a public analysis of ACA-required rebasing cuts to home health care, cuts which home health providers have been fighting. The ACA directed CMS to rebase Medicare pay for home health services, and the home health pay rule included the second of four years of rebasing cuts. The partnership has said the rebasing cuts are so large that many home health providers will be operating at a net loss by 2017. The Medicare Payment Advisory Commission, however, said in comments on the proposed home health pay rule that the rebasing cuts are too small. Berger said a public analysis would fill an information gap created when CMS didn’t publish some expected analyses before implementing the rebasing cuts, and more public information on the cuts could lead to their reconsideration by the HHS secretary. It could also help add support to an ongoing push from lawmakers to replace the rebasing cuts with hospital readmission reforms through the Securing Access Via Excellence (SAVE) Medicare Home Health Act, introduced in the House by Reps. Greg Walden (R-OR) and Tom Price (R-GA). — Michelle M. Stein Health plans extend payment deadlines, states offer sign-up leeway... CMS Gives Last-Minute Extension To Consumers With Long Wait Times HHS tells Inside Health Policy that it handed a last-minute extension to around 500,000 consumers who waited longer than usual when contacting the federal call center Monday night, the deadline to enroll in exchange coverage through healthcare.gov in order to make it effective Jan. 1. Some state-run exchanges signaled they too would offer leeway in enrollment deadlines, and health insurers offered to extend premium payment deadlines beyond Dec. 31. The “several hundred thousand” customers granted the extension by HHS left their contact information, and were among 1.6 million people who called in the last three days, HHS said Tuesday. CMS Principal Deputy Administrator Andy Slavitt said FFM representatives have already begun calling those customers back. The moves aim to address a high volume of consumer demand and stem confusion surrounding healthcare.gov’s first enrollment cut-off for the ACA’s second enrollment period. The federal call center had longer-than-normal wait times, an HHS official told IHP Tuesday (Dec. 16), prompting representatives to ask callers to leave their contact information instead of sitting on the phone. Department workers will return their call “at a convenient time” starting Tuesday, the official said. If they select a plan, coverage will still begin Jan. 1. The call center added 1,000 representatives to handle consumer concerns this year, bringing the total to around 14,000 workers for the second open enrollment period, according to HHS. The department also says it increased the number of Spanish-language staff to 12 percent overall this year from 8 percent last year. The department expected to see a higher volume of calls because of Monday’s deadline. Slavitt added that almost 2.5 million users had selected a plan through the federal marketplace as of midnight Friday, though HHS will not yet release enrollment totals that include numbers later than Dec. 12. America’s Health Insurance Plans says insurers will use CMS data to determine a consumer’s 2015 enrollment status and support those moving to new health insurance plans, hoping to minimize negative impacts on anyone trying to INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 3 navigate the renewal process. Health plans will also give new and returning enrollees more time to pay their January premiums as December winds down, AHIP said in a release Tuesday. Each will work with their call centers to respond to consumers’ questions about their renewal and billing status. January premiums are normally due by Dec. 31, but consumers should check with their insurance provider for dates on a particular grace period. Once a health plan has verified that a current customer has switched carriers, AHIP said, the plan will turn off autobilling, send a revised invoice and refund excess payments if necessary. Multiple plans are contacting enrollees by email, phone or letter if they have switched carriers, putting themselves at most risk for gaps in coverage. The payment leeway comes as a handful of states are also extending their enrollment deadlines for those who want coverage starting Jan. 1: As of Tuesday morning (Dec. 16), New York, Idaho, Minnesota, California and Connecticut had strayed from their original Dec. 15 date. As of Tuesday morning, the deadlines stood at midnight local time in Colorado, DC, Hawaii and Kentucky; Dec. 18 in Maryland; Dec. 19 in Connecticut; Dec. 20 in Idaho, Minnesota and New York; Dec. 21 in California; Dec. 23 in Washington, Rhode Island and Massachusetts; and Dec. 31 in Vermont. The 36 states which use healthcare.gov adhered to a federal deadline of midnight PST, except for 10 pm local time in Alaska. Applications must have already been started by midnight local time Monday (Dec. 15) in California and Connecticut. Those applying for tax credits in Idaho must have done so by midnight local time on Monday. Most states with changed deadlines are trying to accommodate anyone who simply needs more time to hear or understand that they need to enroll or renew coverage by a certain date for their plan to kick in Jan. 1. In New York’s case, the extension aims to help those in western cities who were blindsided by massive snowstorms last month. “The extreme weather that parts of the state experienced in November has effectively cut short the enrollment period for many consumers who are making decisions about 2015 coverage options or enrolling in coverage for the first time,” New York State of Health executive director Donna Frescatore said in a Dec. 12 release. “By extending the deadline - in an already condensed enrollment period - we are ensuring that all New Yorkers have the same opportunity to start the new year with affordable coverage in a plan that’s right for them.” — Rachel S. Karas AHIP CEO: ACA Goals Unachievable If PhRMA Has ‘Blank Check’ America’s Health Insurance Plans CEO Karen Ignagni on Friday (Dec. 12) made another plea for drug makers to work with payers and providers to make pricing of specialty drugs more competitive and transparent, saying that absent such a a move changes in the health care landscape designed to increase quality and access while lowering costs may not be be sustainable in the long term. The Affordable Care Act has created a retail health care market in which consumers are focused on affordable, high-quality care, and government-sanctioned monopolies for innovative drugs create a stumbling block, she said at an IIR-sponsored FDA/CMS Summit in Washington. “The recognition of the problem is the idea that we can no longer continue a system where one sector expects a blank check,” Ignagni said. “That’s the way the system has been organized, and that doesn’t work if the goal is affordability.” Ignagni said that while specialty drugs make up only make up 1 percent of plans’ medication purchases they account for 25 percent of spending. She said plans have been working with providers, consumers and state and federal agencies in order to spur competition, but competition is lacking in the specialty drug market. While there are a number of models that are helping plans control costs such as medical homes, Accountable Care Organizations and bundling, the high price of specialty drugs is a factor that can raise premium prices for consumers, she said. While she didn’t specifically mention any high-priced specialty drugs, her comments come as the debate rages over the high cost of new hepatitis C cures Sovaldi and Harvoni, which combines Sovaldi (sofosbuvir) and ledipsavir. Sovaldi, has been priced by maker Gilead Sciences at $84,000 for a 12-week course, and the price tag of Harvoni, comes in at $94,500. Ignagni noted that state Medicaid directors have been vocal in their belief that the prices for these much-needed, innovative drugs are unsustainable. The National Association of Medicaid Directors wrote Congress in October asking for the federal government to either control the price of specialty drugs or help states pay for them. She said she doesn’t want to stifle much-needed innovation, and understands that asking drug makers to come on board with controlling costs means a hit to their bottom lines. “Because my cost containment is someone else’s revenue reduction,” Ignagni said. The federal government and states can help, she said, by implementing policies that remove barriers to competition, such as the FDA encouraging the development of biosimilars and helping get those product get to the marketplace quickly, she said. One way drug manufacturers can help, Ignagni said, is by increasing transparency with regard to how much it costs to develop a specialty drug. 4 INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 “Now we have to turn to having a conversation about innovation, having a conversation about value,” Ignagni said. “How do we know what is being spent on R&D (research and development) — how do we know that we are subsidizing R&D versus marketing, advertising and other kinds of costs? I think we’ve gotten to the point that to sustain a private market, just as now in the health plan community and hospital community, we need transparency here.” Ignagni said she understands that bringing the demand and supply sides of the equation together to find a way to spur competition to control costs while also fostering innovation is difficult, but she said early efforts to have these conversations with drug makers have been promising. “With out a doubt, moving to a retail market is a change, but I think that with change comes opportunity and certainly challenges,” Ignagni said. “I’m excited about the opportunities for partnership, and the conversations to talk about transparency; to talk about value; to talk about preserving our private sector system; and to talk about doing it together.” — Todd Allen Wilson Premier Tells OIG Hospitals Can Manage Gainsharing, AdvaMed Wary Hospital system Premier applauds HHS’ Office of the Inspector General (OIG) proposal to loosen its strict interpretation of the gainsharing civil monetary penalty rules (CMP), and says the office should allow hospitals to pay providers incentives based on objective quality metrics and benchmarks. But the Advanced Medical Technology Association (AdvaMed), which represents device manufacturers, says looser rules on gainsharing could limit patients’ options and stifle medical device innovation, responding to an OIG proposed rule on the Anti-Kickback statute and civil monetary penalty rules released in October. OIG said in a proposed rule released in October that it is considering defining “reduce or limit services” within the context of the gainsharing CMP rule in a way that more fully recognizes the changing health care landscape. The agency says the clarification is needed given the rapid changes in health care and the focus on quality measures designed to improve quality of care and reduce costs. Under the gainsharing CMP provisions, as interpreted by OIG, hospitals are barred from providing incentives to patients that would cause them to “reduce or limit services” — regardless of whether those services are not medically necessary or align with the latest clinical protocols. OIG says it recognizes that CMS is running several programs and demos like the Medicare Shared Savings Program that exempt participants from the gainsharing provisions, but entities not participating in those programs are in no way exempt. Like the American Hospital Association argued in its comments on the proposed rules, Premier contends that practice changes providers and hospitals make in order to meet the myriad of quality-measures they are required to make — often at the risk of penalties if measures aren’t met — under CMS directives and other means designed to improve care and reduce costs does not mean that services for patients have been reduced or limited, and are often times medically necessary. “Ensuring evidence-based care is being provided while measuring quality improvement should be an on-going process for which physicians are compensated. As long as the programs are resulting in value to the Medicare program through reduced costs and patients are benefiting from better care, the Gainsharing CMP should not act as an impediment,” Premier says in its comments on proposed rule. But AdvaMed says in its comments that OIG should be cautious in relaxing the gainsharing CMP because opening up provisions may lead to reductions in care in order to meet short-term savings. The organization is particularly concerned about hospitals standardizing devices physicians can use. “AdvaMed understands the desire of the OIG to update its guidance on the issue of gainsharing and to interpret the statute in a way that ‘reflects today’s health care landscape.’ However, the OIG must also be able to ensure that any gainsharing programs and arrangements are carefully designed in order to prevent patients from being put at risk from inappropriate incentives that influence clinical decisions, leading to stinting on care or otherwise inappropriate choices,” AdvaMed says in its comments. Premier argues that standardization of certain items — surgical items, medical devices or drugs — can be done in a way that does not harm patient care and allows for the use of non-standardized items when “deemed appropriate for a particular patient.” The hospital system says most hospitals have in place structures like Pharmaceutical and Therapeutics Committees (P and T) that determine what pharmaceutical products should be included on hospitals’ formularies. These types of committees use a methodical approach that uses evidence-based, peer-reviewed literature to make determinations. Premier says these types of committees can be expanded and reworked to make sure any quality and cost savings improvements that rely on incentivizing physicians can work as an effective backstop against abuses of gainsharing. “This type of peer-reviewed, evidence-based cost benefit approach could serve as a model for the identification of appropriate cost saving opportunities and targets. Institutions could set up analogous committees for this purpose, or might wish to utilize existing P and T committee structures,” Premier says. But AdvaMed says presenting physicians with a standardized list of products that they are “encouraged” to choose from may keep doctors from making critical patient-by-patient determinations as to what the best item to use for INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 5 a particular patient would be — thereby reducing or limiting services. “As the OIG considers regulatory language to interpret the Gainsharing CMP, the protections must not be relaxed in such a way as to allow for financial arrangements between hospitals and physicians that prioritize cost-saving measures (whether mandated or ‘encouraged’) at the expense of a physician’s freedom to choose the most clinically appropriate items and services for the patient,” AdvaMed says. — Todd Allen Wilson CMS Proposes Plan For Calculating Home Health Star Ratings CMS will calculate star ratings for home health agencies in part by relying on a number of quality measures already reported by home health agencies, the agency says in a proposal released Thursday (Dec. 11). The agency is looking for feedback from stakeholders, and may hold Open Door Forums on its proposal. Bill Dombi, vice president for law at the National Association for Home Care and Hospice, says that, while the group is still reviewing CMS’ proposal, NAHC is supportive of CMS’ efforts to provide patients useful information about good home health agencies. He also notes that the star ratings are not yet fully developed as the agency is still gathering industry’s comments. CMS says it considered several methods to calculate the star ratings before proposing a method that takes into account 10 of the 27 process and outcome quality measures that home health agencies already report. Proposed measures included in the star ratings need to apply to a significant number of patients, and most home health agencies should have enough data to report them, CMS says in a fact sheet. The measures also need to show variation across agencies, and the providers need to be able to show improvement. The measures shouldn’t vary randomly over time, as well. The process measures CMS proposes to take into account include: timely initiation of care, drug education on all medications provided to the patient or caregiver, influenza immunization for the current flu season, and whether beneficiaries received a pneumococcal vaccine. Proposed outcome measures include: improvement in walking (ambulation), improvement in bed transferring, improvement in bathing, improvement in pain interfering with activity, improvement in shortness of breath (dyspnea), and hospitalization. CMS says the star ratings will be a combination of individual measure rankings and how a home health agency’s performance compares on each measure to the performance of all home health providers. Outcome measures will be riskadjusted, CMS says. Home health star ratings are part of CMS’ plan to adopt star ratings across all Medicare compare websites, the agency says. Nursing homes, physicians, and Medicare Advantage plans have star ratings, and CMS says it plans to implement star ratings for dialysis facilities and hospitals in 2015, as well. Dialysis facilities and patients have criticized CMS for moving forward with the dialysis star ratings program. NAHC is studying the methodology CMS has proposed, while also looking at the pros and cons of some of the other existing star rating systems, Dombi says. Visiting Nurse Associations of America President Tracey Moorhead says the group plans to work with CMS to make sure providers have a say in the development of the star ratings program, and that the program has a testing and review period before it is implemented. “CMS plans to solicit stakeholder feedback on the proposed star rating methodology, including the measures proposed for inclusion,” the agency states in a fact sheet. CMS also says it plans to post a Frequently Asked Questions sheet to its website. The methodology will be finalized based on feedback and technical analysis, the fact sheet says. — Michelle M. Stein CRE: CMS Sends Mixed Message On Rights Of Gay Couples In Medicaid CMS sent mixed messages in a proposal unveiled Thursday (Dec. 11) to revise patient rights to comport with the Supreme Court’s ruling on same sex marriage in United States v. Windsor, according to Bruce Levinson, senior vice president for regulatory intervention at the regulatory watchdog group Center for Regulatory Effectiveness. On one hand, the proposed rule does not change CMS guidance from 2013 that lets states deny Medicaid protections to gay married couples, but Levinson said the rule also opens the door to more substantial policy changes by stating in a separate section that same-sex spouses must be treated equally to opposite-sex spouses. If same-sex spouses aren’t given the protections of a married couple, nursing homes can take their jointly-held assets, leaving spouses of the institutionalized homeless. The average annual cost of nursing homes is more than $80,000, and seniors must spend down their assets to be eligible for Medicaid. Medicaid protects a certain amount of couples’ combined resources so the spouses of the institutionalized may remain at home. If states do not recognize gay couples’ marriages, same-sex spouses could be forced out of their homes, Levinson said. When CMS issued guidance in the fall of 2013 to apply the Windsor ruling, CMS said same-sex spouses and straight married couples are eligible for equal Medicare benefits and followed suit with exchange premium tax credits. Those 6 INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 policies jibe with the Supreme Court’s Defense of Marriage Act ruling because they are federal programs. However, CMS let states adopt their own policies for Medicaid and Children’s Medicaid coverage because of what it called “the unique federal-state relationship that characterizes the Medicaid and CHIP programs.” The CRE challenged CMS for not requiring states to recognize legally married gay couples. Levinson said the proposed rule that CMS issued Thursday (Dec. 11) sends mixed signals. The rule explicitly references the previous guidance CMS sent to states with no indication it has changed. As in the previous guidance, CMS points out that Medicaid is both a federal and state program. “We note that Medicaid eligibility and CoP/CfC policies addressed in this proposed rule are administered by different statutes and are administered by state Medicaid agencies and CMS, respectively,” the Dec. 11 proposed rule states in reference to the earlier guidance. However, in the section of the proposed rule that defines residents’ rights, CMS states that nursing homes would have to honor spousal-impoverishment protections. “The same-sex spouse of a resident must be afforded treatment equal to that afforded to an opposite-sex spouse if the marriage was valid in the jurisdiction in which it was celebrated,” the proposed rule states. — John Wilkerson Stakeholders: CMS’ Proposed Open Enrollment Dates Misaligned Several key stakeholders are urging the administration to scrap its plans to schedule the exchange open enrollment period in the fall and instead align it with tax season. Brian Haile and George Brandes, both former health policy experts with the tax firm Jackson Hewitt, recently wrote to CMS that its case for setting enrollment from Oct. 1 through Dec. 15 in 2015 and beyond is flawed, and that the administration would maximize enrollment by scheduling sign-ups at a time when the IRS is distributing billions in tax refunds and there is no competition with holiday season. The advocacy group Families USA also says that it would be better from a consumer perspective to align enrollment and tax filings times. CMS’ Center for Consumer Information and Insurance Oversight (CCIIO) proposed the new enrollment dates as part of a wide-ranging proposal on health plan benefit and payment parameters for 2016. Comments on that proposal are due next Monday (Dec. 22) In their comment letter, Haile and Brandes, list several reasons why open enrollment should be aligned with tax season, including that consumers have more money at that time as well as more accurate information about their financial situation. “Balancing all of the relevant considerations, CCIIO should begin open enrollment no earlier than December 1, 2015 and ensure that it continues through as much as the tax season as possible,” they conclude. The two also clarify that they are offering advice as private citizens with experience in tax policy and social welfare. Federal Reserve data show that money is tightest for consumers during the final months of the year, while winter and early spring bring more cash, Haile and Brandes write. They note that qualified health plan enrollment closely parallels consumer liquidity as evidenced in the first year of enrollment: Medicaid enrollment was higher in the fall months and QHP selection stronger toward the end of the period. “While we cannot blindly extrapolate from the first years experience, the evidence does support the view that an open enrollment period that continues through the Spring helps to maximize enrollment.” In short, they write, setting it up so that enrollment competes with Christmas consumption is a “fool’s errand,” whereas selling insurance a few weeks or moths later — when consumers actually have cash in their wallets — is a far better bet for success. Plus, consumers appear to be better able to optimize plan selections during the tax filing season, and they enjoy greater access to expect tax advice, Haile and Brandes add. They also point out that tax refunds — and penalties — are especially critical for the low-income population. The two say that CCIIO’s many arguments for the fall-only open enrollment period don’t hold water. Specifically, they argue that CCIIO already disproved one of its own points — that avoiding an enrollment period that crosses calendar years would prevent consumer confusion — with its first two open enrollment periods. CCIIO in the proposal rule had also suggested that the October start date would be beneficial to employees in employer-sponsored plans who may not be able to drop their coverage and enroll outside of their employers’ open enrollment periods. Many open enrollment periods — including the Federal Employee Health Benefits Program — take place in October. But Haile and Brandes say that a better solution would be to allow for a special enrollment period, just as CMS has done for individuals who lose minimal essential coverage during the year. Agents and brokers and consumer advocates have also supported that idea. Rachel Klein of Families USA says the group is pleased with the proposed length of open enrollment, but believes aligning enrollment with tax season does makes sense from a consumer’s perspective. There are definitely some logistical challenges from an insurer’s administrative point of view — due to overlapping INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 7 calendar and plan years— but from a consumer perspective it is a time when people are thinking about finances, income and what their situation is for the coming year. It also makes sense to schedule open enrollment during tax season as the subsides and the penalties are tied to the tax system, she says. Klein stresses that some consumers who are auto-enrolled in the fall may not be aware of any changes to their plan — including network and formulary alterations — until after open enrollment has ended. It is very important that enrollment either be extended or people be allowed a special enrollment period in those cases, she says. — Amy Lotven Spending Bill Exempts Expatriate Health Plans From Certain ACA Requirements Congress tucked into its massive spending bill an amended version of the Expatriate Health Coverage Clarification Act, which exempts such plans from many ACA provisions, including the health insurance tax on plans. The measure, sponsored by Reps. John Carney (D-DE) and Devin Nunes (R-CA), passed the House on April 29 and the Senate over the weekend with the support of various stakeholders, including the Chamber of Commerce. The Chamber told House members that it would consider their stance on the original expatriate health bill as a key vote. After the spending bill passed the House, Democratic Delaware Gov. Jack Markell praised Carney’s work to help ensure that the many Cigna employees who managed expatriate plans from the state would be able to keep their job. “As the Governor has emphasized to the Obama administration on numerous occasions, it does not make sense to treat expatriate insurance plans managed from Delaware the same as domestic insurance plans,” Markell’s office said. The administration, however, said in April that it opposed the initial language “because it would reduce consumer protections and create even more loopholes in the tax code.” The White House added that it remained willing to work with Congress to improve the bill, noting that it had shared some “straightforward changes” to the bill with members. According to Carney’s office, the version of the expatriate measure included in the omnibus differs from the original bill in several ways. It clarifies that group plans will be treated like other employer-sponsored plans for the purposes of satisfying coverage requirements. It also ensures that plans include coverage for inpatient hospital services, outpatient facilities, physician and emergency services. That change is consistent with recent guidance from IRS and a proposed regulation. The amended version also requires that expatriate health insurers be licensed to sell insurance in more than two countries, establish provider networks in at least eight countries, reimburse for health care services in at least eight currencies, maintain call centers in at least three countries, and handle call center inquiries in at least eight languages. The version also defines an expatriate as a skilled employee working outside of the United States for more than six months in a consecutive 12-month period; or “someone who is a member of a group that is not formed primarily for the sale of health insurance and that meets purposes listed in 501(c)(3) or 501(c)(4) of the Internal Revenue Code (e.g., religious missionaries or students) — subject to approval by the Secretary of HHS.” — Amy Lotven Burwell Pushes Enrollment Target; Industry Predicts Higher Totals HHS Secretary Sylvia Burwell reiterated her department’s goal of getting 9.1 million Americans to enroll in and pay for private health insurance by the Feb. 15 deadline, and touted the importance of American faith leaders in helping the administration reach that number during a brief appearance at the United Methodist Building Friday (Dec. 12). Religious leaders are “voices in the community and voices (people) trust,” Burwell said, and play a large role in “encouraging friends and neighbors to make this law work for them.” Some groups predict that final numbers will exceed expectations: Kaiser Family Foundation Senior Vice President Larry Levitt told Bloomberg that the administration should meet its target “quite comfortably,” while Avalere Health Vice President Caroline Pearson said as many as 5 million new customers could join around 6 million who are expected to renew their coverage. The Congressional Budget Office also believes around 13 million people will enroll in private plans sold through government-run exchanges in 2015, though the administration disagrees with that prediction. Burwell will continue her enrollment push with trips to Arizona and Texas this weekend to meet with religious leaders, who HHS is asking to participate in a “Faith Weekend of Action” to convince congregations to sign up ahead of the deadline. Three-quarters of calls to federal exchange help lines were from returning customers in the weeks following Thanksgiving, Burwell said. Re-enrollments will begin at midnight PST, or 3 a.m. EST, on Dec. 16, she added when asked if the department plans to extend its deadline for coverage starting Jan. 1. Burwell also mentioned increased efforts to reach Latinos through Spanish-speaking navigators. She similarly met with newly elected governors in Washington last week to discuss their priorities going forward, including relief from some provisions of the Affordable Care Act. — Rachel S. Karas 8 INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 HHS Partners With PayNearMe To Spread Enrollment Message HHS hopes to reach financially underserved Americans, who prefer cash transactions or do not have bank accounts or credit cards, to convince them to enroll in a health plan by running healthcare.gov ads at the bottom of transaction receipts through a partnership with electronic cash transaction company PayNearMe, HHS Secretary Sylvia Burwell announced Thursday (Dec. 11). HHS says consumers cannot pay for health premiums with PayNearMe at this time, and that issuers would have to have an agreement with PayNearMe to accept the payment. The department believes the company is working to make those transactions possible, though PayNearMe did not return a request for comment by press time. Receipts printed at 7-Eleven, Family Dollar and ACE Cash Express stores across the country that use PayNearMe will include an announcement telling customers about upcoming ACA deadlines and directing them to the federal health exchange site. Those ads will run until the end of the open enrollment period in February. PayNearMe receipts are users’ proof of large transactions like rent, loan and utility payments, which officials believe make them more likely to be kept and thoroughly read than other receipts. “Leveraging developments from technology companies like PayNearMe helps us to reach our consumers where they are, with the information they need to sign up and re-enroll in quality, affordable care through the Health Insurance Marketplace,” Burwell said in the release. “With this partnership, we are using digital platforms to place Open Enrollment information in the hands of consumers who need it.” HHS last year proposed a rule requiring insurers accept all types of payment options, including replenishable debit cards, to ensure the millions of people eligible for insurance subsidies in the exchanges but who lack traditional bank accounts can pay their monthly premiums. HHS’ move came following pressure from the tax preparation firm Jackson Hewitt and state officials who stressed that large portions of the population that the ACA aimed to reach lack a traditional bank account. A 2013 national FDIC survey of unbanked and underbanked households found that 28 percent of households, or 34 million, are financially underserved. — Rachel S. Karas CMS Expects 122 Entities To Seek Relief From Contraceptive Mandate At least 122 religious nonprofits and certain for-profit businesses that disagree with the ACA’s requirement that plans cover some or all contraceptive services with no employee cost-sharing are expected to submit notices of their faith-based objections directly to HHS Secretary Sylvia Burwell, according to recently released documents. Groups may use a new letter template, which CMS debuted Dec. 5, to ask to opt out of the mandate. The model notice is the latest in a series of moves aimed at giving leeway to nonprofits and businesses — like artsand-crafts chain Hobby Lobby, Inc — that say they won’t provide birth control to their employees due to their religious beliefs. “The model notice is really quite simple, as was the option already available,” said Sarah Lipton-Lubet, director of reproductive health programs at the National Partnership for Women & Families. “Now they have two. What more can they want?” In July, the Supreme Court issued an interim order in Wheaton College v. Burwell saying that those objecting to the coverage mandate do not need to fill out a federal form, and can instead submit a notice of objection directly to HHS. HHS would then inform their health insurer or third-party administrator of the objection. The model notice issued last week would satisfy that requirement for all eligible entities. Advocates of religious freedom like the Becket Fund for Religious Liberty, the plaintiff firm in Little Sisters of the Poor v. Burwell, argue that signing a paper to opt one group out of the contraception mandate still authorizes someone else to distribute birth control instead. The nuns and their lawyers say that such an accommodation still violates their beliefs. But Lipton-Lubet disagrees. “I think the administration is really going to great lengths to accommodate these institutions,” Lipton-Lubet said. “There’s probably nothing that will satisfy them because what they’re interested in at the end of the day is denying women access to birth control.” HHS does not know for sure how many entities will contact the administration for an exemption but bases its 122 figure on those involved in related litigation, according to supplemental documents. The administration is seeking stakeholder input on whether collecting the information is practical and necessary for the agency to function; whether the estimated burdens of time and money in providing and processing information are accurate; and ways to improve the information’s quality and minimize burden on those involved. The department estimates organizations will spend around 50 minutes and $53 for clerical, managerial and legal labor to prepare and send self-certification information to federal agencies. Assuming that 122 entities will submit material to HHS and the Department of Labor, which share jurisdiction, the agencies each expect to spend 51 hours and INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 9 an equivalent labor cost of $3,213 in processing the data. That number reflects the correction of a previous error in which HHS failed to split the burden with Labor, showing 110 total hours of associated work instead of 51. Under the Affordable Care Act, employers are required to cover all preventive services without cost-sharing. — Rachel S. Karas Insurers Blast Spending Bill Language Aimed At Blocking Risk Corridors Insurers are upset that the $1.1 trillion federal spending bill released late Tuesday (Dec. 9) includes language that bars CMS from using program management appropriations for the health law’s risk corridors program, which is designed to protect insurers from inaccurate rate setting during the initial years of ACA implementation. Republicans had previously indicated that they would address what they’ve called a “taxpayer bailout” of insurance companies, but prior to release of the spending bill sources had been unclear if the risk corridor language would be in or out. Insurers quickly blasted the decision, and suggested Congress would better serve the public by focusing on issues that would make plans more — not less — affordable. “American budgets are already strained by health care costs, and this change will lead to higher premiums for consumers and make it more difficult to achieve affordability,” America’s Health Insurance Plans spokeswoman Clare Krusing said in an email. “Our focus should be on changes to the law that will lower costs — like repealing the health insurance tax — not those that drive premiums higher,” she added. Incoming Majority Leader Mitch McConnell (R-KY) recently included the insurance tax on a list of provisions he would like to see on the chopping block next year. The risk corridor is one of three premium stabilization initiatives set out in the ACA, and is meant to protect insurers from negative effects of inaccurate rate-setting that leads to revenue that is higher or lower than expected. The program collects money from issuers that earn more than a set margin — originally 3 percent, but which was increased by 2 percent for 2015 to address the fact that non-ACA compliant plans were allowed to continue for longer than expected — and pays that money to issuers who lost more than expected. Initially the program was not designed to be budget-neutral, but following the GOP outcry the administration said that it would design the program in that manner. CMS first said that in the event of a shortfall, any plan not fully reimbursed in year one would be made whole in the following year. Later, the agency said that it would pull funding from other appropriations to cover the program. In October, both Republicans and HHS spun a Government Accountability Office opinion on risk corridors as positive news. The GAO found that fiscal 2014 appropriations language gives HHS authority to fund the program, yet also said that Congress must include certain language in its fiscal 2015 budget for the department to be able to collect and disperse payments in future years. An aide to Marco Rubio (R-FL), one of the most vocal opponents of the so-called “bailout,” praised the spending bill provision. “While the administration will still be able to administer the risk corridor program, at least now they must do it in a budget-neutral fashion, opposed to unlawfully providing for a taxpayer bailout,” Rubio aide Alex Conant told The Hill. — Rachel S. Karas Cures Bill A Dry Run For PDUFA . . . begins on page one and devices, and may bump up research funding at the National Institutes of Health. Upton said last month he expects to have a draft of the 21 st Century Cures bill ready in January, with hopes of getting a full bill to the House floor by Memorial Day. Janet Woodcock, director of FDA’s Center for Drug Evaluation and Research (CDER), said at an earlier panel during the FDA/CMS Summit that the 21 st Century Cures initiative is a “front burner” priority for the agency. PDUFA was first passed in 1992 and updated in 1997, 2002, 2007 and 2012. The next version — PDUFA VI — needs to be passed by Sept. 30, 2017, when the latest version is set to expire. Harvey said PDUFA VI needs to include a focus on modernizing the way clinical trials are done, adding that clinical trials are becoming unsustainable as the cost for a single trial is rising above $1 billion. “We really need to use new technology,” Harvey said. “We need to use 21st century regulations and not 18th or 19th century perspectives to run clinical trials and do it in a more efficient way.” Kay Holcombe, senior vice president for Science Policy at the Biotechnology Industry Organization (BIO), said the 21st Century Cures legislation will also help determine what resources FDA needs going forward that can be built into PDUFA. She said FDA gets “saddled” with a multitude of requirements in PDUFA updates, but finds that user fees don’t cover the costs of meeting those requirements. The 21st Century Cures bill is expected to impose new requirements on the agency, so FDA will know what it needs to fulfill those requirements as PDUFA VI is negotiated, Holcombe said. “It gives us a good base of information as we’re going into PDUFA VI in terms of the resources the agency is going 10 INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 to have to come up with in order to do things out of 21st Century Cures,” Holcombe said. When asked whether industry should be paying more or all of the costs for the drug approval process — user fees account for roughly 70 percent of the costs currently — Holcombe said it is highly dependent on what the benefits for drug makers are. “I don’t want to sound crass, but when you put money on the table you’re kind of wondering what it is that you’re getting back,” Holcombe said. She said industry may be supportive of paying a higher share if that leads to reducing the time and cost associated with developing and producing new drugs. Holcombe also said that the “industry will speak with one voice” during PDUFA negotiations with BIO on equal footing with the pharmaceutical industry — noting that the two groups have already been talking and working with each other in this regard. “BIO very wants to be, obviously, not taking a back seat to PhRMA, but working with PhRMA as an equal partner,” she said. Robert Metcalf, the vice president of Global Regulatory Affairs for Eli Lilly who will be heading the Pharmaceutical Research and Manufacturers of America’s (PhRMA) regulatory affairs arm starting next year, said PhRMA views its relationship with BIO going forward as “very much of a joint partnership across BIO and PhRMA as equals.” Metcalf and patients’ advocates on the panel also said PDUFA VI will build on PDUFA V’s efforts to open up the drug development and regulatory process to patient’s concerns. Jeff Allen, executive director of patient advocacy group Friends of Cancer Research, said PDUFA V built the framework for including patients in the conversation and has created an opportunity for drug makers to build those concerns into their trials. “It has to start earlier than it historically has in terms of understanding what patient outcomes are desired and incorporate those into the experimental process,” Allen said. Metcalf said PDUFA VI will be an opportunity to began incorporating patient input into the development process in a scientific and systematic way. “Patient input can and should be not only a motive, but also scientific as well,” Metcalf said. “That’s the opportunity I see going forward. We’ve got to bring in skills and capabilities that we haven’t necessarily done, like social science capabilities.” — Todd Allen Wilson Hatch, Bennet Bill Sets Up New Drug Class Eligible For 15 Years Exclusivity Sens. Orrin Hatch (R-UT) and Michael Bennet (D-CO) have introduced legislation that picks up on a controversial House proposal to offer 15 years of data protection as an incentive for drug makers to develop therapies that treat one or more unmet medical needs. Brand drug makers back the idea and have urged its inclusion in the House Energy and Commerce Committee’s upcoming 21st Century Cures bill, but some House Democrats, generic drug makers, AARP and pharmacy benefit managers charge the patent idea would merely extend market monopolies of high-priced drugs like Sovaldi. The newly introduced Dormant Therapies Act would offer the 15 years data exclusivity for a new class of drugs called “dormant therapies.” To be eligible, a medication would have to be intended for one or more unmet medical needs, have a suitable clinical trial plan developed by the sponsor, and at the time of request contain an active moiety that is not the same as, or highly similar to, an active moiety for another application. A dormant drug’s patent would be extended for 15 years once FDA accepts the application, mirroring the approach in a bipartisan House bill, Modernizing Our Drug and Diagnostics Evaluation and Regulatory Network (MODDERN) Act, spearheaded by Rep. Leonard Lance (R-NJ). At a hearing this summer, Energy and Commerce health subcommittee chair Joe Pitts (R-PA) suggested it was time to rethink long-standing drug patent law, and the National Health Council, whose members include the innovator pharmaceutical and biologics lobby groups, touted the bipartisan bill as a way to encourage repurposing of drugs that stalled in early clinical trials. But some Democratic lawmakers suggested lawmakers instead turn to other incentives, such as tax credits and public funding for basic research. The 15 years data exclusivity included in the House and Senate bills aims to make up for patent time lost during drug development. “It takes on average 14 years for a compound to make its way through the therapeutic pipeline from discovery, through clinical trials, to formal approval, and eventually to the patient,” says a press release from Hatch’s office. “Because patents last for only 20 years, much of this time is consumed during the lengthy research and development process. The result is companies are investing in research on compounds that can be brought to market quickly, rather than new treatments that could serve people with the most complex medical needs.” In order to receive the designation, sponsors must list all patents and applications for patents that may provide protection for the medicine, a waiver of patent rights to enforce any patent described in that list which may expire after INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 11 the end of the protection period, and any additional information required in order to determine eligibility for designation. Hatch envisions the bill as spurring treatments for such conditions as Alpha-1, ALS, Alzheimer’s, epilepsy, lupus, mesothelioma and multiple sclerosis, the release says. “I’m pleased Sen. Bennet has joined me in this important effort to encourage the development of desperately needed treatments for some of the most troubling diseases and disabilities out there,” said Hatch. “We hope to create a timecertain protection to encourage innovators to capture lost opportunities and bring new and essential products to market for the patients who need them.” Brand drug makers and some patient groups floated similar ideas in comments sent to the House Energy and Commerce Committee for its 21st Century Cures initiative. But generic drug makers, AARP and Rep. Henry Waxman (D-CA) urged Congress to proceed with caution. “Virtually any drug with a novel active ingredient could qualify for this reward,” Waxman said. “Well, that’s giving away too much. That’s going to upset the ability of making drugs affordable and is far more of an incentive that I think is justified.” Pitts recently said company incentives could be included in the upcoming 21st Century Cures bill, a draft of which the panel hopes to unveil in January. Drug makers have sought added exclusivity for a range of drugs, including for small molecule drugs and first-in-class treatments. — Erin Durkin Patty Murray Tapped As Ranking Member Of Senate HELP Committee Sen. Patty Murray (D-WA) will become ranking member of the Senate health committee as the upper chamber transitions to Republican control in the next Congress, confirming expectations that she would take the spot. As chair of the powerful Budget Committee, Murray has aggressively advocated for FDA’s funding and food safety laws. “Senator Murray is committed to looking for innovative ways to expand access to life-saving treatments and cures, while maintaining high scientific standards to ensure safety and consumer protection,” an aide said, responding to an FDA Week inquiry about Murray’s FDA priorities. “She will also continue to push for investments in biomedical research to improve health and well-being for families in Washington state and across the country.” “I plan to work with incoming-Chairman Alexander and my colleagues on both sides of the aisle to make sure that our country is doing everything possible to... improve health care access and affordability... for all workers and all families,” said Murray in a statement. Industry groups in the past have applauded Murray’s support on issues relating to biotechnology and the reauthorization of the Prescription Drug User Fee Act in 2012 (PDUFA). The Pharmaceutical Research and Manufacturers of America also has praised Murray for her support in permanently reauthorizing the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Generally, the association said it was looking forward “to working with Sen. Murray in her new capacity.” The Advanced Medical Technology Association and the Biotechnology Industry Organization expressed the same sentiment in a similar statement — though they declined to specify on what issues they are looking forward to working with her. In 2006, Murray and then-Sen. Hillary Clinton stalled both of President George W. Bush’s nominees for FDA commissioner because of rules regarding over-the-counter contraception. Clinton and Murray double-teamed Bush’s nominees until they were satisfied that contraception could be available to consumers 17 and older without a prescription. — David Hood Appropriators Target RACs . . . begins on page one Hearings and Appeals, and create a process for OMHA to send feedback to CMS and the RACs in order to reduce the number of claims that are likely to overturned once they reach the third level of appeals. “Unintended consequences of RAC audits can reduce patient access to care and jeopardize the economic viability of critical health care providers,” the report says. “CMS has an obligation to find a reasonable balance to eliminate true fraud and abuse while not slowing payment to the majority of honest providers that are negatively impacted by the RAC process.” Appropriators ask CMS to include in its fiscal 2016 budget request a timeline, milestones and “measurable goals” to address concerns with the RACs and reduce the appeals backlog. The RACs and providers have blamed each other for the backlog at third level of appeals, which is handled by OMHA. In response to OMHA’s request for ideas to help address the backlog — which the lawmakers’ report pegs at nearly 750,000 appeals — doctors and hospitals said the driving force behind the backlog is RAC audits, which they say are overly aggressive. The RACs, however, have pointed to “frequent filers” who appeal every RAC denial as the reason behind the sharp uptick in appeals and the backlog. OMHA officials have said they cannot keep up with the number of appeals flooding the system. The appropriations 12 INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 report calls on OMHA to use its 2015 funding to increase its capacity to process a growing caseload and to find a way to handle the current backlog — though lawmakers have previously said that to bring the backlog under control OMHA would need more than 400 new judges. Appropriators direct OMHA to compile a report describing a plan to resolve the backlog, as well. The lawmakers say that CMS also needs to provide the House and Senate with a report on the cross-agency working group that has been looking at the Medicare appeals process and that group’s recommendations. The report should include CMS’ strategy to improve the entire appeals process and an analysis of Medicare audit contractors’ quality of medical reviews. It should also include a timeline and a strategy to eliminate the backlog and steps to address the overturn rate at the third level of appeals, the spending report states. CMS also needs to find a way to “improve stakeholder confidence that Medicare policies are interpreted consistently and transparently throughout the system.” The American Coalition for Healthcare Claims Integrity says it is pleased that lawmakers are calling for more transparency and a consistent application of Medicare policy, but “[w]e are disappointed that the bill contains language sympathetic to an industry responsible for incorrectly billing Medicare” for billions in 2014. HHS recently released a financial report that found the improper payment rate for Medicare fee-for-service climbed to almost 13 percent in fiscal 2014, and the RACs pointed out that the increase in the improper payment rate coincided with CMS’ decision to pause the RACs and limit what audits they could conduct. “The directives to CMS in this bill amount to nothing less than the bullying of a federal agency by a trilliondollar hospital industry committed to dismantling one of the nation’s most effective oversight programs,” a spokesperson for the coalition said. “[P]roviders continue to improperly bill Medicare at staggering rates while advocating for less transparency and a hands-off approach to government oversight.” Melissa Jackson, senior associate director for policy at the American Hospital Association, said the hospitals are pleased Congress “has taken an interest in addressing the problems with the RAC program.” “Congress clearly understands that inappropriate contractor denials have placed a severe strain on the Medicare appeals system. We welcome the report requested from CMS on the Medicare appeals process and look forward to the findings,” Jackson added. One lobbyist following the issue said a requirement for CMS to do anything about the appeals issue is overdue, and the required reports from CMS and OMHA could finally be the point where the agencies have to address and take action around the backlog. — Michelle M. Stein Rx-Price Debate Seeps Into Budget Bill . . . begins on page one guidance clarifying its policy. Although Congress held just one oversight hearing on the program since creating it more than 20 years ago, lawmakers have taken more of an interest of late. The House Energy & Commerce Committee asked the Medicare Payment Advisory Commission to review the program, even though it’s mostly outside MedPAC’s jurisdiction, and Energy & Commerce health subcommittee Chair Joe Pitts (R-PA) implied last week that he’d like to reform the program to free up some money to pay for the committee’s initiative to encourage development of breakthrough drugs and medical devices. Congress has significantly expanded the program since its inception in 1992. Drug makers argue the program no longer lives up to its original mission, and entities that receive the discount are adamant that the program helps the poor get prescription drugs. The Government Accountability Office and the Office of the Inspector General both reported lackluster oversight of the program. The appropriations report asks HRSA to review how the program is working. “There are concerns that HRSA has been unable to demonstrate that the 340B program benefits the most vulnerable patients,” the report states. “In order to best serve the public need, the program should examine its ability to ensure patients’ access to 340B savings for outpatient drugs. HRSA is directed to work with covered entities to better understand the way these entities support direct patient benefits from 340B discounted sales.” A spokesperson for the Safety Net Hospitals for Pharmaceutical Access said the group is pleased that Congress requested an update on the website that HRSA is creating to show drug price ceilings. “Healthcare providers need to know they are not being overcharged by manufacturers,” the spokesperson said. However, SNHPA said there already is ample evidence that safety-net hospitals in the 340b program serve the needy. “Every day the 340B program helps safety-net hospitals treat needy patients across America, just as Congress intended,” a SNHPA release states. The Alliance for Integrity and Reform of 340B welcomed the review. “The program, through which qualifying hospitals and grantee organizations receive what can be substantial discounts on drug purchases, has expanded precipitously among hospitals with little evidence that more needy patients are benefiting.” The other drug-price flashpoint that the appropriations report brings up is hepatitis C. The current cure for the INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 13 infection costs $94,500 for a 12-week course, and Medicaid directors and health plans accuse the drug industry of price gouging. “The agreement encourages CMS to consider the prevalence of chronic viral hepatitis among beneficiaries and the cost of providing care to those who are in the late stages of this disease,” the report states. “The agreement encourages CMS to educate Medicare beneficiaries and healthcare providers about hepatitis C and the need for screening while identifying opportunities to improve the quality of treatments and services.” The Centers for Disease Control and Prevention already has researched the prevalence of hepatitis C among Americans. The CDC estimates that 3.2 million people are infected, but many others believe the true number is higher. — John Wilkerson ICD-10 Delay Might Ride With SGR . . . begins on page one included. The new billing codes are set for implementation in October 2015, following repeated implementation delays by CMS and Congress. The latest delay was attached by lawmakers to the Sustainable Growth Rate patch in March, and some lobbyists say that Congress’ actions on SGR when that patch runs out next March provide the most likely, though certainly not the only, vehicle for another ICD-10 delay. One health technology lobbyist said there is likely to be continued and increased pressure to delay ICD-10. But as long as the current implementation date is in place, the industry will keep aiming to meet that date and assume the new code set will go into effect next year. Upton and Sessions said Wednesday (Dec. 10) that, going into the next session, “we will continue our close communication with the Centers for Medicare and Medicaid Services to ensure that the deadline can successfully be met by stakeholders. This is an important milestone in the future of health care technologies, and it is essential that we understand the state of preparedness at CMS.” Robert Tennant, senior policy adviser with the Medical Group Management Association, said Congress will likely be closely monitoring both the industry and CMS before acting on any ICD-10 delay in 2015, and it’s not something they will take lightly. Doctors have pushed against implementation of ICD-10, and the Texas Medical Association has been urging members to ask lawmakers to delay ICD-10 for two years. Upton and Sessions said that, following the most recent ICD-10 delay, lawmakers “heard from a number of interested parties concerned about falling behind or halting progress.” Hospitals — including the American Hospital Association, America’s Essential Hospitals, Association of American Medical Colleges and others — recently wrote to House and Senate leaders urging them to not add any more delays to ICD-10 implementation, and pushing for October 2015 implementation. One lobbyist said it was House leadership’s decision not to include a delay in the latest spending deal. “A further delay would only add additional costs as existing investments would be further wasted and future costs would grow,” hospitals say in their Dec. 5 letter. CMS plans to hold its first round of end-to-end testing in January, which will allow participants to not only see if their ICD-10 claims get accepted but also see how CMS processes them. Though the testing will focus on those further ahead and prepared to test, the results of the end-to-end testing will either “assuage concerns or fuel the fire,” Tennant said. The tests will give a clearer picture around claims rejections and reimbursements, and many in the industry will be looking to see how quickly CMS can turn around the results of the tests and how transparent the agency will be regarding the readiness level of state Medicaid programs, he added. Congress and stakeholders will also likely be looking at industry surveys to help gauge how ready the health sector is for a move to ICD-10, Tennant said. By March, when Congress is working on the next SGR fix or patch, the industry should have an idea of how testing is going and what preparations look like, Tennant said. While the next SGR fix or patch makes sense as a vehicle to move a delay, it’s not the only option, a health technology lobbyist said. The ICD-10 delay is not likely to cost anything, and because of that such a provision could get inserted into a host of legislative measures moving through the next Congress. The House Energy & Commerce Committee’s 21st Century Cures initiative could be another place where a delay could easily fit, the lobbyist added. But the technology lobbyist points out that last year’s ICD-10 delay was a surprise for those who wanted to implement the new code set. The delay was seen by many as a consolation prize for physicians after Congress couldn’t agree how to pay for a permanent SGR fix. Now, those who are pro-ICD-10 are more organized and ready to take their case to lawmakers, the lobbyist said. If they want to avoid a delay, those who want ICD-10 to be implemented will have to be louder than those who are pushing for a delay, the lobbyist added. Every time a delay goes through, it breeds uncertainty, Tennant said. If another delay is necessary, more money should also be allocated to CMS for ICD-10 preparations, he said. The technology lobbyist said the best ways to help avoid a delay are good education and outreach so that the industry is prepared. But Tennant said that at this point CMS is expected to help the industry make the monumental transition to ICD-10 with out appropriate funding. If CMS does move forward with ICD-10 in October, Tennant said, CMS will need to have some sort of contingency plan or glide path ready to help smooth out the transition to the new code set. — Michelle M. Stein 14 INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 Vit als: A Vitals continued on next page Health P olicy Blog Po Excerpts of Inside Health Policy Blogs Coats, Heller, Scott To Join Senate Finance Committee Sen. Orrin Hatch (R-UT), who is set to take the gavel of the Senate Finance Committee in January, announced on Monday (Dec. 15) that Sens. Dan Coats (R-IN), Dean Heller (R-NV) and Tim Scott (R-SC) will be join the committee for the 114th Congress. “With the addition of these three Senators, the Republican side of this committee will only be stronger, and I am confident we’ll be able to work with our counterparts on the other side of the aisle to help drive a robust agenda to meet the demands of the American people,” Hatch said. Coats, Heller and Scott all say on their websites that medical malpractice reform is a priority, as well as health insurance that can be bought and is portable across state lines. Scott also says states should be permitted to create high risk pools again, while Heller champions reimporting prescription drugs. The full list of Republicans on the Senate Finance committee next session also includes: Sens. Chuck Grassley (IA), Mike Crapo (ID), Pat Roberts (KS), Mike Enzi (WY), John Cornyn (TX), John Thune (SD), Richard Burr (NC), Johnny Isakson (GA), Rob Portman (OH) and Pat Toomey (PA). — Michelle M. Stein Gruber Subpoenaed To Hand Over Pay, Communication Docs By Dec. 24 In a move that continues the saga of embattled MIT economist Jonathan Gruber, House Oversight and Government Reform Committee Chairman Darrell Issa (R-CA) has given Gruber until Dec. 24 to hand over a slew of documents listed in a subpoena issued Thursday (Dec. 11). The committee subpoenaed all of the health care consultant’s documents and communications with federal, state or local government employees related to aspects of his work on the Affordable Care Act. “He has so far been unwilling to fully comply with the Oversight Committee’s repeated requests,” Issa said Friday (Dec. 12). “This week, Dr. Gruber repeatedly refused to answer several key questions, including the amount of taxpayer funds he received for his work on ObamaCare. The American people deserve not just an apology, but a full accounting, which Dr. Gruber must provide.” House Oversight is asking Gruber to provide the following: • All documents and communications to or from any federal, state, or local government employee, including, but not limited to, any document or communication referring or relating to the Affordable Care Act or federal and state health care exchanges. • All documents and communications referring or relating to funding, for research or otherwise, from any federal, state, or local government agency, including,but not limited to, any contracts with a federal, state, or local government agency. • All documents and communications referring or relating to work product produced to any federal, state, or local government agency, for any purpose, including, but not limited to, the results of any and all economic models or simulations. Gruber had already submitted a list of federal grants or contracts awarded in the current and past two fiscal years for his consulting and economic microsimulation modeling work, per an earlier request from the committee. But at a Dec. 9 hearing, Gruber repeatedly deferred to his counsel when asked how much he was paid for his work with state-based exchanges. His lawyers told the committee that they found their earlier submission to be sufficient under their interpretation of the request. Still, lawmakers pressed for more information, saying they believed those contracts totaled millions of dollars. They also asked Gruber whether he had ever discussed with anyone in the administration if the health care reform bill was written in a “tortured” way to make sure the Congressional Budget Office did not score the individual mandate as a tax. Gruber said that he did not recall. — Rachel S. Karas MNsure Urges PreferredOne Enrollees To Seek New Coverage Via Exchange Minnesota health exchange officials said Thursday they are reaching out to more than 28,000 Minnesotans whose current plans are no longer offered on MNsure and encouraging them to shop for new 2015 coverage. The targeting is a result of PreferredOne’s September decision to leave the marketplace, which state officials say impacted about 23,000 residents. PreferredOne customers have the option of purchasing their existing plan off-exchange. However, consumers who held tax credits in 2014 and keep their current plan next year will see their premiums increase by an average 168 percent, according to exchange official, who cites a preliminary Wakely Consulting Group analysis that is set to be finalized next week. Those who did not have tax credits will still see an average increase of about 66 percent. The study also found that those enrollees who return to the marketplace for a new plan are expected to save an average of $311 each month, officials say. “This preliminary analysis from Wakely Consulting confirms what we have been saying from the beginning: it is in the best interest of Minnesotans to shop and compare policies on MNsure,” CEO Scott Leitz said in the release. “MNsure is the only place to get financial help or to qualify for low-cost or nocost plans. Minnesotans who purchased plans through MNsure and don’t return to the exchange will no longer be eligible for financial help. That’s why we want all enrollees to return to MNsure and find a new plan that fits their budget.” Although four Blue Cross and Blue Shield plans and one by HealthPartners will also be discontinued for around 5,000 people, MNsure officials said their outreach is aimed at PreferredOne enrollees. Staff are calling customers to refer them to a broker or a navigator organization; sending postcards with details on avoiding premium hikes; as well as sending emails to any enrollee whose plan will not be offered on the exchange in 2015. — Rachel S. Karas INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 15 FDA: Agency’s Tools Not Strong Enough To Stop Brands’ Misuse Of REMS A key FDA official said Thursday (Dec. 11) that tools Congress gave the agency are not strong enough to address innovator companies’ increasingly aggressive use of Risk Evaluation and Mitigation Strategies (REMS) to block generic drugs and biosimilars from reaching the marketplace. The comments came less than a week after FDA issued a draft guidance aimed at stemming brand companies’ use of REMS to slow generic drug development. The issue came up at an IIR-sponsored FDA/CMS Summit in Washington where FDA officials discussed the agency’s priorities for 2015 and issues they continue to face. “The problem is the use of REMS blocking generic competition,” said John Jenkins, director of the Office of New Drugs in the Center for Drug Evaluation and Research. “Innovators have really become very aggressive in using that strategy [and] hiring the best lawyers to back up that strategy.” Last week, the agency proposed guidance that formalizes a process whereby generic drug makers may ask FDA to write to brand manufacturers verifying generics’ methods of producing drugs are safe and stating that brands cannot use REMS to withhold drug samples needed for generic drug development. But Jenkins said the guidance only addresses one part of the problem. “We issued a guidance around one of the ploys to argue it was unsafe for the generic companies to have access to the REMS-covered medication in order to do their bioequivalent study,” he said. Jenkins said that this is a growing major problem for FDA, and that Congress did not intend REMS to block generics from entering the marketplace. He said Congress had given some tools to the agency to address generic entry, but these tools were not as strong as they could have been. He noted that the Food and Drug Administration Amendments Act (FDAAA) states holders of approved applications cannot use any element to assure safe use to block or delay approval of generics, but says FDA could have been given stronger tools to address the problem. Biologics innovators are also making it difficult for biosimilar manufacturers to obtain samples to do tests, said Jenkins, but he didn’t expand on specific solutions to the problem. Novo Nordisk Vice President for U.S. Regulatory Affairs Robert Clark, said he support Jenkins’ comments and that using REMS in this way is not a responsible practice. Jenkins’ comments appear to track with the generic drug industry’s view of the new FDA guidance as only a first step to addressing the REMS issue. Generic Pharmaceutical Association CEO Ralph Neas applauded the FDA’s guidance, but said that GPhA will continue to push for legislation. Meanwhile, the Pharmaceutical Research and Manufacturers of America continues to highlight why REMS are needed for patient safety. “One thing that is important to understand is that REMS are put in place for important safety reasons,” PhRMA Vice President and Senior Counsel Jeff Francer told FDA Week earlier in the week. “They’re designed extraordinary carefully to protect against very serious risks and it’s important that if generic companies are going to be doing clinical trials on these same drugs, that the protections for patients have to be just as strong in these generic tests as they are in the innovator’s REMS.” Reps. Steve Stivers (R-OH) and Peter Welch (D-VT) introduced legislation in September aimed at stopping alleged misuse of REMS by brand companies. The bill prevents innovators from using REMS to thwart generic drug access. — Erin Durkin SUBSCRIPTIONS: 703-416-8500 or 800-424-9068 [email protected] NEWS OFFICE: 703-416-8577 Fax: 703-416-8543 [email protected] 16 Health Group Publisher: Chief Editor: Managing Editor: Associate Editor: Contributing Editor: Donna Haseley ([email protected]) John Wilkerson ([email protected]) Michelle M. Stein ([email protected]) Todd Allen Wilson ([email protected]) Amy Lotven ([email protected]) Production Manager: Production Specialists: Lori Nicholson ([email protected]) Daniel Arrieta, Michelle Moodhe Inside CMS is published every Thursday by Inside Washington Publishers, P.O. Box 7167, Ben Franklin Station, Washington, DC 20044. Subscription rates: $705 per year in U.S. and Canada; $755 per year elsewhere (air mail). © Inside Washington Publishers, 2014. All rights reserved. Contents of Inside CMS are protected by U.S. copyright laws. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or translated into any language in any form or by any means, electronic or mechanical, without written permission of Inside Washington Publishers. INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 House E&C Seeks Quick Advice On FDA’s LTD Role As It Drafts Cures Bill The House Energy and Commerce Committee this week asked stakeholders to provide detailed feedback by Jan. 5 on key issues surrounding FDA’s controversial plan to regulate laboratory developed tests, including ways Congress potentially could step in to shape FDA and CMS’ regulatory roles over LDTs as part of the committee’s soon-to-be-released draft 21st Century Cures legislation. In a white paper distributed Tuesday (Dec. 9), E&C lawmakers point out that FDA issued a framework to “fundamentally alter the regulatory landscape” of LDTs just a week after the committee held a roundtable in late July looking at how advances in diagnostic testing could speed access to treatments. FDA’s plan spurred questions over the agency’s authority over such tests and whether the framework would duplicate CMS’ already existing oversight of laboratories, the white paper notes. The committee asks stakeholders to weigh in on 11 key regulatory issues — in the process signaling topics lawmakers might try to tackle as part of their medical treatment modernization initiative. Committee Chair Fred Upton (R-MI) recently said he hopes to float a draft bill for comment in January. “As the 21st Century Cures initiative proceeds, with preparations for a discussion draft early in the New Year, the committee appreciates all interested stakeholders’ specific feedback on the following questions by January 5, 2015, in addition to advice on what role Congress should play in addressing any other related issues,” the Dec. 9 white paper states. FDA, after notifying Congress in July of its plan to apply the existing device classification systems to LDTs and to prioritize regulation of “high-risk” tests, followed with formal guidance in October. The framework has since received push back from certain stakeholders, including the American Clinical Laboratory Association. The committee, noting the debate over FDA’s plan, asks for input on the following: • How should the lines be drawn separating the practice of medicine, the actual conduct of a diagnostic test and the development and manufacturing of diagnostic tests? • What should be defined as a “device” subject to FDA regulation, particularly when it comes to tests developed and performed in laboratories? • Are LDT risks different from those of distributed test kits, and is the traditional device classification system appropriate? • Should the medical device concepts of safety and effectiveness apply to both test kits and LDTs? • Would greater reliance on post market processes speed patient access to new diagnostic tests? • Should requirements for submission of a supplemental clearance or approval differ for LDTs and distributed test kits? • Should different standards for dissemination of scientific information apply to diagnostic tests and traditional medical devices; and what standards should apply to labs that develop, perform, and improve these tests? • How can duplication between CLIA and FDA be reduced, such as with the agencies’ quality systems regulations? • How should diagnostic tests for rare diseases or conditions, customized diagnostic tests and diagnostic tests needed for emergency or unmet needs (such as Ebola) be regulated? • Should all current diagnostic tests be “grandfathered” into the marketplace, and what transition process should be used? • What incentives would encourage development of more accurate or more efficient diagnostic tests? CMS vs. FDA regulation. The committee seeks feedback on how the lines between the practice of medicine, the conduct of a diagnostic test and the manufacturing of diagnostic tests should be defined; along with what should comprise a “device” under the Food, Drug and Cosmetic Act subject to FDA regulation versus regulation by CMS. “The Section 1143 guidance documents raise important questions about the relationship between the FFDCA and the Clinical Laboratory Improvement Amendments (CLIA), administered by the Centers for Medicare & Medicaid Services,” says the committee. “Is there overlap between the requirements of the guidance documents and CLIA? For instance, how do FDA’s quality systems regulations compare with CLIA quality systems requirements? Are there areas of duplication where there would be efficiencies to having either CLIA or FDA regulate, rather than both?” Definition of risk. The committee also asks how risk should be defined in the context of FDA’s risk-based approach to diagnostics and whether the types of risks posed by diagnostic tests differ from therapeutic medical devices. “Are these risks different with LDTs compared to distributed test kits? Is the traditional medical device classification system appropriate for these products?” the committee asks. Safety and effectiveness. Lawmakers question whether medical device concepts of safety and effectiveness apply to test kits and LDTs. They also ask whether the balance between premarket review and postmarket controls should be reconsidered, something FDA’s Center for Devices and Radiological Health has contemplated for medical devices broadly. Premarket submission requirements. Lawmakers want advice on when a supplemental premarket submission should be required for a modification, with the white paper stating that stakeholders were confused as to when this is INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014 17 necessary. “Should this differ between LDTs and distributed test kits?” the white paper asks. Labeling, emergency needs and development incentives. Other questions include what should comprise labeling for diagnostic test; how diagnostic tests for rare diseases, customized diagnostic tests, and diagnostic tests for emergency or unmet needs be addressed; how existing products should be handled when transitioning into the new system; and what incentives could be put in place to encourage the development of new, more accurate or more efficient diagnostic tests.— Erin Durkin Drug Compounders To Congress: Stem FDA’s Office-Use Rx Requirements A group of of pharmacy and healthcare provider organizations is pushing congressional health care committees to take legislative steps to stop FDA from requiring patient-specific prescriptions for office-use compounding, complaining the agency’s approach is barring patient access to urgently-needed antibiotics and goes against congressional intent. The letter comes after key brand and generic drug associations conversely threw their weight behind FDA’s enforcement approach last month. The International Academy of Compounding Pharmacists, PCCA (Professional Compounding Centers of America), American Medical Association, and American Pharmacists Association were among the groups to send a letter Wednesday (Dec. 10) to the committees’ chairs and ranking members, insisting FDA’s patient-specific prescription requirement for office-use compounding goes against lawmakers’ intent in the Drug Quality and Security Act (DQSA). “Congress’ multiple statements in the Senate Congressional Record show clear and overwhelming intent that compounded preparations for office-use remain available after the passage of the DQSA,” the letter states. “These numerous statements as well as strong urging from physician and pharmacy stakeholders, did not direct the agency to limit officeuse medication preparation by 503A compounders,” added the letter addressed to the Senate health and House Energy & Commerce committees. The groups ask lawmakers to weigh in legislatively “as soon as possible” to address their concerns with FDA’s policies surrounding office-use and repackaged compounded medications. DQSA set up a new category of outsourcing compounders that register with FDA and are subject to federal quality standards and other requirements. Traditional compounding pharmacies remain under state jurisdiction. But compounders worry that a recent FDA letter laying out its stance against office-use compounding will prompt states to also restrict the practice, and hope lawmakers will change the policy through clarifying legislation as soon as possible. In September, FDA answered a bipartisan House inquiry about whether the agency would allow anticipatory compounding for physicians to use in their practice without having patient-specific prescriptions. FDA affirmed that traditional compounding pharmacies must obtain patient-specific prescriptions . IACP Executive Vice President and CEO David Miller told FDA Week he hopes the group’s letter will lead lawmakers to “fix” the DQSA, either through congressional action or through pressure on FDA to return to its earlier envisioned approach for office-use compounding done by traditional compounders. The letter notes that FDA considered a different approach for office-use compounding in a draft Compliance Policy Guide. “In addition, when FDA considered changes to the Compliance Policy Guide (CPG) for human compounding several years ago, the draft CPG specifically provided for office-use,” the groups state. But the agency’s current position appears to be that patient-specific prescriptions are needed. “This position interferes with the practice of medicine and the doctor-patient relationship,” said Aaron Lopez, senior director of public affairs at PCCA. “PCCA urges Congress to address these concerns regarding patient access to officeuse compounds and repackaged medications as soon as possible, or work with FDA on a responsible regulatory solution.” The groups say access to the following drugs has declined over the past year as a result of FDA’s office-use compounding stance: • Antibiotics for urgent and emergent use in treating ophthalmology patients; • Buffered lidocaine for use in dermatology procedures; • Vascular endothelial growth factor inhibitors; • Injection therapies used to treat erectile dysfunction in urology patients; • Cantharidin to treat viral skin conditions in office by dermatologists and pediatricians; • Injectable methylcobalamin for the treatment of pernicious anemia and other vitamin B-12 deficiencies. The letter comes after the Biotechnology Industry Organization, Generic Pharmaceutical Association, Pharmaceutical Research and Manufacturers of America and Pew Charitable Trusts wrote to the agency saying they support FDA’s enforcement actions and implementation of DQSA. “In particular, FDA should not be impeded by efforts to carve out exemptions to the law that could allow compounding without prescriptions, outside of limited amounts in anticipation of a prescription, by pharmacies that do not comply with FDA regulations and do not meet appropriate quality standards,” the drug groups said. — Erin Durkin 18 INSIDE CMS — www.InsideHealthPolicy.com — December 18, 2014
© Copyright 2026